Abstract
Increasing evidence suggests a role for cyclooxygenase-2 (COX-2) in traumatic brain injury (TBI). In the present study the role of COX-2 in TBI was investigated using COX-2 gene disrupted (COX-2 null) mice and wild type (WT) controls that were subjected to controlled cortical impact (CCI) model of TBI. There was increased expression of COX-2 in ipsilateral hippocampus in WT mice subjected to CCI. CCI resulted in a significant increase in PGE2 concentrations in WT compared to COX-2 null hippocampi. There was a significant increase in TUNEL staining of CA1 neurons 24 h after CCI in WT, but not in COX-2 null mice, compared to sham-operated controls consistent with a protective role for COX-2 in the early phase of injury after TBI. However, there was no difference in lesion volume 21 d after CCI in COX-2 null and WT mice. COX-2 gene disruption did not alter Morris water maze performance. Taken together, these results suggest only a minor role for COX-2 activity in determining outcome after TBI in mouse.
Keywords: Cyclooxygenase, Prostaglandin E2, TUNEL staining, behavior, Morris water Maze
Introduction
TBI is among the leading causes of morbidity and mortality yet there are no effective treatments to limit injury after trauma. Primary mechanical impact causes acute cell damage that initiates a series of secondary disruptive events within minutes to days, causing cell death through necrosis, apoptosis and autophagy that leads to grave neurological deficits (Hatton 2001; Lai et al. 2007). A variety of different mechanisms including oxidative stress and inflammation (Ray et al. 2002), protein aggregation (Smith et al. 2003), edema (Unterberg et al. 2004) and deficits in regional cerebral blood flow (Marklund et al. 2002) have been reported to play a major role in execution of secondary injury in TBI.
Cyclooxygenase (COX) is the obligate enzyme for the metabolism of arachidonic acid to pro-inflammatory and, paradoxically, anti-inflammatory prostaglandins. COX has two major isoforms; COX-1 and COX-2, which have identical enzymatic activity but have different physiological roles. COX-1 is expressed constitutively in almost all cell types where it plays important roles in maintaining normal tissue homeostasis. COX-2 is present in many cell types including brain and is induced by various disease conditions and toxic stimuli. In rat brain COX-2 immunoreactivity is found in neuronal populations of hippocampus, cortex and amygdala, while in human brain COX-2 is predominantly expressed in hippocampus (Belton and Fitzgerald 2003; Hurley et al. 2002; Vane et al. 1998). COX-2 activity has been hypothesized to be important in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and, cerebral ischemia (Alloza et al. 2006; Chang et al. 1996; Drachman and Rothstein 2000; Iadecola et al. 2001; Nakayama et al. 1998; Teismann et al. 2003).
Previous reports have both supported and refuted a role for COX-2 activity in exacerbating injury after TBI. There is increased expression of COX-2 in ipsilateral hippocampus and cortex in TBI (Dash et al. 2000; Gopez et al. 2005; Kunz et al. 2006; Strauss et al. 2000). Some reports have shown protective effects of COX-2 inhibitors against TBI-induced behavioral and cellular alterations (Gopez et al. 2005) while one study has reported deterioration of behavior in a model of TBI (Koyfman et al. 2000). Another study by Kunz and colleagues (Kunz et al. 2006) have reported insignificant effects of COX-2 inhibitors on cell death induced by TBI. SC58125, a COX-2 selective inhibitor, did not produce neuroprotection in a juvenile rat model of TBI (Hickey et al. 2007). Thus, there have been conflicting results in studies with COX-2 inhibitors in TBI models. COX-2 null mice provide an alternate but specific means of testing whether COX-2 activity contributes to neuronal cell death and dysfunction after TBI which has not yet been tested.
Material and methods
All experiments were conducted in accordance with NIH guidelines for use and care of laboratory animals and in accordance to regulations of the University of Pittsburgh Institutional Animal Care and Use Committee.
Breeding and genotyping of COX-2 null mice
Heterozygous COX-2 null mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and bred 7–10 generations to produce a colony. For the current study, female heterozygous colony-bred mice (homozygous female COX-2 null mice are sterile) were bred with COX-2 heterozygous mice to produce WT and COX-2 null mice. PCR was performed using DNA extracted from pup tail snips using phenol/chloroform extraction with the following cycling parameters: 94°C, for 45 seconds; 55°C, for 30 seconds; 72°C for 90 seconds, 30 cycles. Primers were as follows: COX-2 primers - forward 5′ACC TCT GCG ATG CTC TTC C3′ and reverse 5′CAC CAT AGA ATC CTG TCC GG3′; NEO primers - forward 5′CTT GGG TGG AGA GGC TAT TC3′ and reverse 5′AGG TGA GAT GAC AGG AGA TC3′. Reaction products were then separated on a 1.8% agarose gel stained with ethidium bromide, and photographed.
Controlled cortical impact model of brain injury
TBI was induced in male mice using cortical contusion injury (CCI) as described previously (Sinz et al. 1999) with minor modifications (Tehranian et al. 2006). Twelve-week-old male WT and COX-2 null mice were anesthetized with 2% isoflurane in N2O/O2 (2:1) via nose cone and placed in a stereotatic frame (Kopf, Tujunga, CA). A temperature probe was inserted through a burr hole in the left frontal cortex to monitor brain temperature and a rectal probe (Physitemp, Clifton, NJ) was used to measure body temperature. Both brain and body temperatures were maintained at 37.0 ± 0.5 °C for the whole procedure. A 5 mm craniotomy was made over the left parietotemporal cortex with a dental drill and the bone flap was removed. Vertically directed CCI was performed using a pneumatic cylinder (Bimba Co, Monee, IL) with a 3 mm flat-tip impounder at a velocity of 6.0 ± 0.2 m/s and a depth of 1.2 mm with shams undergoing identical surgical procedures except CCI (n = 15 animals/group). Immediately after injury, the bone flap was replaced and sealed with Koldmount Cement (Vernon Benshoff Co., Albany, NY) and the wound sutured. Anesthesia was discontinued, and mice were placed in an oxygen hood for 30 min before they were returned to their cages.
