Abstract
Background
We have demonstrated a decrease in pH in the incisional wound environment, suggesting a possible contribution of low pH to postsurgical pain. In this study, we characterized the acid-responsiveness of nociceptors innervating the plantar aspect of the rat hindpaw 1 day after plantar incision and compared this to plantar skin from unincised control rats.
Methods
Using the rat glabrous in vitro skin-tibial nerve preparation, afferent nerve activities from single mechanosensitive nociceptors were recorded. Differences in mechanosensitivity, spontaneous activity and chemosensitivity of units were evaluated. For chemosensitivity, acid-responsiveness of nociceptors to lactic acid (pH 5.5 to 6.5) was studied.
Results
C-fibers showed dose-dependent, sustained responses to lactic acid. A greater proportion of C-fibers from ≤ 2 mm from the incision was activated by pH 6.0 lactic acid (52.9%) compared to control (14.3%). Total evoked potentials during acid exposure were greater in C-fibers innervating ≤ 2 mm from the incision compared to those in unincised skin. The prevalence of acid responses and total evoked potentials during acid exposure in C-fibers innervating > 2 mm from the incision were not different from control. Few A-fibers responded to lactic acid with a range of pH 5.5 to 6.5 in both incision and control groups. Increased spontaneous activity and mechanosensitivity were also evident.
Conclusions
C-fibers in the vicinity of the incision showed qualitatively and quantitatively greater chemosensitivity to pH 6.0 lactic acid compared to control. This change was localized to ≤ 2 mm from the incision, suggesting increased chemosensitivity of nociceptive C-fibers 1 day after plantar incision.
Introduction
We have developed and characterized models of incisional pain and described a variety of pain related behaviors to better understand mechanisms for postoperative pain.1,2 In a previous study, we have demonstrated that a decrease in pH occurs immediately after incision and is sustained for several days.3 The decreased pH is localized at the incision site, and pain related behaviors are evident during the period of low tissue pH. Because low pH activates and sensitizes nociceptors4 and acid injection causes pain in human volunteers5, decreased pH may contribute to nociception after incision and pain in patients after surgery. Several possible channels or receptors activated by low pH are expressed on nociceptors6–8 suggesting drugs blocking pH responses may be candidate analgesics for patients after surgery.
The pH required to produce sustained activation of nociceptors (pH 5.0 to 6.0) is much lower than the pH of incisions (pH 6.8 to 7.0). Recent studies suggest that mediators such as nerve growth factor (NGF) and lactate may enhance pH response of sensory neurons through various mechanisms.9–11 Both NGF and lactate are increased in the incisional wound environment,12,13 suggesting that they might contribute to sensitization of nociceptors to low pH.
Nociceptor sensitization is a key finding in hyperalgesic, pathologic pain states. In most studies of nociceptor sensitization, heat responsiveness is examined; more recently mechanosensitivity is generating considerable interest.14,15 However, chemosensitivity of nociceptors in pathologic states is rarely evaluated.
In this study using a rat glabrous in vitro skin-nerve preparation, we hypothesized that the acid-responsiveness of nociceptive afferents innervating the plantar aspect of the rat hindpaw 1 day after plantar incision would be greater than the responsiveness of afferents in the sham-operated rats. As a low pH stimulus, we used lactic acid based on our previous study showing that tissue lactate concentration is increased at the same time that pH is decreased and pain behaviors are obvious.3,13 This chemical stimulus may in part simulate the chemical challenge acting on nociceptors in the incisional wound environment in vivo. Using computer-controlled mechanical stimulator, quantitative mechanosensitivity of theses fibers to force-controlled stimuli was also studied.
Materials and Methods
General
All experimental procedures were approved by The University of Iowa Animal Care and Use Committee, Iowa City, Iowa. Rats were treated in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals issued by the International Association for the Study of Pain.16
Forty two adult male Sprague Dawley rats (250–300 g; Harlan, Indianapolis, IN) were used. Rats were housed in groups of two to three in clear plastic cages, with a 12-h light-dark cycle. Food and water were available ad libitum.
Plantar incision
A plantar incision similar to that described previously1 was made under 1.5–2% isoflurane anesthesia delivered via a nose cone. The surgical field was prepared in a sterile manner, and a 1.0-cm longitudinal incision was made in the plantar aspect of the right hindpaw beginning 1.0 cm from the end of heel; skin and fascia were incised. The skin was closed with 5-0 nylon sutures and topical antibiotic ointment was applied to the wound. After surgery, rats were allowed to recover in their cages. Sham-operated rats, without incision, were used as controls. The electrophysiologic recordings were performed one day after incision.
Electrophysiological studies
Preparation
The rat glabrous in vitro skin-nerve preparation, modeled as saphenous nerve-skin preparation,17 has been described elsewhere.14,18 In brief, rats were euthanized in a carbon dioxide chamber; the medial and lateral planter nerves and their innervated territory on the glabrous hindpaw skin were subcutaneously dissected until the nerve and skin could be removed. The skin was placed epidermal side down in the in vitro perfusion chamber, and superfused with modified Krebs–Henseleit solution (in mM: 110.9 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2SO4, 24.4 NaHCO3 and 20.0 glucose, pH 7.4), which was saturated with a gas mixture of 95% oxygen and 5% carbon dioxide. The temperature of the bath solution was maintained at 32 ± 0.5 °C. The plantar nerves were drawn through a small hole into the recording chamber containing a superficial layer of mineral oil and a bottom layer of modified Krebs-Henseleit solution. The nerve was desheathed on a mirror stage, and small filaments were repeatedly split with sharpened forceps to allow single fiber recording to be made using extracellular gold-wire recording electrodes. Neural activity was amplified (DAM50, Harvard Apparatus, Holliston, MA), filtered, and displayed using standard techniques. Amplified signals were led to a digital oscilloscope and an audiomonitor and fed into a personal computer via a data acquisition system (spike2/CED1401 program, Cambridge Electronic Design Ltd., Cambridge, UK).
Identification of afferents
The receptive fields of afferent units were identified by probing the dermis side of the skin with a blunt glass rod; thus, mechanosensitive afferents were recorded. Only units with a clearly distinguished signal to noise ratio (greater than 2:1) were further studied. Once the receptive field was identified, ongoing spontaneous activity was recorded over a 5-min period for each fiber before any modality testing. After recording of spontaneous activity, a standard protocol of mechanical stimulation followed by lactic acid application was performed.
