Abstract
The relationship between micro structural features and macroscopic mechanical properties of engineered tissues was investigated in pure and mixed composite scaffolds consisting of collagen Type I and fibrin proteins containing embedded smooth muscle cells. In order to vary the matrix microstructure, fibrin polymerization in mixed constructs was initiated using either the blood-derived enzyme thrombin or the snake venom-derived enzyme ancrod, each at low and high concentrations. Micro structural features of the matrix were quantified by analysis of high resolution scanning electron micrographs. Mechanical properties of the scaffolds were assessed by uniaxial tensile testing as well as creep testing. Viscoelastic parameters were determined by fitting creep data to Burger’s four-parameter model. Oscillatory dynamic mechanical testing was used to determine the storage modulus, loss modulus, and phase shift of each matrix type. Mixed composite scaffolds exhibited improved tensile stiffness and strength, relative to pure collagen matrices, as well as decreased deformation and slower relaxation in creep tests. Storage and loss moduli were increased in mixed composites compared with pure collagen, while phase shift was reduced. A correlation analysis showed that the number of fiber bundles per unit volume was positively correlated with matrix modulus, strength, and dynamic moduli, though this parameter was negatively correlated with phase shift. Fiber diameter also was negatively correlated with scaffold strength. This study demonstrates how microstructural features can be related to the mechanical function of protein matrices and provides insight into structure-function relationships in such materials. This information can be used to identify and promote desirable micro structural features when designing biomaterials and engineered tissues.
Keywords: collagen, fibrin, mechanical properties, protein matrices, ancrod
1 Introduction
The elucidation of structure-function relationships in tissues is a main goal in the fields of biomechanics and tissue engineering. Matrix microstructure is an important factor in determining the functional characteristics of engineered tissues, contributing to both cellular and mechanical function. Recent efforts in the field of biomechanics have assessed structure-function relationships in synthetic materials [1,2], as well as natural materials [3,4] and native tissues [5,6], by combining both experimental and theoretical approaches. Such studies contribute to our understanding of tissue function under normal and pathological conditions, and provide design principles for the development of engineered tissue substitutes.
The microarchitecture of engineered scaffolds created using reconstituted proteins can be altered using biochemical interventions that affect matrix assembly, remodeling, and stabilization. For example, in pure collagen hydrogels it has been shown that pH and protein concentration [7] and the presence of proteoglycans [8] can alter matrix structure and the resulting mechanical properties. We have used mixtures of collagen Type I and fibrin in combination with vascular smooth muscle cells to create tissue engineered blood vessel constructs that exhibit improved mechanical properties over the pure polymers alone [9,10]. Such scaffolds created from naturally derived proteins have the advantage that cells are able to recognize and bind to them, as well as remodel them as necessary, but it has proven difficult to achieve mechanical properties that are sufficient to withstand the physiological forces found in the vasculature without prolonged in vitro culture periods [11, 12]. There therefore is a need to better understand how fibrillar protein structure leads to macroscopic tissue function.
Fibrinogen is a 340 kDa plasma protein that circulates in the bloodstream. It is a hexamer consisting of two copies of each of the α, β, and γ subunits stabilized by disulphide bonds. The structure is kept soluble under normal physiological conditions by the fact that molecular aggregation is prevented by specific fibrinopeptides at the N-terminus of the α and β chains (called fibrinopeptide A and B, respectively). Injury to the vascular wall releases the enzyme thrombin, which cleaves fibrinopeptides A and B to form monomeric fibrin. This molecule spontaneously polymerizes in a half-staggered manner into insoluble threadlike fibrils. Subsequent further aggregation of fibrin threads laterally forms a meshwork (clot) at the site of injury to prevent further bleeding and promote the wound healing response [13]. Thrombin release also activates the plasma transglutaminase Factor XIII, which crosslinks adjacent fibrin strands to further stabilize the clot.
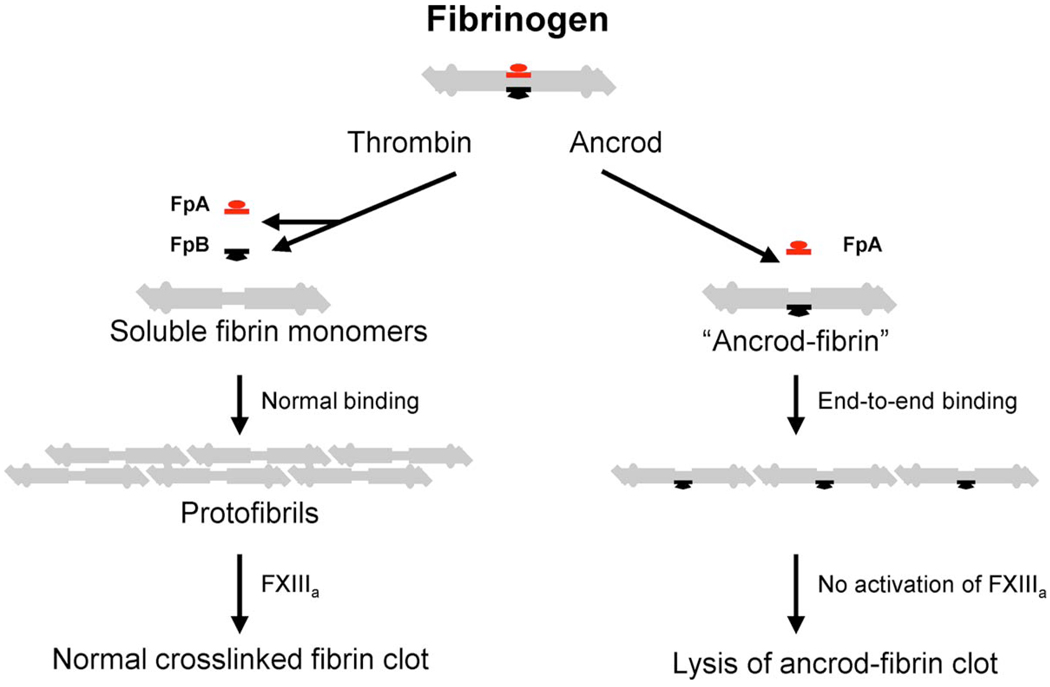
The snake venom thrombinlike enzymes (SVTLEs) are a class of serine proteases that are functionally and structurally related to thrombin, and therefore have been studied intensively in hemostasis research [14, 15]. Ancrod is one member of a subclass of SVTLE that are used as moderators of coagulation. It is derived from the venom of the Malayan pit viper and has selective substrate specificity for fibrinogen. As shown schematically in Fig. 1, ancrod cleaves fibrinopeptide A only from the α chain of the fibrinogen molecule, such that the resulting fibrin aggregates preferentially in the lateral direction instead of in the half-staggered arrangement that is typical of cleavage by thrombin [16]. In addition, ancrod does not activate Factor XIII, and therefore results in clots that are more susceptible to lysis by plasmin and subsequent removal by the fibrinolytic system. The polymerization of fibrinogen using ancrod has been studied in vitro, and it was found that failing to remove fibrinopeptide B from the fibrinogen monomer results in the slow assembly of thin fibrin fibers [17].