Western blot analysis
Western blots were performed as described previously (Tehranian et al. 2006). Briefly, 24 hrs after TBI (n = 3 per group), mouse hippocampal homogenates were prepared and proteins measured (Biorad, Temeculah, CA). Proteins (50 μg) were separated on a 12% SDS-PAGE gel, transferred onto PVDF membranes and incubated overnight with anti-COX-2 polyclonal antibody (Abcam, Cambridge, MA; 1:1000). Proteins were visualized using an enhanced chemiluminescence protein detection kit (Amersham, Arlington Heights, IL) and exposed on BioMax MR film (Kodak, Rochester, NY).
Immunohistochemistry
Wild type mice were anesthetized then transcardially perfused via the left ventricle with 20 ml 0.9% NaCl followed by 20 ml 4% paraformaldehyde 24 hrs after TBI (n = 3 per group). The brains were removed, sectioned at 2-mm intervals, processed and embedded in paraffin then cut 5 μm thick on a microtome, mounted on glass slides and processed for immunohistochemical staining. Brain slices were incubated with polyclonal anti-COX-2 antibody (1:200, Cayman Chemical, Ann Arbor, MI) overnight and immunofluorescence labeled using a Cy3-conjugated secondary antibody (Zymed laboratories, Invitrogen, CA). After secondary antibody application, sections were counterstained with DAPI and coverslipped. Images were captured with a confocal microscope (Olympus Fluoview).
Prostaglandin measurement
Four groups of mice, male, 10–12 weeks old, WT sham, WT TBI, COX-2 null sham and COX-2 null TBI (n = 7–9 per group) were used to compare prostaglandin production. Ipsilateral hemispheres were dissected and rapidly frozen in liquid nitrogen 24 h after TBI. Tissue was weighed and homogenized in 1 ml of ice-cold 0.05M Tris/MeOH (1:3) using a Tissue Tearor (Biospec Products, Inc, Bartlesville, OK). Homogenates were spun 20 min at 14,000 r.p.m., and the supernatants dried using a Speed Vac UVS400 (Savant Instruments, Inc., Holbrook, NY). PGE2 was assayed using an enzyme immunoassay (EIA) kit as described by the manufacturer (Cayman Chemical, Ann Arbor MI). Prostaglandin concentrations were normalized to brain tissue wet weight.
TUNEL labeling
TUNEL labeling was performed as described previously (Clark et al. 2001). Briefly, animals were sacrificed 24 h after surgery, brains were harvested and cut into 10μM sections. Sections were incubated in 300 U/ml TdT (terminal tranferase) and 20 nmol/ml biotin-16-dUTP (Boehringer Mannheim, Mannheim, Germany) and then incubated in avidin-biotin complex (ABC standard kit, Vector Labs, Burlingame, CA) and DNA strand breaks were visualized with diaminobenzidine (DAB, Vector Labs). In animals with intact hippocampi, TUNEL-positive cells were counted in CA1, CA2 and CA3 regions of hippocampus (n = 9 per group). All cells exhibiting apoptotic morphology were considered TUNEL-positive.
Measurement of hemispheric and lesion volume
Brains from sham and CCI injured WT and COX-2 null mice (n = 16–18 per group) were perfusion fixed via the left ventricle 3 weeks after injury, cut into 10 μm thick slices at 0.5 mm intervals and stained with hematoxylin and eosin. The volume of remaining normal hemisphere was determined using MCID Imaging analysis software (St Catharines, Ontario, Canada) as previously described (Tehranian et al. 2006). Hemispheric volumes were calculated by multiplying slice area by slice interval thickness times the number of slices. Lesion volume was calculated by subtracting the volume of the ipsilateral hemisphere from the contralateral hemisphere.
Morris water maze performance
Morris water maze performance in mice was evaluated as described previously (Sinz et al. 1999; Whalen et al. 1999). On days 14–18 post injury (n = 9–12 per group) the hidden platform version of the Morris water maze was used. A white pool (83 cm diameter, 60 cm deep) filled with 24°C water to 29 cm depth was situated in a room with several extra maze cues located on the walls. Contained within the pool was a 10-cm round goal platform, located 1 cm below the surface of the water approximately 15 cm from the southwest wall. A video tracking system mounted above the pool (Chromotrack 3.0, San Diego Instruments, San Diego, CA) recorded the swim speeds of the mice. Each mouse was subjected to a series of four trials per day. For each trial, mice were randomized to one of four starting locations and were placed in the pool. Mice were given a maximum of 120 s to find the submerged platform with latency recorded. Mice were placed in a 37°C incubator for 4 min between trials. To test for potential nonspecific deficits in visual and motor function, a visible platform task was performed on D19 and 20. The platform was raised 2 cm above the water surface with mice randomized to starting locations. Animals whose D20 visible platform scores were greater than 2 SD from the sham group 2-day average were removed from the study.
Statistical analysis
All data are expressed as mean ± S.E. Morris water maze data was analyzed using repeated measures ANOVA with Tukey’s post-hoc analysis. All other results were analyzed using Student’s t-test with p < 0.05 considered statistically significant.
