Abstract
Age-related changes in the neural organization of spatial information are required to account for much of the senescent loss in human scotopic spatial vision, specifically declines in the high spatial frequency cut-off of the contrast sensitivity function and enlargements of the area over which there is complete spatial summation (Ricco’s area). These results are consistent with hypothesized enlargements of ganglion cell receptive field centers during adulthood. This hypothesis was tested with 50 subjects (19–88 years) by measuring contrast thresholds for two low spatial frequency gratings (0.3 and 1.2 cycles per degree) at a series of scotopic mean illuminance levels. Contrast sensitivity increased with retinal illuminance and then reached a plateau, corresponding to the onset of Weber-like behavior. No age-related change in the light level associated with the onset of Weber-like behavior was found at either spatial frequency. This result is inconsistent with proposed age-related enlargements of ganglion cell receptive field centers under scotopic conditions.
Keywords: Aging, Scotopic vision, Psychophysics, Ganglion cell receptive fields
1. Introduction
Data of two forms demonstrate that there are age-related changes in the neural organization of spatial information under scotopic conditions. These results include senescent enlargements of the area of complete spatial summation (Schefrin, Bieber, McLean, & Werner, 1998), and a loss in contrast sensitivity to low frequency sinusoidal gratings with an accompanying decline in the high spatial frequency cut-off across adulthood (Schefrin, Tregear, Harvey, & Werner, 1999). Further analyses suggest that there are at least two scotopic spatial mechanisms tuned to low spatial frequencies and that these mechanisms undergo different rates of senescence (Peterzell, Schefrin, Tregear, & Werner, 2000; Schefrin et al., 1999).
The purpose of this study was to psychophysically test the hypothesis that age-related enlargements of receptive field centers (RFCs) of ganglion cells receiving rod input underlie the changes in scotopic spatial mechanisms described above. The paradigm used to test this hypothesis is based on a combination of contrast sensitivity results obtained from humans along with electrophysiological recordings obtained from feline ganglion cells. It has been shown that contrast sensitivity to a wide range of spatial frequencies under photopic conditions (Van Ness & Bouman, 1967) and to low spatial frequencies under mesopic (Smith, 1973) and scotopic conditions (Daitch & Green, 1969) increases monotonically with mean luminance level and then remains essentially constant. The range of luminance levels over which contrast sensitivity is constant is an example of Weber-like behavior. Psychophysical data show that the onset of Weber-like behavior occurs at a lower mean luminance level for lower relative to higher spatial frequency gratings. An explanation of this latter finding has been put forward by Enroth-Cugell and Shapley (1973) based upon their physiological recordings from feline ganglion cells receiving rod input. They found that the activity of an automatic gain control for steady-state light adaptation located within the retina was dependent upon the level of background flux associated with the stimulus. For stimuli that are larger than RFCs of ganglion cells, flux is the product of retinal illumination times the area of the cell’s receptive field center. Thus, at any given ambient light level, ganglion cells with larger RFCs that are presumably tuned to relatively lower spatial frequencies will be more light adapted than their counterparts with smaller RFCs that are maximally sensitive to higher spatial frequencies. As a result of this difference in the state of light adaptation between these types of ganglion cells, the onset of Weber- like behavior associated with the threshold detection of luminance-varying sinusoidal gratings will occur at a lower ambient light level for lower relative to higher spatial frequency gratings. Thus, if there is an age-related increase in the size of ganglion cell RFCs, one may expect that Weber-like behavior for a particular spatial frequency grating would occur at a lower luminance level in older versus younger observers. This was tested psychophysically and the results do not support the hypothesis that there are age-related enlargements of RFCs of ganglion cells tuned to relatively low spatial frequencies under scotopic conditions.
2. Methods
2.1. Subjects
Fifty subjects (27 female and 23 male) ranging in age from 19 to 88 years participated in this experiment. A thorough medical history revealed that at the time of testing all subjects were in good ocular health. No subjects were experiencing systemic complications, nor were they taking medications known to interfere with normal visual functioning. The Bailey-Lovie Log MAR Chart #4 was used to measure best-corrected distance acuities on all subjects. Forty-eight of the fifty subjects demonstrated corrected Snellen acuities of 20/25 or better. The visual acuities for the two oldest subjects were at least 20/30. An experienced clinician examined subjects younger than 60 years of age using direct ophthalmoscopy; dilated Slit Lamp examinations were performed on subjects older than 60 years of age. Based on these evaluations, all subjects were determined to be free from retinal abnormalities. Five of the older subjects demonstrated a few scattered punctate lenticular opacities. Finally, all subjects were trichromatic based upon the AO HRR pseudoisochromatic plates, Farnsworth Panel D-15 test, F-2 plate, and Neitz anomaloscope.
