Abstract
Bone loss due to osteoporosis or disuse such as in paraplegia or microgravity is a significant health problem. As a treatment for osteoporosis, brief exposure of intact animals or humans to low magnitude and high frequency (LMHF) mechanical loading has been shown to normalize and prevent bone loss. However, the underlying molecular changes and the target cells by which LMHF mechanical loading alleviate bone loss are not known. Here, we hypothesized that direct application of LMHF mechanical loading to osteoblasts alters their cell responses, preventing decreased bone formation induced by disuse or microgravity conditions. To test our hypothesis, preosteoblast 2T3 cells were exposed to a disuse condition using the Random Positioning Machine (RPM) and intervened with an LMHF mechanical load (0.1-0.4g at 30Hz for 10-60 min/day). Exposure of 2T3 cells to the RPM decreased bone formation responses as determined by alkaline phosphatase (ALP) activity and mineralization even in the presence of a submaximal dose of BMP4 (20ng/ml). However, LMHF mechanical loading prevented the RPM-induced decrease in ALP activity and mineralization. Mineralization induced by LMHF mechanical loading was enhanced by treatment with bone morphogenic protein 4 (BMP4) and blocked by the BMP antagonist noggin, suggesting a role for BMPs in this response. In addition, LMHF mechanical loading rescued the RPM-induced decrease in gene expression of ALP, runx2, osteomodulin, parathyroid hormone receptor 1, and osteoglycin. These findings suggest that preosteoblasts may directly respond to LMHF mechanical loading to induce differentiation responses. The mechanosensitive genes identified here provide potential targets for pharmaceutical treatments that may be used in combination with low level mechanical loading to better treat osteoporosis or disuse-induced bone loss.
Keywords: bone loss, mineralization, mechanical loading, osteoporosis, BMP4, osteoblasts
Introduction
Musculoskeletal pathologies associated with decreased bone mass, including osteopenia, osteoporosis, disuse-induced bone loss, and microgravity-induced bone loss, affect millions of Americans annually. Bone loss is particularly dangerous since it is at first asymptomatic but can lead to severe fractures of bones, typically those in the hip, spine, and wrist [Gass and Dawson-Hughes, 2006]. While osteoporosis usually affects the elderly, it can afflict both men and women of any age. Additionally, bone loss occurs in spaceflight, rendering astronauts at-risk for fractures during long term space travel. On average, astronauts lose 1-2% of bone mass per month during space missions [Lang et al., 2004], and there are no known countermeasures that can effectively mitigate this bone loss.
It has long been regarded that mechanical stimuli are anabolic to bone. High magnitude, low frequency impact such as running has been recognized to increase bone and muscle mass [Galloway and Jokl, 2000; Snow-Harter et al., 1992; Vuori, 1995]. However, the opposite stimulus, a low magnitude and high frequency (LMHF) mechanical load experienced in activities as low impact as standing, has also been shown to be anabolic to bone [Rubin et al., 2001a]. This type of signal is transmitted by the musculature to the skeleton during the majority of each day, assisting in maintenance of posture and other non-strenuous activities. If it is true that muscle atrophy (sarcopenia) parallels aging [Lee et al., 2006; Lees et al., 2006; Rosenberg, 1997], then these intrinsic, LMHF mechanical signals would diminish with it, leaving the musculoskeletal system without potentially key signals critical to the regulation and retention of skeletal mass and morphology.
Recently, an LMHF mechanical loading device has been developed to treat bone loss by applying a low magnitude mechanical signal at a high frequency to the whole animal or human. The LMHF mechanical load has been shown to be effective in treating musculoskeletal pathologies in a number of subjects during research and clinical trials, including animals [Rubin et al., 2001b], children with musculoskeletal diseases such as cerebral palsy or muscular dystrophy [Gray et al., 1992; Ward et al., 2004], young women with low bone mass [Gilsanz et al., 2006], and post-menopausal osteoporotic women [Rubin et al., 2004]. While several pre-clinical and clinical trials have demonstrated that the LMHF mechanical loading affects bone formation in vivo, the target tissues of the mechanical load and underlying molecular changes mediating the responses are not known. In this manuscript, we focus on the effects of LMHF loading on bone formation using preosteoblast cells in continuation of our previously published work [Pardo et al., 2005; Patel, 2007].
Here, we hypothesized that an LMHF mechanical loading applied directly to preosteoblasts prevents decreased bone formation responses in vitro by altering gene expression and cell function. To test our hypothesis, preosteoblast 2T3 cells were exposed to the Random Positioning Machine (RPM), a model used for disuse [Pardo et al., 2005], and intervened with LMHF mechanical signals (0.1-0.4g acceleration at 30Hz frequency) for 10-60 min per day. We developed an in vitro method to apply LMHF mechanical loading to osteoblast cells, examined various in vitro bone formation markers, and performed bone morphogenic protein (BMP) inhibitor studies. We found that LMHF loading stimulated an osteogenic response that required the expression of BMPs, inducing alkaline phosphatase (ALP) activity, enhancing subsequent mineralization, and increasing osteogenic gene expression.
Materials and Methods
Cell culture
Murine 2T3 preosteoblast cells were kindly provided to us by Dr. Steve Harris, University of Texas Health Science Center at San Antonio. The cells were cultured in a growth medium (α-minimal essential medium) containing 10% fetal bovine serum (Atlanta Biologicals) with 100 units/ml of penicillin and 100μg/ml of streptomycin and grown in a standard humidified incubator (37°C, 5% CO2) as previously described by us [Pardo et al., 2005; Patel, 2007]. For mineralization experiments, the growth medium was supplemented with fresh ascorbic acid (50 μg/ml) and β-glycerolphosphate (5 mM) with or without BMP2 or BMP4 (0-50 ng/ml) and/or noggin (100 ng/ml). For the initial seeding of cells, we followed an Opticell seeding protocol as previously published by us [Pardo et al., 2005]. During re-feeding, each Opticell was filled with 14ml of fresh growth medium with freshly prepared supplements. For short term (3 days) experiments, the medium was changed at the start of the experiment, and there was no need for another medium change. For long term (>3 days) experiments, the Opticells were removed for a medium change from their experimental conditions for approximately 20 minutes every three days for the duration of the experiment as previously published by us [Pardo et al., 2005].
