Summary
The pathogenesis of immune-mediated drug-induced liver injury (DILI) following halogenated anesthetics, carbamazepine, or alcohol has not been fully elucidated. Detecting cytochrome P4502E1 (CYP2E1) IgG4 autoantibodies in anesthetic DILI patients suggests a role for interleukin IL-4 in this hapten-mediated process. We investigated IL-4-mediated mechanisms using our model of experimental DILI induced by immunizing BALB/c (WT) and IL-4−/− (KO) mice with S100 liver proteins covalently modified by a trifluoroacetyl chloride (TFA) hapten formed following halogenated anesthetic metabolism by CYP2E1. WT mice developed more hepatitis, TFA and S100 antibodies (p<0.01), as well as T cell proliferation to CYP2E1 and TFA (p<0.01) than KO mice. Additionally, WT CD4+T cells adoptively transferred hepatitis to naïve Rag−/− mice (p<0.01). Pro-inflammatory cytokines were expectedly decreased in TFA hapten-stimulated KO splenocyte supernatants (p<0.001); however, IL-2 and interferon-γ (p<0.05), as well as IL-6 and IL-10 (p<0.001) levels were elevated in CYP2E1-stimulated KO splenocyte supernatants, suggesting dual IL-4-mediated proinflammatory and regulatory responses. Anti-IL-10 administered to KO mice increased hepatitis, TFA and CYP2E1 antibodies in KO mice confirming a critical role for IL-4. This is the first demonstration of dual roles for IL-4 in the pathogenesis of immune-mediated DILI by suppressing autoantigen-induced regulatory responses while promoting hapten-induced pro-inflammatory responses.
Keywords: CYP2E1, trifluoroacetyl chloride, TFA, DILI, idiosyncratic acute liver failure, IL-4
Introduction
Drug-induced liver injury (DILI) from idiosyncratic drug reactions account for approximately 13% of acute liver failure cases in the United States [1–3] representing the third largest cause of acute liver failure. Acute liver failure from acetaminophen or idiosyncratic drug reactions requires liver transplantation in 28% of patients and results in death in 50% of cases [2;3]. Evidence suggests that a type of DILI that follows the administration of halogenated volatile anesthetics, tienilic acid, dihydralizine, carbamazepine or alcohol is immune-mediated [4;5]. However, precise pathogenic mechanisms responsible for the initiation or subsequent development of hepatitis have not been fully elucidated.
Halogenated volatile anesthetics such as isoflurane, desflurane or halothane trigger immune responses to complex antigens formed from native hepatic proteins such as cytochrome P450 2E1 (CYP2E1) [6;7] that have become covalently modified by a trifluoroacetyl chloride (TFA) hapten. This hapten is formed during the oxidative metabolism of the halogenated anesthetic by CYP2E1 [8–10]. Immune responses to these complex antigens occurring in secondary lymphoid organs generate cytokines that promote the development of inflammation in the liver [11]. Additionally, TFA – haptenated native liver proteins induce both TFA antibodies, as well as autoantibodies to CYP2E1 [6;12–14].
CYP2E1 autoantibodies of the IgG4 subclass produced in the sera of persons with halogenated volatile anesthetic DILI form circulating, non-precipitating immune complexes that are believed to directly damage hepatocytes in these persons [15;16]. Detecting CYP2E1 autoantibodies of the IgG4 subclass strongly suggests a role for IL-4 in the development of these autoantibodies [17] and suggests that IL-4 promotes the development of immune-mediated DILI. Landmark studies clearly show that IL-4 and CD4+T cells can induce B cell proliferation and isotype switching from IgM to IgG4 and IgE [17;18], which promotes the development of allergic and autoimmune diseases [19;20]. However, the mechanism of how IL-4 or any of the other cytokines initiates or regulates immune responses to autoantigens or neoantigens formed by drug haptens is not known. Additionally, while the formation neoantigens has been strongly implicated in the initiation of immune-mediated DILI [6;10–12;14;21], precise mechanisms for these processes have not been explored.
We have previously described a novel model of the initiation of experimental immune-mediated DILI induced by immunizing BALB/c mice with S100 proteins covalently modified with TFA haptens [11;22]. Hepatitis is evident at 3 weeks while serum transaminase elevation occurs by 12 weeks. The hepatic inflammatory infiltrate consists of neutrophils, eosinophils, T and B lymphocytes as well as NK and NKT cells [11].
Using this model of immune-mediated DILI, we have investigated the role of the CYP2E1 autoantigen [6;12;14], the TFA hapten [8–10], and IL-4 in the initiation of immune-mediated DILI by using susceptible BALB/c (WT) and IL-4 deficient (KO) mice. We verified that initiation of immune responses to CYP2E1 and TFA subsequently promote the development of anesthetic DILI by adoptively transferring hepatitis to female naïve Rag−/− mice. We have found critical roles for CD4+T cell reactions to key antigens CYP2E1 and TFA in the initiation of anesthetic DILI by suppressing regulatory cytokine responses to the CYP2E1 autoantigen and by inducing proinflammatory cytokine responses to TFA haptenated proteins. This is the first demonstration of dual roles for IL-4 in the pathogenesis of autoimmune phenomena in immune-mediated DILI using a single animal model.
Results
Splenocytes from BALB/c (WT) proliferate in response to CYP2E1 and TFA
Previous studies demonstrated CYP2E1 autoantibodies in anesthetic DILI patients, identifying CYP2E1 as the primary autoantigen in anesthetic DILI [6;12]. Additional studies detected TFA antibodies in anesthetic DILI patients and strongly suggested that TFA was the primary hapten responsible for covalent modification of native proteins that trigger immune responses resulting in DILI [8–10]. However, the lack of an animal model of immune – mediated, anesthetic DILI hampered studies investigating roles for CYP2E1 or TFA in the pathogenesis of anesthetic DILI. Hence, we first investigated the roles of CYP2E1 and TFA in the initiation phase of our model of immune – mediated, anesthetic DILI [11].
We measured proliferation to CYP2E1 and TFA using mixed splenocyte cultures that were collected from WT mice two weeks following TFA-S100 immunizations (TFA-S100/CFA) and then re-stimulated with CYP2E1 or with TFA coupled to a non-self protein keyhole limpet hemocyanin (KLH-TFA) for 72h. We found that proliferation to CYP2E1 and KLH-TFA was significantly higher in splenocytes from TFA-S100 – immunized mice stimulated with 25 or 15µg/ml of antigen (p<0.05, Figure 1A and B) but not 10 or 5µg/ml when compared to CFA – immunized mice. Splenocytes from WT mice did not demonstrate responses to KLH without the TFA hapten (results not shown).
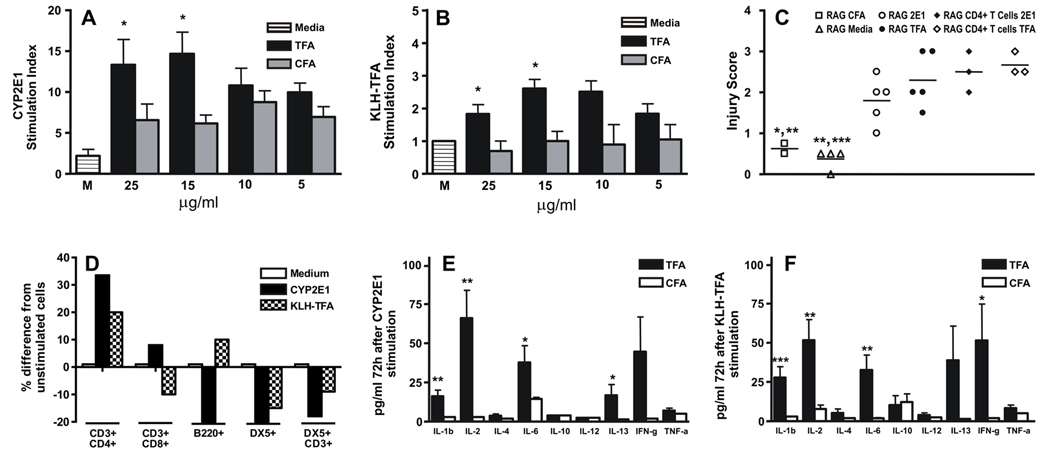
Figure 1.