Written informed consent was obtained following the Tenets of Helsinki and with the approval of Institutional Review Boards at the University of Colorado, Boulder, and the University of California, Davis.
2.2. Apparatus and stimuli
A detailed description of the apparatus and protocol used to measure contrast thresholds has been published (Schefrin et al., 1999) so only a brief description will be presented here. The stimuli presented on a computer monitor and a dim red fixation light from an LED were viewed by the subjects at optical infinity through a 6× astronomical telescope. A 15 mm field stop was placed before the telescope so that its 2.5 mm diameter image formed the exit pupil of this optical system and was coincident with the center of the subject’s natural pupil. Subjects were aligned with the exit pupil using a chin rest and forehead restraint. Earlier work by Loewenfeld (1979) has shown that a 2.5 mm exit pupil is smaller than natural pupils across the age range of our subjects and under our experimental conditions. Thus, the effective pupil area was constant across all subjects.
The stimuli consisted of 0.3 and 1.2 cycle per degree (cpd) horizontally oriented sinusoidal gratings, centered at 6° nasal along the horizontal meridian. To limit the representations of the stimuli within the spatial and temporal frequency domains, the gratings were presented within a circular, 2-D Gaussian envelope (in sine phase) and a 1-s Gaussian temporal envelope in which the maximal contrast was attained 0.5 s from the start of the trial. The number of physical cycles was held constant at eight within the central portion of the stimulus because previous studies have demonstrated that human scotopic and photopic contrast sensitivities to sinusoidal gratings are affected by the number of physical cycles present within the stimulus (Howell & Hess, 1978; Savage & Banks, 1992).
The video signals from a Macintosh computer to a high-resolution monitor were combined by a video attenuator (Pelli & Zhang, 1991) to drive the green phosphor (λd = 548 nm) with a minimum of 12-bit level resolution. Interposing calibrated neutral density filters between the monitor and the telescope controlled the mean luminance of the monitor. By convolving the calibrated radiometric output of the monitor with the scotopic luminosity function (Wyszecki & Stiles, 1982), corrected for age-related changes in ocular media density (Werner, 1982), and assuming an equivalent pupil area for all our observers (see above), we calculated that the difference in retinal illuminance across the age range of our subjects would be ~0.1 log scotopic Troland. Because of this small difference between the modeled retinal illuminances of the youngest and oldest observers, we did not measure directly the lens density of individual subjects.
2.3. Procedure
Subjects adapted to the dark for 30 min prior to testing. At each tested light level, subjects adapted for 1 min by viewing a blank screen of the same average luminance as the sinusoidal gratings. Contrast thresholds were measured using a maximum-likelihood (Harvey, 1986, 1997), 2-alternative temporal forced-choice procedure. Contrast thresholds for both test gratings at every luminance level corresponded to a detection probability of 75%, based on a logistic psychometric function.
3. Results
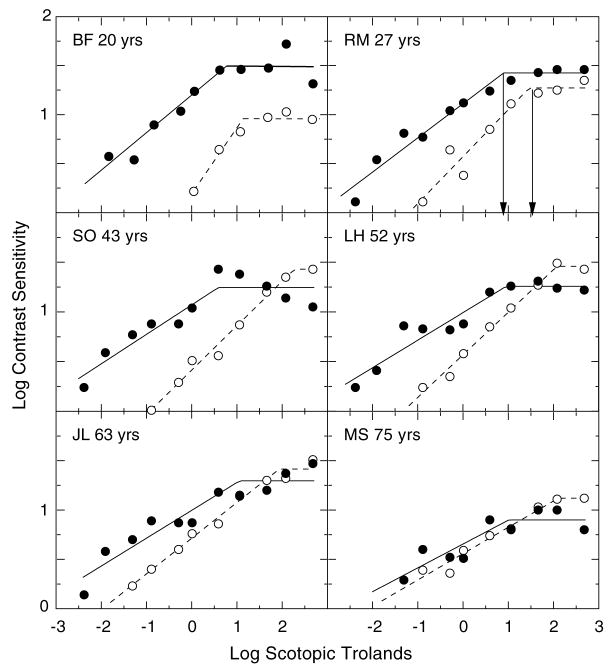
In Fig. 1, the upper, middle and lower panels show typical data measured on younger, middle-age and older subjects, respectively. In each panel, contrast sensitivities of an individual observer to the 0.3 and 1.2 cpd gratings over a range of mean luminance levels are represented by the filled and unfilled symbols, respectively. These data are consistent with earlier psychophysical results collected under photopic conditions showing that the onset of Weber-like behavior, denoted by the plateau of the function, occurs at a lower mean luminance level for lower relative to higher spatial frequency gratings (Van Ness & Bouman, 1967). In addition, the data are consistent with our previous results (Schefrin et al., 1999) demonstrating that the shape of the contrast sensitivity function is low pass at a relatively low mean luminance level (−0.85 log scotopic Tds) for subjects covering a broad age range.
