Abstract
DARPP-32 is a dual function protein kinase/phosphatase inhibitor which plays a vital role in striatal signaling. The phosphorylation of DARPP-32 at T34 is essential for mediating the effects of both psychostimulant and antipsychotic drugs; however these drugs are known to have opposing behavioral and clinical effects. We hypothesized that these drugs exert differential effects on striatonigral and striatopallidal neurons which comprise distinct output pathways of the basal ganglia. To directly test this idea, we developed novel BAC transgenic mice which allow analysis of DARPP-32 phosphorylation selectively in striatonigral and striatopallidal neurons. Using this new methodology we show that cocaine, a psychostimulant, and haloperidol, a sedation-producing antipsychotic, exert differential effects on DARPP-32 phosphorylation in the two neuronal populations which can explain their opposing behavioral effects. Furthermore, we find that a variety of drugs that target the striatum have cell-type specific effects which previous methods were not able to discern.
INTRODUCTION
Since its discovery twenty-five years ago, many studies have established that DARPP-32 (Dopamine and cAMP regulated phospho-protein of 32kD) is a key regulator of signaling in striatal neurons1. DARPP-32 function depends on its relative state of phosphorylation at two main regulatory sites, T34 and T75. When DARPP-32 is phosphorylated at T34 by protein kinase A (PKA) it becomes a potent inhibitor of protein phosphatase 1 (PP-1), which in turn regulates the phosphorylation state of several classes of effector proteins including transcription factors, ionotropic receptors, and ion channels2. PKA also phosphorylates and activates protein phosphatase 2A (PP-2A), which dephosphorylates DARPP-32 at T753, 4. When phosphorylated at T75 by cdk5, DARPP-32 becomes an inhibitor of PKA signaling, thereby relieving inhibition of PP-15.
Biochemical studies have shown that a variety of therapeutic agents and drugs of abuse can regulate DARPP-32 phosphorylation at T34 and T756. Acute administration of psychostimulants such as cocaine enhances dopamine signaling and has been shown to increase T34 phosphorylation7, 8. Antipsychotics such as haloperidol block dopamine signaling via antagonism of D2 receptors but also increase T34 phosphorylation to a similar degree9. Despite this similar biochemical effect, psychostimulants and antipsychotics have opposing behavioral and clinical outcomes.
Regarding this paradox, we hypothesized that these drugs selectively target different subpopulations of striatal cells, namely striatonigral and striatopallidal neurons. These neurons are morphologically similar and are expressed homogeneously throughout the striatum; however they are known to differ in their efferent projections and their expression of different sub-types of neurotransmitter receptors. Striatonigral neurons project via the direct pathway to the output nuclei of the basal ganglia and have been shown to preferentially express dopamine type 1 receptors (D1R)10. D1 receptors are coupled to Gs/olf proteins which upon activation stimulate adenylyl cyclase, promote the formation of cAMP, and activate PKA11, 12. Based on the classical model of the basal ganglia, activation of the direct pathway is expected to facilitate locomotor behavior13. In contrast, striatopallidal neurons project via the indirect pathway and exert an opposing influence on the output of the basal ganglia resulting in inhibition of locomotion13. Striatopallidal neurons express high levels of type 2 dopamine receptors (D2R) as well as adenosine A2A receptors10, 14. D2 receptors are coupled to Gi proteins which upon stimulation inhibit the formation of cAMP thereby decreasing PKA activity12.
Direct evidence for the hypothesis that cocaine and haloperidol differentially target these two neuronal populations has been lacking due to the inability to distinguish phosphorylation responses in these neurons. Traditional biochemical experiments have the limitation that they examine a mixed population of cells and results of these studies represent an average of signaling events within the striatum. To overcome this, we developed novel BAC transgenic mice which express differentially tagged DARPP-32 protein selectively in D1R-enriched striatonigral and D2R-enriched striatopallidal neurons. Using two different epitope tags and a selective immunoprecipitation protocol, we purified phosphorylated DARPP-32 protein from D1R- or D2R-expressing neurons from mouse striatal tissue. This new methodology allows a quantitative side-by-side comparison of phosphorylation events occurring selectively in two distinct neuronal populations in vivo.
We have applied this technique to analyze phosphorylation responses to several classes of drugs which target the striatum, namely dopamine receptor agonists, psychostimulants, and antipsychotics. Our findings revealed that although cocaine and haloperidol both increased DARPP-32 T34 phosphorylation, cocaine increased T34 phosphorylation only in striatonigral neurons while haloperidol acted selectively on striatopallidal neurons. Additionally, we observed distinct cell-type specific patterns of phosphorylation for different sub-classes of psychostimulant and antipsychotic drugs. Furthermore, we found that dopamine receptor agonists exert different effects in vivo compared with acute striatal slices, revealing the complexity of dopamine signaling in the brain.
RESULTS
Targeting striatonigral and striatopallidal neurons
To study DARPP-32 phosphorylation selectively in striatonigral and striatopallidal neurons, we generated BAC transgenic mice that express c-terminal Flag tagged DARPP-32 and Venus fluorescent protein under the control of the Drd1a (D1R) promoter and mice which express c-terminal Myc tagged DARPP-32 and ECFP under the control of the Drd2 (D2R) promoter (Fig. 1a). The Internal Ribosome Entry Site (IRES) in these constructs allowed separate translation of tagged DARPP-32 and fluorescent marker proteins from the same mRNA transcript. We chose the D1R and D2R promoters to target striatonigral and striatopallidal neurons since the levels of these receptors have been shown to be differentially expressed in the two populations of adult neurons. Using the Flag and Myc tags, we selectively immunoprecipitated DARPP-32 from D1R- and D2R-expressing neurons and analyzed phosphorylation from whole brain and striatal slices in a cell-type specific manner.
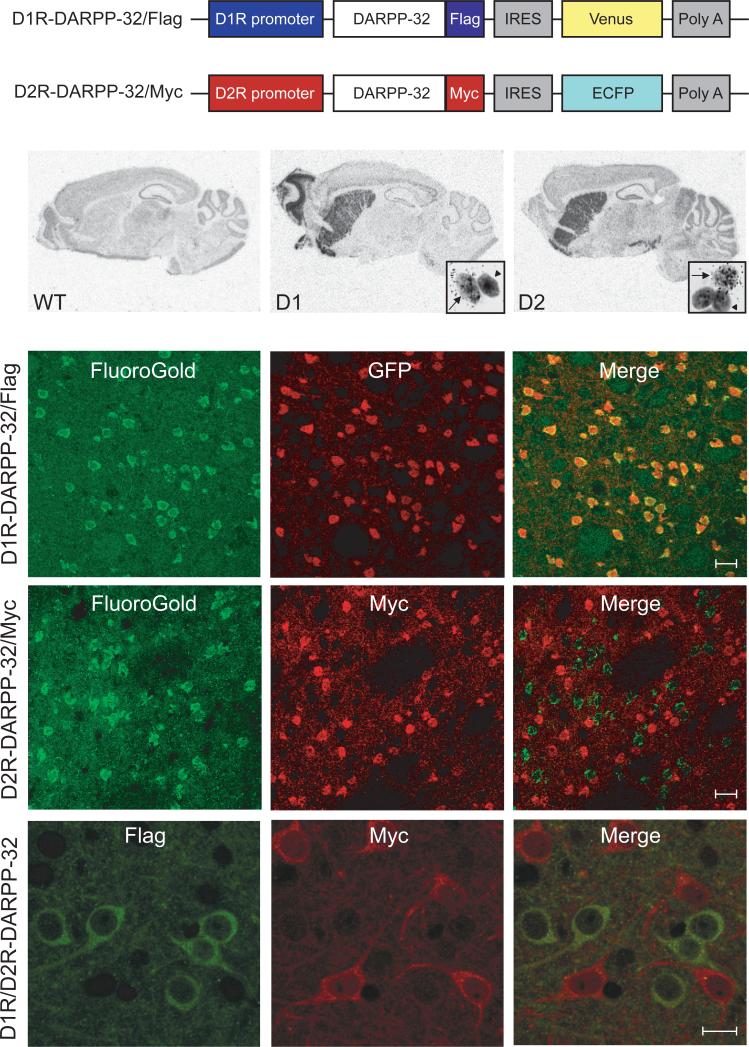
Figure 1. Generation of D1R-DARPP-32/Flag and D2R-DARPP-32/Myc mice.