Immune responses in WT splenocytes have a key role in experimental DILI. Stimulation indices were elevated in splenocyte cultures re-stimulated with the indicated concentrations of either (A) CYP2E1 or (B) KLH-TFA after isolation from TFA-S100 when compared to CFA-immunized WT mice (*p<0.05, Mann-Whitney U. Mean ± SEM of pooled data from individual mice. Experiments were performed in triplicate with n = 4 mice/group). (C) Hepatitis was evident in naïve female Rag −/− mice 10 days after receiving, by adoptive transfer, 1 × 107 TFA-S100-immunized WT mixed splenocytes or CD4+ T cells re-stimulated with CYP2E1 (RAG 2E1 or RAG CD4+ T cells 2E1) or with TFA (RAG TFA or RAG CD4+ T cells TFA) when compared to RAG CFA or RAG Media (*p<0.05, **p<0.01, Mann-Whitney U. Experiments were performed in triplicate with n = 2 mice/group). (D) There were 30 and 20% increases in CD3+CD4+ T cell numbers, 8% increases and decreases in CD3+CD8+ T cell numbers, 20% decreases and 10% increases in B cell numbers, 24 % and 15% decreases in DX5+ NK cell numbers and 18 and 9 % decreases in DX5+CD3+ NKT cell numbers following stimulation with CYP2E1 and TFA, respectively. Shown are mean of pooled data from individual mice. Experiments were performed in duplicate with N = 4 mice/group. (E, F) Cytokine levels in splenocyte supernatants by ELISA prior to transfer, showed significantly elevated IL-1β, IL-2, IL-6 and IL-13 in supernatants from (E) CYP2E1-re-stimulated WT splenocytes (*p<0.05, **p<0.01), while supernatants from (F) TFA-stimulated WT splenocytes had significantly elevated IL-1β, IL-2, IL-6 and IFN-γ when compared to CFA-immunized mice (*p<0.05, **p<0.01, ***p<0.001, Mann-Whitney U. Mean ± SE of pooled data from individual mice. Experiments were performed in duplicate with N = 5 mice/group).
CD4+T cells from WT mice induce hepatitis in Rag-/- mice by adoptive transfer
To confirm that T cell proliferation was associated with the development of DILI from anesthetics, we adoptively transferred hepatitis to naïve Rag−/− mice. We found that WT mixed splenocytes or CD4+ T cells isolated 14 days after TFA-S100 immunizations and re-stimulated for 72h with 10µg/ml of either CYP2E1 or TFA could adoptively transfer hepatitis to Rag-/- mice, which was histologically evident after 10 days, while mixed splenocytes stimulated with medium alone or from CFA-immunized mice could not (Figure 1C). These results strongly suggested that immune responses to CYP2E1 and to TFA generated by CD4+ T cells and possibly even CD8+ T, or NK cells as well as cytokines may induce anesthetic DILI.
CYP2E1 and TFA induce CD3+CD4+Tcell proliferation but reduce NK and NKT cells
Proliferation of T and B lymphocytes as well as NK and NKT cells upon exposure to self antigens has been strongly implicated in the development of several autoimmune diseases [23–26]. To identify which cells were responsible for proliferation to the key antigens in experimental anesthetic DILI, we analyzed the percent composition of these cells in mixed splenocyte cultures 72h following in vitro culture. We found that there were 30 and 20% increases in CD3+CD4+ T cells following stimulation CYP2E1 and TFA, respectively, when compared to splenocytes cultured in medium alone. Conversely, stimulation with CYP2E1 induced 24 and 18% decreases in DX5+NK cells and DX5+CD3+NK T cells, while KLH-TFA stimulation decreased these cells by 15 and 9%, respectively (Figure 1D). These findings suggested that increases in CD4+ T cells as well as decreases in potentially protective NK or NKT cells induced by CYP2E1 and TFA may have a role in the initiation of anesthetic DILI.
When analyzing CD8+ T cells we found that re-stimulation with CYP2E1 increased CD3+CD8+ T cells by 8%, while TFA stimulation decreased these cells by a similar amount when compared to medium alone (Figure 1D). Analysis of B cells showed that CYP2E1 decreased their numbers by 20% while TFA increased the composition of B cells in splenocyte cultures by 10% (Figure 1D). Since interpretation of the immune responses to CYP2E1 were less clear while antibody responses to TFA haptens had been well documented in both rodents and humans, these analyses suggested to us that the CYP2E1 autoantigen may have had an important role in the initiation of CD8+T cell responses while the TFA hapten had a more important role in the initiation of antibody responses in experimental anesthetic DILI.
CYP2E1 and TFA induce Th1 and Th2 responses in TFA-S100 – immunized WT mice
To explain adoptive transfer of hepatitis to T cell-deficient Rag−/− mice by mixed splenocytes as well as purified CD4+ T cells from WT mice immunized with TFA-S100 and re-stimulated for 72h with CYP2E1 or TFA, we measured supernatant cytokines from these splenocyte cultures treated as previously described. Similar to our previous findings [11], cytokines associated with TFA-S100-immunization and re-stimulation by CYP2E1 demonstrated significantly increased levels of primarily Th1 but also Th2 cytokines (Figure 1E) while re-stimulation with TFA induced significant increases in Th1 cytokines alone (Figure 1F). Interestingly supernatant levels of the Th2 cytokine IL-13 in TFA re-stimulated cultures approached but did not reach statistical significance. These studies suggested that the generation of CD4+ T cells by CYP2E1 and TFA by CYP2E1, during the initiation phase of our model triggered significantly elevated levels of primarily Th1 cytokines in response to CYP2E1 and TFA; however, Th2 cytokines generated in response to CYP2E1 may also have had a role in the initiation of anesthetic DILI. Even so, neither the cell-type induced by CYP2E1 or TFA nor the cytokines generated by these cells completely clarified the role of cells and cytokines in the initiation of DILI.
TFA-S100 significantly increases splenic and hepatic levels of IL-4 in WT mice
We had previously described cytokine levels in the liver at 3 weeks in WT mice immunized with CFA ± TFA-S100 [11]; however, we could not confirm a role for a particular cytokine in this process. To determine the role for the autoantigen, hapten or adjuvant in the pattern of cytokine expression after three weeks we determined whether there were significant differences in cytokine expression in TFA-S100 – immunized mice when compared to CFA and S100 controls.
We confirmed that hepatic inflammation was significantly higher in TFA-S100 – when compared to CFA – immunized mice (Figures 2A and B). We found significantly higher levels of IL-4 in mixed splenocyte culture supernatants isolated one week following immunization with TFA-S100 and re-stimulation with CYP2E1 or TFA when compared to cultures in medium alone (Figure 2C), while IL-4 levels in spleen culture supernatants were not different when comparing CFA or S100 – immunized mice. These findings suggested to us that as early as one week following the first immunization with our haptenated autoantigen, IL-4 expression is triggered by the critical DILI autoantigen, CYP2E1 as well as the critical DILI hapten TFA. We also found that livers from TFA-S100 – immunized mice had significantly higher levels of IL-4, in addition to IL-1β, IL-6, IL-13 and the chemokine MIP-2 after 3 weeks (Figure 2D). Taken together with our studies in patients, the present investigations suggest a critical role for IL-4 in the initiation and pathogenesis of immune – mediated DILI. However, whether this constituted a protective or destructive role was not clear. So next we wanted to directly assess the role of IL-4 by using IL-4 deficient mice. Interestingly, we also detected higher hepatic IL-10 levels in CFA - immunized mice when compared to the other groups which could support our findings of diminished inflammation in CFA-immunized mice and further confirm the S100 model [22], as well as our own, as models of hepatic inflammation.
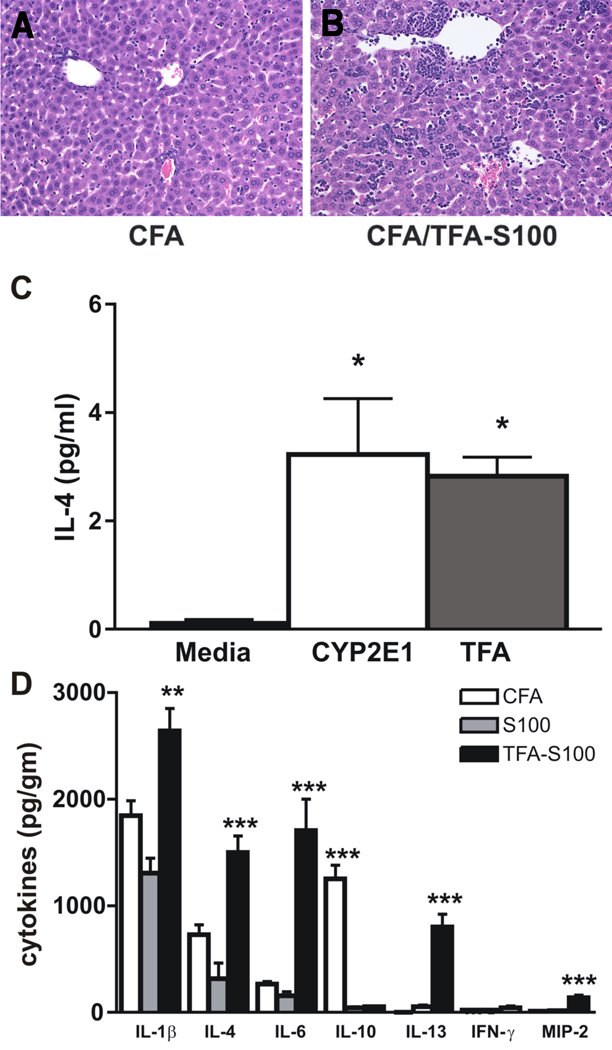
Figure 2.