Results
Hippocampal COX-2 expression after TBI
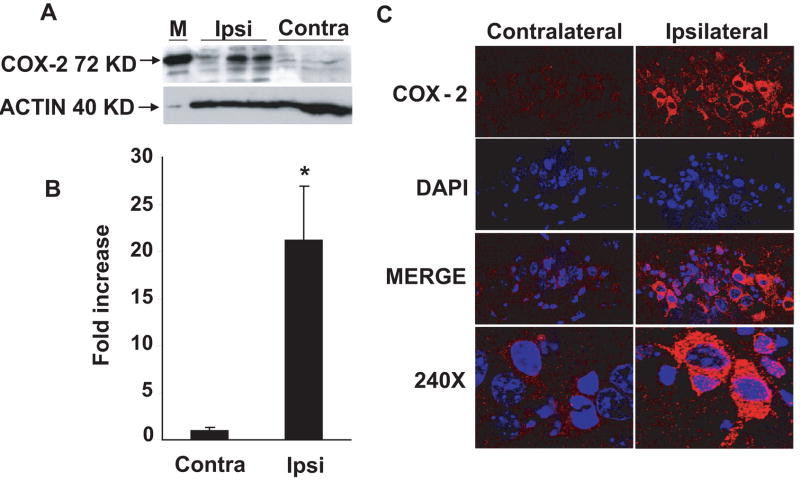
Western blot analysis of hippocampus for COX-2 protein showed robust ipsilateral expression relative to a weak band corresponding to basal levels of COX-2 protein in contralateral tissue after TBI in WT mice (Fig 1A). Densitometric analysis indicates a 15-fold increase in COX-2 expression in ipsilateral as compared to contralateral hippocampus (Fig 1B). Immunohistochemical staining reveals a robust staining consistent with high levels of COX-2 expression in the CA3 region of ipsilateral hippocampus 24 h after TBI in WT mice (Fig 1C). Faint staining was observed in CA1, and no staining at all in the CA2 region of hippocampus.
Figure 1.
Effect of controlled cortical impact-induced traumatic brain injury (TBI) upon COX-2 expression in mouse hippocampus after 24h. (A) Western blot showing COX-2 protein expression in ipsilateral and contralateral hippocampi. (B) Bar diagram showing densitometeric analysis of western blot. (C) Representative confocal photomicrographs showing the expression of COX-2 in ipsilateral and contralateral hippocampi of wild type mice. Double immunofluorescence staining of the fresh frozen sections against COX-2 (red) against DAPI (a nuclear marker stain, blue) showed robust expression in CA3 region of ipsilateral hippocampi. The data are represented mean ± S.E.; n= 3 animals per group. *p <0.04.
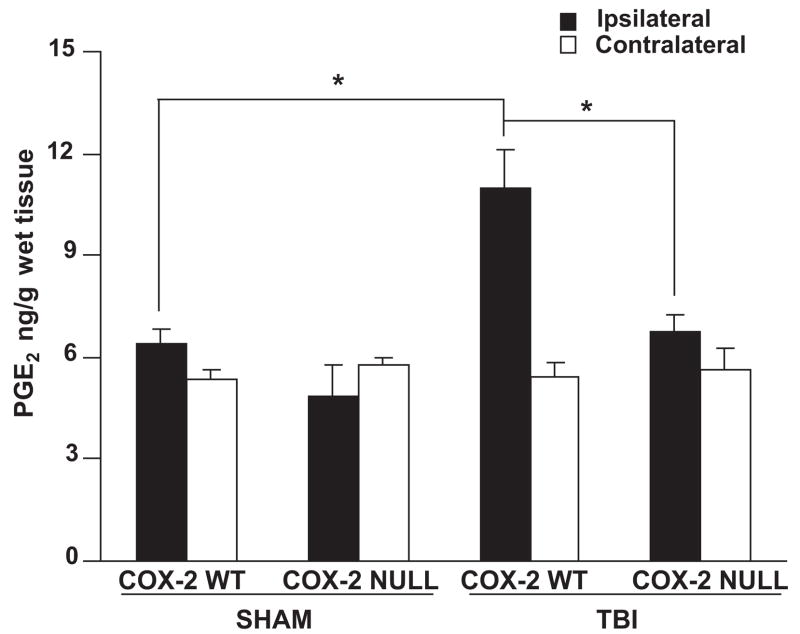
PGE2 production in COX-2 null and WT mice after TBI
TBI resulted in significant increases in the levels of PGE2 in ipsilateral hemisphere of WT mice as compared to COX-2 null mice 24h after TBI; PGE2 concentration was significantly higher in ipsilateral hemisphere of WT mice 24h after TBI as compared to sham. Furthermore, PGE2 concentrations between sham WT or sham COX-2 null mice were not significantly different either in ipsilateral or contralateral hemispheres (Fig 2).
Figure 2.
Effect of genetic disruption of COX-2 on hemispheric PGE2 concentrations in sham or controlled cortical impact-induced traumatic brain injury in mice after 24 h. PGE2 levels in COX-2 WT and COX-2 null mice were quantified as described in methods. Data are represented as mean ± S.E., n = 7–9 animals per group. *P<.05. WT: wild type; TBI: traumatic brain injury. * p <0.01.