(a) Schematic of the modified shuttle vectors used to generate D1R-DARPP-32/Flag and D2R-DARPP-32/Myc mice. IRES, internal ribosome entry site; Poly A, polyadenylation sequence; Venus, venus fluorescent protein; ECFP, enhanced cyan fluorescent protein. (b) In situ hybridization using a 35S labeled antisense probe against GFP which recognizes both Venus and ECFP mRNA shows the expression of the constructs in sagittal brain sections from wild-type (WT), D1R-DARPP-32/Flag (D1), and D2R-DARPP-32/Myc (D2) mice. Insets show magnification of GFP positive striatal cells labeled with silver grains (arrows) and unlabeled cells (arrowheads) after dipping sections in emulsion. (c) FluoroGold retrograde tracer was injected into the substantia nigra pars reticulata. Double immunoflourescence (IF) in striatal sections reveals co-localization of Fluoro-Gold (green) and GFP (red) in the D1R-DARPP-32/Flag mice, scale bar = 20μm. (d) There is no co-localization of Fluoro-Gold (green) and Myc (red) in striatal sections from the D2R-DARPP-32/Myc mice, scale bar = 20μm. (e) IF staining using Flag (green) and Myc (red) antibodies in striatal sections from D1R/D2R-DARPP-32 double transgenic mice showing no co-localization, scale bar = 10μm.
To confirm expression of these constructs in the correct brain areas, we performed in situ hybridization with a 35S-labeled antisense probe against GFP that recognized both Venus and ECFP mRNA (Fig. 1b). There was strong striatal expression seen in both D1R-DARPP-32/Flag and D2R-DARPP-32/Myc mice. The D1R-DARPP-32/Flag mice showed additional expression in the olfactory tubercle and deep layers of the cortex while the D2R-DARPP-32/Myc mice showed expression in midbrain dopaminergic nuclei. The expression patterns of our constructs reflected the known patterns of D1 and D2 receptor expression in mouse brain confirming accurate expression of the transgenes.
At the cellular level, we found that only a subset of striatal cells were positive for GFP mRNA in the D1R-DARPP-32/Flag (36.7% −188/512 cells) and D2R-DARPP-32/Myc (41.4% - 147/355 cells) mice (Fig. 1b). To confirm that these labeled cells were indeed striatonigral or striatopallidal neurons, we took advantage of the fact that these cell types have different projection patterns. Striatonigral neurons send distal projections to the substantia nigra pars reticulata (SNpr) while striatopallidal neurons project more proximally to the globus pallidus. We injected Fluoro-Gold (FG) retrograde tracer distally into the SNpr which was selectively taken up by axon terminals of striatonigral neurons and sent via retrograde transport to cell bodies in the striatum.
Double immunofluorescence in striatal sections revealed near 100% co-localization of FG and GFP in the D1R-DARPP-32/Flag mice (Fig. 1c) and no co-localization of FG and Myc in D2R-DARPP-32/Myc mice (Fig. 1d). These results confirmed that the D1R construct was expressed selectively in striatonigral neurons, whereas the D2R construct was excluded from these cells. Since medium spiny neurons are known to project either to the substantia nigra or the gloubs pallidus, we conclude that the D2R-DARPP-32/Myc construct was expressed in striatopallidal neurons.
After confirming selective targeting of the D1R and D2R constructs, the D1R-DARPP-32/Flag and D2R-DARPP-32/Myc mice were crossed to generate double transgenic mice. Co-staining with Flag and Myc antibodies in striatal sections from D1R/D2R-DARPP-32 mice confirmed that expression of the tagged constructs was indeed restricted to different striatal cell populations (Fig. 1e).
Cell-type specific DARPP-32 immunoprecipitations
Double transgenic D1R/D2R-DARPP-32 mice were used for all biochemical experiments because they allow direct comparison of DARPP-32 phosphorylation in D1R and D2R-expressing neurons in response to drug treatments within the same mouse. A schematic outline of the in vivo biochemical experiments is shown in Figure 2a. The success of this method relied on accurate expression of tagged DARPP-32 protein as well as high specificity of the Flag and Myc immunoprecipitations (IP's).
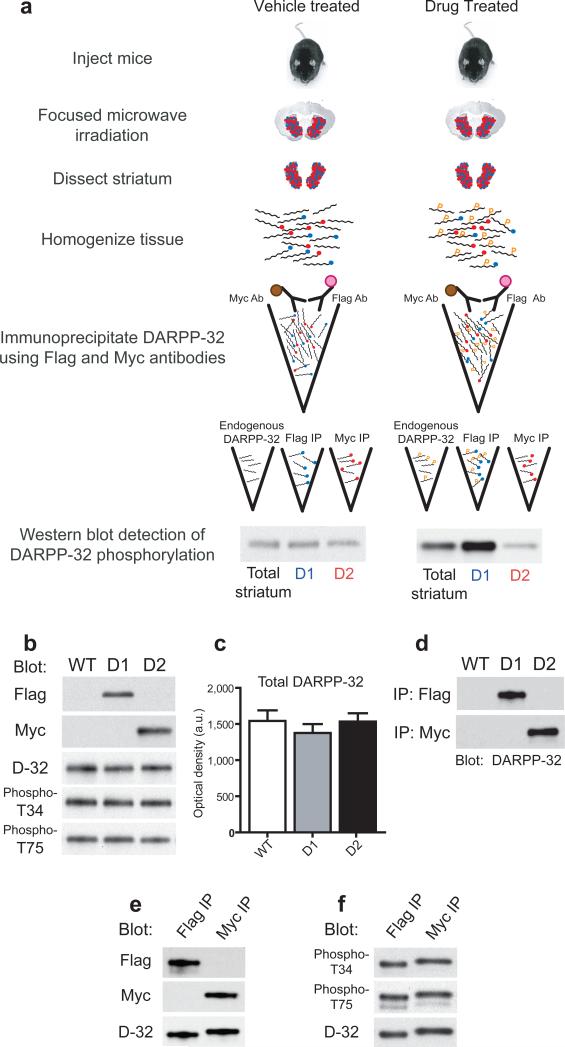
Figure 2. Experimental design and tagged DARPP-32 immunoprecipitation.