Feedback-controlled mechanical stimulation
To determine quantitative mechanosensitivity, a servo force-controlled mechanical stimulator (Series 300B Dual Mode Servo System, Aurora Scientific, Aurora, Ontario, Canada)19 was used. A flat-ended cylindrical metal probe (tip diameter 0.7 mm) attached to the tip of the stimulator arm was placed just close to the most sensitive spot of the receptive field so that no force was generated. First, computer-controlled ramp-shaped force stimuli were applied at 60-s interval to measure the mechanical threshold of the nociceptors. Each force ramp started from zero to 40 and 80 mN, respectively, in 5 s. Then the ascending series of compressive loads (5–120 mN range of force) were applied to evaluate the suprathreshold mechanosensitivity. Since the neural responses of cutaneous mechanosensitive nociceptors to mechanical stimuli are more highly correlated with compressive stress (force) than compressive strain (displacement),19,20 sustained force-controlled stimuli (100 ms rise time, 1.9 s duration of sustained force plateau) were applied at 60-s intervals (see discussion).
Chemical stimulation
After mechanical stimulation, chemosensitivity was assessed using lactic acid. To restrict the chemical stimuli to the isolated receptive field, a small metal ring (5 mm internal diameter), which could seal by its own weight, was used. In some cases, inert silicone grease was added to ensure a waterproof seal.
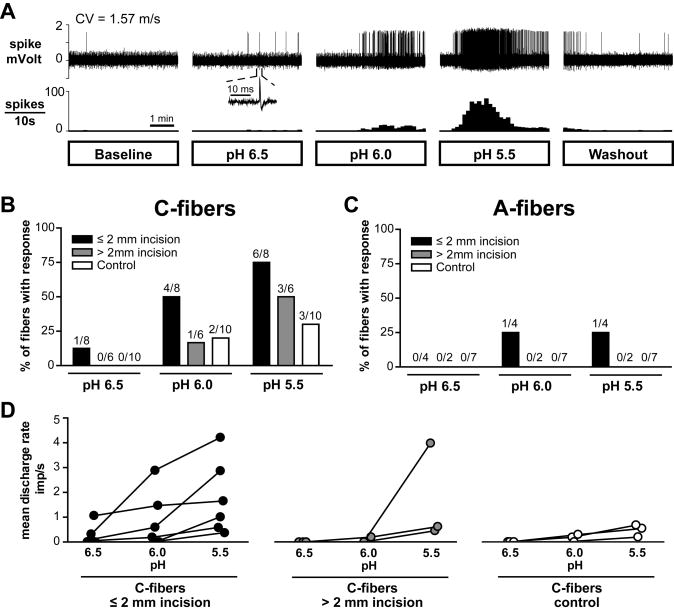
After recording baseline for 5 min, the metal ring was placed and the modified Krebs-Henseleit solution inside the ring was removed with a syringe. Then either pH 6.0 lactic acid (15 mM; 32 °C) or control solution (Krebs–Henseleit solution equilibrated with room air; pH 7.4; 32 °C) was applied to the receptive filed for 5 min, followed by 5-min washout (Fig. 1). Thirty one units (16 C- and 15 A-fibers) from incised rats and 19 units (11 C- and 8 A-fibers) from control rats were tested with pH 6.0 lactic acid. Sixteen units from incised rats and 14 units from control rats were tested with control solution. In the next 20 units (14 C- and 6 A-fibers) from incised rats and 17 units (10 C- and 7 A-fibers) from control rats, 15 mM lactic acid with increasing acidity (pH 6.5, 6.0 and 5.5; 32 °C) was sequentially tested for 5 min in each unit, to further characterize the acid-responsiveness and evaluate pH-dependencies in the response. The interval between each lactic acid application was 15 min. In a separate group of 5 acid-responsive C-fibers ≤ 2 mm from the incision, pH 6.0 lactic acid (15 mM; 32 °C) was repeatedly applied (three times) for 5 min at 5-min intervals in each unit, to evaluate the reproducibility of pH response and the potential for tachyphylaxis. To avoid sensitization/desensitization of nociceptors, fibers having receptive fields in the previously studied area were avoided for subsequent recording.
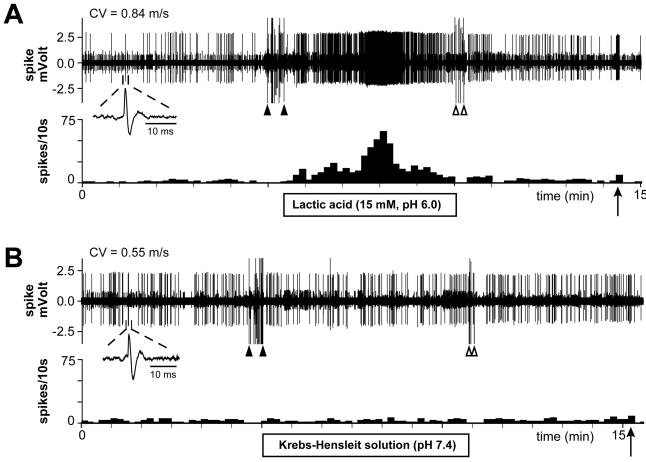
Fig. 1.
Sample recordings showing the experimental protocol used to test the acid- responsiveness of nociceptors one day after incision. Responses of two single C-fibers with receptive fields ≤ 2 mm from the incision to pH 6.0 lactic acid (A) and to control Krebs–Hensleit solution (B). The upper and lower panels in each figure show the digitized oscilloscope tracings and spike density histograms (bin width = 10 s), respectively. Insets display the action potentials of these units. Artifacts produced by placing and removal of the metal ring are marked by two black arrowheads and two white arrowheads, respectively. Black arrows indicate manual mechanical stimuli applied to the receptive fields of the unit. CV = conduction velocity.