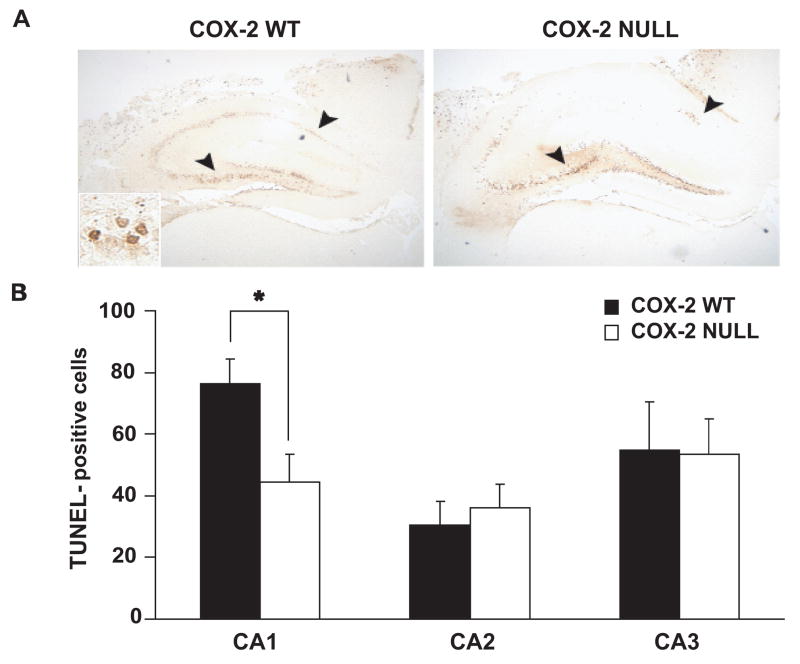
TUNEL labeling in COX-2 null and WT mice after TBI
There were significantly fewer TUNEL-positive neurons in the ipsilateral hippocampal CA1 region of COX-2 null mice 24 h after TBI than in WT mice. There was no statistically significant difference in numbers of TUNEL-positive neurons between COX-2 WT and COX-2 null mice as observed in ipsilateral CA2 or CA3 regions of hippocampus (Fig. 3A&B). There were no TUNEL-positive cells neurons in contralateral hippocampus of either genotype (data not shown).
Figure 3.
A. Effect of genetic disruption of COX-2 on hippocampal neuronal TUNEL-staining 24 h after controlled cortical impact-induced brain injury. Many neurons within CA1 and CA3 ipsilateral to CCI TUNEL-stain in both COX-2 WT and COX-2 null mice (4X). Inset: CA3 (40X). B. TUNEL positive counts in CA1, CA2 and CA3 regions of both the genotypes. Data are represented as mean ± S.E., n = 9 animals per group. WT: wild type; TBI: traumatic brain injury. * p <0.01.
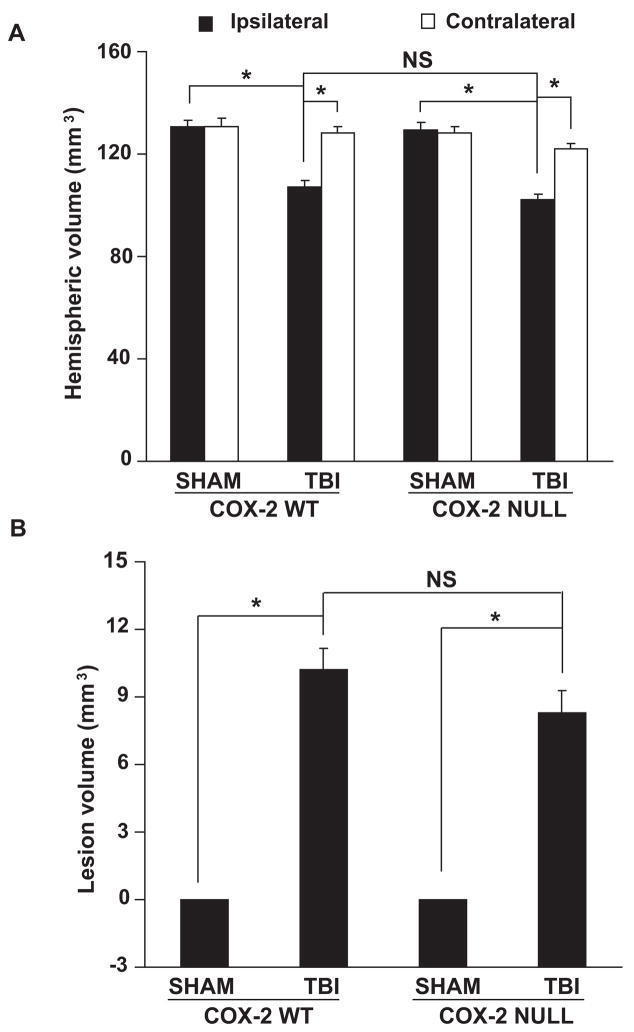
Hemispheric and lesion volume in COX-2 null and WT mice after TBI
There was an increase in lesion volume and a decrease in ipsilateral hemispheric volume in both COX-2 null and WT mice subjected to TBI compared to their respective sham operated hemispheric volumes, but there were no significant differences in lesion volume and hemispheric volume between COX-2 null mice and WT mice subjected to TBI (Fig 4A&B). Thus, the COX-2 gene deletion does not have developmental effects that alter brain volume as have been reported in some other transgenic lines (Tehranian et al. 2006).
Figure 4.
Hemispheric brain volume (A) and lesion volume (B) 21 d after controlled cortical impact-induced brain injury in COX-2 WT and COX-2 null mice. Data are expressed as means ± SE. NS= non-significant. n= 16–18 animals per group. WT: wild type; TBI: traumatic brain injury. * p < 0.01.