Fig. 1.
Schematic of the fibrinogen molecule and its interactions with thrombin and ancrod. Adapted from Ref. [16].
The goal of the present study was to harness the proteolytic effect of ancrod on fibrinogen to alter fibrin fiber morphology in collagen-fibrin composite matrices. We prepared such protein matrices containing vascular smooth muscle cells and used either thrombin or ancrod to polymerize the fibrin phase, while simultaneously polymerizing the collagen phase by changing the temperature and pH. Our expectation was that ancrod-catalyzed fibrin matrices would have a microstructure distinct from thrombin-catalyzed scaffolds. By correlating the morphology of the matrices at the microscale and to their macroscopic tissue properties, we aimed to develop an understanding of the microstructural characteristics of collagen-fibrin composites that contribute to enhanced mechanical properties in tissue engineered scaffolds.
2 Methods and Materials
2.1 Cell Culture and Construct Preparation
Rat aortic smooth muscle cells (RASMCs) were isolated from adult rats by tissue isolation, dissection, and collagenase digestion. Cells were cultured under standard conditions in 50/50 DMEM/F12 medium supplemented with fetal bovine serum (FBS) and antibiotics. All RASMC used in construct preparation were at passage 4–10 and were used at a concentration of 1.0 × 106 cells/ml. All cell culture reagents were obtained from Mediatech (Herndon, VA).
Bovine collagen Type I (MP Biomedicals, Solon, OH) was dissolved in cold 0.02 N acetic acid solution at 4.0 mg/ml. Bovine fibrinogen (Sigma Chemical Co., St. Louis, MO) was dissolved in cold culture medium at 4.0 mg/ml. To prevent fibrin degradation by plasmin, the fibrinogen solution was supplemented with 4.0 mg/l of ɛ-amino caproic acid (ACA). Thrombin enzyme (Sigma) and ancrod enzyme (Viprinex™, Neurobiological Technologies Inc., Emeryville, CA) were dissolved in phosphate buffered saline (PBS) at a concentration of 25.0 U/ml.
Pure collagen scaffolds were created by combining FBS (10% v/v), 5X DMEM (20% v/v) and complete medium (10% v/v) to a pellet of collected cells. The appropriate volume of 0.1 N NaOH solution (10% v/v) was added immediately prior to the collagen solution (50% v/v), and the cell-protein solution was thoroughly mixed.
Collagen-fibrin composites were created by suspending collected cells in culture medium (25% v/v) containing the appropriate concentration of fibrin-polymerizing enzyme (thrombin or ancrod), and then adding FBS (10% v/v), 5X DMEM (10% v/v) and 0.1 N NaOH (5% v/v). Fibrinogen solution (25% v/v) and collagen solution (25% v/v) were promptly added and thoroughly mixed. Mixed collagen-fibrin constructs were made using two concentrations of thrombin: 0.01 and 0.10 units of thrombin/mg fibrinogen (UT/mg F), referred to as mixed thrombin high and mixed thrombin low constructs, respectively. Similarly, mixed scaffolds were prepared with 0.01 and 0.10 units of ancrod/mg fibrinogen (UA/mg F) and were referred to as mixed ancrod low and mixed ancrod high, respectively. Pure collagen and mixed thrombin low are our standard matrix formulations, and were considered the controls in this study.
After mixing, the cell-protein suspensions were poured into glass test tubes containing inserts to produce an annular mold. The mold insert consisted of an inner tubular glass rod surrounded by rubber stoppers at a specified distance such that the final construct volume was 3.7 ml. Constructs were allowed to gel for 2–3 h at 37°C. After gelation, constructs were removed and cultured under static conditions in complete medium containing 2.0 mg/ml ACA for 7 days. The total initial protein concentration in all construct types was 2.0 mg/ml.
2.2 Scanning Electron Microscopy and Matrix Morphometry
Scanning electron microscopy was used to examine scaffold micro structure after 7 days of static culture. Constructs were fixed in 4% (v/v) glutaraldehyde solution for 1 h and then serially dehydrated in 30%, 50%, 70%, 90%, 95% (2×), and 100% (2×), ethanol solutions for 20 min each. Dehydrated samples were processed in a critical point drier (Tousimis Corp, Rockville, MD) to preserve their microstructure. Specimens were mounted on aluminum stubs, sputter-coated with gold-palladium and imaged on a high resolution field emission SEM (Carl Zeiss, Peabody, MA).
High resolution (75 kX) electron micrographs were used for quantitative analysis of scaffold structure using IMAGEPRO™ software (Media Cybernetics, Silver Spring MD). Twelve micrographs from four separate ring samples were used to determine fibril diameter, length between nodes of intersection, fibril bundle size, and number of bundles per unit volume.
Bulk gel compaction over time in culture is a characteristic of collagen and fibrin matrices, and was measured digitally imaging the constructs on days 1,3, and 7. Images were analyzed to determine construct dimensions using IMAGETOOL™ software. Two measurements along the length and five diameter measurements were used to calculate tubular gel volume. Gel compaction was expressed as a percentage of the original volume on day 0.
2.3 Uniaxial Tensile Testing
All mechanical characterization was performed on day 7 using a mechanical testing system (EnduraTEC Inc., Minnetonka, MN) equipped with both a screw-driven actuator for tensile testing and an electromagnetic actuator for high frequency tests. Three or four ring segments of 5 mm length were cut from each of three tubular constructs for testing. Ring segments were imaged to obtain exact dimensions before being mounted on stirrup grips for testing. Samples were preconditioned with 3 cycles of 20% strain and were subsequently strained to failure at 0.3 mm/s. Engineering stress and strain were calculated using the force and displacement data and the initial cross-sectional area of each sample. Material modulus was defined as the slope of the linear region of the stress-strain plot and ultimate tensile stress (UTS) was defined as the highest stress attained prior to failure. Maximum force to failure was also determined as a sample property.