In another group of 10 C-fibers from sham control skin, the incision was made during recording to evaluate whether the tissue disruption caused by incision affects acid sensitivity by providing better access of lactic acid to nociceptive nerve terminals. First, after recording baseline activity for 5 min, pH 6.0 lactic acid was applied to the receptive filed for 5 min and the response was measured. This was followed by 5-min washout. Then an incision was made approximately 1 mm from the receptive field. Then 10 min later, the unit was tested with pH 6.0 lactic acid again, and the responses before and after the incision were compared.
Lactic acid for chemical stimulation was made by replacing NaHCO3 (24.4 mM) normally contained in modified Krebs-Henseleit solution with L-lactic acid (Sigma, St. Louis, MO; 85% to a final concentration of 15mM). The pH of lactic acid was measured and adjusted to pH 6.0 with a few drops of 1N NaOH before application. To further increase or decease pH, additional 1N NaOH or 1N HCl was added. The final osmolarity of the lactic acid solution was 312 mOsm; the sodium concentration was 125 mM.
Conduction velocity and fiber categorization
The conduction velocity was always measured at the end of the experiment. The conduction velocity of each unit was determined by monopolar electrical stimulation (5–20 V, 0.5–2.0 ms duration, 0.2–1.0 Hz) into the most mechanosensitive site in the receptive filed. Then the distance between the receptive field and the recording electrode (conduction distance) was divided by the latency of the action potential. Afferent fibers conducting slower than 2.5 m/s were classified as C-fibers, those conducting between 2.5 and 24 m/s as Aδ-fibers, and those conducting faster than 24 m/s as Aβ-fibers.21 Units were classified as mechanosensitive nociceptors on the basis of their graded response throughout the innocuous and noxious range of mechanical force stimuli. Rapidly adapting fibers were not studied.
Data analyses
Action potentials collected on a computer were analyzed off-line with a template-matching function of Spike 2 software (Cambridge Electronic Design Ltd.). If more than one fiber was present in a recording, data were analyzed only if the spike shapes and amplitudes were different and could be easily discriminated. If a unit discharged at a rate of 0.1 imp/s or more without any intentional stimuli, it was categorized as spontaneously active. For chemical responses, unit discharges were counted in 10-s bins and total responses were averaged during baseline, during acid application and after washout. A unit was considered activated (responsive) when it discharged greater than 0.1 imp/s during chemical stimulation. If background activity was present, the unit was regarded as responsive if the activity was increased at least two standard deviations greater than the background activity during the chemical stimulation period. To count impulses generated by acid in a unit with spontaneous activity, background activity was subtracted from the evoked responses during stimulation. Responses to pH 6.0 lactic acid and control Krebs–Henseleit solution were compared in 50 units. Acid dose-response curves were generated in 37 units. To analyze responses to pH 6.0 lactic acid, units from both groups were combined. For mechanical responses, activity was counted in 1-s bins. Mechanical threshold was determined as the lowest force which elicited the first action potential in responses to ramp-shaped force. If background activity was present, threshold was determined by the lowest force which increased background activity by at least two standard deviations greater than the background average for 10 s (1-s bins). For the suprathreshold mechanosensitivity, total spikes during the 1.9 s sustained force were analyzed. Background activity was subtracted from any evoked responses; thus, assuming background activity was sustained during the stimulus period.
Statistics
Conduction velocity of afferent units was compared by unpaired t-test. A Fisher’s exact test was used to compare the percentage of acid-responsive fibers, the percentage of spontaneously discharging fibers and the percentage of fibers responding to each force level. Total evoked potentials or average discharge rates during acid application and the mechanical thresholds were compared by Kruskal-Willis followed by Dunn’s test. The pH-dependent responses to lactic acid were analyzed using linear association test and two-way ANOVA with repeated measures on one factor. The responses of C-fibers to repeated application of pH 6.0 lactic acid were analyzed using Friedman’s test. Mechanical thresholds were compared by one-way ANOVA followed by Scheffé post hoc test, and the stimulus-response relationship for the mechanical responses was compared using two-way ANOVA with repeated measures on one factor; significant main effects of incision group or interactions were followed by separate one-way ANOVAs and Scheffé post hoc test at each force level. Data are presented as mean ± SE or median [range]. Statistical analysis was performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
General properties of afferents
A total of 132 mechanosensitive nociceptors were studied. Seventy-two fibers (47 C-, 24 Aδ- and 1 Aβ-fibers) were studied from 25 incised rats; 60 fibers (39 C- and 21 Aδ-fibers) were studied from 22 unincised, sham control rats. There was no difference in the conduction velocity of afferents between the incised and the unincised groups (C-fibers, 1.15 ± 0.08 m/s vs. 1.11 ± 0.11 m/s; Aδ-fibers, 5.16 ± 0.61 m/s vs. 5.66 ± 0.81 m/s). The conduction velocity of one Aβ-nociceptor identified from an incised rat was 25.6 m/s.
The receptive fields of all fibers were located in glabrous hindpaw skin. For analyses, we subdivided those having receptive fields ≤ 2 mm and those having receptive fields > 2 mm from the incision. This was based on our previous study, which showed that most C-fibers sensitized by heat were localized to ≤ 2 mm from the incision, in vitro, 1 day after plantar incision.14 Of 47 C-fibers and 25 A-fibers (Aδ- and Aβ-fibers) recorded from incised rats, 30 C- and 13 A-fibers had receptive fields ≤ 2 mm from the incision.