(a) Schematic of the procedure for in vivo biochemical experiments using D1R/D2R-DARPP-32 double transgenic mice. One feature of this method is the ability to incubate striatal homogenates simultaneously with Myc and Flag antibodies by differential coupling of these antibodies to magnetic beads (brown circles) and affinity gel (pink circles), respectively. Blue circles represent Flag tags, red circles represent Myc tags. “P” indicates phosphorylated protein. (b) Equal amounts of striatal homogenate from wild-type (WT), D1R-DARPP-32/Flag (D1), and D2R-DARPP-32/Myc (D2) single transgenic mice were blotted with Flag, Myc, total DARPP-32, phospho-T34, and phospho-T75 antibodies. (c) Quantification of the average optical density of total DARPP-32 bands from wild-type (WT), D1R-DARPP-32 (D1), and D2R-DARPP-32 (D2) mice (n=3−4 mice per group). Data were normalized to GAPDH loading control (not shown). (d) Striatal homogenates from wild-type (WT), D1R-DARPP-32/Flag (D1), and D2R-DARPP-32/Myc (D2) mice were incubated with Flag (upper panel) and Myc (lower panel) IP antibodies. Equal amounts of IP'd sample was loaded in each lane and blotted with an antibody against total DARPP-32. (e) Striatal homogenate prepared from D1R/D2R-DARPP-32 double transgenic mice was incubated with Flag or Myc antibodies and IP eluates were blotted with either Flag or Myc antibody. Membranes were striped and re-probed with total DARPP-32 antibody (bottom panel) showing equal loading of IP's. (f) Representative blot of the basal level of T34 and T75 phosphorylation in Flag and Myc IP'd samples from D1R/D2R-DARPP-32 mice detected by phospho-specific antibodies. The phospho-T34 blot was stripped and re-probed with total DARPP-32 antibody (bottom panel).
Western blotting of striatal homogenates revealed Flag and Myc positive bands at 32−34kD in the D1R-DARPP-32/Flag and D2R-DARPP-32/Myc mice, respectively (Fig. 2b). However, we found no detectable change in total DARPP-32, phospho-T34, or phospho-T75 protein levels in the transgenic mice (Fig. 2b,c). Subsequent analysis comparing levels of tagged DARPP-32 with endogenous DARPP-32 revealed that the Flag tagged DARPP-32 represented approximately 3% of endogenous DARPP-32, while the Myc-tagged DARPP-32 represented 1% of endogenous DARPP-32 (Supplemental Figure 1). DARPP-32 is expressed at very high levels in the striatum and the difference in expression between endogenous and tagged DARPP-32 was due to differences in strength between the DARPP-32 and D1R and D2R promoters used to express the constructs. Tagging a small percentage of the DARPP-32 in these neurons was advantageous as we could study cell-type specific changes in phosphorylation without perturbing the endogenous system. Indeed, we did not observe any biochemical or behavioral phenotype resulting from expression of the constructs. The fact that basal T34 and T75 phosphorylation levels were unaltered further indicates that there were no major signaling alterations in these neurons due to the expression of the tagged proteins.
We developed an immunoprecipitation (IP) protocol using antibodies against the Flag and Myc tags which allowed selective purification of DARPP-32 from D1R and D2R-expressing striatal neurons (Fig. 2d). In double transgenic mice we confirmed that there was no contaminating Flag tagged protein present in the Myc IP sample and that there was no Myc contamination in the Flag IP sample (Fig. 2e). We observed a small size shift in the DARPP-32 bands from the IP eluates due to the presence of the Flag and Myc tags (Fig. 2e,f and Supplemental Figure 1). Importantly, we were able to detect basal levels of T34 and T75 phosphorylation in both the Flag and Myc IP samples showing that the tagged DARPP-32 could be phosphorylated in vivo (Fig. 2f).
Effects of D1 and D2 receptor agonists in striatal slices
To establish the validity of the methodology, we analyzed the effects of selective D1R and D2R agonists on DARPP-32 phosphorylation in striatonigral and striatopallidal neurons. Drugs were first tested in acutely prepared striatal slices since the effects of D1R and D2R agonists on DARPP-32 phosphorylation have been well established in this system3, 15. Stimulation of Gs/olf -coupled D1 receptors activates the cAMP/PKA/PP-2A cascade which increases phosphorylation of T34 and decreases phosphorylation of T753, 4, 15. Our results in total striatal homogenates were in agreement with previous studies as the D1R agonist SKF 81297 caused a significant increase in T34 phosphorylation (441.3% ± 64.6% of control, p<.01) and a trend towards a decrease in T75 phosphorylation (87.4% ± 8.3% of control; Fig. 3a,b).
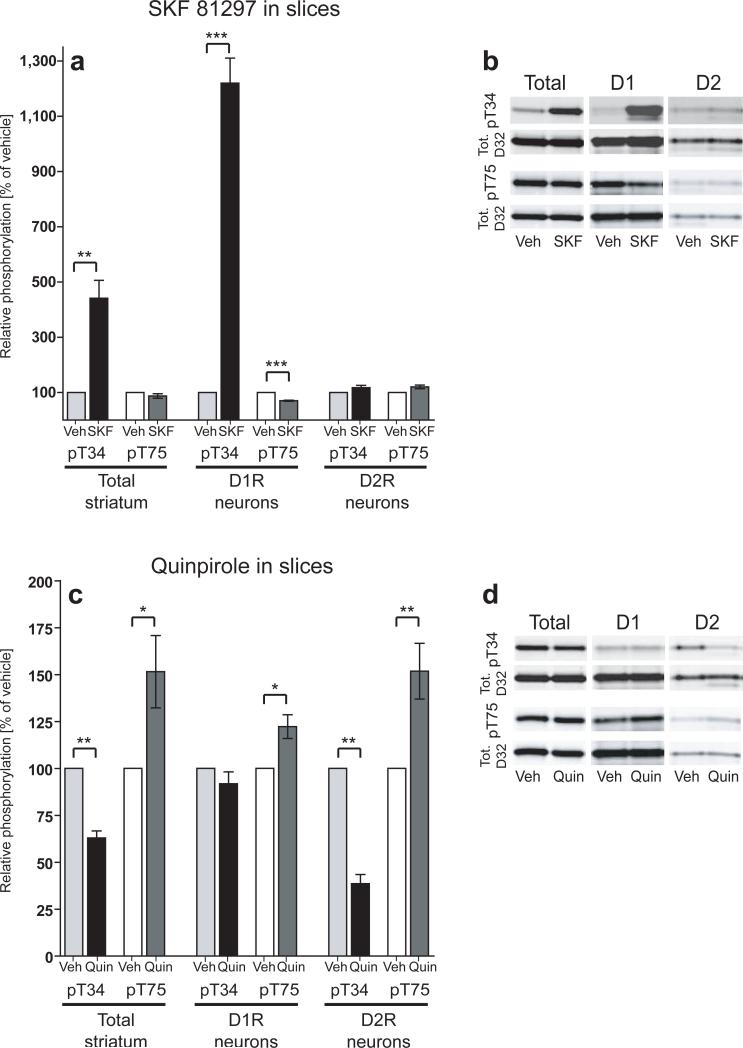
Figure 3. Effects of D1R and D2R agonists on DARPP-32 phosphorylation in striatal slices.