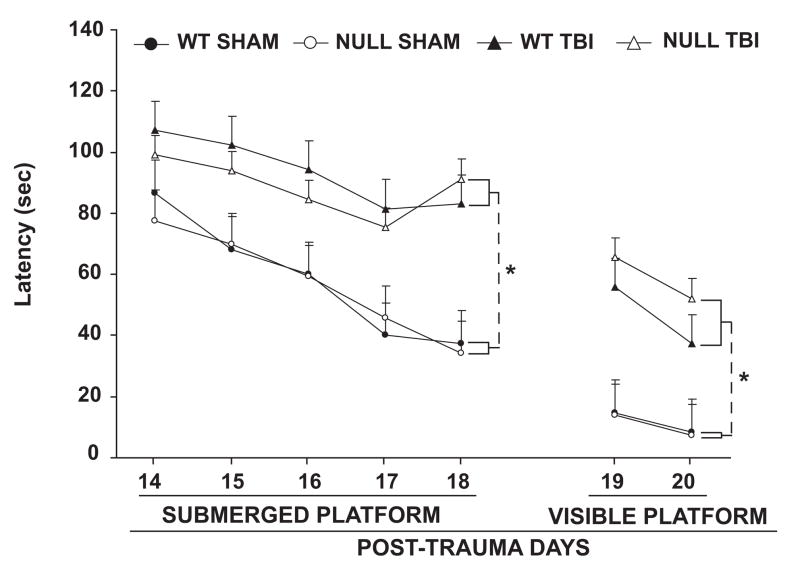
Morris water maze performance in COX-2 null and WT mice after TBI
There was no significant difference in Morris water maze performance afforded by COX-2 gene disruption in either the sham or TBI groups as compared to WT. There were significant differences in latencies between sham operated and TBI mice, but no significant differences between WT and COX-2 null mice within these groups (Fig 5). TBI had a significant effect on swim speed, but there was no difference in swim speeds between COX-2 null or WT mice within the TBI and sham operated groups (data not shown). While water maze performance improved for all groups when the platform was visible, both injured groups had significantly greater swim latencies than that of sham-injured groups.
Figure 5.
Effect of genetic disruption of COX-2 on Morris water maze performance in controlled cortical impact-induced traumatic brain injury in mice after 14 days. The figure represents latency (sec) to reach submerged and visible platforms in water maze. Data are expressed as means ± S.E. n = 9–12 animals per group. WT: wild type; TBI: traumatic brain injury. * p<0.02. [repeated measures ANOVA]
Discussion
The major result of this study is that genetic disruption of COX-2 does not improve overall histological or functional outcome in mice subjected to TBI. We observed a significant decrease in the number of TUNEL-positive cells in the CA1 region of the ipsilateral hippocampus of COX-2 null mice after TBI as compared to WT; however there was no significant difference in TUNEL-positive cells in CA2 or CA3 regions. This early marker of cell death was not translated into any long term behavioral or histological difference in outcome. To the best of our knowledge, it is the first report showing the effect of genetic disruption of the COX-2 gene on histological changes and functional deficits in mice subjected to TBI.
COX-2 expression was increased in ipsilateral cortex and hippocampus of rats subjected to TBI (Dash et al. 2000; Gopez et al. 2005; Kunz et al. 2006; Strauss et al. 2000). In concordance with these results we have found a robust expression of COX-2 in ipsilateral hippocampi of WT mice that were subjected to TBI. In contrast to these reports, COX-2 expression is not increased after in vitro TBI (Morrison et al. 2000). The current study found that there was increased concentration of PGE2 after TBI in WT mice as compared to COX-2 null mice, consistent with the hypothesis that increased production of prostaglandins exacerbates injury after TBI. PGE2 levels have been shown to be increased after traumatic brain injury and the selective COX-2 inhibitors, nimesulide and DFU have been shown to decrease enhanced concentrations of PGE2 (Gopez et al. 2005). Another study (Hickey et al. 2007) reported that SC58125 decreased the levels of TBI-induced PGE2 in juvenile rats.
Cell death after TBI may be executed through both necrotic and apoptotic mechanisms (Ray et al. 2002). TUNEL staining, a marker of DNA fragmentation is a hallmark for both necrosis and apoptosis (Gavrieli et al. 1992) and is reported to be enhanced after TBI (Clark et al. 2001). COX-2 inhibitors have been shown to decrease TUNEL-positive staining in various models of brain injury (Chu et al. 2004; Kaloustian et al. 2007; Kunz and Oliw 2001). Kunz and colleagues (Kunz et al. 2002) have observed co-localization of COX-2 expression with TUNEL-positive staining in dentate gyrus and cortex in a rat model of fluid percussion injury, suggesting a direct role of COX-2 in cell death after TBI. The same group observed that rofecoxib produced an insignificant decrease in TUNEL staining in ipsilateral cortex and dentate gyrus at 12h and 24h after lateral fluid percussion brain injury in rats, however, this effect had vanished after 72h (Kunz et al. 2006). In our study there was a significant decrease in the number of TUNEL-positive cells in the ipsilateral CA1 region of hippocampus, but no significant differences in CA2 and CA3 regions. This suggests that COX-2 expression may contribute to cell death in a circumscribed population of neurons, but that COX-2 is not expressed in all neurons destined to die. Our results are supported by a report showing that indomethacin, a non-selective COX-2 inhibitor, ameliorated ischemic neuronal damage in CA1 hippocampus of gerbils (Sasaki et al. 1988).