TFA-S100 significantly increases splenic and hepatic levels of IL-4 in WT mice. (A, B) WT mice demonstrated significantly more hepatitis three weeks following TFA-S100/CFA immunizations when compared to immunization with CFA alone. Shown are representative liver tissue sections stained with H&E (0.5 µm, Magnification 64x). (C) Analysis of supernatants from cultured mixed splenocytes obtained one week following TFA-S100 immunization showed significantly higher levels of IL-4 when re-stimulated with either CYP2E1 or TFA in comparison with non-stimulated splenocytes (media); *p<0.05, Mann-Whitney U. Mean ± SEM of pooled data from individual mice. Experiments were run in duplicate with N = 4 mice/group. (D) Cytokine expression in liver tissue supernatants from CFA and S100 controls as well as TFA-S100 – immunized BALB/c mice at 3 weeks confirmed mixed Th1 (IL-1β and IL-6) and Th2 (IL-4 and IL-13) cytokines, as well as increased expression of the neutrophil chemoattractant MIP-2 and diminished levels of IL-10 (**p<0.01, ***p <0.001, Mann-Whitney U. Mean ± SEM of pooled data from individual mice. Experiments were run in duplicate with N=5 mice/group).
IL-4 has a critical role in the development of experimental anesthetic DILI
The detection of elevated IL-4 in the splenocyte culture supernatants as well as the liver one week and three weeks following TFA-S100 immunizations, respectively, strongly suggested a role for IL-4 in this process. To directly assess the role of IL-4 in the development of anesthetic DILI, we induced experimental DILI in WT and IL-4-/- (KO) mice and evaluated hepatitis in histological sections, supernatant cytokines in the liver and spleen, as well as serum antibodies after 3 weeks.
We found that hepatic inflammation/injury was higher in WT when compared to KO mice (3.3 ± 0.3 versus 1.6 ± 0.2, mean ± S.E., p<0.01, Figures 3A–C). Hepatic inflammation/injury was not significantly different in WT or KO mice given CFA alone (results not shown). Serum analyses for anti-TFA hapten antibodies demonstrated decreased IgG1 (p<0.001), but not IgG2a subclass antibodies (Figure 3D) in KO mice, showing that IL-4 increased serum TFA IgG1 antibodies and suggested that IL-4 had a role in the generation of B cell responses to the TFA hapten. Analyses of serum for anti-S100 autoantibodies showed significantly decreased IgG1 and IgG2a subclass autoantibodies to S100 in KO mice (p<0.001, Figure 3D) suggesting that IL-4 also increased B cell responses to this antigen and may be responsible for the development of these autoantibodies which had also been previously demonstrated in WT mice [11].
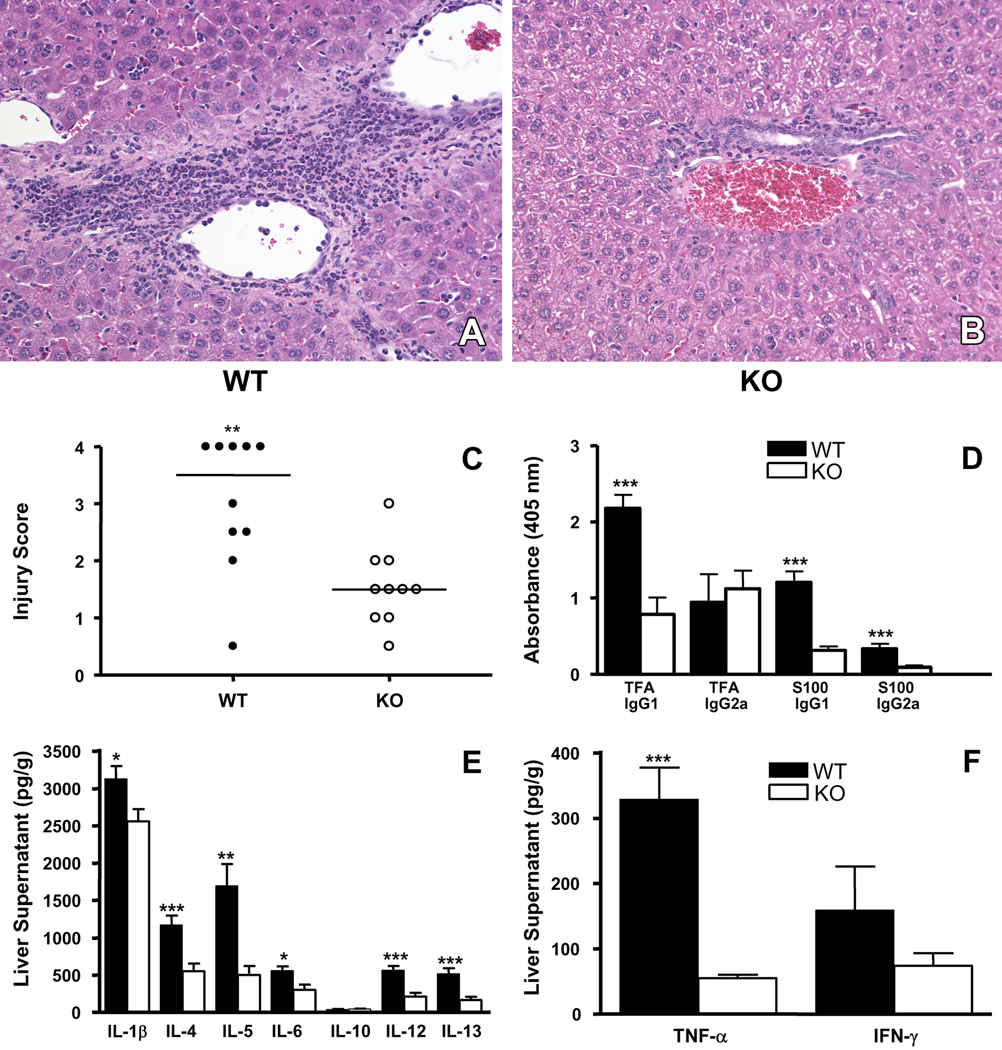
Figure 3.
IL-4 has a critical role in the development of experimental anesthetic DILI. Representative H & E histological section from (A) WT and (B) IL-4−/− (KO) mice demonstrating significantly more hepatitis in WT mice (A and B, 0.5µm, representative data, 64x magnification). (C) Composite injury scores in WT and KO mice showed increased hepatitis in WT when compared to KO mice (**p<0.01, Mann-Whitney U. Experiments were performed in duplicate with N=5 mice/group). (D) Sera analysis for TFA and S100 antibodies showed significantly elevated TFA IgG1 antibodies as well as anti-S100 IgG1 and IgG2a antibodies in WT compared to KO mice (***p<0.001, ANOVA with Tukey’s post test. Mean ± SEM of pooled data from individual mice. Experiments were performed in duplicate with N=5 mice/group). (E, F) Analysis of cytokines in liver tissue supernatants showed that WT mice had significantly elevated levels of IL-1β, IL-4, IL-5, IL-6, IL-12p70, IL-13 and TNF-α when compared to KO mice (*p<0.05, **p<0.01, ***p<0.001, Mann-Whitney U. Mean ± SEM of pooled data from individual mice. Experiments were performed in duplicate with N=5 mice/group ).
When analyzing liver cytokine levels following TFA-S100 immunizations, we found that KO mice had significantly lower IL-12 and IL-13 (p<0.001), IL-5 (p<0.01) as well as IL-1β and TNF-α (p<0.05) when compared to WT mice (Figure 3E and F) suggesting that IL-4 may have increased hepatic inflammation by increasing hepatic proinflammatory as well as regulatory cytokines. Cytokine levels in spleen were not significantly different between groups at this three-week time point (results not shown). These results showed that IL-4 had a critical role in the development of anesthetic DILI; however, whether these immune responses inferred a role for IL-4 in the initiation of experimental DILI needed to be addressed [11].