Random Positioning Machine (RPM)
A desktop RPM manufactured by the Dutch Space Agency was used to simulate disuse or microgravity conditions as previously described by us [Pardo et al., 2005]. The RPM simulates microgravity by continuously moving the gravity vector in three dimensions, which is a method called gravity-vector averaging. In essence, these randomized gravity vectors cancel each other out over time, thus producing a simulated 0-g environment relative to the cells on the RPM. OptiCell disks seeded with cells were mounted on the center of the platform located on the inner frame, and the RPM was operated in random modes of speed and direction via a computer user interface with dedicated control software inside a humidified incubator (5% CO2 at 37°C). In addition to simulated microgravity, cells treated with our RPM system experience a low level of shear stress and strain as we have shown previously using a computational modeling study. Our model predicts a minor portion of the cells close to the long frames of the OptiCell disk showing significantly less than 1 dyn/cm2 of shear stress for a brief moment (<4 s/min) and <200 microstrains of mechanical strain for a fraction of the duration of the RPM's rotation. However, the levels of these forces are significantly lower than the reported force magnitudes (as low as 2 dyn/cm2 shear stress and 500 microstrains) needed to stimulate signaling in osteoblasts [Kapur et al., 2003; Kaspar et al., 2000; McAllister et al., 2000; Wadhwa et al., 2005]. However, our results need to be interpreted with the caution that at least some of the observed RPM effects may be due to mechanical forces other than simulated microgravity. We only performed mechanical force characterization under the RPM conditions because there was no visible fluid movement in the other experimental groups, including the mechanically loaded group.
Low Magnitude, High Frequency (LMHF) Mechanical Loading Platform
LMHF mechanical signals were delivered to the cells using a vertically oscillating platform custom manufactured by Juvent, Inc (Figure 1A). The Opticells were secured to the top of the platform, ensuring a connection between the two. Then the platform and cells were placed into a humidified incubator for the duration of loading. The magnitude of mechanical loading is defined as a peak-to-peak load (Figure 1B), and cells were exposed to 0.1-0.4g acceleration (where 1.0g is Earth's gravitational field) at 30 Hz frequency for 10-60 minutes per day for 3-21 days (Figure 1C). The loading platform dimensions are 17″×17″, and the loading conditions were controlled through a program installed on a laptop.
Figure 1. LMHF mechanical loading using a custom built platform.
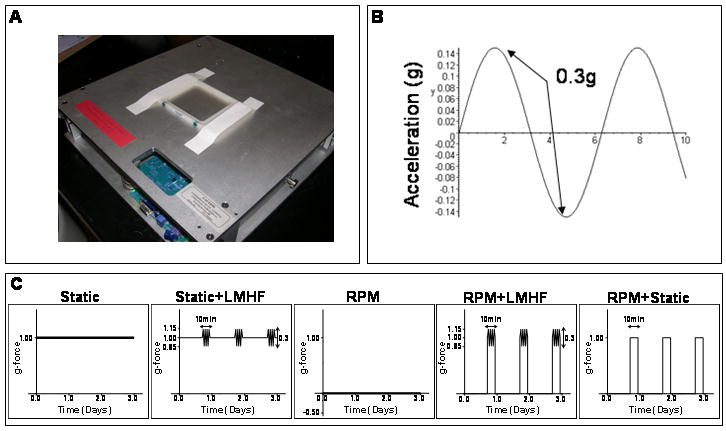
Confluent 2T3 cells grown in OptiCell chambers were exposed to LMHF loading using a platform (A) designed to produce dynamic, vertical oscillations, where the peak to peak acceleration is the magnitude of the load (B). Cells were exposed to five different experimental conditions (C) with varying g-loads as shown on the y-axis using the LMHF platform and/or the RPM.
Experimental Design
Confluent 2T3 cells grown in OptiCells were exposed to static (1g) or RPM conditions with or without a brief, daily LMHF mechanical load inside a humidified incubator (5% CO2 at 37°C). As shown in Figure 1C, 2T3 cells were exposed to five different conditions: 1) Static: cells were exposed to control 1g conditions continuously throughout the experimental duration, 2) Static+LMHF: cells were exposed to static 1g conditions and treated once with LMHF mechanical loading for 10-60 minutes/day, 3) RPM: cells were continuously rotated on the RPM, simulating a disuse or microgravity environment, 4) RPM+LMHF: cells were exposed to the RPM and removed once from the RPM for treatment with LMHF mechanical loading as in the Static+LMHF group, and 5) RPM+Static: Since the RPM+LMHF group not only was treated with the LMHF mechanical loading but also was returned to static 1g conditions briefly, we employed the RPM+Static group as a control. This group of cells was exposed to the RPM and intervened with the static 1g conditions daily (10-60 min/day).
Cell Proliferation Assay
To determine cell counts, attached cells were collected by trypsinization after experimental treatments, and cell numbers were determined using an aliquot of cell suspension and counted with a Coulter counter. Briefly, at least three 1:10 dilutions in a saline solution were made from each sample, and each separate dilution was counted three times with the Coulter counter (size>5μm). These readings were averaged to determine the final cell count for each sample. Coulter counter readings were verified using the standard hemocytometer protocol.
Whole cell lysate and alkaline phosphatase (ALP) enzyme activity
Following the experiment, cells were washed twice with ice-cold phosphate buffered saline (PBS) and lysed in 500 μl of a lysis buffer containing 0.5% Triton X-100, 1 mM MgCl2, and 10 mM Tris-HCl. Samples were stored at -80°C until needed. Alkaline phosphatase (ALP) activity was determined using a Diagnostics ALP assay kit (Sigma), as previously described by us [Pardo et al., 2005; Patel, 2007].
Alizarin Red Stain
Following the experiment, cells were washed twice with ice-cold PBS and fixed in 70% ethanol for 15 minutes. Cultures were stained for two minutes with a 1% Alizarin red solution for calcium detection. Following the stain, cultures were rinsed with 0.01%HCl in ethanol solution and then rinsed with dH20. The plates were dried overnight before being quantified for percent mineralization using ImageJ analysis software. Graphs express mineralization as a percent of the experimental control.