2.4 Creep Testing and Model Fitting
Creep measures the time-dependent deformation in response to an applied static load (or stress). A 0.2 g force was applied to ring segments at a rate of 0.2 g/s, and displacement was recorded while the construct was allowed to creep for 300 s. The load then released and displacement was recorded for an additional 300 s. Construct dimensions were used to calculate the applied stress, σ0, and strain as a function of time, ɛ(t), was plotted.
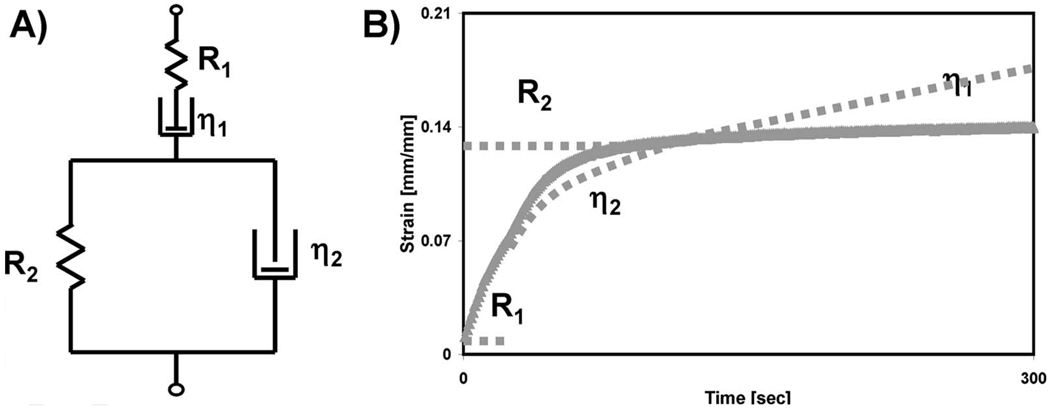
Burger’s four-element model was used to analyze the data collected from the creep portion of the strain-time plot [18]. This constitutive model consists of a spring and dashpot in series (i.e., a Maxwell unit) further connected in series with a spring and dashpot in parallel (i.e., a Voigt unit), as shown schematically in Fig. 2(a). The model parameters represent characteristics of the stress-strain relationship, and therefore the time-dependent behaviors that are evident in the creep curves. R1 and R2 are elastic parameters, while the η1 and η2 are coefficients of viscosity. Figure 2(b) shows diagrammatically how specific portions of the creep plot are related to Burger’s parameters. Individual parameters from Burger’s model for each experimental creep plot were obtained using a custom MATLAB™ script that applied an unconstrained nonlinear function to minimize the sum of the square of the errors between the experimental and predicted creep data.
Fig. 2.
(a) Spring and dashpot representation of Burger’s four-parameter model. (b) Example of a creep plot and parameters that govern each region.
2.5 Oscillatory Dynamic Mechanical Analysis
Dynamic mechanical analysis (DMA) was used to determine the behavior of ring samples in response to an oscillatory strain. After a load hold value of 0.2 g force was reached, a 10% strain was imposed while the frequency was varied from 0.1 Hz to 10 Hz. The corresponding force response was recorded. Values for dynamic amplitude and phase were calculated by custom DMA software (ENDURATEC) and converted to viscoelastic properties using Fourier transform analysis. The tangent of the phase angle was calculated and the initial ring segment dimensions were used to convert the stiffness and phase information into viscoelastic parameters. Storage modulus (E′), loss modulus (E″) moduli, and the phase shift (δ) for each ring segment were determined.
The force and strain values used in creep and DMA testing were chosen to be in the linear viscoelastic region for the collagen-based constructs. The creep test duration was chosen based on previously published work [19]. A frequency sweep was applied because this DMA mode is more appropriate for biological tissue, and the test frequency values were chosen based on previous studies in the literature [20,21].
2.6 Statistical Analysis
Data were collected from four independent experiments, each using a separate cell batch. Comparison of data was performed using analysis of variance (ANOVA) with Tukey’s test. Values of p < 0.05 were considered significant. Error bars on all graphs represent the standard error of the mean. In the figures, an asterisk (×) represents a significant difference when compared with the collagen control, and a dagger symbol (†) is used to represent a significant difference when compared with the standard mixed thrombin low construct.
3 Results
3.1 Gel Compaction
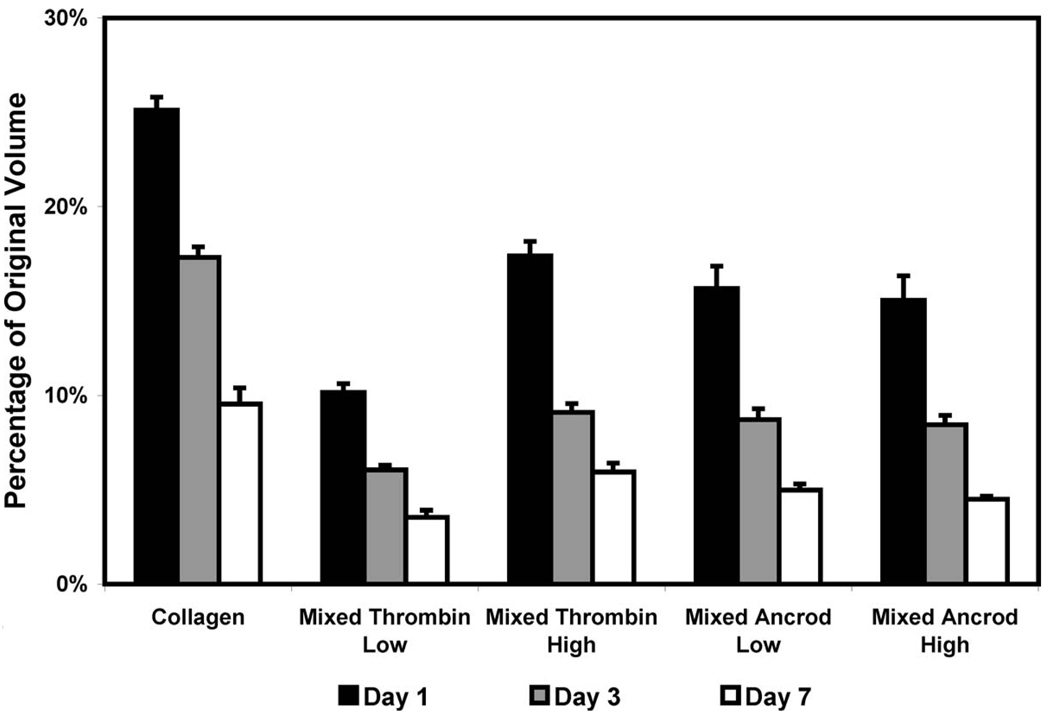
The degree of gel compaction, represented as a percentage of the original volume, is shown in Fig. 3. All constructs compacted to less than 30% of their original volume by day 1, and continued to compact progressively over the remaining time in culture. Collagen constructs compacted to approximately 10% of their original volume by day 7. All collagen-fibrin mixed composite scaffolds compacted significantly more than pure collagen scaffolds. Mixed thrombin low constructs compacted more rapidly than other matrix types to about 4% of their original volume, which was statistically different from the mixed thrombin high constructs (6%). Mixed scaffolds prepared with ancrod compacted to approximately 5% of their original volume by day 7, and there was no statistically significant difference between the volumes of these scaffolds based on ancrod concentration.