(a,c) Bar graphs represent group averages of DARPP-32 phosphorylation from striatal slices incubated with vehicle, SKF 81297 (10 μM, 5 min.), or quinpirole (1 μM, 10 min.). Phospho-T34 and phospho-T75 data were normalized to total DARPP-32 levels and expressed as percent of vehicle control. The left panels represent data from endogenous non-tagged DARPP-32 (Total striatum), the center panels represent data from Flag-tagged DARPP-32 (D1R neurons), and the right panels represent data from Myc-tagged DARPP-32 (D2R neurons). In each experiment 6 striatal slices from 1 mouse were divided into vehicle, SKF 81297, and quinpirole treatment groups and slices from 3 mice were pooled for the analysis of DARPP-32 phosphorylation. Data represent means from 4−8 independent experiments. *, p<.05, **, p<.01, ***, p<.001. (b,d) Representative western blots from treated slices. T34 and T75 phospho-specific blots (top panels) were stripped and re-probed with total DARPP-32 antibody (bottom panels).
We examined phosphorylation responses in D1R and D2R neurons and found that in D1R neurons, SKF 81297 induced a robust 12-fold increase (1220% ± 90.3% of control, p<.001) in T34 phosphorylation and a significant decrease in T75 phosphorylation (70.3% ± 2% of control, p<.001; Fig. 3a,b). There were no changes in phosphorylation at either site in D2R neurons (Fig. 3a,b).
Application of the D2R agonist quinpirole also produced effects on DARPP-32 phosphorylation which were largely cell-type specific (Fig. 3c,d). Activation of Gi-coupled D2 receptors inhibits the cAMP/PKA/PP-2A signaling cascade resulting in a decrease in T34 phosphorylation and an increase in T75 phosphorylation in total striatal homogenates3. Using D1R/D2R-DARPP-32 mice, we replicated these findings and showed that these effects were specific to D2R neurons (T34 decreased to 38.4% ± 5% of control, p<.01 and T75 increased to 151.7% ± 14.9% of control, p<.01; Fig. 3c,d). In D1R neurons, there was no change in T34 phosphorylation; however, quinpirole induced a small increase in T75 phosphorylation (122.2% ± 6% of control, p<.05; Fig. 3c,d). This effect can be explained by the stimulation of D2 autoreceptors in the slice which would decrease dopamine release16, thereby indirectly affecting DARPP-32 phosphorylation in D1R neurons.
Effects of D1 and D2 receptor agonists in vivo
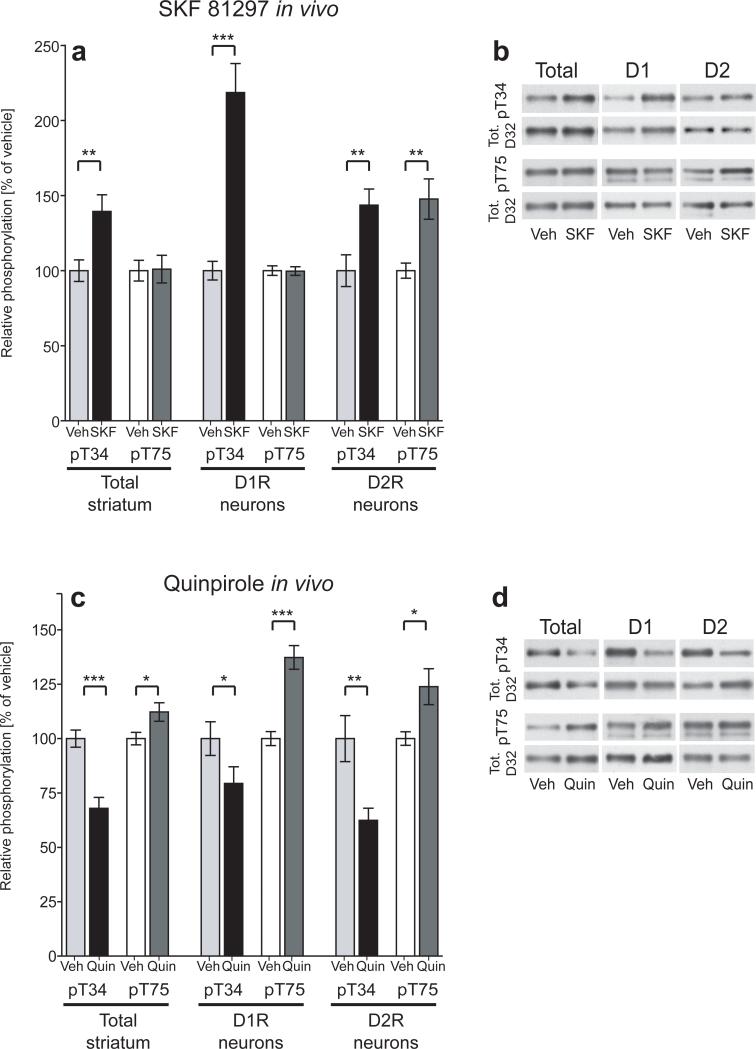
Similar to striatal slices, injection of the D1R agonist SKF 81297 in vivo resulted in a large increase in T34 phosphorylation which occurred selectively in D1R neurons (219% ± 19.3% of control, p<.001; Fig. 4a,b). In contrast to slices, there were moderate but significant increases in both T34 and T75 phosphorylation seen in D2R neurons in vivo (144% ± 10.7% of control, p<.01 for T34; and 148% ± 13.4% of control, p<.01 for T75; Fig. 4a,b). Because these effects were observed in vivo but not in striatal slices, it is likely that the changes observed in D2R neurons were due to stimulation of extra-striatal D1 receptors which could indirectly influence signaling in the striatum.
Figure 4. Effects of D1R and D2R agonists on DARPP-32 phosphorylation in vivo.
(a,c) Bar graphs represent group averages of DARPP-32 phosphorylation from D1R/D2R-DARPP-32 mice injected i.p. with vehicle, SKF 81297 (5mg/kg), or quinpirole (0.2 mg/kg) and sacrificed 15 minutes later. Phospho-T34 and T75 data were normalized to total DARPP-32 levels expressed as percent of vehicle control. The left panels represent data from endogenous non-tagged DARPP-32 (Total striatum), the center panels represent data from Flag-tagged DARPP-32 (D1R neurons), and the right panels represent data from Myc-tagged DARPP-32 (D2R neurons). Each bar represents the mean of 7−12 mice from 2−3 independent experiments. *, p<.05, **, p<.01, ***, p<.001. (b,d) Representative western blots from drug treated D1R/D2R-DARPP-32 mice. T34 and T75 phospho-specific blots (top panels) were stripped and re-probed with total DARPP-32 antibody (bottom panels).