Fourier Transform Infrared (FTIR) Spectroscopy
After 15-21 days of exposure to the stimulus, cells were scraped in 100% ethanol and dried at 50°C overnight. Samples were mixed with potassium bromide (Sigma), pressed into pellets, and analyzed with a Nexus 470 FTIR spectrometer (ThermoNicolet, Madison, WI), which was equipped with a deuterated triglycine sulfate (DTGS) detector. A nitrogen purge was performed, and sixty four scans were acquired [Gersbach et al., 2004].
RNA isolation, reverse transcription, and quantitative real time PCR
Total RNA was prepared and amplified as previously described by us [Pardo et al., 2005; Patel, 2007]. Briefly, total RNA was prepared using the RNeasy Mini Kit (Qiagen) and reverse transcribed by using random primers and a Superscript-II kit (Life Technology). The synthesized and purified cDNA was amplified using a LightCycler (Roche Applied Science), and mRNA copy numbers were determined based on standard curves generated with the genes of interest and normalized against 18S ribosomal RNA. The primer pairs for quantitative real time PCR for alkaline phosphatase (ALP), runt homology domain transcription factor 2 (runx2), bone morphogenic protein 4 (BMP4), osteomodulin (omd), and parathyroid hormone receptor 1 (pthR1) were previously published by us [Pardo et al., 2005; Patel, 2007] and for osteoglycin (ogn) by others [Xing et al., 2005]. Real time PCR for the listed genes was carried out in PCR buffer as described previously by us [Pardo et al., 2005; Patel, 2007].
Statistical analysis
Data are expressed as mean ± SEM with at least 3 independently carried out studies. Statistical analysis was performed using the Student's t-test. A significance level of p<0.05 was considered statistically significant.
Results
LMHF mechanical loading did not alter cell morphology or cell number of 2T3 preosteoblasts
Confluent 2T3 cells exposed to Static, Static+LMHF, RPM, or RPM+LMHF conditions for 3 days showed no apparent change in cell morphology after stimulus exposure as shown in Figure 2A. Additionally, cell number did not change between static and experimental groups with exposure to LMHF mechanical loading or RPM (Figure 2B). These results suggest that the RPM or LMHF mechanical loading did not significantly affect cell shape or growth.
Figure 2. LMHF mechanical loading did not alter morphology or cell number of 2T3 cells.
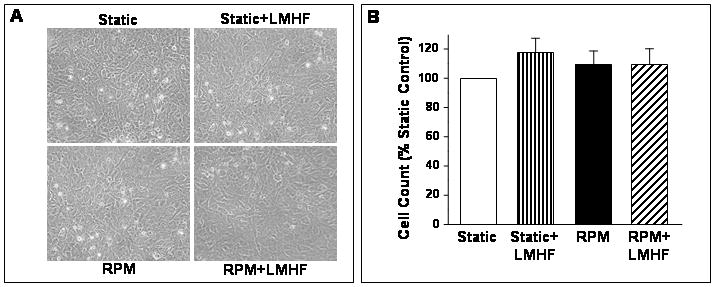
Confluent 2T3 cells were exposed to Static, Static+LMHF, RPM, or RPM+LMHF conditions for 3 days. LMHF loading was applied to the appropriate groups at 0.3g for 10 min/day. Cultured cells were photomicrographed with a phase microscope at the end of the experiment (A), and cell proliferation was assessed by counting cells using a Coulter counter and graphed as % of static control. Data are expressed as mean ± SEM (n=5-11, p>0.15).
LMHF mechanical loading stimulated mineralization in a magnitude-dependent manner
Preosteoblast 2T3 cells show no apparent mineralization in an experimental time frame of 15-21 days unless osteogenic factors such as BMP2 or BMP4 are added to the culture medium. Here, we examined whether an LMHF mechanical load could stimulate in vitro mineralization of 2T3 cells without supplementing the culture medium with exogenous BMPs. As expected, static cells cultured for 21 days in the absence of added BMPs (Static) did not show any significant calcium deposits as identified by Alizarin Red staining (Figure 3A and B). Also as expected, supplementing the media with 20 ng/ml of BMP4 (Static+BMP4) induced a dramatic increase in calcium deposits (Figure 3A and B). Exposure of 2T3 cells to LMHF mechanical loading alone (without exogenously added BMP4) significantly increased calcium deposits by 10 to 40-fold over static conditions (Static+LMHF). Additionally, the effect of LMHF signals was magnitude-dependent, as calcium deposition significantly increased when cells were loaded at levels as low as 0.1g up to 0.4g. To further demonstrate whether LMHF loading induced physiologically relevant mineralization in 2T3 cells, FTIR analysis was performed. Figure 3C is a representative FTIR analysis showing adsorption bands at 1650 cm−1 (Amide I, C=O) and 1530 cm−1 (Amide II, N-H and C-N), which represent bonds in the extracellular matrix and lipid content. Additionally, mineral samples had peaks at 1030 cm−1 (P-O), at 870 cm−1 (C-O), and a split peak at 600 cm−1 (P-O). These bands are characteristic of carbonate-containing, poorly crystalline hydroxyapatite. These results suggest that LMHF loading alone can stimulate mineralization of 2T3 cells without requiring exogenously added BMPs.
Figure 3. LMHF mechanical loading induced mineralization in a magnitude-dependent manner.
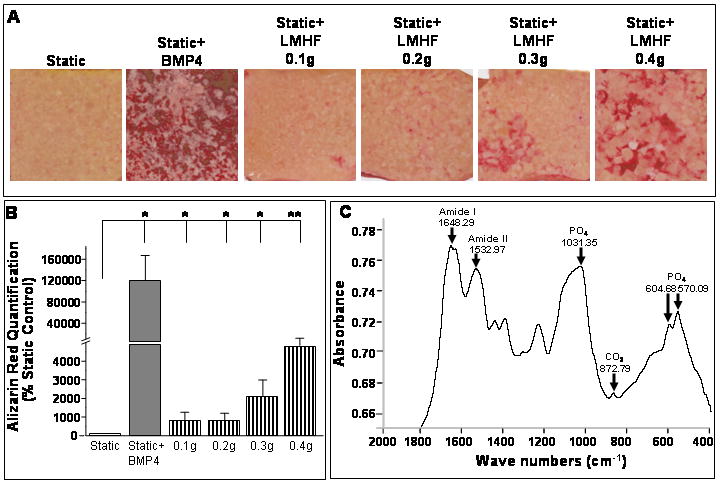
Confluent 2T3 cells were exposed to Static, Static+BMP4, or Static+LMHF conditions at varying loading magnitudes (0.1-0.4g for 10 min/day) for 21 days and stained for calcium deposition using Alizarin Red. The Opticell membrane was scanned (A), and quantification (B) was determined using imaging software and graphed as a % of static control. Data are expressed as mean ± SEM (n=6-10, *p<0.01, **p<0.05).