Fig. 3.
Compaction as a percentage of the original volume for pure collagen and mixed composite scaffolds
3.2 Scanning Electron Microscopy
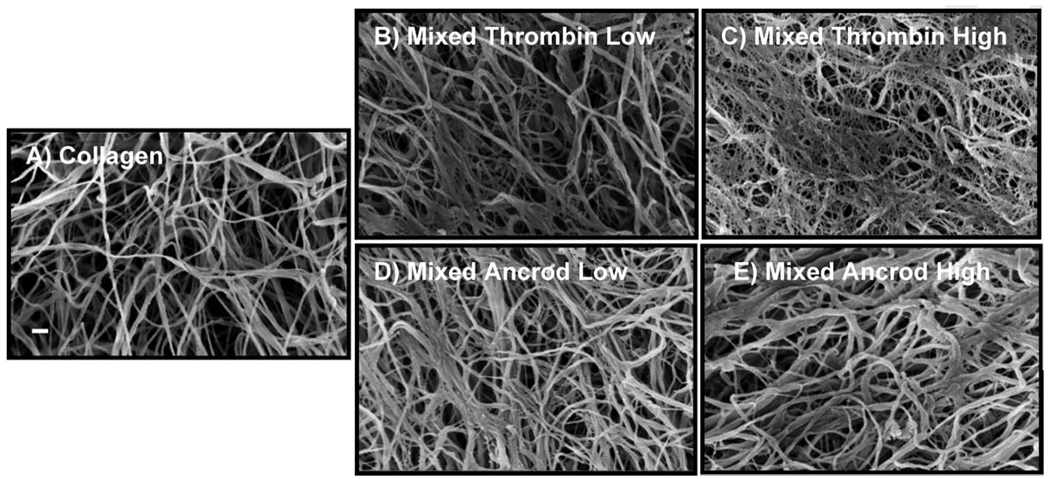
The microstructure of all scaffolds was characterized by randomly oriented fibrils in an intertwined network, as shown in the representative micrographs in Fig. 4. Though the morphology of the matrices was similar in general, there were distinct differences at the microscale that emerged upon close examination and quantification. Pure collagen scaffolds (Fig. 4(a)) had a more open and porous structure than mixed constructs. Matrices containing thrombin-fibrin (Figs. 4(b) and 4(c)) had more distinct fibrillar structure, and an increase in. thrombin concentration caused a more highly interconnected structure. These mixed scaffolds exhibited a fibrin meshwork surrounding the collagen fibrils. Matrices containing ancrod-fibrin (Figs. 4(d) and 4(e)) showed a more solid web structure, with fibrin fibrils aggregated in a parallel fashion. This morphology was more obvious at higher ancrod concentration, resulting in decreased porosity of the matrix.
Fig. 4.
Scanning electron micrographs of (a) pure collagen and (b)–(e) mixed matrices prepared with varying enzyme and concentration. Scale bar in (a) is 200 nm.
3.3 Matrix Morphometry
Figure 5 shows structural parameters that were quantified by image analysis of scanning electron micrographs. The average diameter of the protein fibers (Fig. 5(a)) was largest for pure collagen fibers (~55 nm) and was significantly lower in mixed matrices, ranging from 38 nm to 42 nm. Mixed scaffolds prepared with thrombin had similar average fiber diameters, while mixed ancrod scaffolds were significantly different. In addition, mixed ancrod scaffolds had larger fiber diameters when compared with the standard mixed thrombin low.
Fig. 5.
Quantitative measurements for collagen and mixed scaffolds. (a) Fiber diameter, (b) length between nodes, (c) number of bundles per volume, and (d) width of bundles. * is the statistical difference from collagen, and † is the statistical difference from mixed thrombin low.
Length between fiber nodes (Fig. 5(b)) was largest in the pure collagen scaffold, averaging ~342 nm. This parameter decreased in the mixed composite scaffolds, ranging from 194 nm in the mixed ancrod high scaffold to 285 nm in the mixed thrombin low scaffold. Each comparative (i.e., mixed thrombin high, mixed ancrod low, and mixed ancrod high) mixed scaffold had a value for node length, which was significantly smaller than the standard mixed thrombin low scaffold.
The number of bundles/mm3 (Fig. 5(c)) was found to vary significantly depending on scaffold type. The number of fiber bundles was smallest in pure collagen scaffolds and increased significantly in all mixed scaffolds. The number of bundles per volume increased with an increase in the enzyme concentration in the mixed ancrod scaffolds.
Bundle width (Fig. 5(d)) was measured to assess the extent of fibril aggregation in each scaffold type. Constructs made with ancrod exhibited significantly larger fiber bundles than other matrices, measuring 160 nm and 169 nm for mixed ancrod low and high matrices, respectively. The next largest value was in the pure collagen scaffold, with bundles approximately 139 nm in diameter. The smallest bundles were found in mixed scaffolds prepared with thrombin, which were of similar size at about 130 nm.