IL-4 initiates proliferation to CYP2E1 and TFA
To uncover critical pathogenic mechanisms induced by IL-4, we assessed T cell proliferation in splenocytes collected from WT and KO mice two weeks after immunization with TFA-S100 and stimulated for 72h with CYP2E1 or TFA. We found that proliferation to CYP2E1 and KLH-TFA was higher in WT mice at all doses (p<0.01, Figure 4A and B). This result demonstrated that IL-4 had a critical role in the initiation of immune responses to the key antigens responsible for the development of anesthetic DILI. We also found that splenocyte supernatants from KO mice re-stimulated with the TFA hapten had lower proinflammatory (IL-1β, p<0.01; IL-2, TNF-α, p<0.05), IL-4 effector (IL-13, p<0.05) and acute phase cytokines (IL-6, p<0.05) when compared to WT mice (Figure 4D), suggesting that IL-4 had a critical role in the secretion of these cytokines following recognition of TFA-haptenated proteins by splenocytes from TFA-S100-immunized WT mice. In sharp contrast, when we measured cytokines in splenocyte supernatants from KO mice re-stimulated with CYP2E1, we found higher levels of IL-2 (p<0.05), IL-6 (p<0.001), and regulatory cytokines IL-10 (p<0.001) and IFN-γ (p<0.05) (Figure 4C). Taken together with the demonstration of significantly diminished proliferation to CYP2E1 during the initiation phase (Figure 4A), this surprising finding suggested that IL-4 may promote the development of immune-mediated anesthetic DILI by suppressing IL-10 or IFN-γ regulatory responses to the CYP2E1 autoantigen during the initiation of this process.
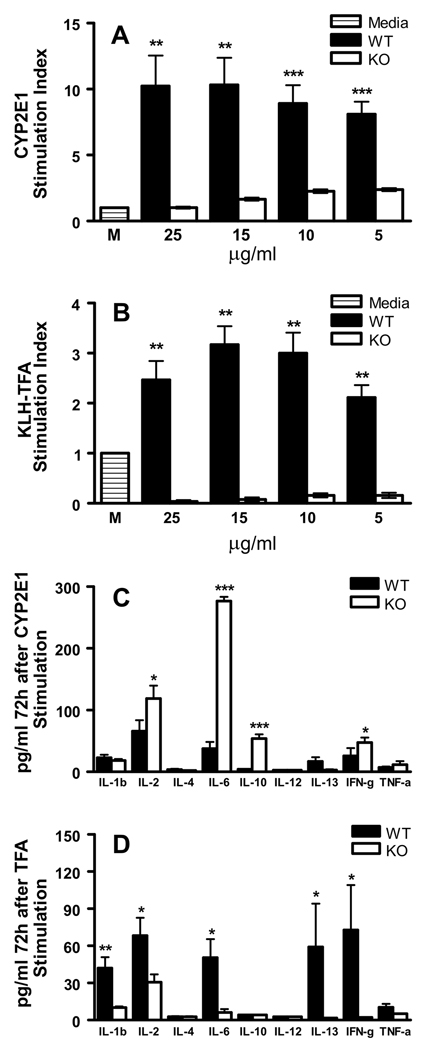
Figure 4.
IL-4 promotes T cell priming in experimental anesthetic DILI. After 72h, proliferation to (A) CYP2E1 and (B) TFA was significantly higher in WT splenocytes when compared to KO mice at all CYP2E1 or TFA doses (**p<0.01, ***p<0.001, Mann-Whitney U. Mean ± SEM of pooled data from individual mice. Experiments were run in triplicate with N = 5 mice/group). Supernatants from (C) CYP2E1-stimulated KO splenocyte cultures had significantly elevated concentrations of IL-2, IL-6, IL-10 and IFN-γ (*p<0.05, ***p<0.001 while (D) TFA-stimulated KO splenocyte cultures had significantly lower concentrations of IL-1β, IL-2, IL-6, IL-13 and IFN-γ, when compared to WT cultures (*p<0.05, **p<0.01, Mann-Whitney U. Mean ± SEM of pooled data from individual mice. Experiments were run in duplicate with N = 5 mice/group).
IL-4 induced proliferation to CYP2E1 does not suppress regulatory T cells
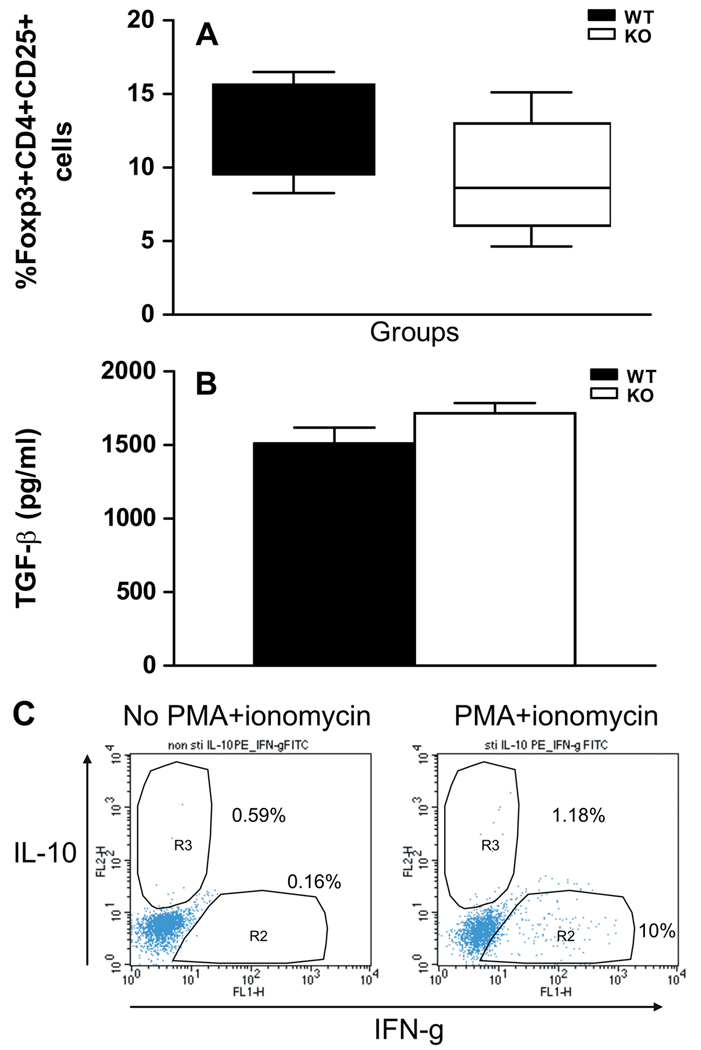
To investigate possible mechanisms for IL-10 production in our splenocyte cultures, we determined whether immune responses to the CYP2E1 autoantigen were being down-regulated by IL-10-producing Foxp3+CD25+ regulatory T cells [27], induced (Tr1 or Th3) regulatory T cells [28] or ordinary T cells performing regulatory functions. Two weeks following TFA-S100 immunizations, we found that there were no significant differences in the percent of Foxp3+CD25+CD4+ T cells when comparing WT and KO mice (Figure 5A), suggesting that decreases in naturally occurring or induced regulatory T cells during initiation of immune responses to CYP2E1 did not account for decreased proliferation to key antigens and the decreased amount of immune-mediated hepatitis in KO mice. In fact, amounts of these cells were somewhat decreased in KO mice, which may be expected since prior studies suggested a requirement for IL-4 in the maturation of antigen-specific Foxp3+CD25+CD4+ T cells [29].
Figure 5.
IL-4 suppression of responses to CYP2E1 does not involve regulatory T cells. (A) The percentages of Foxp3+CD4+CD25+ T cells were not significantly different in KO (9.4 ± 4.0%) when compared to WT (12.3 ± 3.2%) mice (Mean ± SEM of pooled data from individual mice. Experiments were run in duplicate with N = 4 mice/group). (B) TGF-β levels were not significantly different in splenocytes isolated from KO (1716 ± 156.3pg/ml) or WT (1511 ± 241.6pg/ml) mice and re-stimulated for 72h with 10µg/ml CYP2E1 Mean ± SEM of pooled data from individual mice. Experiments were run in duplicate with N = 5 mice/group. (C) Representative flow cytometric analysis of intracellular IL-10 and IFN-γ levels in splenocytes isolated from TFA-S100 – immunized KO mice and activated with PMA and ionomycin. No IFN-γ and IL-10 double positive cells were detected, although IFN-γ single positive and a few IL-10 single positive cells were seen.