LMHF mechanical loading stimulated and RPM inhibited mineralization in 2T3 cells in a BMP4 concentration-dependent manner
We next investigated the effects of LMHF loading and the RPM on 2T3 cell mineralization in a BMP4 concentration-dependent manner. We carried out experiments for a shorter duration of 15 days (rather than the 21 day experiments in Figure 3) to detect differences among BMP4 treated groups. 2T3 cells were exposed to Static, Static+LMHF, or RPM conditions and treated with or without recombinant BMP4 in concentrations varying from 0-50 ng/ml (Figure 4). BMP4 alone induced mineralization in static cultures at 20 or 50 ng/ml treatment, while 10 ng/ml showed a moderate but not statistically significant increase. The LMHF loading significantly augmented the BMP4 effect (Static+LMHF) at 20 ng/ml of BMP4 by more than two-fold above the corresponding static group. We also found that the LMHF loading tended to enhance the effect of 10 ng/ml BMP4 above that of the corresponding static group, although it did not reach statistical significance due to high variation among samples. The RPM prevented mineralization at submaximal concentrations of BMP4 (1 to 20ng/ml) compared to those of both the Static and Static+LMHF groups. This anti-osteogenic effect of RPM in the presence of submaximal BMP4 concentrations was also observed when cells were treated with 20 ng/ml BMP2 (data not shown). However, when treated with a higher concentration of BMP4 (50 ng/ml), 2T3 cells showed maximal mineralization in all three groups (Static, Static+LMHF, and RPM), and no differences were observed among them. These results suggest that BMP4 and LMHF loading provide pro-mineralization effects in an additive manner, and the RPM prevents the pro-mineralization effect of submaximal concentration of BMPs (up to 20 ng/ml). However, treating cells with a supermaximal BMP4 concentration (50 ng/ml) overrides the RPM and LMHF effects, suggesting a key role for BMPs as a mediator of mineralization in response to either mechanical condition.
Figure 4. LMHF loading induced and RPM inhibited mineralization in 2T3 cells in a BMP4 concentration-dependent manner.
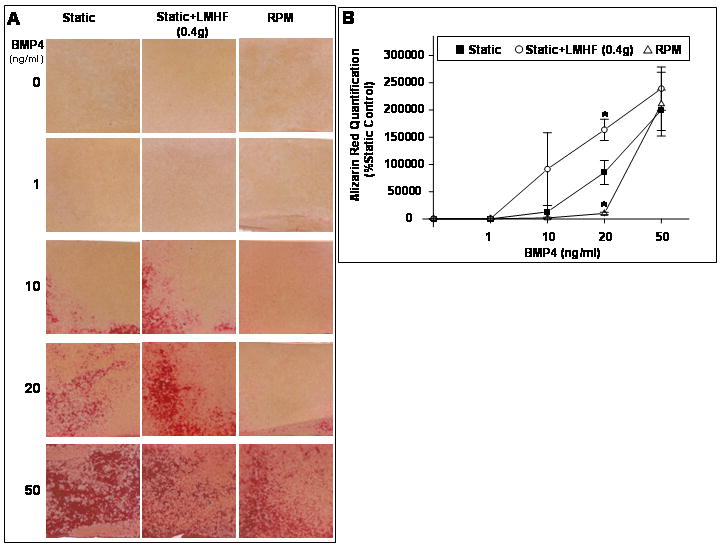
Confluent 2T3 cells were exposed to Static, Static+LMHF, or RPM conditions in the presence of varying concentrations of BMP4 for 15 days. LMHF was applied to the appropriate groups at a magnitude of 0.4g for 10 min/day. Cell cultures were stained with Alizarin Red (A), and the intensity was quantified (B) as in Figure 3. Data are expressed as mean ± SEM (n=4, *p<0.05).
LMHF mechanical loading prevented RPM-induced inhibition of 2T3 cell mineralization
Previously, we have shown that exposure of 2T3 cells to either the RPM or Rotating Wall Vessel (RWV), which are simulators of disuse or microgravity conditions, decreases bone formation responses as determined by ALP activity and osteogenic gene expression [Pardo et al., 2005; Patel, 2007]. Here, we examined whether daily LMHF loading of 2T3 cells could prevent the inhibition of mineralization induced by the RPM. For this study, 2T3 cells were exposed to Static, Static+LMHF, RPM, RPM+LMHF, and RPM+Static conditions in the presence of BMP4 (20 ng/ml) for 15 days and stained with Alizarin Red. As shown in Figure 5, LMHF loading of static cells (Static+LMHF) increased mineralization by 50% above the Static group, while the RPM dramatically inhibited mineralization by ∼90% of the Static group. This RPM-induced inhibition was completely normalized to the level of the Static+LMHF group by exposing the cells to LMHF loading for 10 min/day (RPM+LMHF). However, exposure of the RPM group to static conditions for 10 min/day (RPM+Static) could not rescue the inhibition of mineralization by the RPM (Figure 5), suggesting a specific effect by LMHF loading and not by the static 1g conditions. These results suggest that RPM inhibits mineralization of 2T3 cells, which can be completely rescued by exposure to brief, daily LMHF loading.
Figure 5. LMHF loading prevented inhibition of mineralization caused by RPM.