Response to chemical stimulation
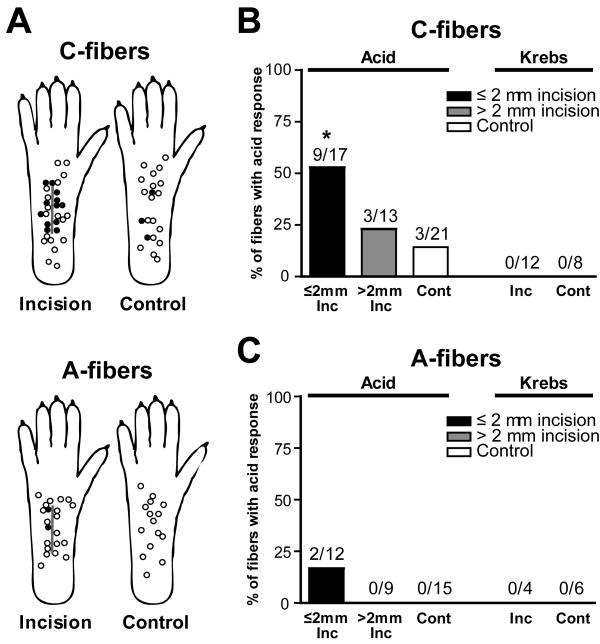
Chemical responses to pH 6.0 lactic acid were evaluated in 17 C-fibers ≤ 2 mm from the incision, 13 C-fibers > 2 mm from the incision and 21 sham control C-fibers. An example of an acid-responsive C-fiber with the receptive filed ≤ 2 mm from the incision is shown in Fig. 1A. A greater proportion of C-fibers ≤ 2 mm from the incision was responsive (52.9%, 9 of 17; P < 0.05 by Fisher’s exact test; Fig. 2A and B) compared to sham control (14.3%, 3 of 21). The prevalence of responsive C-fibers innervating > 2 mm from the incision was not different from sham control (23.1%, 3 of 13; Fig. 2B). For A-fibers, 12 fibers ≤ 2 mm from the incision, 9 fibers > 2 mm from the incision and 15 sham control fibers were tested for their responsiveness to pH 6.0 lactic acid. Few A-fibers responded to pH 6.0 lactic acid: 2 of 12 fibers (16.7%) innervating ≤ 2 mm from the incision were activated; no unit innervating > 2mm from the incision or sham control paw was responsive (Fig. 2A and C). In a separate group of 16 units (12 C- and 4 A-fibers) from incised skin and 14 units (8 C- and 6 A-fibers) from sham control skin, responses to pH 7.4 Krebs–Henseleit solution were tested, and none were excited. (Fig. 1B and Fig. 2B and C). Among 12 C-fibers from incised skin tested with pH 7.4 control solution, 8 fibers were from ≤ 2 mm from the incision.
Fig. 2.
Location and percentage of nociceptors responsive to pH 6.0 lactic acid one day after incision. (A): Distribution of the receptive fields of C- and A-fibers with or without responsiveness to pH 6.0 lactic acid, for incised and sham control rats. Each circle represents the center of a unit’s mechanoreceptive field. Solid and open circles represent receptive fields of afferents with and without acid-responsiveness, respectively. (B, C): Percentage occurrence of acid-responsive units in C-fibers (B) and A-fibers (C) when tested with pH 6.0 lactic acid (* P < 0.05 vs. sham control, Fisher’s exact test). No units were excited by control Krebs–Hensleit solution. Inc = incision; Cont = control.
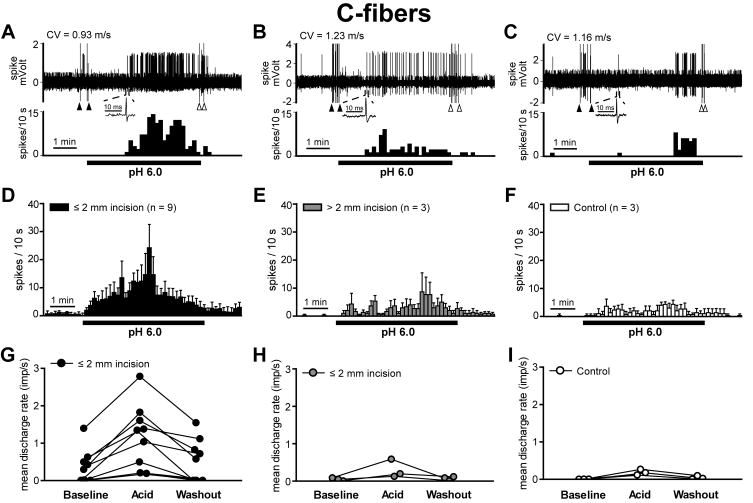
Since the majority of units activated by lactic acid were C-fibers, responses of theses units to pH 6.0 lactic acid were further quantified by counting activity in 10-s bins and by averaging responses during baseline, acid application and washout period (Fig. 3). Fig. 3A–C shows the sample recording trace of a responsive unit during lactic acid application for incision ≤2mm, incision >2mm and sham control. The mean spike density histograms during 5-min acid exposure are shown in Fig. 3D–F. Units discharged in a sustained manner throughout the acid application period. Total evoked potentials during acid exposure were greater in C-fibers innervating ≤ 2 mm from the incision (Fig. 3D; median 179 [61–514] imp; P < 0.05 by the Kruskal-Willis followed by Dunn’s test) compared to those of sham control (Fig. 3F; median 50 [34–78] imp). Total evoked potentials of C-fibers having receptive fields > 2mm from the incision (Fig. 3E; median 57 [35–174] imp) were not different from sham control. The individual magnitude of C-fiber responses is shown in Fig. 3G–I. In C-fibers having receptive fields ≤ 2 mm from the incision (Fig. 3G), the median rate during acid application (median 1.34 [0.20–2.78] imp/s) was greater than that of sham control C-fibers (Fig. 3I; median 0.17 [0.11–0.26] imp/s; P < 0.05 by the Kruskal-Willis followed by Dunn’s test).
Fig. 3.
Summary of the magnitude of the C-fiber response to pH 6.0 lactic acid. (A–C): Sample recordings from three single C-fibers innervating ≤ 2 mm from the incision (A), > 2 mm from the incision (B) and sham control skin (C). The upper and lower panels show the digitized oscilloscope tracings and spike density histograms (bin width = 10 s), respectively. Insets display the action potentials of these units. Artifacts produced by placing and removal of the metal ring are marked by two black arrowheads and two white arrowheads, respectively. CV = conduction velocity. (D–F): Mean spike density histograms of C-fibers innervating ≤ 2 mm from the incision (D, n = 9), > 2 mm from the incision (E, n = 3) and sham control skin (F, n =3). If the unit was spontaneously active before acid exposure, background activity was subtracted from the raw response data for each bin (bin width = 10 s). (G–I): Mean discharge rate of each acid-responsive C-fiber ≤ 2 mm from the incision (G), > 2 mm from the incision (H) and sham control (I) is shown. Five min periods before, during and after lactic acid application were used to calculate the mean discharge rate. Imp = impulse.