Instead, immune responses to the CYP2E1 autoantigen by KO mice may have indicated the presence of IL-10 producing Tr1 cells [28] or recently described conventional IL-10-producing Th1 cells performing regulatory functions [30;31]. Since TGF-β expression is one of the hallmarks of Tr1 cells [27], we measured TGF-β levels in splenocyte supernatants. We found that TGF-β levels were not increased in supernatants from KO mice splenocyte cultures re-stimulated with CYP2E1 (Figure 5B) suggesting that suppressed proliferation to CYP2E1 was unlikely due to Tr1 cells. TGF-β levels were also similar in KO mice splenocyte supernatants re-stimulated with KLH-TFA when compared to WT mice (results not shown). Next we determined whether re-stimulation of TFA-S100 primed splenocytes with CYP2E1 induced Foxp3 negative IFN-γ and IL-10 double positive T cells. However, we were not able to detect Foxp3 negative IFN-γ and IL-10 double positive cells following CYP2E1 stimulation or PMA and ionomycin, while IL-10 and IFN-γ-singly positive cells were present (Figure 5C).
Our results suggested that during the initiation of anesthetic DILI, the source of IL-10 and diminished proliferation to CYP2E1 in KO mouse TFA-S100-primed splenocytes was most likely T cells performing regulatory functions. Interestingly, we did not find significant numbers of NK or NKT cells in these same cultures (results not shown). Nonetheless, we needed to clarify the role of IL-10 in the initiation of experimental anesthetic DILI.
Blocking IL-10 increases experimental anesthetic DILI in KO mice
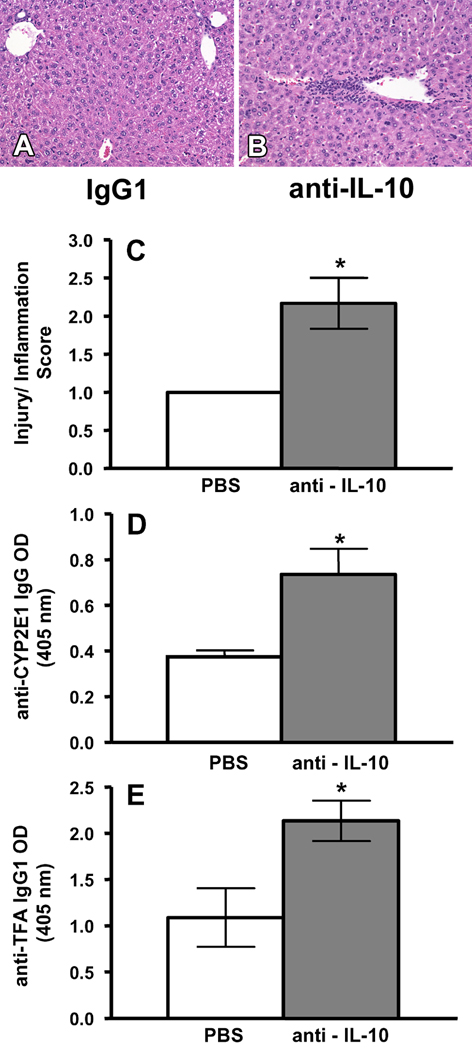
To confirm a role for IL-10 in regulating the development of anesthetic DILI, KO mice received immunizations with TFA-S100 as previously described as well as 250 µg of anti IL-10 or 200 µl PBS on days 14, 16, 18 and 20. Twenty-one days after the initial immunizations, hepatitis was assessed by histology, and sera were assessed for anti-CYP2E1 autoantibodies as well as anti-TFA antibodies.
Hepatitis was increased in KO mice treated with anti-IL-10 when compared to KO mice treated with PBS following TFA-S100 immunizations (p<0.05, Figure 6A – C). Treatment of KO mice with anti-IL-10 following TFA-S100 immunizations also increased levels of anti-CYP2E1 IgG and TFA IgG1 antibodies when compared to PBS/TFA-S100 – treated mice (p<0.05, Figure 6D and E). This finding was very important since we had previously demonstrated that anti-TFA IgG1 antibodies correlate with hepatitis in this model [11]. Moreover anti-CYP2E1 autoantibodies had been detected in anesthetic DILI patients [14;15].
Figure 6.
Anti-IL-10 increases hepatitis, as well as CYP2E1 and TFA antibodies in KO mice. TFA-S100 immunization in combination with injection of (A) 200µl PBS or (B) 250µg anti-IL-10 blocking antibodies on days 14, 16, 18 and 20 increased the severity of hepatitis in comparison with the control. Representative data are shown (64x magnification). (C) Histology scores (2.2 ± 0.3 vs. 1.0 ± 0.5, mean ± SE, *p<0.05, (D) sera anti-CYP2E1 IgG autoantibodies (0.736 ± 0.111 vs. 0.374 ± 0.028, mean ± SE, *p<0.05) and (E) anti-TFA IgG1 antibodies (2.134 ± 0.319 vs. 1.089 ± 0.317, mean ± SE, *p<0.05) were observed for anti-IL-10– vs PBS -treated female KO mice respectively (Mann-Whitney U. Pooled data from individual mice. Experiments were performed in duplicate with N = 4 mice/group).
Discussion
We have shown that analogous to clinical findings, IL-4 driven immune responses to CYP2E1 and TFA have critical roles in the initiation and severity of experimental anesthetic DILI. Surprisingly, re-stimulation of IL-4 deficient mouse (KO) splenocytes with CYP2E1 induces significantly elevated levels of IL-2, IL-6, IL-10 and IFN-γ suggesting that IL-10 - or even IFN-γ – producing T cells may have a role in down-regulating T cell proliferation to CYP2E1. Blocking IL-10 in KO mice increased hepatitis as well as CYP2E1 and TFA antibodies in KO mice confirming a regulatory role for IL-10 in the development of experimental anesthetic DILI. Our studies strongly suggest dual roles for IL-4 in the development of immune-mediated anesthetic DILI by down-regulating protective responses to the CYP2E1 autoantigen while promoting pro-inflammatory responses to TFA.
Previous clinical investigations have determined that immune responses to CYP2E1 and TFA have critical roles in the development of anesthetic DILI. These elegant studies clearly demonstrated antibodies to CYP2E1 and TFA in the serum of persons who developed anesthetic DILI or Id-ALF [6;9;12]. Since a hapten-based immune-mediated mouse model of DILI is a recent development [11], we can now determine the exact roles for these key proteins and directly investigate the pathophysiology responsible for the development of immune-mediated DILI.
No previous study has identified critical cytokines associated with the initiation of anesthetic DILI, in mice or patients. However, elevated acute phase cytokines have been demonstrated in toxicity models following the administration of intra-peritoneal halothane to guinea pigs [32] and large doses of intraperitoneal halothane to mice [33], suggesting that these studies represent important models of anesthetic Id-ALF from direct toxicity by halothane. We believe that our immune-mediated approach may parallel what occurs during the clinically quiescent phase of anesthetic DILI in patients, where hepatitis is undetectable because of normal or mildly elevated serum transaminase levels which are late findings. Demonstrating immune responses to key antigens associated with anesthetic DILI in patients (Figure 1) as well as a critical role for IL-4 (Figure 2 – Figure 4), which has been clearly implicated by the demonstration of IgG4 autoantibodies during active anesthetic DILI [15;16] firmly supports the translational significance of this model.
We have demonstrated significantly elevated TNF-α and IL-1β, as well as IL-6, IL-10, IL-13, and IFN-γ in the splenic priming phase of our model prior to the development of significant hepatitis. TNF-α and IL-1β have significant roles in the development of DILI following acetaminophen [34]. Moreover, these same cytokines have been demonstrated in patients with infectious hepatitis and in autoimmune hepatitis models [11]. Our studies agree with these previous findings and suggest that toxic and autoimmune responses, as well as Th1, Th2 and regulatory cytokines have roles in the initiation phase of immune-mediated DILI. More importantly our studies directly correlate the production of these cytokines with key antigens that have been strongly associated with the development of anesthetic DILI [6;9;10;12;14;15].