3.4 Tensile Mechanical Properties
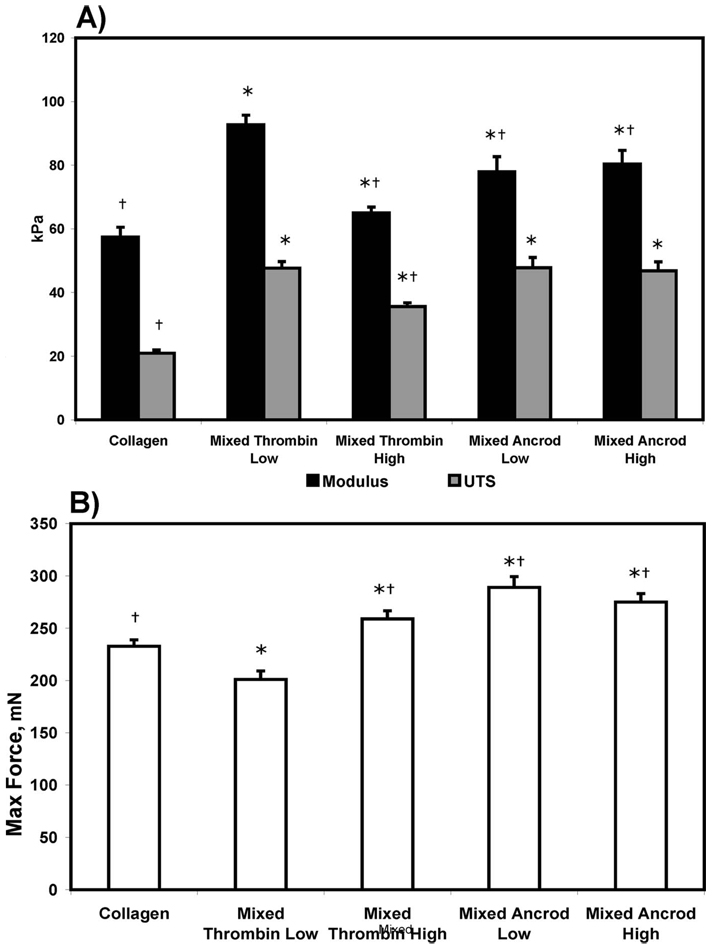
The results of uniaxial tensile testing are shown in Fig. 6. The material modulus and ultimate tensile strength (Fig. 6(a)) for the control collagen scaffolds were 57.4 kPa and 20.9 kPa, respectively. All of the mixed composites showed significantly increased properties when compared with the pure collagen scaffolds. Mixed thrombin low composites demonstrated the highest values of modulus (92.8 kPa) and UTS (47.6 kPa), while the mixed thrombin high showed significantly lower values (65.0 kPa and 35.6 kPa, respectively). Mixed ancrod composites displayed modulus values (approximately 80 kPa) that were intermediate between mixed thrombin low and high, however the UTS values were on par with mixed thrombin low. There was no statistical difference in modulus and UTS values as a result of the different ancrod concentrations.
Fig. 6.
(a) Material modulus, UTS, and (b) maximum force at failure for pure collagen and the mixed composites constructs. * is the statistical difference from collagen, and † is the statistical difference from mixed thrombin low.
Maximum force to failure (Fig. 6(b)) was highest in constructs made with ancrod and was lowest in mixed thrombin low composites (201 mN), which was significantly lower than the collagen control (233 mN). However, the other mixed composites all exhibited increased force to failure. The value of maximum force in the mixed thrombin high composite (259 mN) was significantly increased over mixed thrombin low. Maximum load was approximately equal in both ancrod scaffolds, at about 280 mN.
3.5 Creep Testing
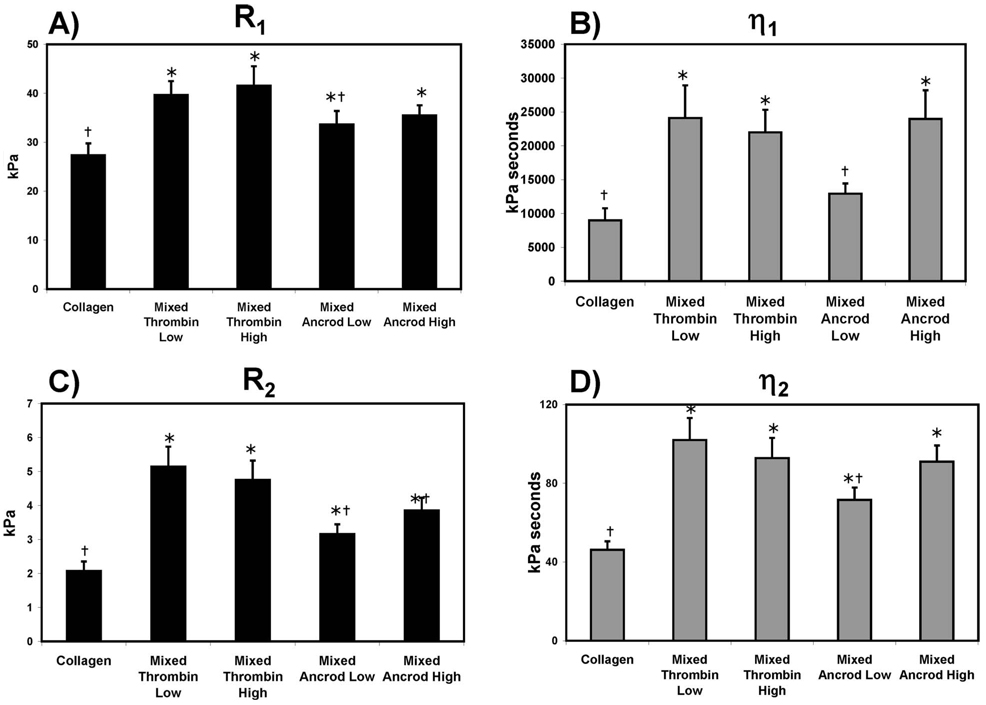
Average values for creep parameters determined by analysis of Burger’s model are shown in Fig. 7. R1, an elastic parameter reflective of instantaneous elastic response (Fig. 7(a)), was decreased in pure collagen scaffolds relative to all mixed scaffolds, indicating an increased ability to deform instantaneously. R1 was similar in all mixed scaffolds, however, there was a statistically significant decrease in this parameter for mixed ancrod low scaffolds relative to mixed thrombin low scaffolds. η1 (Fig. 7(b)) represents the unrecoverable strain response and the continuous creeping behavior in the long term, with a lower value for this parameter indicating an increased propensity to creep over time, η1 was lowest in collagen and mixed ancrod low scaffolds, while values for the other mixed scaffolds were statistically similar.
Fig. 7.