Genetic disruption of COX-2 in mice does not affect lesion volume after TBI in our studies and is supported in an earlier report by Koyfman and colleagues (Koyfman et al. 2000) which indicates ineffectiveness of nimesulide in reducing brain edema in a rat model of closed head trauma. Latency to reach the submerged and visible platforms in the Morris water maze overall remained the same for WT and COX-2 null mice after TBI, and a similar trend was observed with the swim speeds. These results are similar to the lack of an effect of SC58125 upon water maze performance in a juvenile rat TBI model (Hickey et al. 2007). Consistent with these findings, celecoxib has been reported to be ineffective in decreasing spatial memory impairment, as assessed by Morris water maze, in a lateral cortical injury rat model of TBI (Dash et al. 2000). COX-2 inhibition by nimesulide has shown no significant changes in neurological severity scores in a rat model of closed head trauma (Koyfman et al. 2000) while it has been reported to improve cognitive outcome and motor function in a model of diffuse traumatic brain injury in rats (Cernak et al. 2002). Gopez and colleagues (Gopez et al. 2005) report improved memory performance using post-injury treatment with DFU, a COX-2 selective inhibitor, after a lateral cortical impact injury in rats.
These contradicting protective effects elicited by various COX-2 selective inhibitors (DFU, Nimesulide, celecoxib and rofecoxib) in many types of TBI in rats may be the result of COX-2 independent effects of these inhibitors or induction of alternative routes of arachidonic acid metabolism by these drugs. Several COX-2 independent mechanisms of action of COX-2 selective inhibitors have been previously reported (Han and Roman 2006; Kardosh et al. 2004; Miyamoto et al. 2006; Yoon et al. 2003; Zhong et al. 2004). Celecoxib has been reported to prevent experimental autoimmune encephalomyelitis in COX-2 deficient mice by the inhibition of inflammatory cells into the CNS and by inhibiting the expression of various adhesion molecules, chemokines and inflammatory mediators (Han and Roman 2006; Kardosh et al. 2004; Miyamoto et al. 2006; Yoon et al. 2003; Zhong et al. 2004). Celecoxib has been shown to inhibit interlukin 1β secretion by inhibition of AKT kinase and endoplasmic reticulum Ca2+ ATPase (Alloza et al. 2006). Zhong and colleagues (Zhong et al. 2004) have reported the inhibitory effect of NS398 on HIF-1α and HIF-1 transcriptional activity under normoxic and hypoxic conditions in COX-2 negative hct116 cells. Various NSAIDs have been reported to have an inhibitory role in NO-induced apoptosis and differentiation of articular chondrocytes by blocking the downstream signaling cascade of p38 kinase and PKC, such as NFκB and caspase-3 activation (Yoon et al. 2003). COX-2 inhibitors also inhibited amyloid precursor protein (APP) processing and generation of amyloid-β peptide in various cell lines that that were transfected with human mutants APP751 and APP695 and these effects were independent of COX-2 activity (Weggen et al. 2001). Since many of the above mechanisms have a pronounced role in TBI (Ray et al. 2002), we assume that protective effects shown by selective COX-2 inhibitors in previous studies might have been caused due to some or all of these mechanisms.
When COX-2 activity is inhibited, increased arachidonic acid substrate is available for the CYP P450 epoxygenase and lipoxygenase metabolic pathways that may lead to the increased formation of epoxyeicosatrienoic acids (EETs) and lipoxins respectively. EETs are present in brain and act as potent vasodilators (Ellis et al. 1990) and possess anti-inflammatory and anti-thrombotic effects (Larsen et al. 2006). EETs increase baseline cerebral blood flow in rats (Alkayed et al. 1996) and have been shown to protect against cerebral ischemia in mice (Zhang et al. 2007). Lipoxins are known to be anti-inflammatory mediators (Machado et al. 2006; Schwab and Serhan 2006; Serhan and Savill 2005). These protective effects of EETs and lipoxins may also be responsible for the COX-2 independent protective effects of selective COX-2 inhibitors in various types of TBI in rats. Thus the acute administration of COX-2 inhibitors may have complex effects on arachidonic acid administration other than simply decreasing the production of COX-2 regulated prostaglandins.
There are a number of limitations to the current study. Life long depletion of COX-2 activity may induce compensatory changes in other proteins, such as increased expression of other isoforms of COX. A recent study (Toscano et al. 2007) has reported that genetic disruption of COX-2 in mice affects the transcriptional regulation of many genes in brain that are involved in mitochondrial function, lipid metabolism, cytokine signaling and GABA neurotransmission. These compensatory or alterative changes in gene expression could potentially confound the results.
Mouse models of TBI also have some limitations. In order to produce a robust behavioral effect with CCI in these studies, a severe impact was used that ablates most of the ipsilateral hippocampus by 21 d. Thus, this model was not designed to detect long term histological changes in hippocampus. Behavioral evaluation of mice after TBI is also limited since most of the behavioral testing systems are designed for rats; thus sensitivity for detecting differences in mice may be limited (Frick et al. 2000). Ultimately, strategies for acutely altering expression or activity of COX-2 by non-pharmacological means such as inducible knock-outs and knock-ins of COX-2 may provide a more definitive test of the hypothesis that COX-2 activity exacerbates injury after TBI. Such studies are beyond the scope of the present experiments.