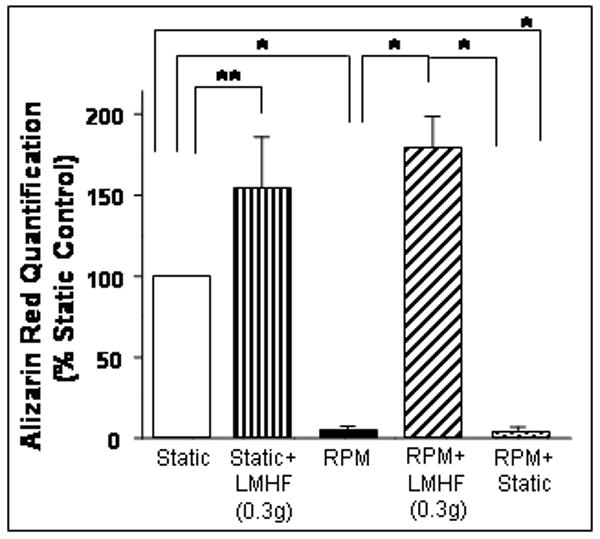
2T3 cells were exposed to Static, Static+LMHF, RPM, RPM+LMHF, and RPM+Static conditions in the presence of BMP4 for 15 days. LMHF was applied to the appropriate groups at 0.3g for 10 min/day. Alizarin Red staining was quantified and data are expressed as mean ± SEM (n=6, *p<0.005, **p<0.05).
LMHF mechanical loading requires BMPs to induce mineralization in 2T3 cells
The result in Figure 3 suggested that the osteogenic effect of LMHF may be mediated by BMPs. To further test this hypothesis, we examined whether the specific BMP antagonist noggin could block the osteogenic response induced by LMHF loading. As shown in Figure 6, LMHF loading, BMP4 (20 ng/ml), and BMP4+LMHF loading significantly increased mineralization compared to the Static group. However, treatment with noggin (100 ng/ml) completely blocked mineralization in all groups (Static, Static+LMHF, Static+BMP4, and Static+BMP4+LMHF). This result suggests that LMHF mechanical loading requires the expression of BMPs to induce mineralization in 2T3 cells.
Figure 6. LMHF loading induced mineralization in a BMP-dependent manner.
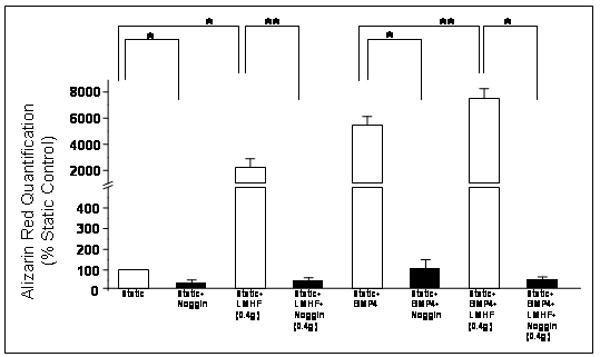
2T3 cells were exposed to Static, Static+LMHF, Static+BMP4, and Static+LMHF+BMP4 with or without noggin for 18-21 days. LMHF loading was applied to the appropriate groups at 0.4g for 10 min/day. Alizarin Red staining was quantified and data are expressed as mean ± SEM (n=6-10, *p<0.005, **p<0.05).
LMHF mechanical loading increased ALP activity in a magnitude- and time-dependent manner
In addition to using mineralization as a late marker for in vitro bone formation, we examined whether LMHF mechanical loading regulated ALP activity, an early in vitro bone formation marker for osteoblast differentiation. LMHF mechanical loading applied to 2T3 cells for 10 min/day for 3 days induced an increase in ALP activity in a magnitude-dependent manner, where 0.3g and 0.4g induced significant changes compared to static control (Figure 7A). Next, we investigated the time-dependent effects of LMHF mechanical loading, where at least 10 min/day of LMHF loading was required to observe a significant effect on ALP activity (Figure 7B). Exposure to 0.3g LMHF mechanical loading for as few as 10 min/day for 3 days was sufficient to increase ALP activity in 2T3 cells while 30 minutes or 1 hour per day did not further increase ALP activity compared to 10 min/day. These results suggest that LMHF mechanical loading increases ALP activity in a magnitude- and time-dependent manner.
Figure 7. LMHF loading increased alkaline phosphatase (ALP) activity in a magnitude- and time-dependent manner.
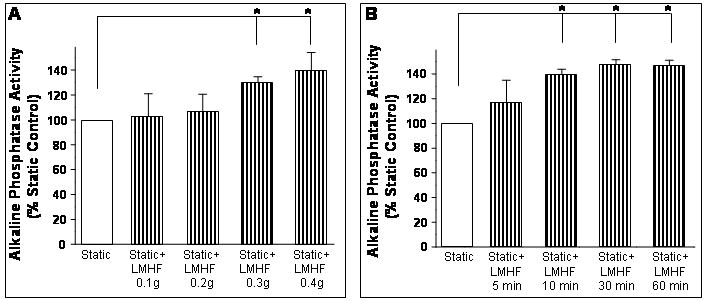
2T3 cells were exposed to Static or Static+LMHF conditions at varying loading magnitudes (A) or varying treatment-time/day (B), and cell lysates were obtained. After 3 days exposure, ALP activity was determined using a colorimetric assay, normalized to total protein, and graphed as a % of static control. Data are expressed as mean ± SEM (n=6, *p<0.05).
LMHF mechanical loading prevented inhibition of ALP activity induced by the RPM
Previously, we have shown that exposure of 2T3 cells to either the RPM or RWV induces decreased bone formation responses as determined by ALP activity [Pardo et al., 2005; Patel, 2007]. Here, we examined whether LMHF mechanical loading could prevent ALP activity inhibition induced by exposing 2T3 cells to the RPM. As expected, RPM exposure (3 days) decreased ALP activity by 30% of static control (Figure 8). This RPM-induced decrease was completely normalized to static level by exposing cells to LMHF mechanical loading (RPM+LMHF) at either 0.3g (Figure 8A) or 0.4g (Figure 8B) for 10 min/day. In contrast, exposure to static conditions for 10 min/day (RPM+Static) was not able to prevent RPM-inhibition of ALP activity (Figure 8B), suggesting a specific effect of the mechanical loading and not the static 1g conditions. In addition, LMHF mechanical loading (Static+LMHF) for 10 min/day at both 0.3g and 0.4g increased ALP activity by 30% above static control as shown in Figure 8. These results are consistent with the aforementioned mineralization studies.
Figure 8. LMHF mechanical loading prevented inhibition of alkaline phosphatase activity caused by RPM.