Model parameters derived from creep testing of pure collagen and mixed composites constructs: (a) R1, (b) R2, (c) η2, and (d) η1 for * is the statistical difference from collagen, and † is the statistical difference from mixed thrombin low
R2 and η2 reflect the behavior of the scaffolds in the transient region of the creep curve. R2 (Fig. 7(c)) is related to the magnitude of strain, while η2 reflects the duration of the transient region. Substantial deformation and relaxation in the transition region lead to lower values of these parameters. R2 was significantly lower in pure collagen compared with all mixed scaffolds, and mixed scaffolds prepared with ancrod had significantly lower values when compared with thrombin-activated scaffolds. The value of R2 was independent of enzyme concentration. Collagen matrices also had the lowest value for η2 (Fig. 7(d)), indicating increased relaxation time. Among the composite scaffolds, mixed ancrod low demonstrated a significant decrease in η2 when compared with the standard mixed thrombin low, as well as mixed ancrod high.
3.6 Dynamic Mechanical Analysis
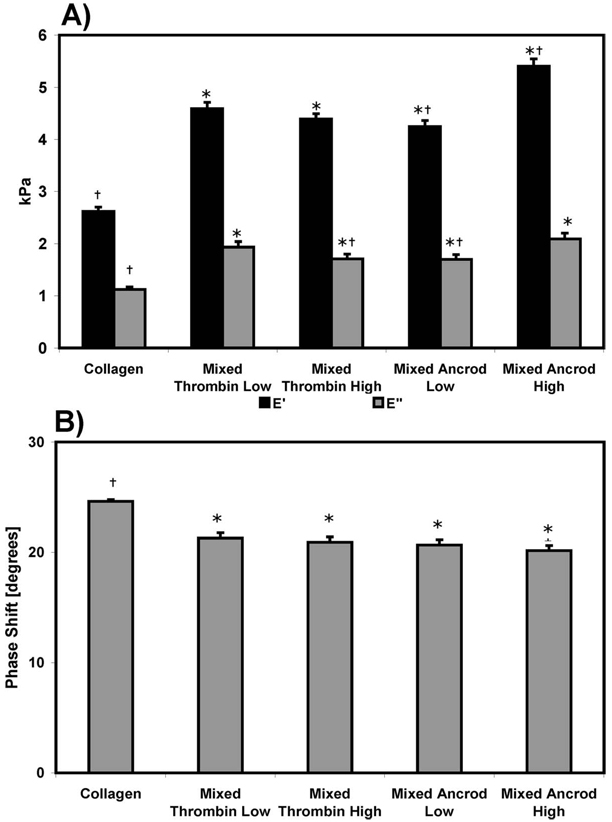
Storage (E′) and loss (E″) moduli, as well as phase shift, were averaged across the range of frequencies and plotted in Fig. 8. All mixed scaffolds exhibited significantly increased values for E′ and E″ (Fig. 8(a)), relative to pure collagen. Increased ancrod enzyme concentration lead to a higher value of E′ in mixed ancrod high scaffolds, though there was no statistically significant difference between mixed thrombin low and high. E″ was significantly decreased in mixed thrombin high relative to mixed thrombin low. A plot of the phase shift (δ) averaged across frequencies (Fig. 8(b)) showed an increased lag in pure collagen matrices, when compared with mixed constructs. However, all scaffolds exhibited phase shift behavior more typical of elastic materials (i.e., δ closer to 0 deg) than viscous fluids.
Fig. 8.
(a) Storage and loss moduli, and (b) phase shift for pure collagen and the mixed composites constructs. * is the statistical difference from collagen, and † is the statistical difference from mixed thrombin low.
3.7 Correlation Analysis
In order to further bring out potential relationships between microstructural features and mechanical properties, a correlation analysis was performed and the results are shown in Table 1. In this analysis, values of the correlation coefficient (R) in the range of |0.5|–|1.0| were considered moderate to strong. These values of R were statistically significant, as determined by a t-test for Pearson’s coefficient. The analysis showed a relatively strong positive correlation between the number of bundles per volume and both viscoelastic moduli, and a more moderate positive correlation with tensile modulus and strength. A negative correlation was observed between the number of bundles per volume and the phase shift, and the fiber diameter was negatively correlated with the ultimate tensile stress.
Table 1.
Correlation of microstructural features and tensile and viscoelastic properties
| E′ | E″ | Phase shift | R1 | R2 | η1 | η2 | Modulus | UTS | Max force | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fiber diameter | −0.45 | −0.40 | 0.19 | −0.25 | −0.30 | −0.21 | −0.33 | −0.47 | −0.50 | −0.38 |
| Length between nodes | −0.34 | −0.23 | 0.26 | −0.03 | 0.07 | −0.02 | 0.10 | −0.43 | −0.47 | −0.37 |
| Number of bundles/mm3 | 0.88 | 0.86 | −0.77 | 0.32 | 0.37 | 0.32 | 0.20 | 0.61 | 0.63 | 0.37 |
| Width of Bundles | 0.05 | 0.06 | 0.00 | 0.13 | 0.04 | −0.12 | −0.01 | 0.11 | 0.07 | −0.01 |
4 Discussion
Matrix microstructure is an important factor in determining both the biological and mechanical functions of engineered tissues. Our approach to the structure-function paradigm was to study the relationship between microstructure and macroscopic function in naturally derived protein gels composed of a mixture of collagen and fibrin proteins. One objective of this study was to analyze the role of selected microstructural features in determining mechanical properties, by quantifying structural features and correlating them with tensile and viscoelastic properties. A second objective was to improve the mechanical properties of protein-based engineered tissues by manipulating scaffold polymerization kinetics and the resulting matrix microarchitecture.
The role of environmental conditions on fibrin clot structure, particularly in the biological milieu, has been studied extensively in order to more fully understand hemostasis (see, e.g., Refs. [22,23]). Most of this work has focused on factors such as temperature, pH, and ionic strength, which are known to affect fibrin clot structure. In previous work, we have shown that the concentration of thrombin in cell-seeded fibrin hydrogels affects matrix microstructure and tensile mechanical properties [24]. In the present study, we selectively altered the micro structure of collagen-fibrin mixed composites in a controlled fashion, by changing both the type and concentration of enzyme used to polymerize the fibrin component, and observed consequent changes in mechanical behavior.