In conclusion, the current study suggests that the genetic deletion of COX-2 plays only a minimal role in determining behavioral and histological outcome after TBI in mouse. COX-2 disruption diminished TUNEL staining in CA1 region 24 h after TBI. These results suggest that COX-2 plays a role in exacerbating early cell death in CA1 in the early time period after injury, effects that could be more robust at less severe injury levels. But COX-2 null mice did not have improved histological or behavioral outcome at 21 days, suggesting that the protection afforded by COX-2 deletion was transient in this model and limited to CA1. Previous reports have shown that some, but not all, COX-2 inhibitors afford histological and behavioral protection after TBI. This may be due the nonspecific effects of COX-2 inhibitors and differences in severity and location of TBI in the various animal models used.
Acknowledgments
The authors thank Ms. Pat Strickler for administrative support. This work was supported by to the Department of Veterans Affairs/Department of Defense, Traumatic Brain Injury Initiative Merit Review Program (SHG) and the NIH R01 NS37459 (SHG).
Abbreviations
- DFU
5,5-dimethyl-3-3(3-fluorophenyl)-4-(4-methylsulfonal)phenyl-2(5H)-furanone
- APP
amyloid precursor protein
- CCI
controlled cortical impact
- COX-2
cyclooxygenase-2
- EETs
epoxyeicosatrienoic acids
- PGE2
prostaglandin E2
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end- labeling
- TBI
traumatic brain injury
References
- Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol. 1996;271(4 Pt 2):H1541–1546. doi: 10.1152/ajpheart.1996.271.4.H1541. [DOI] [PubMed] [Google Scholar]
- Alloza I, Baxter A, Chen Q, Matthiesen R, Vandenbroeck K. Celecoxib inhibits interleukin-12 alphabeta and beta2 folding and secretion by a novel COX2-independent mechanism involving chaperones of the endoplasmic reticulum. Mol Pharmacol. 2006;69(5):1579–1587. doi: 10.1124/mol.105.020669. [DOI] [PubMed] [Google Scholar]
- Belton O, Fitzgerald DJ. Cyclooxygenase isoforms and atherosclerosis. Expert Rev Mol Med. 2003;5(9):1–18. doi: 10.1017/S1462399403005842. [DOI] [PubMed] [Google Scholar]
- Cernak I, O’Connor C, Vink R. Inhibition of cyclooxygenase 2 by nimesulide improves cognitive outcome more than motor outcome following diffuse traumatic brain injury in rats. Exp Brain Res. 2002;147(2):193–199. doi: 10.1007/s00221-002-1245-z. [DOI] [PubMed] [Google Scholar]
- Chang JW, Coleman PD, O’Banion MK. Prostaglandin G/H synthase-2 (cyclooxygenase-2) mRNA expression is decreased in Alzheimer’s disease. Neurobiology of aging. 1996;17(5):801–808. doi: 10.1016/0197-4580(96)00110-8. [DOI] [PubMed] [Google Scholar]
- Chu K, Jeong SW, Jung KH, Han SY, Lee ST, Kim M, Roh JK. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab. 2004;24(8):926–933. doi: 10.1097/01.WCB.0000130866.25040.7D. [DOI] [PubMed] [Google Scholar]
- Clark RSB, Chen M, Kochanek PM, Watkins SC, Jin KL, Draviam R, Nathaniel PD, Pinto R, Marion DW, Graham SH. Detection of single- and double-strand DNA breaks after traumatic brain injury in rats: comparison of in situ labeling techniques using DNA polymerase I, the Klenow fragment of DNA polymerase I, and terminal deoxynucleotidyl transferase. J Neurotrauma. 2001;18(7):675–689. doi: 10.1089/089771501750357627. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J Neurotrauma. 2000;17(1):69–81. doi: 10.1089/neu.2000.17.69. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Rothstein JD. Inhibition of cyclooxygenase-2 protects motor neurons in an organotypic model of amyotrophic lateral sclerosis. Annals of neurology. 2000;48(5):792–795. [PubMed] [Google Scholar]
- Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol. 1990;259(4 Pt 2):H1171–1177. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11(16):3461–3465. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Narayan RK, Strauss KI. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56(3):590–604. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Roman J. COX-2 inhibitors suppress lung cancer cell growth by inducing p21 via COX-2 independent signals. Lung Cancer. 2006;51(3):283–296. doi: 10.1016/j.lungcan.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hatton J. Pharmacological treatment of traumatic brain injury: a review of agents in development. CNS Drugs. 2001;15(7):553–581. doi: 10.2165/00023210-200115070-00005. [DOI] [PubMed] [Google Scholar]
- Hickey RW, Adelson PD, Johnnides MJ, Davis DS, Yu Z, Rose ME, Chang YF, Graham SH. Cyclooxygenase-2 activity following traumatic brain injury in the developing rat. Pediatric research. 2007;62(3):271–276. doi: 10.1203/PDR.0b013e3180db2902. [DOI] [PubMed] [Google Scholar]
- Hurley SD, Olschowka JA, O’Banion MK. Cyclooxygenase inhibition as a strategy to ameliorate brain injury. J Neurotrauma. 2002;19(1):1–15. doi: 10.1089/089771502753460196. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, Morham S, Ross ME. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci U S A. 2001;98(3):1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaloustian S, Wann BP, Bah TM, Falcao S, Dufort AM, Ryvlin P, Godbout R, Rousseau G. Celecoxib after the onset of reperfusion reduces apoptosis in the amygdala. Apoptosis. 2007;12(11):1945–1951. doi: 10.1007/s10495-007-0122-4. [DOI] [PubMed] [Google Scholar]
- Kardosh A, Blumenthal M, Wang WJ, Chen TC, Schonthal AH. Differential effects of selective COX-2 inhibitors on cell cycle regulation and proliferation of glioblastoma cell lines. Cancer Biol Ther. 2004;3(1):55–62. doi: 10.4161/cbt.3.1.571. [DOI] [PubMed] [Google Scholar]