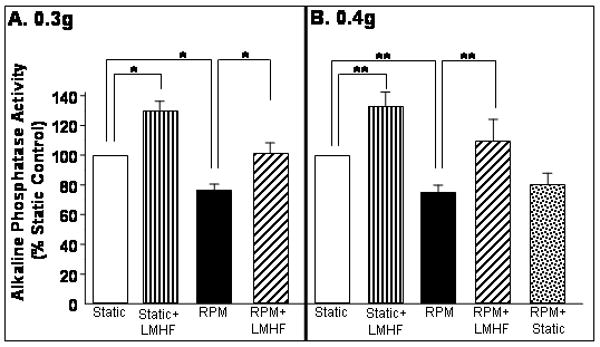
2T3 cells were exposed to Static, Static+LMHF, RPM, RPM+LMHF, and RPM+Static conditions for 3 days. LMHF was applied to the appropriate groups at 0.3g (A) or 0.4g (B) for 10 min/day. ALP activity was measured using a colormetric assay, normalized to total protein, and graphed as a % of static control. Data are expressed as mean ± SEM (n=6-9 *p<0.01, **p<0.05).
LMHF mechanical loading rescued RPM-induced decrease in osteogenic gene expression
We have previously identified genes in 2T3 cells that change in response to the RPM and the RWV [Pardo et al., 2005; Patel, 2007]. Here, we investigated alterations in a subset of these osteogenic genes induced by LMHF loading. Consistent with our previous findings, real time PCR data (Figure 9) showed that RPM exposure for 3 days significantly inhibited the expression of ALP, runx2, osteomodulin, parathyroid hormone receptor 1, and osteoglycin. LMHF loading significantly increased the expression of all the tested genes except for osteoglycin in the Static+LMHF group (Figure 9). Moreover, LMHF loading prevented the RPM-induced decrease in expression of ALP, runx2, osteomodulin, pthr1, and osteoglycin (RPM+LMHF). RPM exposure did not decrease BMP4 mRNA level compared to static control. However, LMHF loading of static cells (Static+LMHF) increased BMP4 level by ∼two-fold above Static control and increased BMP4 level by ∼2.5-fold in RPM-exposed cells (RPM+LMHF) compared to the RPM (Figure 9). These data demonstrate that LMHF regulates expression of osteogenic genes, providing insight into the molecular changes induced by LMHF mechanical loading in 2T3 cells.
Figure 9. LMHF loading rescued RPM-induced decrease in osteogenic gene expression.
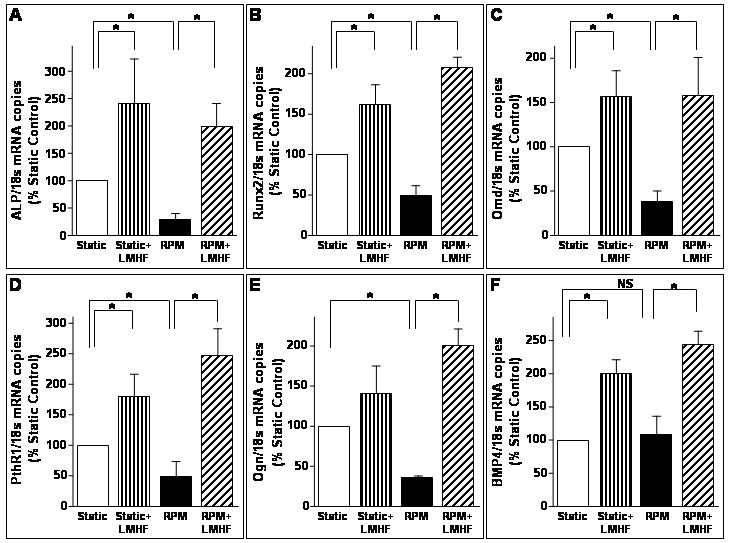
Confluent 2T3 cells were exposed to Static, Static+LMHF, RPM, and RPM+LMHF conditions for 3 days. LMHF loading was applied to the appropriate groups at 0.3g for 10 min/day. Total RNA was obtained from the cell lysates, purified, and reverse transcribed to obtain cDNA. Quantitative real time PCR was performed for ALP (A), runx2 (B), osteomodulin (C), parathyroid hormone receptor 1 (D), and osteoglycin (E) using 18s rRNA as an internal control. Data are expressed as mean ± SEM (n=3-10, *p<0.05).
Discussion
One of the most significant findings of the current study is that cultured osteoblasts can directly sense and respond to an extremely low magnitude and high frequency mechanical loading, leading to osteogenic changes. While both osteoblast-like and osteocyte cells have been previously studied for their mechanosensitive capabilities, in this study, we focused on osteoblasts [Malone et al., 2007; Zhang et al., 2006]. The in vitro data presented here suggest that osteoblasts could at least in part be responsible for the anabolic effects observed in vivo in both animals and humans [Gray et al., 1992; Rubin et al., 2001b; Ward et al., 2004]. These results are significant because it suggests that low impact loading in animals and humans may directly stimulate osteoblasts and subsequent bone formation responses. It is widely accepted that bone is responsive to signals that create peak strain magnitudes of 2,000-3,500 microstrains (με), such as those created from physical activities like running [Murfee et al., 2005; Rubin and Lanyon, 1984a; Rubin et al., 2001c]. However, muscle contractions during standing impose strains in the spectral range of 10-50 Hz of at least two orders of magnitude lower than high impact or strenuous activities [Rubin et al., 2001c] and have recently been shown to normalize bone loss [Rubin et al., 2004; Rubin et al., 2002; Rubin et al., 2001b]. Previous studies have shown that in cortical bone tissue, application of 2,000 με at a frequency of 0.5 Hz (high magnitude and low frequency) maintained bone mass [Rubin and Lanyon, 1984b; Rubin et al., 2001c]. When the frequency was increased to 1 Hz, only a strain of 1,000 με was needed to maintain bone mass, and at 30 Hz, only 70 με (low magnitude, high frequency) was needed to inhibit bone resorption [Rubin and Lanyon, 1985; Rubin et al., 2001c]. Thus, bone response to mechanical loading appears to correlate with the product of frequency and load magnitude, meaning small strains induced by a low force could stimulate bone formation and maintain bone mass if applied at a high frequency. However, the underlying molecular changes regulating how such a low level signal can be anabolic to bone tissue and which cells or tissues sense and mediate the response are unknown.