Increased thrombin concentration yielded finer fibrin fibrils and a more meshlike mixed composite, as is evident in the electron micrograph images of the two types of mixed thrombin matrices. The length between nodes was significantly decreased, while the number of bundles per volume was significantly increased in the mixed thrombin high composite. The average fiber diameter was not different between mixed composites made with different thrombin levels, and this probably was due to the influence of the relatively large collagen fibers in these mixed matrices, which masked the effect of thrombin on fiber diameter.
Ancrod has been investigated as an antithrombotic and anti-adhesion therapy [25,26], but has not previously been used as a polymerization agent for creating protein-based engineered tissues. In the present study we focused on using ancrod to laterally polymerize fibrin in mixed collagen-fibrin scaffolds. The combination of ancrod-fibrin with collagen in the mixed matrices produced a micro structure that was more weblike with ancrod-fibrin fibrils adhered to the collagen fibrils, as opposed to the thrombin-fibrin structure, which created a separate but intertwined matrix structure with the collagen network. The overall result was a more dense fiber network with reduced porosity. These differences were significant when quantified and compared with the mixed thrombin low scaffold. In addition, all measured microstructural features were significantly different when the ancrod enzyme concentration was increased.
Tensile characterization of mixed protein scaffolds demonstrated that their mechanical properties were influenced by the method of fibrin polymerization. As in our previous work, collagen-fibrin mixed composite scaffolds exhibited higher properties than pure collagen scaffolds. Mixed thrombin low constructs exhibited the highest properties, and increasing the thrombin concentration decreased both strength and stiffness. Ancrod-polymerized mixed matrices had values of stiffness, which were reduced, but strength that was equivalent to the mixed thrombin low scaffold. Importantly, our study allows these differences to be related to matrix microstructure. Quantitative morphometry revealed significant differences in the micro structural features between scaffold types, and a correlation analysis was performed to determine which of these features affected matrix mechanical properties.
A modest negative relationship between fiber diameter and ultimate strength was observed. Strength is a very structure sensitive property in traditional composite materials, and in this study collagen/fibrin fibrils were the lowest level of structure. Statistically, thinner fibers will have fewer flaws in the fiber structure, and therefore the overall matrix will exhibit higher strength. The in vitro formation of fibrin is governed by kinetic factors (including enzyme type) which can lead to deviations in the normal aggregation of this monomer. The environment in which the fibrinopeptides on the fibrinogen monomer are enzymatically cleaved may lead to normal, slowed, or arrested formation of fibrin fibrils, which will contribute to the homogeneity of the fibril structure and further will be manifested as changes in macroscopic properties.
The number of fiber bundles per volume also was significantly different between scaffold types, and was positively correlated with tensile properties. The differences in morphology were a direct result of variations in the fibrin polymerization kinetics, which altered aggregation of the fibrin protein and its interaction with collagen. Increases in the number of bundles per volume were linked to increases in modulus and UTS. The hierarchical organization of the extracellular matrix is critical in determining the mechanical function of tissues, and fiber bundling in the protein matrices in this study was a form of larger-scale organization. These hierarchical structures contributed to all tensile properties, including stiffness, because bundling resulted in increased arrangement of the individual fibrils, and because bundled fibers can bear additional force per unit area than larger monolithic fibers.
Biological tissues are viscoelastic in nature, and therefore characterization of their time-dependent properties can provide insight into both their structure and their function. In this study the creep behavior of matrices was quantified by mathematically modeling the strain response with respect to time. The instantaneous deformation, which results after the step increase in load, is represented by the elastic response parameter R1. This parameter was lowest in pure collagen, but was unchanged across mixed scaffold types. Of particular interest in this study was the transitory and long-term behavior of the matrices. Components of the Voigt unit, R2 and η2, are interdependent parameters, which govern the behavior in the transient region of the creep curve. R2 is related to the magnitude of deformation, while η2 is related to the time required to relax to a constant strain rate. The coefficient of viscosity, η1, symbolizes the unrecoverable strain response and represents the gradual deformation (creep), which occurs in the long term. Mixed scaffolds demonstrated decreased deformation (R2), slower relaxation (η2), and a decreased propensity to creep in the long term (η1) when compared with the pure collagen scaffold. The correlation between microstructural features and creep parameters was weak, however, it is likely that the presence of fibrin in mixed scaffolds altered the transition behavior of these matrices through collagen-fibrin interactions. Stretching of fibrin fibrils between collagen fibrils as the matrix deforms would account for the observed decrease in deformation and slowed transition in mixed matrices, and also explains their decreased propensity to creep with increased amounts of time.
Viscoelastic characterization using oscillatory dynamic mechanical analysis revealed increases in stored and dissipated energy that occurred in all mixed scaffolds when compared with pure collagen. This behavior suggests that fibrin adds recoil to the matrices because of its more elastic character. This effect is reflected in a decrease in phase shift, and the interaction between collagen and fibrin may also contribute to increased energy storage. The increased loss modulus in mixed scaffolds is most likely a result of the viscous interactions that occur as collagen and fibrin biopolymer chains slide past each other. Strong positive correlations were found between the number of bundles per volume and the storage and loss moduli, and a strong negative correlation was observed between the number of bundles per volume and the phase shift. These correlations reinforce the idea that specific protein-protein interactions (bundling) at the microscale result in macroscopic property changes.
The goal of this study was to understand relationships between specific microstructural features of protein composite matrices and the macroscopic mechanical properties of tissue engineered constructs made from these materials. The use of the snake venom-derived enzyme ancrod allowed us to modulate the microstructure of the matrices while keeping other conditions constant between treatments, and to compare those matrices with similar materials made using thrombin. The changes in mechanical properties that resulted were not large in magnitude, but illuminated the role of matrix microstructure in the determination of macroscopic function. Such analyses are important because they help biomaterials scientists and tissue engineers to identify and promote microscale features that result in more fully functional materials. Therefore the type of information we have generated can be used to improve design strategies in creating new biomaterials and engineered tissues.
Acknowledgment
Ancrod enzyme was generously provided by Neurobiological Technologies Inc. This work was supported in part by the American Heart Association and the National Institute for Biomedical Imaging and Bioengineering.