- Koyfman L, Kaplanski J, Artru AA, Talmor D, Rubin M, Shapira Y. Inhibition of cyclooxygenase 2 by nimesulide decreases prostaglandin E2 formation but does not alter brain edema or clinical recovery after closed head injury in rats. J Neurosurg Anesthesiol. 2000;12(1):44–50. doi: 10.1097/00008506-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Kunz T, Marklund N, Hillered L, Oliw EH. Cyclooxygenase-2, prostaglandin synthases, and prostaglandin H2 metabolism in traumatic brain injury in the rat. J Neurotrauma. 2002;19(9):1051–1064. doi: 10.1089/089771502760341965. [DOI] [PubMed] [Google Scholar]
- Kunz T, Marklund N, Hillered L, Oliw EH. Effects of the selective cyclooxygenase-2 inhibitor rofecoxib on cell death following traumatic brain injury in the rat. Restor Neurol Neurosci. 2006;24(1):55–63. [PubMed] [Google Scholar]
- Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13(3):569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- Lai Y, Hickey RW, Chen Y, Bayir H, Sullivan ML, Chu CT, Kochanek PM, Dixon CE, Jenkins LW, Graham SH, Watkins SC, Clark RS. Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600551. [DOI] [PubMed] [Google Scholar]
- Larsen BT, Gutterman DD, Hatoum OA. Emerging role of epoxyeicosatrienoic acids in coronary vascular function. Eur J Clin Invest. 2006;36(5):293–300. doi: 10.1111/j.1365-2362.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12(3):330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- Marklund N, Sihver S, Langstrom B, Bergstrom M, Hillered L. Effect of traumatic brain injury and nitrone radical scavengers on relative changes in regional cerebral blood flow and glucose uptake in rats. J Neurotrauma. 2002;19(10):1139–1153. doi: 10.1089/08977150260337958. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Mizuno M, Oka N, Kusunoki S, Yamamura T. Selective COX-2 inhibitor celecoxib prevents experimental autoimmune encephalomyelitis through COX-2-independent pathway. Brain. 2006;129(Pt 8):1984–1992. doi: 10.1093/brain/awl170. [DOI] [PubMed] [Google Scholar]
- Morrison B, 3rd, Eberwine JH, Meaney DF, McIntosh TK. Traumatic injury induces differential expression of cell death genes in organotypic brain slice cultures determined by complementary DNA array hybridization. Neuroscience. 2000;96(1):131–139. doi: 10.1016/s0306-4522(99)00537-0. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998;95(18):10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002;17(4):1137–1152. doi: 10.14670/HH-17.1137. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Nakagomi T, Kirino T, Tamura A, Noguchi M, Saito I, Takakura K. Indomethacin ameliorates ischemic neuronal damage in the gerbil hippocampal CA1 sector. Stroke; a journal of cerebral circulation. 1988;19(11):1399–1403. doi: 10.1161/01.str.19.11.1399. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6(4):414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Sinz EH, Kochanek PM, Dixon CE, Clark RS, Carcillo JA, Schiding JK, Chen M, Wisniewski SR, Carlos TM, Williams D, DeKosky ST, Watkins SC, Marion DW, Billiar TR. Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J Clin Invest. 1999;104(5):647–656. doi: 10.1172/JCI6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Uryu K, Saatman KE, Trojanowski JQ, McIntosh TK. Protein accumulation in traumatic brain injury. Neuromolecular Med. 2003;4(1–2):59–72. doi: 10.1385/NMM:4:1-2:59. [DOI] [PubMed] [Google Scholar]
- Strauss KI, Barbe MF, Marshall RM, Raghupathi R, Mehta S, Narayan RK. Prolonged cyclooxygenase-2 induction in neurons and glia following traumatic brain injury in the rat. J Neurotrauma. 2000;17(8):695–711. doi: 10.1089/089771500415436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranian R, Rose ME, Vagni V, Griffith RP, Wu S, Maits S, Zhang X, Clark RS, Dixon CE, Kochanek PM, Bernard O, Graham SH. Transgenic mice that overexpress the anti-apoptotic Bcl-2 protein have improved histological outcome but unchanged behavioral outcome after traumatic brain injury. Brain Res. 2006;1101(1):126–135. doi: 10.1016/j.brainres.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100(9):5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano CD, Prabhu VV, Langenbach R, Becker KG, Bosetti F. Differential gene expression patterns in cyclooxygenase-1 and cyclooxygenase-2 deficient mouse brain. Genome Biol. 2007;8(1):R14. doi: 10.1186/gb-2007-8-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129(4):1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Clark RS, Dixon CE, Robichaud P, Marion DW, Vagni V, Graham SH, Virag L, Hasko G, Stachlewitz R, Szabo C, Kochanek PM. Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab. 1999;19(8):835–842. doi: 10.1097/00004647-199908000-00002. [DOI] [PubMed] [Google Scholar]
- Yoon JB, Kim SJ, Hwang SG, Chang S, Kang SS, Chun JS. Non-steroidal anti-inflammatory drugs inhibit nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes independent of cyclooxygenase activity. J Biol Chem. 2003;278(17):15319–15325. doi: 10.1074/jbc.M212520200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Willard M, Simons J. NS398 reduces hypoxia-inducible factor (HIF)-1alpha and HIF-1 activity: multiple-level effects involving cyclooxygenase-2 dependent and independent mechanisms. Int J Cancer. 2004;112(4):585–595. doi: 10.1002/ijc.20438. [DOI] [PubMed] [Google Scholar]