Fig. 1.
Log contrast sensitivity for six observers plotted as a function of log retinal illuminance (scotopic trolands). Successive rows show data from younger, middle-age and older individuals. Filled and unfilled symbols represent sensitivities for the 0.3 and 1.2 cpd gratings, respectively. Solid and dashed lines show corresponding bilinear functions fitted to each data set using a least-squares criterion. The arrows in the upper right-hand panel denote the retinal illuminances associated with the onsets of Weber-like behavior for the two spatial frequencies.
The data from each subject, at each spatial frequency, were fitted simultaneously by two straight lines using a Marquardt least-squares algorithm in KaleidaGraph v.3.08 d. The first linear function was unconstrained in its slope; the second function was constrained to a slope of zero. The intersection of the two functions defined the retinal illuminance corresponding to the onset of Weber-like behavior. These intersections are denoted by arrows for the subject in the upper right-hand panel of Fig. 1. Goodness of fit was evaluated by r2 (mean=0.92, SD=0.1). For reference, r2 for the function fitted to observer LH (0.3 cpd) in Fig. 1 is equal to the mean of the sample.
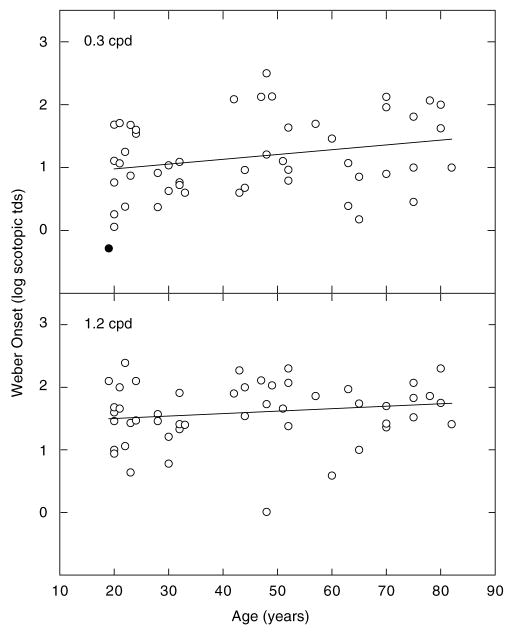
In the upper and lower panels of Fig. 2, the mean illuminance level corresponding to the onset of Weber-like behavior is plotted as a function of age for the 0.3 and 1.2 cpd gratings, respectively. The lines in each panel represent the linear regression fit to each set of data. The slopes of these functions were not statistically different from zero (p > 0.05), and r2 in each case was 0.0. (In the upper panel, the filled symbol represents data collected from one subject who was determined to be a statistical outlier using the studentized deleted residual test. This data point was not used in any subsequent analyses of the data collected at 0.3 cpd.) These analyses indicate that there is no significant age-related change in the mean luminance levels associated with the onset of Weber-like behavior for either the 0.3 cpd grating or the 1.2 cpd grating. It is important to note that neither of these analyses supports the initial hypothesis that during adulthood there is a progressive enlargement of RFCs of ganglion cells that receive rod input.
Fig. 2.
Onsets of Weber-like behavior plotted as a function of age. Upper and lower panels show data for 0.3 and 1.2 cpd gratings, respectively. The line passing through each set of symbols represents the linear regression fitted to these points; the slopes are not significantly different from zero.
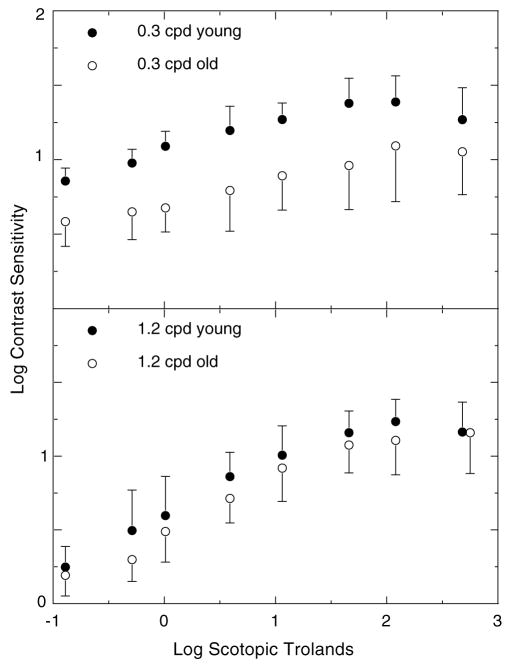
Fig. 3 shows mean contrast sensitivities as a function of retinal illuminance for the nine youngest (mean age=21 yr; 1 SD=0.9 yr) and nine oldest subjects (mean age=75 yr; 1 SD=4.3 yr). Consistent with our previous results (Schefrin et al., 1999) these functions show a loss in contrast sensitivity for the 0.3 cpd but not the 1.2 cpd grating at scotopic illuminance levels. A two-way ANOVA testing the effects of age and spatial frequency upon contrast sensitivity indicated that were significant main effects for spatial frequency (F1,32 =21.56, p < 0.0001) and age (F1,32 =46.34, p < 0.0001), as well as a significant age x spatial frequency interaction (F1,32 =5.42, p < 0.0264).