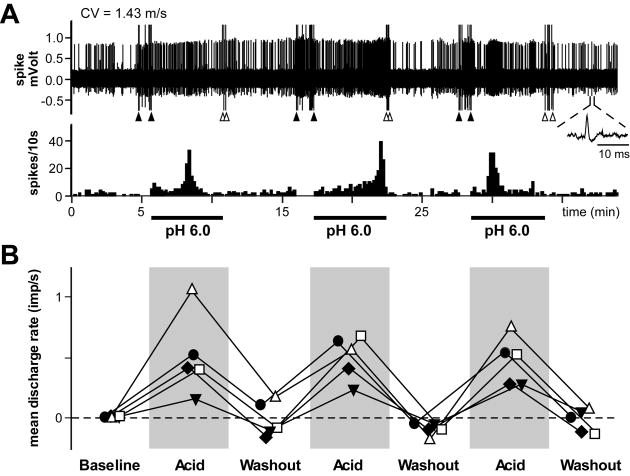
In a separate group of 5 acid-responsive C-fibers ≤ 2 mm from the incision, pH 6.0 lactic acid was repeatedly applied (three times) for 5 min at 5-min intervals (Fig. 4). The magnitude of responses of C-fibers to repeated pH 6.0 lactic acid was reproducible, and no tachyphylaxis was noted (Fig. 4B); the mean discharge rates during repeated application of pH 6.0 lactic acid were not significantly different.
Fig. 4.
Repeated application of pH 6.0 lactic acid to acid-responsive C-fibers ≤ 2 mm from the incision. (A): Sample recording from one single C-fiber. After a 5-min baseline, pH 6.0 lactic acid (15 mM; 32 °C) was repeatedly applied (three times) for 5 min at 5-min intervals, followed by 5-min washout. The upper and lower panels show the digitized oscilloscope tracings and spike density histograms (bin width = 10 s), respectively. Inset displays the action potential of this unit. CV = conduction velocity. (B): Mean discharge rate of each acid-responsive C-fiber ≤ 2 mm from the incision during repeated application of pH 6.0 lactic acid. The solid circles represent data from fiber in (A). Five min periods of baseline, acid application and washout were used to calculate the mean discharge rate. All five units were spontaneously active before acid exposure, and background activity was subtracted from the response data afterwards. Imp = impulse.
Chemical responses of nociceptors to lactic acid of three different pH levels are summarized in Fig. 5. Sample recordings of an acid-responsive C-afferent from an incised rat during the application of 15 mM lactic acid with different pH (pH 6.5, 6.0 and 5.5) are shown in Fig. 5A. C-fibers showed pH-dependent responses to lactic acid: greater acidity activated more C-fibers (P < 0.05, linear by linear association test; Fig. 5B), and generated greater discharge rates during acid application (P < 0.05, two-way ANOVA with repeated measures on one factor; Fig. 5D). Few A-fibers responded to lactic acid with a range of pH 6.5 to 5.5 in both incision and sham control groups (Fig. 5C).
Fig. 5.
Summary of chemical responses of nociceptors to lactic acid of three different pH levels. (A): Sample recordings from one single C-fiber innervating ≤ 2 mm from the incision. After a 5-min baseline, 15 mM lactic acid with pH 6.5, 6.0 and 5.5 was sequentially applied for 5 min, followed by 5-min washout. The interval between each acid application was 15 min. The upper and lower panels show the digitized oscilloscope tracings and spike density histograms (bin width = 10 s), respectively. Inset displays the action potential of this unit. CV = conduction velocity. (B, C): Prevalence of acid-responsive units in C-fibers (B) and A-fibers (C). (D): Mean discharge rate of each acid-responsive C-fiber during application of 15 mM lactic acid with three different pH levels. Imp = impulse.
In another group of 10 C-fibers from sham control skin, 9 were not activated by pH 6.0 lactic acid before the incision, and these 9 units remained unresponsive after the incision. The prevalences of responsive units both before and after the incision (10.0%, 1 of 10) were not different from sham control (23.1%, 3 of 13). One of 10 fibers was activated by pH 6.0 lactic acid before the incision, and the discharge rates during acid application before and after the incision were the same (0.13 imp/s and 0.11 imp/s, respectively). Therefore, acid responsiveness did not change immediately after incision.
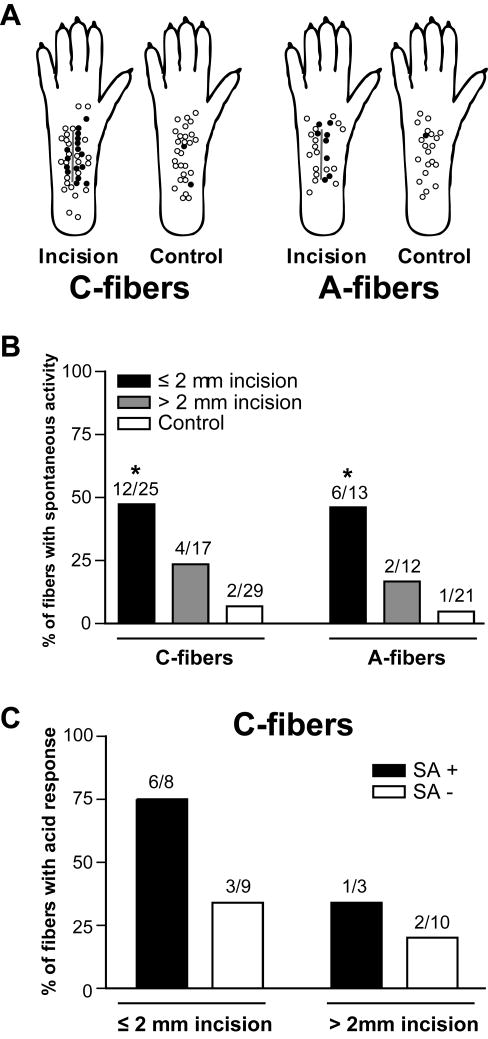
Spontaneous discharge
The distribution of receptive fields of C- and A-nociceptors with or without spontaneous activity is shown in Fig. 6A. The prevalence of spontaneously discharging C-fibers (48.0%, 12 of 25) and A-fibers (46.2%, 6 of 13) innervating ≤ 2 mm from the incision was greater than that of sham control C-fibers (6.9%, 2 of 29; P < 0.01 by Fisher’s exact test) and A-fibers (4.8%, 1 of 21; P < 0.01 by Fisher’s exact test), respectively (Fig. 6B). C- and A-fibers from > 2mm from the incision showed no difference in the prevalence of spontaneous activity compared to sham control. Among C-fibers innervating ≤ 2 mm from the incision, 6 of 8 (75%) spontaneously discharging units were activated by pH 6.0 lactic acid vs. 3 of 9 (33.3%) units without spontaneous discharge (Fig. 6C). This difference was not significant. The relationship between spontaneous activity and acid sensitivity was not analyzed in A-fibers, because few A-fibers were responsive to pH 6.0 lactic acid.