Detecting increased IL-4 in splenocyte supernatants and subsequently in the liver of TFA-S100 – immunized mice when compared to S100 and CFA – immunized control mice clearly indicates a critical role for IL-4 in a hapten – mediated inflammatory process in the liver (Figure 2C and D). Previous studies have correlated IL-4 with the development of immune-mediated T cell hepatitis [35] where IL-4 in conjunction with CD4+ T cells induces liver injury by activating the Jak1/STAT6 pathway in hepatocytes and NKT cells resulting in the production of eotaxin and IL-5 [36]. Similar to these studies we have found that IL-4 is required for the initiation of anesthetic DILI. We also show that KO mice develop significantly less hepatitis as well as hepatic cytokine responses (Figure 3). However, we did not find significant differences in hepatic eotaxin when comparing TFA-S100-immunized WT and KO mice (results not shown). Additionally, we demonstrate significant decreases in splenic NK and NKT cells presumably triggered by CYP2E1 and TFA. Thus our studies show a direct effect of immune responses to these antigens on the survival of potentially protective NK and NKT cells, and further suggest that additional regulatory pathways may influence the development of immune-mediated DILI from anesthetics or other drugs that use similar mechanisms. Along these lines, IL-4 can increase mRNA and protein expression of CYP2E1 in hepatocytes [37;38]. In this way IL-4 may have a similar role in the development of halogenated volatile anesthetic DILI in susceptible persons by increasing metabolism of halogenated volatile anesthetics, thus creating more reactive haptens and subsequently more neoantigens that could initiate anesthetic DILI.
Surprisingly we have found increased levels of IL-2, IL-6 IL-10 and IFN-γ in splenocytes from TFA-S100 – immunized KO mice stimulated with CYP2E1 (Figure 4C). This finding suggests to us that IL-4 may have an additional role in the suppression of potentially protective responses by IL-10-producing T cells propagated by pathogenic T cells that secrete IL-2, similar to previously described Th1 cells performing regulatory functions [30]. While we did not find significantly increased antigen-specific Foxp3+CD25+CD4+ regulatory T cells, this may have been affected by the deficiency of IL-4 in our KO mouse, since IL-4 is required for the maturation of these cells [29]. While our findings clearly suggest that IL-4 has a role in the development of anesthetic DILI in our model and in patients, it is clear that some KO mice can develop reasonable amounts of hepatitis. This finding could suggest that the primary role for IL-4 is in the initiation phase while an alternative role later in disease may be regulated by additional immunomodulatory mechanisms induced by CYP2E1, IL-10 or even IFN-γ. Another possibility is that suppression of CYP2E1 immune-mediated responses in the initiation of this model by IL-10 may promote the development of chronic, but decreased disease in KO mice, a mechanism recently suggested in the development of chronic cutaneous leishmaniasis [31].
We cannot ignore our detection of IL-4 in the liver of KO mice (Figure 3E) and wonder whether or not IL-4 contributed to the development of hepatitis in some KO mice (Figure 3C). Expectedly, IL-4 levels are below levels of detection in the spleen. We have contacted Jackson Laboratories, the source of our mice and confirmed our mice to be IL-4 deficient. Even so, these experiments have been repeated several times by us with similar results. We have concluded that these levels may not be checked by others and the IL-4 deficiency status may have been assumed in all organs. We elected to keep the IL-4 levels as we detected them in the Results section, since this is truly what we found.
Our studies suggest that in immune-mediated DILI from halogenated volatile anesthetics or other drugs that generate drug haptens, CD4+ and possibly CD8+ T cell responses to key antigens such as CYP2E1 and TFA are primarily responsible for the initiation of immune responses that result in hepatitis. Although CD4+T cells generated during the initiation of anesthetic DILI trigger both Th2 and Th1 cytokines, IL-4 has a key role in the initiation of this process. In the absence of IL-4, IL-10-producing T cells diminish immune responses to CYP2E1, suggesting that IL-4 may directly or indirectly decrease the activation or survival of these T cells. Blocking IL-10 in KO mice increases hepatitis as well as anti-CYP2E1 autoantibodies and anti-TFA antibodies confirming a critical role for IL-10 in these processes (Figure 7). This is the first demonstration for dual roles for IL-4 in experimental immune-mediated DILI that may partially explain the development of autoimmune symptoms in patients with anesthetic DILI [15] or immune-mediated DILI from other drugs that use similar mechanisms. Ongoing investigations will further define roles of IL-4, neoantigens, additional immune cells and other cytokines such as IFN-γ in immune-mediated DILI from halogenated volatile anesthetics or other drugs using similar mechanisms such as, tienilic acid, dihydrazaline, carbamazepine, and alcohol.
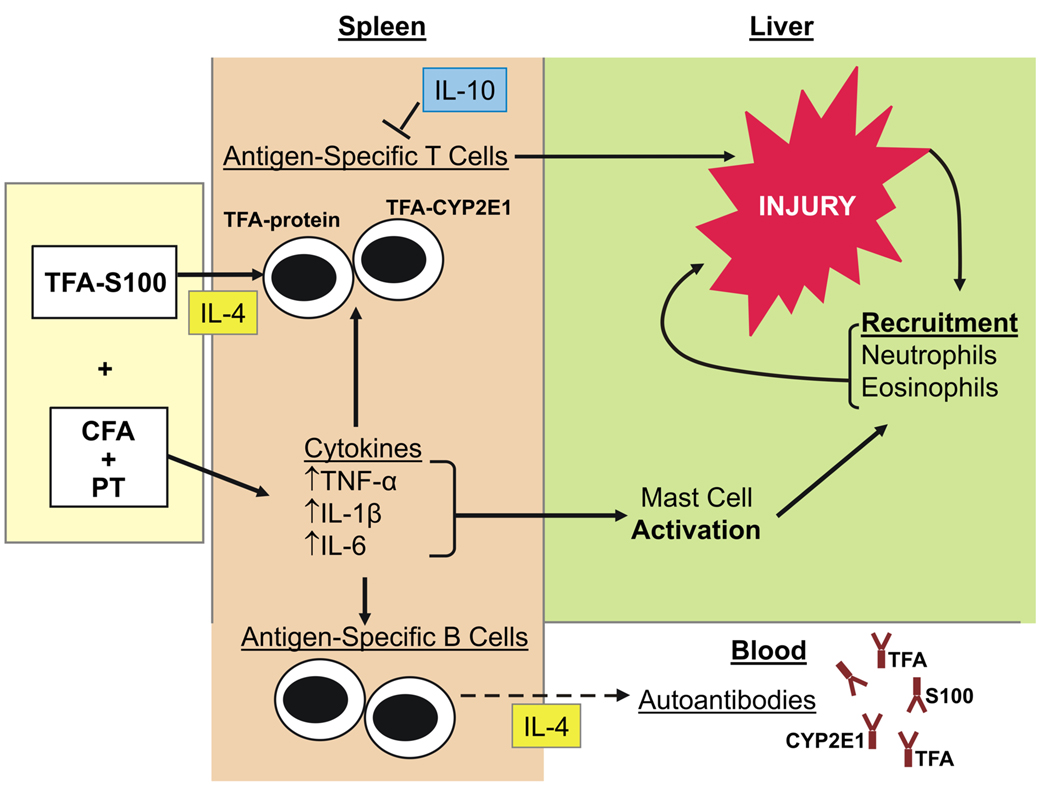
Figure 7.
Proposed mechanism of immune-mediated DILI. In our model of experimental (murine), immune-mediated, anesthetic DILI, BALB/c mice develop an IL-4-promoted hypersensitive response to the TFA hapten and S100 liver proteins resulting in splenocyte TNF-α, IL-1β, IL-6 production. IL-4-dependent splenocyte proliferation is also demonstrated in response to human CYP2E1 and TFA through suppression of IL-10 and possibly IFN-γ regulatory responses. Immune responses to CYP2E1, S100 and TFA also activate mast cells that recruit neutrophils to the liver. Directly or indirectly through IL-10, a predominantly IL-4-driven Th2-type response leads to S100 and CYP2E1 autoantibody production as well TFA hapten antibodies.