Injection of the D2R agonist quinpirole in vivo also resulted in changes in DARPP-32 phosphorylation in both cell types (Fig. 4c,d). As seen with slices, quinpirole significantly decreased T34 phosphorylation (67.9% ± 4.9% of control, p<.001 in total homogenates; 62.4% ± 5.6% of control, p<.01 in D2R neurons) and increased T75 phosphorylation (112.2% ± 4.2% of control, p<.05 in total homogenates; 123.9% ± 8.2% of control, p<.05 in D2R neurons) in total striatal homogenates and in D2R neurons (Fig. 4c,d). Interestingly, quinpirole also caused a significant decrease in T34 phosphorylation (79.4% ± 7.7% of control, p< .05) and an increase in T75 phosphorylation (137.3% ± 5.4% of control, p<.001) in D1R neurons (Fig. 4c,d). This D2R agonist effect on D1R neurons in vivo can be explained by the presence of D2 autoreceptors on dopaminergic nerve terminals which negatively regulate the release of dopamine 16, 17. Decreased dopamine binding at D1 receptors would deactivate the cAMP/PKA/PP-2A cascade resulting in decreased T34 phosphorylation and increased T75 phosphorylation as observed here.
Differential effects of cocaine and haloperidol in vivo
To address the paradox of how psychostimulants and antipsychotics can produce similar effects on T34 phosphorylation in total striatum, we injected D1R/D2R-DARPP-32 mice acutely with cocaine or haloperidol. Cocaine blocks the dopamine transporter increasing the availability of dopamine at the synapse. Increased dopamine is expected to activate both Gs/olf-coupled D1 receptors and Gi-coupled D2 receptors. It has been shown previously that acute cocaine increases T34 phosphorylation and decreases T75 phosphorylation in total striatum3. We reproduced these findings; however, cell-type specific analysis revealed differential and opposing effects in D1R and D2R neurons (Fig. 5a). Specifically, we observed a robust increase in T34 phosphorylation in D1R neurons (192% ± 10.5% of control, p<.001) but a significant decrease in T34 phosphorylation in D2R neurons (72.5% ± 3.3% of control, p<.01; Fig. 5a). Conversely, T75 phosphorylation was decreased in D1R neurons (65% ± 4.4% of control, p<.001) and increased in D2R neurons (123.4% ± 9.3% of control, p<.05; Fig. 5a).
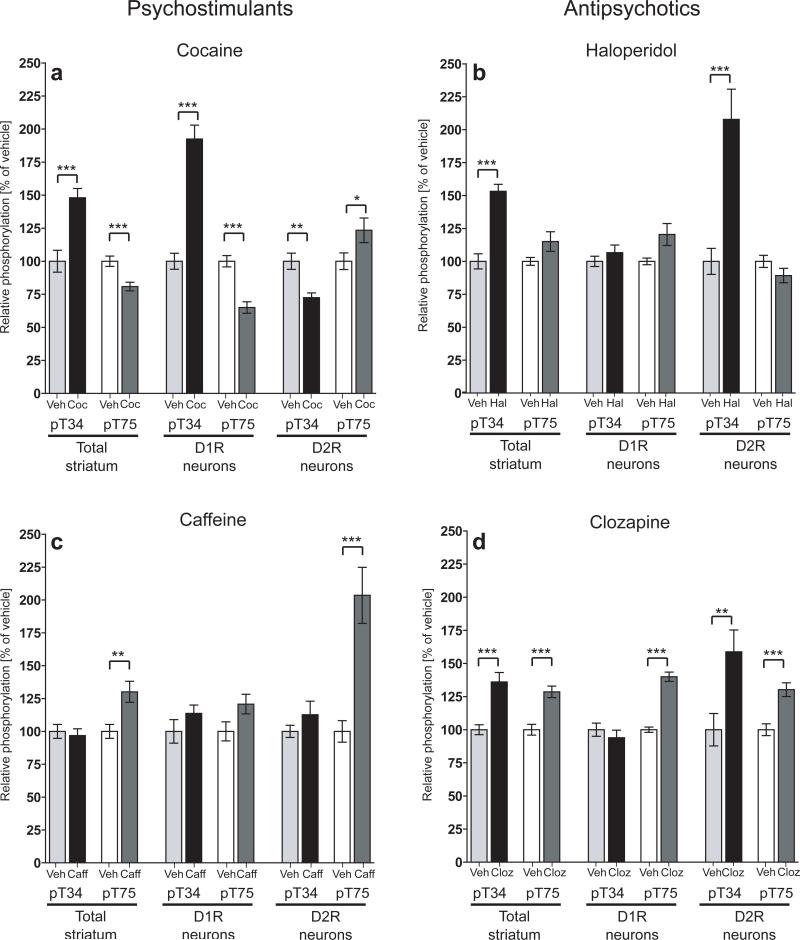
Figure 5. Differential regulation of DARPP-32 phosphorylation by psychostimulants and antipsychotics.
D1R/D2R-DARPP-32 mice were injected i.p. with either (a) cocaine (20 mg/kg), (b) haloperidol (1 mg/kg), (c) caffeine (7.5 mg/kg), (d) clozapine (5 mg/kg), or vehicle and sacrificed 15 minutes later. Phospho-T34 and T75 data were normalized to total DARPP-32 levels and expressed as percent of control. The left panels represent data from endogenous non-tagged DARPP-32 (Total striatum), the center panels represent data from Flag-tagged DARPP-32 (D1R neurons), and the right panels represent data from Myc-tagged DARPP-32 (D2R neurons). Each bar represents the mean of 7−14 mice from 2−4 experiments. *, p<.05, **, p<.01, ***, p<.001.
Haloperidol is a typical antipsychotic known to be a strong antagonist of D2 receptors18. Injection of haloperidol resulted in a markedly different pattern of DARPP-32 phosphorylation compared with cocaine (Fig. 5b). Although haloperidol induced an increase in T34 phosphorylation in the total striatal sample similar to that found with cocaine (153.2% ± 5.2% of control, p<.001), this increase was highly specific to D2R neurons (207.8% ± 22.9% of control, p<.001) as there was no change in T34 phosphorylation in D1R neurons (Fig. 5b). Additionally, there was no significant regulation of T75 phosphorylation in either cell type. These results directly show that although cocaine and haloperidol both stimulate DARPP-32 T34 phosphorylation, this effect is restricted to different cell populations.
Cell-type specific effects of caffeine and clozapine
Cocaine and haloperidol act primarily on the dopaminergic system. Caffeine is a psychostimulant drug which has been shown to affect adenosine signaling via antagonism of A2A receptors19. A2A receptors are highly expressed in the striatum and selectively localized to striatopallidal neurons where they positively regulate the cAMP/PKA/PP-2A cascade, thereby opposing D2R signaling in these cells20. In agreement with previous studies21, we found that caffeine increased T75 phosphorylation in total striatal homogenates of D1R/D2R-DARPP-32 mice (130.1% ± 8% of control, p<.01; Fig. 5c). The effect of caffeine on T75 phosphorylation was found to be highly specific for D2R neurons (203.5% ± 21.3% of control, p<.001), as there was no effect on either phosphorylation site in D1R neurons (Fig. 5c).