Fig. 6.
Spontaneous discharge of mechanosensitive nociceptors one day after plantar incision. (A): Distribution of the receptive fields of C- and A-fibers with or without spontaneous activity. Solid circles represent receptive fields of afferent units with spontaneous activity, and open circles represent those without spontaneous activity. (B): Prevalence of spontaneous discharge in C- and A-fibers (* P < 0.01 vs. sham control, Fisher’s exact test). (C): Relationship between spontaneous activity and acid sensitivity in C-fibers from incised rat. SA = spontaneous activity.
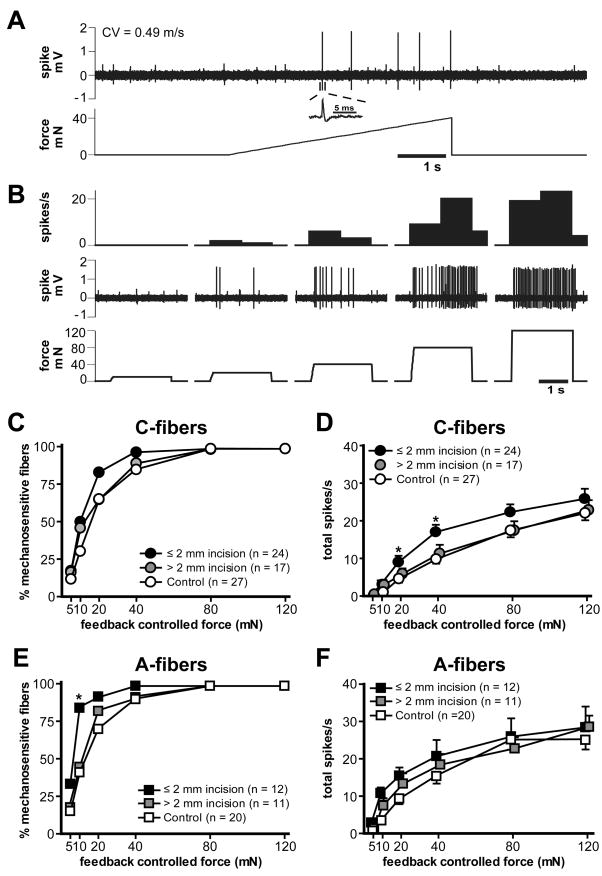
Response to mechanical stimulation
The example traces in Fig. 7A and B show the responses of a single C-nociceptor to the computer-controlled mechanical stimuli. A ramp-shaped force stimulus was used to determine mechanical threshold. (Fig. 7A), and then the ascending series of sustained force stimuli was applied to evaluate the suprathreshold mechanosensitivity (Fig. 7B). A summary of mechanical responses of C- and A-fibers 1 day after incision is shown in Fig. 7C–F. Mean response threshold of C-fibers innervating ≤ 2 and > 2 mm from the incision was 14.2 ± 2.4 mN and 20.7 ± 4.7 mN, respectively. The mean mechanical threshold of sham control C-fibers was 23.1 ± 3.8 mN. There was no difference in mechanical threshold among the groups. The force-response curve of C-fibers showed increases in responses to greater mechanical forces (P < 0.001 by two-way ANOVA with repeated measures on one factor; Fig. 7D). When compared with sham-operated control, C-fibers which had receptive fields ≤ 2 mm from the incision showed greater responses to 20 and 40 mN stimuli (P < 0.05 by one-way ANOVA followed by Scheffé post hoc test; Fig. 7D). The mean threshold of A-fibers innervating ≤ 2 and > 2 mm from the incision was 9.6 ± 3.4 mN and 15.4 ± 6.5 mN, respectively. The mean threshold of control A-fibers was 16.5 ± 3.5 mN. There was no difference in the mean mechanical thresholds of A-fibers among the groups. When the percentage of mechanosensitive fibers was compared at each force stimulus level, a greater percentage of A-fibers innervating ≤ 2 mm from the incision responded to 10 mN stimulus (83.3 %, 10 of 12; P < 0.05 by Fisher’s exact test; Fig. 7E) compared to control (40%, 8 of 20). A-fibers showed increases in responses to greater mechanical forces (P < 0.001 by two-way ANOVA with repeated measures on one factor), but there was no difference in force-response curves among three groups (Fig. 7F).
Fig. 7.
Mechanical responses of afferent units one day after plantar incision. (A): Sample recordings of the mechanical responses of a control C-fiber to the ramp-shaped force stimulus. The upper and lower panels show the digitized oscilloscope tracings and the force stimulus applied, respectively. The mechanical threshold of this unit was 16.4 mN. Inset displays the action potential of this unit. CV = conduction velocity. (B): Responses of the same unit to the ascending series of sustained force stimuli, showing greater discharge response to higher force stimuli. The upper, middle and lower panels show the spike density histograms (bin width = 1 s), raw spike discharges and the force stimuli applied, respectively. (C, D): The percentage mechanosensitivity (C) and stimulus-response function (D) of C-fibers in sham control (n = 27), ≤ 2 mm from the incision (n = 24) and > 2 mm from the incision (n = 17) (* P < 0.05 vs. sham control, one-way ANOVA followed by Scheffé post hoc test). (E, F): The percentage of mechanosensitivity (E) and stimulus-response function (F) of A-fibers in control (n = 20), ≤ 2 mm from the incision (n = 12) and > 2 mm from the incision (n = 11) (* P < 0.05 vs. control, Fisher’s exact test).