The current study provides insight into the potential cellular and molecular changes regulating how such low level mechanical loads could prevent or normalize bone loss. To examine the underlying changes of the observed in vivo anabolic effects in response to LMHF loading, we used the RPM to induce a disuse response in vitro using 2T3 cells. As expected, the RPM decreased ALP activity (Figure 8) and mineralization (Figure 5), and LMHF treatment increased both markers in static cells (Static+LMHF) in a magnitude-dependent manner (Figures 3 and 7). Moreover, LMHF loading prevented the RPM inhibition of ALP activity (Figure 9) and mineralization (Figure 5), preventing decreased bone formation responses induced by disuse or simulated microgravity conditions. The effects of both the RPM and LMHF loading were much more dramatic on mineralization of 2T3 cells than ALP activity. This may be because ALP activity is an early indicator of bone formation and is transient [Lian and Stein, 1995], making it a less sensitive marker of osteogenesis especially in response to mechanical stimuli. In our previous reports, we observed a more dramatic decrease in ALP activity in 2T3 preosteoblasts. For an unknown reason, the magnitude of this decrease has changed over time. However, we always observe an inhibition of osteoblast differentiation marked by ALP activity, gene expression, and mineralization, despite alterations in the magnitude of these changes. [Pardo et al., 2005] Together, these findings suggest that osteoblasts may directly respond to the LMHF signal at least in vitro by induction of osteogenic markers.
Importantly, we found that LMHF loading alone can induce mineralization of 2T3 cells (Figure 3) in a magnitude-dependent manner, and up to 20 ng/ml of BMP4 or BMP2 supplementation further increased this response in an additive manner (Figure 4). However, a supermaximal concentration (50 ng/ml) of BMP4 added to the cells was able to overcome both the RPM inhibition and LMHF loading increase (Figure 4). More importantly, treatment with the BMP antagonist noggin completely blocked mineralization induced by the LMHF stimulus. One of the limitations of this study was the range of low magnitude force due to a physical limitation of the LMHF device. Nevertheless, we tested LMHF loading magnitudes of 0.1-0.4g, and observed a 10 to 40-fold increase in mineralization in a magnitude-dependent manner with exposure to LMHF loading. Together, these results suggest that LMHF loading requires BMPs to induce osteogenic responses in osteoblasts at least in vitro, and future studies could investigate more detailed mechanistic roles of key BMPs in this process. Moreover, these data suggest that while either BMP4 treatment or LMHF loading alone may be used, a combination of the mechanical and humoral stimuli may be a better therapeutic modality to prevent or normalize bone loss. However, it is important to point out that chronic systemic supplementation of BMP4 has been shown to induce hypertension at least in a mouse model [Miriyala et al., 2006], suggesting caution in its use.
The current study reveals some of the osteogenic genes that are regulated by LMHF and/or the RPM. We have previously shown by DNA microarray analysis that RPM and RWV inhibits expression of a subset of osteogenic genes [Pardo et al., 2005; Patel, 2007]. Here, we investigated alp, runx2, pthr1, osteomodulin, osteoglycin, and BMP4. Runx2 is considered a “master gene” that plays a critical role in the formation of the skeleton, and without runx2, there is a complete lack of skeleton formation in a mouse model [Katagiri and Takahashi, 2002; Komori et al., 1997]. Parathyroid hormone related protein plays a role in calcium mobilization and has been linked to loss of bone density in space-flown rats [Torday, 2003], while osteomodulin (omd) and osteoglycin (ogn) belong to the small leucine rich proteoglycan (SLRP) family, members of which have been hypothesized to play roles in bone matrix formation and mineralization [Buchaille et al., 2000]. Here, we show that LMHF loading induced the expression of parathyroid hormone receptor 1 (pthr1), omd, and ogn (Static+LMHF) and prevented the RPM-inhibition of their expression (RPM+LMHF) (Figure 9).
We found that regulation of BMP4 by the RPM and LMHF was unique compared to the other genes. BMP4 is involved in the development of the skeleton, including cartilage formation and various joint developments [Tsumaki et al., 2002; Wijgerde et al., 2005]. The RPM did not alter BMP4 mRNA levels, which is consistent with our previous report [Pardo et al., 2005]. However, LMHF loading induced an increase in BMP4 mRNA in both static (Static+LMHF) and RPM-exposed cells (RPM+LMHF). This result suggests that the mechanism of action of the RPM and LMHF loading are distinct. While the decreased bone formation response of osteoblasts in response to the RPM is not dependent on changes in BMP4 mRNA level, LMHF loading seems to be BMP-dependent. Together, these data suggest that LMHF regulates expression of osteogenic genes, providing molecular changes that could potentially explain how LMHF loading stimulates bone formation responses in 2T3 cells. The mechanosensitive genes identified here provide potential targets for pharmaceutical treatments that may be used in combination with LMHF mechanical loading to more effectively treat bone pathologies.
In this study, we show that osteoblasts respond directly to an LMHF mechanical load. It has not been reported how the LMHF may be sensed by and transmitted in the cells to induce osteogenic responses. However, potential mechanotransduction pathways may involve integrins [Iqbal and Zaidi, 2005], stretch-activated channels and the ensuing influx of extracellular calcium [Iqbal and Zaidi, 2005], cell deformations and cytoskeleton [Ingber, 1999; Ingber, 2006]. Elucidating the mechanosensors and mechanotransduction pathways would be interesting future work.
In summary, we have revealed that LMHF mechanical loading, which has been shown to prevent bone loss in animals and humans, elicits cellular and molecular changes in osteoblasts that may mediate bone formation responses to extremely low magnitude loading. We have found that the RPM inhibits markers of bone formation, such as ALP activity and mineralization, as well as critical gene expression such as runx2. However, LMHF mechanical loading prevents these RPM-induced effects, and LMHF loading requires the expression of BMPs to induce osteogenic effects. While it has been previously shown that LMHF loading normalizes bone loss in vivo, this research provides insight into how such low level mechanical loading may prevent or normalize bone loss in animals and humans. Finally, these data combined with previously published reports using LMHF loading strongly suggest that this mechanical loading platform may be also used as a novel countermeasure in spaceflight.