Clozapine is an example of an atypical antipsychotic drug which exhibits less activity at D2 receptors and greater binding of other neuromodulatory receptors such as serotonin receptors18. Despite differences in their receptor profiles, haloperidol and clozapine both increased T34 phosphorylation in total striatal homogenates (153.2% ± 5.2% of control, p<.001 for haloperidol and 140.7% ± 7.7% of control, p<.001 for clozapine, Fig. 5b and d). As with haloperidol, the clozapine-induced increase in T34 phosphorylation was specific to D2R neurons, although at the doses tested here, this increase was smaller than with haloperidol (158.8% ± 16.4% of control, p<.001 for clozapine, versus 207.8% ± 22.9% of control, p<.01 for haloperidol, Fig. 5b and d). Interestingly, there were also moderate but significant increases in T75 phosphorylation in both D1R and D2R neurons with acute clozapine treatment which were not seen with haloperidol (140% ± 3.5% of control, p<.001 in D1R neurons; 130.2% ± 5.2% of control, p<.001 in D2R neurons, Fig. 5d) most likely reflecting the broader spectrum of receptors targeted directly by clozapine18.
DISCUSSION
The study of biochemical processes in the striatum has long been hindered by the difficulty in selectively studying signaling responses in striatonigral and striatopallidal neurons due to the fact that they are morphologically homogeneous and anatomically intermixed. To overcome this obstacle we have developed a novel methodology which allows cell-type specific analysis of DARPP-32 phosphorylation in these two neuronal populations. Using this technique we have resolved the paradox that psychostimulants and antipsychotics cause the same biochemical change in the striatum but have opposing behavioral and clinical effects. We showed that the psychostimulant cocaine increases DARPP-32 T34 phosphorylation selectively in striatonigral neurons, while the antipsychotic haloperidol increases T34 phosphorylation only in striatopallidal neurons. Since these two populations are coupled to opposing output pathways, the differential regulation of these neurons can explain the drugs’ opposing behavioral effects. Furthermore, we found that other types of psychostimulant and antipsychotic drugs also resulted in differential patterns of DARPP-32 phosphorylation in striatonigral and striatopallidal neurons which could not be shown with conventional techniques. Additionally, we found that while D1 and D2 receptor agonists selectively target striatonigral or striatopallidal neurons in striatal slices, administration of either of these drugs in vivo affected DARPP-32 phosphorylation in both cell populations.
The importance of studying cell-type specific responses in the striatum is exemplified by our results with cocaine which provide experimental support for the classical model of the basal ganglia. This model states that the striatonigral and striatopallidal pathways exert opposing influence on the basal ganglia's control of locomotor function13. A hallmark behavioral response to cocaine is a robust induction of locomotor activity22. It was previously shown that acute cocaine caused an increase in DARPP-32 T34 phosphorylation and a decrease in T75 phosphorylation, a pattern associated with an increase in excitability in striatal neurons3, 23. If this effect occurred in all striatal cells, then both pathways would be activated, effectively canceling out their behavioral effects. We observed that the cocaine-induced increase in T34 and decrease in T75 phosphorylation occurred selectively in D1R-striatonigral neurons, while an opposite phosphorylation pattern was seen in D2R-striatopallidal neurons. This differential regulation of signaling in striatonigral and striatopallidal neurons shows how cocaine, a dopamine potentiator, can modulate signaling in both circuits in ways expected to result in a cooperative effect on the basal ganglia's control of locomotor behavior.
In contrast to cocaine, the typical antipsychotic haloperidol can cause behavioral sedation and catalepsy18. Despite their opposing behavioral effects, cocaine and haloperidol have both been shown to increase T34 phosphorylation in the striatum3, 24. Here we show that although both drugs increased T34 phosphorylation in total striatal samples, the increase was restricted to D1R neurons with cocaine and to D2R neurons with haloperidol. Haloperidol is expected to act on D2R-expressing striatopallidal neurons since it has been shown to have high antagonist activity at D2 receptors18. Striatopallidal neurons also express A2A receptors which counteract D2R signaling by increasing cAMP and PKA activity20. Haloperidol blocks the inhibitory effect of D2 receptor activation, shifting the balance towards positive regulation of cAMP by A2A receptors25. This could account for the observed increase in T34 phosphorylation in D2R neurons.
The psychostimulant caffeine, known to be an antagonist of A2A receptors19, also selectively targeted D2R-striatopallidal neurons. In contrast to haloperidol which increased T34 phosphorylation, caffeine increased T75 phosphorylation in D2R neurons. Since T34 and T75 oppositely regulate the function of DARPP-32, namely inhibition of PP-1 or PKA, respectively2, the differential regulation of these sites by haloperidol and caffeine suggests a biochemical mechanism for the drugs’ opposing behavioral effects.
Clozapine is an example of an atypical antipsychotic drug which has low D2R activity but has affinity for several classes of serotonergic, adrenergic, muscarinic, and histaminergic receptors18. Although haloperidol and clozapine both increased T34 phosphorylation selectively in D2R neurons, the increase was larger with haloperidol than with clozapine. Clozapine also had additional effects on T75 phosphorylation which were present in both D1R and D2R neurons, possibly due to effects on 5-HT6 receptors which are expressed in both cell types.
The regulation of T34 phosphorylation in D2R neurons may represent a common mechanism of action of antipsychotics. Indeed, it has been suggested that many classes of antipsychotic drugs, regardless of their primary receptor targets, can affect D2R signaling to some degree18. The very high level of T34 activation in D2R neurons in response to haloperidol may exceed the beneficial level and reach levels associated with unwanted locomotor side effects. In accordance with this idea, clinical studies have shown that in vivo D2 receptor occupancy by antipsychotics exceeding 80% is often associated with extra-pyramidal side effects26. The more limited increase in T34 phosphorylation balanced by increases in T75 seen with clozapine likely reflects this drug's ability to target several different pathways.
In addition to the study of psychostimulants and antipsychotics, we examined the effects of selective D1R and D2R agonists in vivo and in striatal slices. In acute striatal slices, where afferent and efferent connections are removed our results followed predictions that D1R agonists would act selectively on striatonigral neurons while D2R agonists would target striatopallidal neurons. However, in vivo, these agonists affected DARPP-32 phosphorylation in both cell types. This can be explained by the presence of extra-striatal D1 and D2 receptors which would be targeted by an in vivo injection. The impact of these receptors is readily seen in our results obtained with the D2R agonist quinpirole. In addition to stimulation of post-synaptic D2 receptors, quinpirole activates pre-synaptic D2 autoreceptors on dopaminergic terminals which inhibit the release of dopamine16, 17. Decreased dopamaine would produce an effect on D1 receptors similar to blockade of D1 receptors, namely a decrease in the cAMP/PKA/PP-2A signaling cascade. Decreased PKA activity would result in decreased in T34 phosphorylation while decreased PP-2A activity would cause an increase in T75 phosphorylation in D1R neurons, as observed here. Although dopaminergic terminals are severed in striatal slices decreasing overall dopaminergic tone, it is likely that stimulation of some pre-syanptic receptors by quinpirole further reduces dopamine release resulting in the small effect on T75 phosphorylation observed in D1R neurons in striatal slices. In D2R neurons, direct activation of post-synaptic D2 receptors by quinpirole overrides any change in dopamine levels induced pre-synaptically.