Discussion
The major finding of the present study is that, 1 day after plantar incision, a greater proportion of C-fibers are activated by pH 6.0 lactic acid in vitro, and total evoked potentials during lactic acid exposure are greater in C-fibers ≤ 2 mm from the incision compared to sham control. These data are the first to demonstrate the chemical sensitization of C-nociceptors after incision. Consistent with previous study,14 more nociceptors have spontaneous activity in incised skin. Our data also suggest evidence for mechanical sensitization of C- and A-nociceptors ≤ 2 mm from the incision when tested with force-controlled mechanical stimuli.
In the present study, 15 mM lactic acid with pH 6.5, 6.0 and 5.5 was used to assess the chemosensitivity of skin nociceptors in vitro. Previously, we have demonstrated that incision of the plantar hindpaw, the gastrocnemius muscle, and the paraspinal region increases the tissue lactate concentration at the same time that pH is decreases and pain behaviors are increased.3,13 These results suggested that decreases in pH and increases in lactate together could contribute to pain caused by incisions. Our current data showing increased chemosensitivity of C-nociceptors to lactic acid after incision further supports the possibility that cofactors such as lactate or others might facilitate nociceptor activation by low pH and contribute to postsurgical pain.
Lactate was shown to enhance the response of acid sensing ion channel 3 (ASIC-3) to low pH in vitro by the mechanism of decreasing of divalent ions in the extracellular media.11 ASIC-3 is expressed on nociceptors and is a candidate to mediate acid-induced nociception,22, 23 in addition to transient receptor potential vanilloid receptor 1 (TRPV1).24 In isolated sensory neurons and ASIC-3 expressing cells, 15 mM lactate produced more than a 70% increase in current evoked by a reduction in pH to 7.0. In the same system, when applied at pH 8.0 or 7.4, lactate produced no depolarization and no current.11 Likewise, although we did not test the response of nociceptors to 15 mM lactate at neutral pH separately in the present study, it is unlikely that the activation of nociceptors during lactic acid stimulation is through direct activation by lactate itself. The relationship between lactate and pH response in the in vitro skin-nerve preparation warrants future study: the contributions of proton and lactate to the activation of nociceptors by lactic acid need to will be further evaluated.
Consistent with a previous study,4 few A-fibers responded to lactic acid with a range of pH 6.5 to 5.5 in both incisions and sham control groups, and majority of nociceptors responded to lactic acid in a pH-dependent manner were C-fibers. Acid-responsive C-nociceptors showed sustained and reproducible excitation throughout the acid application period, in agreement with previous studies.4,25 Among 21 C-fibers from control skin, 3 units (14.3%) were responsive to pH 6.0 lactic acid; this is somewhat lower than the prevalence of responsive C-units from previous studies.4,25 In the saphenous in vitro skin-nerve preparation of the rat, 27.5–46.8% of C-units were responsive to the carbon dioxide-saturated synthetic interstitial fluid (pH 6.1). The difference among studies in the criteria defining responsiveness of nociceptor to stimulant solutions could have contributed to this discrepancy. Compared to the present study which used minimum discharge rate of 0.1 imp/s (30 spikes/5 min) as a response criterion, previous studies either did not follow any arbitrary response criterion of a minimum increase in discharge rate25 or used lower criterion value.4 Also, the difference in the composition of stimulant solution could make difference in the chemical response of units. For example, when exposed to C-fibers, carbon dioxide-saturated synthetic interstitial fluid produced significantly shorter latencies and somewhat greater mean responses than phosphoric acid of the same pH 6.1.4
In the present study, chemical sensitization was localized to the C-fibers in the vicinity of the incision. C-fibers ≤ 2 mm from the incision showed qualitatively and quantitatively greater responses to pH 6.0 lactic acid compared to sham control or units > 2 mm from the incision. In our previous study, we have also shown that heat sensitization of C-fibers are localized to ≤ 2 mm from the incision.14 These data suggest the possible contribution of the wound environment and the released mediators to the incision-induced peripheral sensitization of nociceptors. One example of these mediators is NGF, which was shown to be increased in skin after incision.26 NGF immunoreactivity was found adjacent to the incision, and when examined using Western blot on postoperative day 2, the increase in NGF was in the area immediately surrounding the incision.12 The sensitization of TRPV1 by NGF was shown in vitro.9 In the experiment using isolated sensory neurons and TrkA-and TRPV1-expressing cells, activation of TRPV1 by protons was potentiated by NGF through the mechanism of promoting the trafficking of the TRPV1 to the surface membrane. TRPV1 is a non-selective cation channel gated by capsaicin, noxious heat and protons,7 and the sensitization of TRPV1 by NGF could also be possible mechanism of heat sensitization of C-fibers ≤ 2 mm from the incision.14,27 NGF was also shown to be a key element for both the basal expression and the transcriptional regulation of the ASIC-3 encoding genes.10,28 An increase in NGF level enhanced ASIC-3 encoding gene expression, causing an increase in ASIC current amplitude in sensory neurons and an increase in the number of ASIC-expressing neurons. Another example of mediators that might contribute to the chemical sensitization of nociceptors is prostaglandin E2. Prostaglandin E2 was shown to be increased on 1 and 3 days after skin wounding in mice,29 and to sensitize TRPV1 responses through EP1 receptors in TRPV1-expressing cells and mouse sensory neurons.30 Decreased pH in the wound environment could also be related to the increased responsiveness of nociceptors to lactic acid. It was demonstrated that acid itself (pH 6.5–6.7) sensitized TRPV1 to more acidic solutions in vitro, in a study using TRPV1-expressing HEK293 cells.31 The slope of the acid concentration-effect curve was greater and EC50 for acid activation was smaller in cells pre-incubated at pH 6.7 compared to those pre-incubated at pH 7.4. Other inflammatory mediators released after tissue damage caused by incision could also contribute to the peripheral chemical sensitization of nociceptors. Facilitation of pH response by inflammatory soup (composed of bradykinin, serotonin, histamine and prostaglandin) was shown in the rat skin and dorsal root ganglion cells.25,32 The competitive TRPV1 antagonist capsazepine was found to abolish the inflammatory facilitation of the sustained pH response in dorsal root ganglion cells.33 However, unlike the findings in cultured dorsal root ganglion cells, the augmentation of the nociceptive pH response by inflammatory soup was not blocked by capsazepine in the rat skin.34 This findings suggested that the potentiation of the pH response by inflammatory soup may be mediated though different mechanisms in nociceptive terminals compared to dorsal root ganglion cells. To better understand the underlying mechanism of the chemical sensitization of nociceptors observed in the present study, the possible contribution and interaction of other mediators with lactic acid needs to be further explored.