Materials and Methods
Materials
ACK Lysing Buffer and PBS were from Quality Biological (Gaithersburg, MD); alkaline phosphatase-conjugated rabbit anti-mouse IgG1 and IgG2a and IgG2b and Golgi Stop™ from BD Biosciences (Woburn MA); alkaline phosphatase substrate kit, from Bio Rad; mouse cytokine/chemokine 22-plex pre-mixed LINCOplex kit for Millipore (St. Charles, MO); Difco Bacto Adjuvant Complete Freund H37 Ra (CFA) from Fisher Scientific (Pittsburgh, PA); fetal calf serum (FCS), L-glutamine, HEPES Buffer Solution (1M), minimum essential medium (MEM) penicillin-streptomycin, RPMI 1640 and Trypan Blue Stain, from Invitrogen™, (Carlsbad, CA); goat anti-mouse IgG (heavy and light chains) alkaline phosphatase conjugate (AKP, Chemicon International, Temecula, CA); Gill’s Number 1 hematoxylin, from Sigma-Aldrich (St. Louis, MO); human CYP2E1 from Gentest, BD Biosciences (Woburn, MA); Immulon 2HB® microtiter 96-well plates, from ISC BioExpress (Kaysville, UT); ketamine and xylazine, from, from Penn Veterinary Supply, Inc (Lancaster, PA); methyl 3H thymidine, from Perkin-Elimer (Boston, MA); PE anti-mouse/rat Foxp3 Staining Set, from eBioscience (San Diego, CA); pertussis toxin, from List Biologicals (Campbell, CA); rat anti mouse IL-10 monoclonal antibody (clone JESS-2A5), Rat IgG1 Isotype control, and Quantikine cytokine ELISA kits, from R&D Systems (Minneapolis, MN).
Mice
Eight to 10 week-old, female, inbred BALB/c (WT), IL-4 −/− (KO) mice on BALB/c background as well as Rag −/− mice on BALB/c background, purchased from the Jackson Laboratory (Bar Harbor, Maine) were maintained under pathogen-free conditions our animal facility. The IL-4 deficient mice were derived from targeted deletion of IL-4 performed in a BALB/cJ – derived ES cell line. Targeted ES cells were then injected into BALB/c blastocysts. The colony has been maintained by brother sister mating of offspring from these blastocysts. This colony was derived solely on a BALB/cJ background. The Rag1tm1Mom mutant strain was developed by Dr. Peter Mombaerts in the laboratory of Dr. Susumu Tonegawa at the Center for Cancer Research, Massachusetts Institute of Technology. A replacement targeting vector with the Pgk-neo marker was used. Homologous recombination of the targeting vector resulted in a 1356 bp deletion in the 5' end of the coding sequence. The 129S7/SvEvBrd AB1 ES cell line was used. The BALB/c congenic strain was generated by Dr. Bob Coffman by backcrossing mice carrying the Rag1tm1Mom mutation 7 times to BALB/cAnNTac inbred mice. The approximate control is the BALB/cJ mouse. The strain is maintained by homozygous sibling matings. Approval for all procedures was obtained from the Animal Care and Use Committee of the Johns Hopkins University.
Induction of hepatitis with TFA haptenated cytosolic S-100 (TFA-S100)
To induce hepatitis, WT or KO mice were immunized s.c. with two doses of 200µg of syngenic S100 covalently altered by TFA on days 0 and 7, emulsified in an equal volume of CFA at the base of the neck, and 500ng of Pertussis toxin (PT) i.m. in the right hind flank on day 0 as previously described [11].
Anti – IL10 Administration
Experimental anesthetic DILI was induced in KO mice as described [11]. In addition, mice received 250µg /200µl PBS anti-IL-10, isotype control or 200µl PBS on days 14, 16, 18 and 20. The mice were killed three weeks after the initial immunization.
Proliferation Assays
One or two weeks after the initial TFA-S100 immunization, whole splenocytes were isolated and 2.5 × 105cells/ 100 µl PBS/2%FCS were loaded into 96-well plates with 100 µl aliquots of Media, Con A, 25, 15, 10 or 5µg/ml CYP2E1, trifluoroacetylated keyhole limpet hemocyanin (KLH-TFA) or KLH alone. Plates were incubated for 72h at 37°C, 5% CO2, 95% air (humidified), pulsed with 10µl, 1µCi/ well methyl 3H thymidine and then stored in −20°C. Experiments were performed at least in duplicate with N=4 mice/group.
FACS analysis of the cellular composition of splenocyte culture supernatants
Splenocytes were isolated 2 weeks following initial TFA-S100 immunization, re-stimulated with media ± CYP2E1 or KLH-TFA (10µg/ml), incubated for 72h at 37°C, 5% CO2, 95% (humidified), pooled by stimulation antigen, counted, washed with PBS/2%FCS, and stained with the antibody combinations described below. For intracellular cytokine staining cells were treated with Golgi-Stop™ at 24h, harvested by 48h, pooled by antigen stimulation, permeabilized and fixed. (Experiments were run in duplicate with N=4 mice/group)
We utilized 1:100 dilutions of the following FITC/PE/CyChrome™ or APC-labeled antibody combinations: CD4(L3T4, clone H129.19)/CD8a(Ly-2, clone 53.7.7)/CD3e(clone 145-2C11); CD49b Pan-NK Cells (clone DX5)/CD45R B220(clone RA3–6B2)/CD3e(clone 145-2C11); CD11c(clone HL3)/ CD8a(Ly-2, clone 53.7.7)/CD3e(clone 145-2C11); F4/80(clone BM8)/Ly-6G Gr-1(clone RB6–8C5)/CD45(clone 30-F11) and CD4(L3T4, clone H129.19)/Foxp3(clone FJK-16s) /CD25(clone PC61). For intracellular cytokine staining, CD4+ T cells were stained with 1:100 dilutions of the following FITC/PE, CyChrome™ antibody: IFN-γ(clone XMG1.2)/ IL-10(clone JES5–16E3)/CD4+(G3T4, clone H129.19). All antibodies were from BD Biosciences Pharmingen (San Diego, CA) except F4/80 and Foxp3 which were from eBiosciences (San Diego, CA). Cells were fixed with PBS/4%paraformaldeyde and analyzed by flow cytometry within three days.
Adoptive Transfer Experiments
Whole splenocytes were isolated from CFA or TFA-S100 – immunized mice as described above and cultured for 72h in media ± CYP2E1 or KLH-TFA (10µg/ml). The CD4+T cells used for adoptive transfer experiments were obtained using magnetic beads MACS® Cell Separation System (Miltenyi Biotech, Inc., Auburn, CA) as per manufacturers instructions on whole splenocytes from TFA-S100 – immunized BALB/c mice cultured in media or re-stimulated with either CYP2E1 or TFA (10 µg/ml). Prior to adoptive transfer, splenocytes or CD4+ T cells/ml isolated from in vitro whole splenocyte cultures were washed several times in PBS and viable cells counted. 1 × 107 cells/ ml were injected into separate Rag-/- mice using the tail vein. Ten-14d later the mice were killed and livers assessed for hepatitis. Experiments were done in triplicate with N=2 mice/group.
Histological analysis
Liver tissue sections (0.5µm thick) were fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin (H&E) and scored for inflammation and injury using a previously described grading system [11]: Grade 0, no portal tract, lobular inflammation or hepatocyte necrosis; Grade 1, minor periportal or lobular inflammation without necrosis; Grade 2, periportal or lobular inflammation involving <50% of the liver section; Grade 3, periportal or lobular inflammation involving ≥50% of the liver section; Grade 4, inflammation with bridging necrosis. Experiments were performed in duplicate with N=5 mice/group.
ELISA to detect TFA or S-100 antibodies
Blood collected on day 21 following the initial immunization was allowed to clot at RT, centrifuged at 2000 rpm for 15min at 4°C, serum was removed and antibodies were detected by ELISA as previously described [11] using 0.5µg/100µl BALB/c S-100, TFA bound to ovalbumin or CYP2E1. Secondary antibodies consisted of goat anti-mouse IgG (heavy and light chains) alkaline phosphatase conjugate (AKP), mouse IgG1 AKP or mouse IgG2a AKP in PBS/2%FCS (1:1000). The OD (405 nm) of TFA autoantibodies was determined after a 30min incubation with substrate, while S-100 and CYP2E1 autoantibody levels were detected after 60min. Experiments were performed in duplicate with N=5 mice/group.
Cytokine measurements
Liver and spleen samples from individual mice were snap-frozen and stored at –80°C until homogenized using 10% weight/volume in 2% MEM [11]. Cytokine levels in individual organs were standardized by converting cytokine levels to pg/g of tissue. Splenocyte culture supernatants were collected and stored at −80°C until tested. Cytokines were measured using Quantikine cytokine ELISA kits purchased from R&D Systems (Minneapolis, MN). Experiments were performed in duplicate with N=5 mice per group.
Statistical Analysis
Statistical analyses were performed using GraphPad® Prism Version 3.02 for Windows (GraphPad® Software, Incorporated, San Diego, CA). Histology scores, proliferation assays and cytokines were analyzed using Mann-Whitney U. Antibodies were analyzed by ANOVA with Tukey’s post-test. A p value < 0.05 was considered significant. *p<0.05; **p<0.01; ***p<0.001.