Regarding extra-striatal D1 receptors, it is known that they are expressed in the cortex and limbic regions which send glutamatergic projections to the striatum27, 28. Alterations in striatal glutamate release would result in complex changes in both T34 and T75 phosphorylation which could impact signaling in both striatonigral and striatopallidal neurons29. D1 receptors are also located on striatonigral terminals and stimulation of these receptors can increase GABA release to the ventral tegmental area30. This would result in decreased striatal dopamine levels and could account for the observed increase in T34 phosphorylation in D2R neurons. The ability of D1R agonists to act on D2R neurons is not unprecedented as a previous study showed that in vivo injection of a different D1 agonist, SKF 82958, resulted in cFos activation in both striatonigral and striatopallidal neurons31. These findings reveal the complexity of dopamine signaling in vivo, and highlight the advantage of genetic targeting of these neurons as pharmacological manipulations may indirectly affect signaling in both cell populations.
Conclusions
In addition to the drugs tested here, there are a variety of agents which have been shown to regulate DARPP-32 phosphorylation in striatal neurons6. For many of these drugs it is not known whether they differentially affect signaling in striatonigral or striatopallidal neurons. The importance of selectively analyzing responses in these two neuronal populations is becoming increasingly clear as recent studies have shown important differences between D1R and D2R-expressing neurons with regard to their synaptic properties and regulation of dendritic spine number32-34. We have expanded on these studies by developing a novel BAC transgenic mouse method which allows cell-type specific analysis of biochemical signaling events in these neurons. In principle, this methodology can be applied to study any type of post-translational modification on any protein in any cell type of interest. We expect that future studies utilizing cell-type targeted approaches will be vital to a thorough understanding of cell signaling in the basal ganglia and other brain systems.
METHODS
Generation of tagged DARPP-32 BAC transgenic mice
All animal protocols were performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Rockefeller University Institutional Animal Care and Use Committee.
D1R-DARPP-32/Flag and D2R-DARPP-32/Myc BAC mice were generated according to the GENSAT bacterial artificial chromosome (BAC) modification protocol35. Detailed protocols for these procedures can be found at www.gensat.org. For the D1R-DARPP-32/Flag mouse, a 532bp segment of the Drd1a promoter corresponding to the region immediately adjacent to the start codon was placed 5’ to mouse DARPP-32 cDNA containing a c-terminal Flag tag (DYKDDDDK) in a shuttle vector containing the IRES-Venus sequence. This modified shuttle vector was recombined into the BAC containing the full Drd1a promoter. BAC DNA from positive co-integrates was injected into pronuclei from FVB/N oocytes to generate transgenic mice.
For the D2R-DARPP-32/Myc mouse, a 484bp segment of the Drd2 promoter corresponding to the region immediately adjacent to the start codon was placed 5’ to mouse DARPP-32 cDNA containing a c-terminal Myc tag (SEQKLISEEDL). Venus cDNA was excised from the vector and replaced with ECFP cDNA (Clontech). This modified vector was recombined in to the Drd2 BAC and prepared as described above.
Litters were screened for positive transgenics by PCR genotyping from tail DNA using the following primers: D1R-DARPP-32/Flag mice: Forward-AGGTCCTGAAAGGCAGCAG, Reverse-CTTATCGTCGTCGTCCTTGTAGTC. D2R-DARPP-32/Myc mice: Forward-GAGGATGAAGAGGAGGACGA, Reverse-CAGAAATCAATTTTTGTTCAGAGG. Positive mice were backcrossed more than 10 generations to the C57Bl/6 background. Backcrossed D1R/DARPP-32/Flag and D2R-DARPP-32/Myc mice were bred to obtain double transgenic mice expressing both Flag- and Myc-tagged DARPP-32. Male mice heterozygous for both Flag and Myc were used for all experiments.
In situ hybridization
12μm sagittal sections of fresh frozen brain from wild-type and transgenic mice were processed for in situ hybridization with a 35S labeled antisense riboprobe against full-length GFP using a protocol described previously36. Briefly, slides were fixed in 4% paraformaldehyde, and washed twice in 4x SSC buffer. Sections were acetylated for 10 minutes and then dehydrated in a series of ethanols and incubated overnight at 55°C in a humidity chamber with 2 × 106 cpm of probe per slide mixed in hybridization buffer. The next day slides were washed 4 times in 4x SSC and incubated with RNAse A for 15 minutes at 37°C. Slides were then washed with a series of SSC buffers of decreasing concentration. Sections were dehydrated, dried, and placed on Kodak MR film for 10 days.
To visualize GFP labeling at the cellular level, slides were dipped in Kodak NTB emulsion and left in the dark for 6 weeks. Slides were developed and counterstained with cresyl violet. Pictures of labeled cells were taken on a Zeiss microscope using a 63x water objective and AxioVision software.
Fluoro-Gold Retrograde Labeling
6−8 week old D1R-DARPP-32/Flag and D2R-DARPP-32/Myc mice were anesthetized sodium pentobarbital. The mice were placed in a stereotaxic apparatus (Kopf) and injected bilaterally with 1.0ul of 0.8% Fluoro-Gold (Fluorochrome, LLC) into the SNpr at a rate of 250nl/minute. Coordinates for injection into the SNpr were A/P −2.9, M/L +/−1.5, D/V −4.7 from Bregma. Mice were perfused 5 days following the injection.
Immunohistochemistry
Mice were perfused transcardially with 1x PBS and 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde for one hour, cryoprotected in sucrose, and frozen at −80°C until sectioning. 12μm coronal sections were blocked for one hour in 2% normal serum and incubated overnight at 4°C with antibodies against Fluoro-Gold (1:3500, Chemicon), GFP (1:1000, gift of Dr. Myriam Heiman), Flag (1:2000, Sigma), or Myc (1:2500 or 1:10,000, Novus). Slides were washed and incubated with the appropriate fluorescent secondary antibody (1:200) at room temperature for one hour (Cy2, Cy3, FITC, or Rhodamine-Red conjugated, all from Jackson ImmunoResearch). Fluorescent images were taken on a Zeiss confocal microscope using 40x and 63x oil objectives.
Flag and Myc-tagged DARPP-32 immunoprecipitations
Bilateral striata from one mouse (in vivo experiments) or six striatal slices (slice experiments) were sonicated in 500ul IP lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, Complete Mini protease inhibitors (Calbiochem), and Halt phosphatase inhibitors (Pierce)). Homogenates were spun down for 20 minutes at 4°C at 13K rpm. Supernatant was removed and 50 uL/IP of washed EZView Red anti-Flag M2 affinity gel (Sigma) was added along with 45 uL/IP of anti-Myc antibody (Novus) coupled to magnetic beads (Dynabeads M-280 Tosylactivated, Invitrogen). The homogenate/antibody mixture was gently rotated overnight at 4°C. The Myc antibody was coupled to the Dynabeads following the protocol in the product manual; 3ug of Myc antibody was added for every 5ul of magnetic beads.
Following the overnight incubation, the homogenate/antibody mixture was placed on a magnetic particle concentrator (“MPC”, Invitrogen) for 2 minutes to separate the Myc magnetic beads from the Flag affinity gel. The supernatant containing the unbound homogenate and Flag affinity gel was removed to a new tube and kept on ice. The Myc magnetic beads were washed three times in 1x PBS using the MPC to separate the beads each time. After the final wash, 30uL of non-reducing sample buffer (Pierce) was added and the beads were boiled for two minutes. Eluted supernatants were removed from the beads reduced with B-mercaptoethanol. Samples were stored at −80°C until used for immunoblotting.