References
- 1.Lin AS, Barrows TH, Cartmell SH, Guldberg RE. Microarchitectural and Mechanical Characterization of Oriented Porous Polymer Scaffolds. Biomaterials. 2003;24(3):481–189. doi: 10.1016/s0142-9612(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 2.Engelmayr GC, Sacks MS. A Structural Model for the Flexural Mechanics of Nonwoven Tissue Engineering Scaffolds. ASME J. Biomech. Eng. 2006;128(4):610–622. doi: 10.1115/1.2205371. [DOI] [PubMed] [Google Scholar]
- 3.Thomopoulos S, Fomovsky GM, Holmes JW. The Development of Structural and Mechanical Anisotropy in Fibroblast Populated Collagen Gels. ASME J. Biomech. Eng. 2005;127(5):742–750. doi: 10.1115/1.1992525. [DOI] [PubMed] [Google Scholar]
- 4.Wang DH, Makaroun M, Webster MW, Vorp DA. Mechanical Properties and Microstructure of Intraluminal Thrombus From Abdominal Aortic Aneurysm. ASME J. Biomech. Eng. 2001;123(6):536–539. doi: 10.1115/1.1411971. [DOI] [PubMed] [Google Scholar]
- 5.Stylianopoulos T, Barocas VH. Multiscale, Structure-Based Modeling for the Elastic Mechanical Behavior of Arterial Walls. ASME J. Biomech. Eng. 2007;129(4):611–618. doi: 10.1115/1.2746387. [DOI] [PubMed] [Google Scholar]
- 6.Sokolis DP, Kefaloyannis EM, Kouloukoussa M, Marinos E, Boudoulas H, Karayannacos PE. A Structural Basis for the Aortic Stress-Strain Relation in Uniaxial Tension. J. Biomech. 2006;39:1651–1662. doi: 10.1016/j.jbiomech.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Tensile Mechanical Properties of Three-Dimensional Type I Collagen Extracellular Matrices With Varied Microstructure. ASME J. Biomech. Eng. 2002;124(2):214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- 8.Stuart K, Panitch A. Influence of Chondroitin Sulfate on Collagen Gel Structure and Mechanical Properties at Physiologically Relevant Levels. Biopolymers. 2008;89:841–851. doi: 10.1002/bip.21024. [DOI] [PubMed] [Google Scholar]
- 9.Rowe SL, Stegemann JP. Interpenetrating Collagen-Fibrin Composite Matrices With Varying Protein Contents and Ratios. Biomacromolecules. 2006;7(11):2942–2948. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of Engineered Vascular Constructs Made From Collagen, Fibrin, and Collagen-Fibrin Mixtures. Biomaterials. 2004;25(17):3699–3706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 11.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional Tissue-Engineered Blood Vessels From Bone Marrow Progenitor Cells. Cardiovasc. Res. 2007;75(3):618–628. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Isenberg BC, Williams C, Tranquillo RT. Small-Diameter Artificial Arteries Engineered In Vitro. Circ. Res. 2006;98(1):25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 13.Mosesson M, Siebenlist K, Meh D. The Structure and Biological Features of Fibrinogen and Fibrin. Ann. N. Y. Acad. Sci. 2001;936:11–30. doi: 10.1111/j.1749-6632.2001.tb03491.x. [DOI] [PubMed] [Google Scholar]
- 14.Marsh N, Williams V. Practical Applications of Snake Venom Toxins in Haemostasis. Toxicon. 2005;45:1171–1181. doi: 10.1016/j.toxicon.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Castro H, Zingali R, Albuquerque M, Pujol-Luz M, Rodrigues C. Snake Venom Thrombin-Like Enzymes: From Reptilase to Now. Cell. Mol. Life Sci. 2004;61:843–856. doi: 10.1007/s00018-003-3325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illig K, Ouriel K. Ancrod: Understanding the Agent. Semin Vasc. Surg. 1996;9:303–314. [PubMed] [Google Scholar]
- 17.Shen L, Hermans J, McDonagh J, McDonagh R. Role of Fibrinopeptide B Release: Comparison of Fibrins Produced by Thrombin and Ancrod. Am. J. Physiol. 1977;232:H629–H633. doi: 10.1152/ajpheart.1977.232.6.H629. [DOI] [PubMed] [Google Scholar]
- 18.Berglund J, Nerem R, Sambanis A. Viscoelastic Testing Methodologies for Tissue Engineered Blood Vessels. ASME J. Biomech. Eng. 2005;127:1176–1184. doi: 10.1115/1.2073487. [DOI] [PubMed] [Google Scholar]
- 19.Gentleman E, Livesay GA, Dee KC, Nauman EA. Development of Ligament-Like Structural Organization and Properties in Cell-Seeded Collagen Scaffolds In Vitro. Ann. Biomed. Eng. 2006;34(5):726–736. doi: 10.1007/s10439-005-9058-4. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan L, Weiss J, Wessman M, Hoying J. Design and Application of a Test System for Viscoelastic Characterization of Collagen Gels. Tissue Eng. 2004;10:241–252. doi: 10.1089/107632704322791880. [DOI] [PubMed] [Google Scholar]
- 21.Mavrilas D, Sinouris E, Vynios D, Papageorgakopoulou N. Dynamic Mechanical Characteristics of Intact and Structurally Modified Bovine Pericardial Tissues. J. Biomech. 2005;38:761–768. doi: 10.1016/j.jbiomech.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Ferry J, Morrison P. Preparation and Properties of Serum and Plasma Proteins. VII. The Conversion of Human Fibrinogen to Fibrin Under Various Conditions. J. Am. Chem. Soc. 1947;69:388–400. doi: 10.1021/ja01194a066. [DOI] [PubMed] [Google Scholar]
- 23.Bale M, Mosher D. Effects of Thrombospondin on Fibrin Polymerization and Structure. J. Biol. Chem. 1986;261:862–868. [PubMed] [Google Scholar]
- 24.Rowe SL, Lee SY, Stegemann JP. Influence of Thrombin Concentration on the Mechanical and Morphological Properties of Cell-Seeded Fibrin Hydrogels. Acta Biomater. 2007;3:59–67. doi: 10.1016/j.actbio.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hennerici M, Kay R, Bogousslavsky J, Lenzi G, Verstraete M, Orgogozo J the ESTAT investigators. Intravenous Ancrod for Acute Ischaemic Stroke in the European Stroke Treatment With Ancrod Trial: A Randomised Controlled Trial. Lancet. 2006;368:1871–1878. doi: 10.1016/S0140-6736(06)69776-6. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury S, Hubbell J. Adhesion Prevention With Ancrod Released Via a Tissue-Adherent Hydrogel. J. Surg. Res. 1996;61:58–64. doi: 10.1006/jsre.1996.0081. [DOI] [PubMed] [Google Scholar]