Acknowledgements
DBN is supported by NIHR21DK075828. This work is supported in part by a grant from the American Autoimmune Related Diseases Association, Mr. and Mrs. Joseph Scoby and The Gail I Zuckerman Foundation.
Abbreviations used in this article
- ALF
acute liver failure
- CYP2E1
cytochrome P450 2E1
- DILI
drug-induced liver disease
- H & E
hematoxylin and eosin
- PT
pertussis toxin
- RT
room temperature
- TFA
trifluoroacetyl chloride
Footnotes
Conflicts of Interest
None
References
- 1.Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, Stribling R, Crippin JS, Flamm S, Somberg KA, Rosen H, McCashland TM, Hay JE, Lee WM. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl.Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann.Intern.Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Masubuchi Y, Bourdi M, Reilly TP, Graf ML, George JW, Pohl LR. Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochem.Biophys.Res.Commun. 2003;304:207–212. doi: 10.1016/s0006-291x(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 4.Castell JV. Allergic hepatitis: a drug-mediated organ-specific immune reaction. Clin.Exp.Allergy. 1998;28(Suppl 4):13–19. 13-9. [PubMed] [Google Scholar]
- 5.Adkinson NF, Jr, Essayan D, Gruchalla R, Haggerty H, Kawabata T, Sandler JD, Updyke L, Shear NH, Wierda D. Task force report: future research needs for the prevention and management of immune-mediated drug hypersensitivity reactions. J.Allergy Clin.Immunol. 2002;109:S461–S478. doi: 10.1067/mai.2002.122214. [DOI] [PubMed] [Google Scholar]
- 6.Bourdi M, Chen W, Peter RM, Martin JL, Buters JT, Nelson SD, Pohl LR. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem.Res.Toxicol. 1996;9:1159–1166. doi: 10.1021/tx960083q. [DOI] [PubMed] [Google Scholar]
- 7.Mackay IR, Toh BH. Autoimmune hepatitis: the way we were, the way we are today and the way we hope to be. Autoimmunity. 2002;35:293–305. doi: 10.1080/08916930290015610. [DOI] [PubMed] [Google Scholar]
- 8.Neuberger J, Mieli-Vergani G, Tredger JM, Davis M, Williams R. Oxidative metabolism of halothane in the production of altered hepatocyte membrane antigens in acute halothane-induced hepatic necrosis. Gut. 1981;22:669–672. doi: 10.1136/gut.22.8.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pohl LR, Thomassen D, Pumford NR, Butler LE, Satoh H, Ferrans VJ, Perrone A, Martin BM, Martin JL. Hapten carrier conjugates associated with halothane hepatitis. Adv.Exp.Med.Biol. 1991;283:111–120. doi: 10.1007/978-1-4684-5877-0_12. 111-20. [DOI] [PubMed] [Google Scholar]
- 10.Njoku D, Laster MJ, Gong DH, Eger EI, Reed GF, Martin JL. Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury. Anesth.Analg. 1997;84:173–178. doi: 10.1097/00000539-199701000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Njoku DB, Talor MV, Fairweather D, Frisancho-Kiss S, Odumade OA, Rose NR. A novel model of drug hapten-induced hepatitis with increased mast cells in the BALB/c mouse. Exp.Mol.Pathol. 2005;78:87–100. doi: 10.1016/j.yexmp.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol.Pharmacol. 1996;50:573–582. [PubMed] [Google Scholar]
- 13.Martin JL, Kenna JG, Martin BM, Thomassen D, Reed GF, Pohl LR. Halothane hepatitis patients have serum antibodies that react with protein disulfide isomerase. Hepatology. 1993;18:858–863. doi: 10.1002/hep.1840180417. [DOI] [PubMed] [Google Scholar]
- 14.Njoku DB, Greenberg RS, Bourdi M, Borkowf CB, Dake EM, Martin JL, Pohl LR. Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesth.Analg. 2002;94:243–249. doi: 10.1097/00000539-200202000-00003. table. [DOI] [PubMed] [Google Scholar]
- 15.Njoku DB, Mellerson JL, Talor MV, Kerr DR, Faraday NR, Outschoorn I, Rose NR. Role of CYP2E1 immunoglobulin G4 subclass antibodies and complement in pathogenesis of idiosyncratic drug-induced hepatitis. Clin.Vaccine Immunol. 2006;13:258–265. doi: 10.1128/CVI.13.2.258-265.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson JS, Rose NR, Martin JL, Eger EI, Njoku DB. Desflurane hepatitis associated with hapten and autoantigen-specific IgG4 antibodies. Anesth.Analg. 2007;104:1452–1453. doi: 10.1213/01.ane.0000263275.10081.47. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gascan H, Gauchat JF, Roncarolo MG, Yssel H, Spits H, de Vries JE. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J.Exp.Med. 1991;173:747–750. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gascan H, Gauchat JF, Aversa G, Van Vlasselaer P, de Vries JE. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J.Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- 19.Zhang K, Clark EA, Saxon A. CD40 stimulation provides an IFN-gamma-independent and IL-4-dependent differentiation signal directly to human B cells for IgE production. J.Immunol. 1991;146:1836–1842. [PubMed] [Google Scholar]
- 20.Svensson L, Nandakumar KS, Johansson A, Jansson L, Holmdahl R. IL-4-deficient mice develop less acute but more chronic relapsing collagen-induced arthritis. Eur.J.Immunol. 2002;32:2944–2953. doi: 10.1002/1521-4141(2002010)32:10<2944::AID-IMMU2944>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Pohl LR, Kenna JG, Satoh H, Christ D, Martin JL. Neoantigens associated with halothane hepatitis. Drug Metab Rev. 1989;20:203–217. doi: 10.3109/03602538909103537. [DOI] [PubMed] [Google Scholar]
- 22.Lohse AW, Manns M, Dienes HP, Meyer zum Buschenfelde KH, Cohen IR. Experimental autoimmune hepatitis: disease induction, time course and T-cell reactivity. Hepatology. 1990;11:24–30. doi: 10.1002/hep.1840110106. [DOI] [PubMed] [Google Scholar]
- 23.Greve B, Weissert R, Hamdi N, Bettelli E, Sobel RA, Coyle A, Kuchroo VK, Rajewsky K, Schmidt-Supprian M. I kappa B kinase 2/beta deficiency controls expansion of autoreactive T cells and suppresses experimental autoimmune encephalomyelitis. J.Immunol. 2007;179:179–185. doi: 10.4049/jimmunol.179.1.179. [DOI] [PubMed] [Google Scholar]
- 24.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J.Immunol. 2003;171:462–468. doi: 10.4049/jimmunol.171.1.462. [DOI] [PubMed] [Google Scholar]
- 25.La CA, Van KL, Fu DS. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Van KL, La CA, Price M, Campagnolo DI, Collins M, Young DA, Vollmer TL, Shi FD. Autoreactive T cells mediate NK cell degeneration in autoimmune disease. J.Immunol. 2006;176:5247–5254. doi: 10.4049/jimmunol.176.9.5247. [DOI] [PubMed] [Google Scholar]
- 27.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J.Clin.Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills KH, McGuirk P. Antigen-specific regulatory T cells--their induction and role in infection. Semin.Immunol. 2004;16:107–117. doi: 10.1016/j.smim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J.Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 30.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J.Exp.Med. 2007;204:273–283. doi: 10.1084/jem.20062175. %19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J.Exp.Med. 2007;204:285–297. doi: 10.1084/jem.20061886. %19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourdi M, Amouzadeh HR, Rushmore TH, Martin JL, Pohl LR. Halothane-induced liver injury in outbred guinea pigs: role of trifluoroacetylated protein adducts in animal susceptibility. Chem.Res.Toxicol. 2001;14:362–370. doi: 10.1021/tx000244x. [DOI] [PubMed] [Google Scholar]
- 33.You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- 34.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 35.Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J.Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 36.Moscat J, Rennert P, Diaz-Meco MT. PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death.Differ. 2006;13:702–711. doi: 10.1038/sj.cdd.4401823. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, Beaune P, Guillouzo A. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol.Pharmacol. 1993;44:707–715. [PubMed] [Google Scholar]
- 38.Lagadic-Gossmann D, Lerche C, Rissel M, Joannard F, Galisteo M, Guillouzo A, Corcos L. The induction of the human hepatic CYP2E1 gene by interleukin 4 is transcriptional and regulated by protein kinase C. Cell Biol.Toxicol. 2000;16:221–233. doi: 10.1023/a:1007625925095. [DOI] [PubMed] [Google Scholar]