Acknowledgments
This work was supported in part by funding from National Institute of Health grants HL71014 (HJ), HL075209 (HJ), and AR43498 (CR). We would like to thank Tommy Wilson from Juvent, Inc. for his help with the mechanical loading platform as well as Chih-Wen Ni at Georgia Tech and Emory University for helpful comments during these studies. Additionally, we wish to thank Andres Garcia, Blaise Porter, Ben Keselowsky, Charles Gersbach, and Jennifer Phillips at Georgia Tech for their assistance in FTIR and helpful comments on mineralization experiments.
References
- Buchaille R, Couble ML, Magloire H, Bleicher F. Expression of the small leucine-rich proteoglycan osteoadherin/osteomodulin in human dental pulp and developing rat teeth. Bone. 2000;27:265–70. doi: 10.1016/s8756-3282(00)00310-0. [DOI] [PubMed] [Google Scholar]
- Galloway MT, Jokl P. Aging successfully: the importance of physical activity in maintaining health and function. J Am Acad Orthop Surg. 2000;8:37–44. doi: 10.5435/00124635-200001000-00004. [DOI] [PubMed] [Google Scholar]
- Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006;119:S3–S11. doi: 10.1016/j.amjmed.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Gersbach CA, Byers BA, Pavlath GK, Garcia AJ. Runx2/Cbfa1 stimulates transdifferentiation of primary skeletal myoblasts into a mineralizing osteoblastic phenotype. Exp Cell Res. 2004;300:406–17. doi: 10.1016/j.yexcr.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–74. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- Gray B, Hsu JD, Furumasu J. Fractures caused by falling from a wheelchair in patients with neuromuscular disease. Dev Med Child Neurol. 1992;34:589–92. doi: 10.1111/j.1469-8749.1992.tb11489.x. [DOI] [PubMed] [Google Scholar]
- Ingber D. How cells (might) sense microgravity. Faseb J. 1999;13(Suppl):S3–15. doi: 10.1096/fasebj.13.9001.s3. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20:811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun. 2005;328:751–5. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- Kapur S, Baylink DJ, Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32:241–51. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- Kaspar D, Seidl W, Neidlinger-Wilke C, Ignatius A, Claes L. Dynamic cell stretching increases human osteoblast proliferation and CICP synthesis but decreases osteocalcin synthesis and alkaline phosphatase activity. J Biomech. 2000;33:45–51. doi: 10.1016/s0021-9290(99)00171-2. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–59. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- Lee WS, Cheung WH, Qin L, Tang N, Leung KS. Age-associated decrease of type IIA/B human skeletal muscle fibers. Clin Orthop Relat Res. 2006;450:231–7. doi: 10.1097/01.blo.0000218757.97063.21. [DOI] [PubMed] [Google Scholar]
- Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol. 2006;290:C609–15. doi: 10.1152/ajpcell.00408.2005. [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995;15:118–40. [PMC free article] [PubMed] [Google Scholar]
- Malone AM, Batra NN, Shivaram G, Kwon RY, You L, Kim CH, Rodriguez J, Jair K, Jacobs CR. The role of actin cytoskeleton in oscillatory fluid flow-induced signaling in MC3T3-E1 osteoblasts. Am J Physiol Cell Physiol. 2007;292:C1830–6. doi: 10.1152/ajpcell.00352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TN, Du T, Frangos JA. Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun. 2000;270:643–8. doi: 10.1006/bbrc.2000.2467. [DOI] [PubMed] [Google Scholar]
- Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation. 2006;113:2818–25. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- Murfee WL, Hammett LA, Evans C, Xie L, Squire M, Rubin C, Judex S, Skalak TC. High-frequency, low-magnitude vibrations suppress the number of blood vessels per muscle fiber in mouse soleus muscle. J Appl Physiol. 2005;98:2376–80. doi: 10.1152/japplphysiol.01135.2004. [DOI] [PubMed] [Google Scholar]
- Pardo SJ, Patel MJ, Sykes MC, Platt MO, Boyd NL, Sorescu GP, Xu M, van Loon JJWA, Wang MD, Jo H. Simulated microgravity using the Random Positioning Machine inhibits differentiation and alters gene expression profiles of 2T3 preosteoblasts. Am J Physiol Cell Physiol. 2005;288:C1211–1221. doi: 10.1152/ajpcell.00222.2004. [DOI] [PubMed] [Google Scholar]
- Patel MJ. Identification of Mechanosensitive Genes in Osteoblasts by Comparative Microarray Studies Using the Rotating Wall Vessel and the Random Positioning Machine. Journal of Cellular Biochemistry. 2007;101:587–99. doi: 10.1002/jcb.21218. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–51. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001a;412:603–4. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, Qin YX. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–57. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. Faseb J. 2001b;15:2225–9. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol. 1984a;107:321–7. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984b;66:397–402. [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–7. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Sommerfeldt DW, Judex S, Qin Y. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov Today. 2001c;6:848–858. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]
- Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res. 1992;7:761–9. doi: 10.1002/jbmr.5650070706. [DOI] [PubMed] [Google Scholar]
- Torday JS. Parathyroid hormone-related protein is a gravisensor in lung and bone cell biology. Adv Space Res. 2003;32:1569–76. doi: 10.1016/S0273-1177(03)90397-8. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Nakase T, Miyaji T, Kakiuchi M, Kimura T, Ochi T, Yoshikawa H. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J Bone Miner Res. 2002;17:898–906. doi: 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- Vuori I. Exercise and physical health: musculoskeletal health and functional capabilities. Res Q Exerc Sport. 1995;66:276–85. doi: 10.1080/02701367.1995.10607912. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Embree MC, Kilts T, Young MF, Ameye LG. Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarthritis Cartilage. 2005;13:817–27. doi: 10.1016/j.joca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–9. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- Wijgerde M, Karp S, McMahon J, McMahon AP. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev Biol. 2005;286:149–57. doi: 10.1016/j.ydbio.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, Mohan S. Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem. 2005;96:1049–60. doi: 10.1002/jcb.20606. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ryder KD, Bethel JA, Ramirez R, Duncan RL. PTH-induced actin depolymerization increases mechanosensitive channel activity to enhance mechanically stimulated Ca2+ signaling in osteoblasts. J Bone Miner Res. 2006;21:1729–37. doi: 10.1359/jbmr.060722. [DOI] [PubMed] [Google Scholar]