In the present study, when tested with force-controlled mechanical stimuli, C- and A-fibers in the vicinity of the incision showed modest evidence for mechanical sensitization in vitro, 1 day after plantar incision. This result agrees with our previous study using in vitro mouse glabrous skin-tibial nerve preparation which revealed mechanical sensitization of nociceptors 1 day after plantar incision; the responses to suprathreshold mechanical stimulation were increased in low threshold Aδ- and C-fibers.15 On the other hand, we did not identify sensitization of C-fibers after incision in our previous study which used length-controlled mechanical stimuli.14 The discrepancies between these results seem to be in part related to the differences in stimulus patterns. Previously, it has been shown that the neural responses of nociceptors to compressive mechanical stimuli are more highly correlated with stress than displacement.19,35 In the present study, with our recent modification of mechanical stimulus pattern, neuronal responses during constant, sustained compressive stress were able to be characterized by applying constant force.
In conclusion, this study indicates chemical sensitization of C-fibers in vitro, 1 day after plantar incision. C-fibers in the vicinity of the incision showed qualitatively and quantitatively greater responses to pH 6.0 lactic acid compared to control. Previously, we have demonstrated increased lactate and decreased pH in the incisional wound environment, and increased chemosensitivity of nociceptors to lactic acid after incision support the possibility that lactate, as a cofactor, may facilitate nociceptor activation by low pH and contribute to postsurgical pain. C- and A-nociceptors close to the incision also showed spontaneous discharge and mechanical sensitization, in vitro.
Acknowledgments
This work was supported by the Department of Anesthesia at the University of Iowa and by National Institutes of Health, Bethesda, Maryland grants GM-55831 to T.J.B.
Footnotes
Presented, in part, at Neuroscience 2008 in Washington, D.C., November 16, 2008
References
- 1.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 2.Pogatzki EM, Niemeier JS, Brennan TJ. Persistent secondary hyperalgesia after gastrocnemius incision in the rat. Eur J Pain. 2002;6:295–305. doi: 10.1053/eujp.2002.0339. [DOI] [PubMed] [Google Scholar]
- 3.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–75. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 4.Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993;154:113–6. doi: 10.1016/0304-3940(93)90184-m. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 7.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–8. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24:4211–23. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–70. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–70. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 12.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–35. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 13.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. J Pain. 2007;8:59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain. 2004;112:204–13. doi: 10.1016/j.pain.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. Pain. 2008;138:380–91. doi: 10.1016/j.pain.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 17.Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett. 1986;66:141–6. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- 18.Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89:187–98. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 19.Khalsa PS, LaMotte RH, Grigg P. Tensile and compressive responses of nociceptors in rat hairy skin. J Neurophysiol. 1997;78:492–505. doi: 10.1152/jn.1997.78.1.492. [DOI] [PubMed] [Google Scholar]
- 20.Khalsa PS, Zhang C, Qin YX. Encoding of location and intensity of noxious indentation into rat skin by spatial populations of cutaneous mechano-nociceptors. J Neurophysiol. 2000;83:3049–61. doi: 10.1152/jn.2000.83.5.3049. [DOI] [PubMed] [Google Scholar]
- 21.Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–99. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- 22.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–83. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 23.Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24:10974–9. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 25.Steen KH, Steen AE, Reeh PW. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J Neurosci. 1995;15:3982–9. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a transient receptor potential type V1 receptor antagonist, and morphine on pain behavior after plantar incision. Anesthesiology. 2008;108:1100–8. doi: 10.1097/ALN.0b013e31817302b3. [DOI] [PubMed] [Google Scholar]
- 28.Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2003;278:48907–13. doi: 10.1074/jbc.M309468200. [DOI] [PubMed] [Google Scholar]
- 29.Kapoor M, Kojima F, Yang L, Crofford LJ. Sequential induction of pro-and anti-inflammatory prostaglandins and peroxisome proliferators-activated receptor-gamma during normal wound healing: a time course study. Prostaglandins Leukot Essent Fatty Acids. 2007;76:103–12. doi: 10.1016/j.plefa.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bianchi BR, Lee CH, Jarvis MF, El Kouhen R, Moreland RB, Faltynek CR, Puttfarcken PS. Modulation of human TRPV1 receptor activity by extracellular protons and host cell expression system. Eur J Pharmacol. 2006;537:20–30. doi: 10.1016/j.ejphar.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Kress M, Reeh PW, Vyklicky L. An interaction of inflammatory mediators and protons in small diameter dorsal root ganglion neurons of the rat. Neurosci Lett. 1997;224:37–40. doi: 10.1016/s0304-3940(97)13450-4. [DOI] [PubMed] [Google Scholar]
- 33.Vyklicky L, Knotkova-Urbancova H, Vitaskova Z, Vlachova V, Kress M, Reeh PW. Inflammatory mediators at acidic pH activate capsaicin receptors in cultured sensory neurons from newborn rats. J Neurophysiol. 1998;79:670–6. doi: 10.1152/jn.1998.79.2.670. [DOI] [PubMed] [Google Scholar]
- 34.Habelt C, Kessler F, Distler C, Kress M, Reeh PW. Interactions of inflammatory mediators and low pH not influenced by capsazepine in rat cutaneous nociceptors. Neuroreport. 2000;11:973–6. doi: 10.1097/00001756-200004070-00015. [DOI] [PubMed] [Google Scholar]
- 35.Ge W, Khalsa PS. Encoding of compressive stress during indentation by group III and IV muscle mechano-nociceptors in rat gracilis muscle. J Neurophysiol. 2003;89:785–92. doi: 10.1152/jn.00624.2002. [DOI] [PubMed] [Google Scholar]