Flag IP/homogenate mixtures were spun down for 30 seconds at 13,000 rpm and unbound supernatant was removed to a fresh tube. This represented the total striatum sample. 1ul of this unbound supernatant was used in a BCA protein assay (Pierce) to determine protein concentration. Flag affinity gel was washed three times in 1x PBS and applied to the MPC to remove any residual magnetic beads. Flag IP's were eluted and stored at −80°C as described above.
In addition to the anti-Myc-coupled Dynabeads, anti-Myc-coupled agarose (Novus) was also used for the Myc IP's with identical results. For this protocol, Myc-agarose was incubated with the striatal homogenate for 6 hours at 4°C. The unbound supernatant was subsequently incubated with Flag-affinity gel overnight at 4°C. Beads were washed and eluted as described above.
Immunoblotting
Flag IP, Myc IP and total striatum samples were loaded onto 10.5−14% Tris-HCl gels, separated by electrophoresis and transferred to PVDF membranes. Membranes were blocked for one hour in 5% milk in 1xTBS/Tween-20 and incubated overnight at 4°C with primary antibodies against phospho-T34 (1:5000)37, phospho-T75 (1:7500)5, Myc (1:750, Cell Signaling) or Flag (1:5000, Sigma). Antibody binding was revealed by HRP conjugated goat anti-rabbit or mouse IgG (1:10,000) and the ECL detection method (PerkinElmer) with Kodak BioMax film. Membranes were stripped (Chemicon, Re-blot plus strong stripping buffer) and reprobed using a monoclonal antibody against DARPP-32 which is not phosphorylation state specific38. Total DARPP-32 antibody binding was revealed by HRP conjugated goat anti-mouse IgG (1:10,000). Quantification of bands was done by densitometry, using NIH Image software, version 1.52.
Striatal slice preparation
6−8 week old D1R/D2R-DARPP-32 mice were killed by decapitation. The brains were rapidly removed and placed in ice-cold, oxygenated Krebs–HCO3− buffer (124 mM NaCl, 4 mM KCl, 26 mM NaHCO3, 1.5 mM CaCl2, 1.25 mM KH2PO4, 1.5 mM MgSO4 and 10 mM d-glucose, pH 7.4). Coronal slices (350 μm) were prepared using a vibrating blade microtome, VT1000S (Leica Microsystems). Striata were dissected from the slices in ice-cold Krebs–HCO3− buffer. Each slice was placed in a polypropylene incubation tube with 2 ml fresh Krebs–HCO3− buffer containing adenosine deaminase (10 μg/ml). The slices were preincubated at 30 °C under constant oxygenation with 95% O2/5% CO2 for 60 min. The buffer was replaced with fresh Krebs–HCO3− buffer after 30 min of preincubation. Slices were treated with drugs as specified in each experiment (all from Sigma). After the drug treatments, slices were transferred to Eppendorf tubes, frozen on dry ice, and stored at −80°C until used in the Flag and Myc immunoprecipitation protocol described above.
In vivo drug treatments
6−8 week old male D1R/D2R-DARPP-32 double transgenic mice were used for all experiments. Mice were injected intraperitoneal with drug or vehicle and sacrificed 15 minutes later using focused microwave irradiation. Brains were removed and striata were rapidly dissected and frozen at −80°C until processed for immunoprecipitation. Drugs used: SKF 81297 5mg/kg (Tocris); Quinpirole 0.2 mg/kg, Caffeine 7.5 mg/kg, Cocaine 25 mg/kg, Haloperidol 1mg/kg, Clozapine 5 mg/kg (all from Sigma).
Statistics
Optical density of western blot bands from each treatment group were compared to the appropriate vehicle treated group using unpaired, student's t-tests. Summary data on bar graphs display the mean; error bars represent standard error of the mean.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by the National Institutes of Health grants MH074866 & DA10044, The Picower Foundation, The Simons Foundation, The Peter J. Sharp Foundation, DHHS grant 90AZ2791-03, and DOD/USAMRAA grants DAMD17-02-1-0705 and W81XWH-05-1-0146.
REFERENCES
- 1.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 2.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 3.Nishi A, et al. Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci U S A. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn JH, et al. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibb JA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 6.Svenningsson P, et al. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 7.Zachariou V, et al. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. Cocaine self-administration in mice is inversely related to phosphorylation at Thr34 (protein kinase A site) and Ser130 (kinase CK1 site) of DARPP-32. J Neurosci. 2006;26:2645–2651. doi: 10.1523/JNEUROSCI.3923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakansson K, et al. Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem. 2006;96:482–488. doi: 10.1111/j.1471-4159.2005.03558.x. [DOI] [PubMed] [Google Scholar]
- 10.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 11.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 12.Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- 13.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 14.Fink JS, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 15.Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pothos EN, Przedborski S, Davila V, Schmitz Y, Sulzer D. D2-Like dopamine autoreceptor activation reduces quantal size in PC12 cells. J Neurosci. 1998;18:5575–5585. doi: 10.1523/JNEUROSCI.18-15-05575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22:8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 19.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 20.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 21.Lindskog M, et al. Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature. 2002;418:774–778. doi: 10.1038/nature00817. [DOI] [PubMed] [Google Scholar]
- 22.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 23.Yan Z, et al. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- 24.Pozzi L, et al. Opposite regulation by typical and atypical anti-psychotics of ERK1/2, CREB and Elk-1 phosphorylation in mouse dorsal striatum. J Neurochem. 2003;86:451–459. doi: 10.1046/j.1471-4159.2003.01851.x. [DOI] [PubMed] [Google Scholar]
- 25.Svenningsson P, et al. Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience. 1998;84:223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- 26.Farde L, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 27.Jin LQ, Wang HY, Friedman E. Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J Neurochem. 2001;78:981–990. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- 28.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Nishi A, et al. Glutamate regulation of DARPP-32 phosphorylation in neostriatal neurons involves activation of multiple signaling cascades. Proc Natl Acad Sci U S A. 2005;102:1199–1204. doi: 10.1073/pnas.0409138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- 31.Le Moine C, Svenningsson P, Fredholm BB, Bloch B. Dopamine-adenosine interactions in the striatum and the globus pallidus: inhibition of striatopallidal neurons through either D2 or A2A receptors enhances D1 receptor-mediated effects on c-fos expression. J Neurosci. 1997;17:8038–8048. doi: 10.1523/JNEUROSCI.17-20-08038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day M, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 33.Lee KW, et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci. 2008;28:1185–1197. doi: 10.1523/JNEUROSCI.3908-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong S, Yang XW, Li C, Heintz N. Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication. Genome Res. 2002;12:1992–1998. doi: 10.1101/gr.476202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svenningsson P, et al. Cellular distribution of adenosine A2A receptor mRNA in the primate striatum. J Comp Neurol. 1998;399:229–240. doi: 10.1002/(sici)1096-9861(19980921)399:2<229::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemmings HC, Jr., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.