Fig. 3.
Mean log contrast sensitivity for groups of younger (filled symbols) and older (unfilled symbols) observers plotted as a function of log retinal illuminance in separate panels for 0.3 and 1.2 cpd gratings. Error bars represent ±1 SEM.
Another parameter that we analyzed was the slope of the ascending limb of the contrast sensitivity vs. retinal illuminance functions from subjects in Fig. 3. For all but one observer, the slope associated with the 1.2 cpd grating data was steeper than the slope calculated for 0.3 cpd data. In addition, the mean difference in slopes for the 0.3 and 1.2 cpd gratings was nearly the same for each group of subjects. These observations were supported by a two-way ANOVA that examined the effects of age and spatial frequency of the stimulus upon the slopes of the measured data. There was a significant main effect of spatial frequency (F1,32 =47.68, p < 0.0001), but no significant effect of age, nor was there a significant interaction between spatial frequency and age.
4. Discussion
The primary goal of this experiment was to test the hypothesis that there is an age-related increase in the RFCs of ganglion cells that mediate spatial vision under scotopic conditions. An expectation within the context of our hypothesis is that enlargements of RFCs would lead to the onsets of Weber-like behavior at lower retinal illuminance levels for older compared to younger subjects. As can be seen in both panels of Fig. 2 showing estimates of the onsets of Weber-like behavior across the age span of our subjects, this hypothesis is clearly not supported. Thus, for our experimental conditions, in which retinal illuminance is essentially the same for all subjects, there is no evidence of an age-related increase in the size of RFCs.
Although the initial hypothesis of this study was rejected by our findings, the data are fundamental to understanding the senescence of scotopic vision and constrain various explanations of age-related changes in spatial mechanisms. The data collected in this experiment confirm and extend our earlier results showing that over a range of scotopic light levels, age-related losses in contrast sensitivity are greater at relatively lower spatial frequencies than at higher spatial frequencies. The sensitivity loss for the 0.3 but not the 1.2 cpd gratings (Fig. 3) strongly implies that neural changes in specific spatial mechanisms mediating scotopic vision contribute to age-related declines in contrast sensitivity. Retinal illuminance was controlled, but it was not possible to control age-related increases in intraocular light scatter (Hennelly, Barbur, Edgar, & Woodward, 1998; IJspeert, de Waard, van den Berg, & de Jong, 1990; Westheimer & Liang, 1995). Light scatter does decrease contrast sensitivity, but this will affect high spatial frequencies somewhat more than low (Hennelly et al., 1998), the opposite of the age-related changes in contrast sensitivity. The results in Fig. 3 clearly contradict predictions based on senescent increases in light scatter. A second preneural factor that possibly could influence our data collected at 0.3 cpd is positive defocus at low luminance levels. Bedell (1987) and Coletta and Maggisano (1998) have demonstrated increasing losses in contrast sensitivity to low spatial frequencies as the mean luminance of gratings changes from photopic to scotopic light levels. They attribute this loss in sensitivity to increases in the effects of optical aberrations at low light levels due to a lessening of the Stiles–Crawford effect. However it is unlikely that the effects of positive defocus can account for the sensitivity loss to the 0.3 cpd grating. It has been shown that for small effective pupil diameters such as that used in this study there is very little difference in the Stiles–Crawford effect at varying photopic to scotopic light levels (Crawford, 1972), and as we demonstrated in our earlier work using the same experimental apparatus and procedure there was no difference in contrast sensitivity to a 0.4 cpd grating when measured on three subjects (19–43 years of age) with or without cycloplegia (Schefrin et al., 1999).
The results shown in Fig. 3 also lend themselves to analyses regarding possible sites of age-related changes in light adaptation under scotopic conditions. Sloane, Owsley, and Jackson (1988) measured contrast sensitivities and then compared the slopes between younger and older observers for a series of temporally modulated low to middle spatial frequency gratings over a range of mesopic to photopic mean luminance levels. A primary interpretation of their results is that differences in the slopes of functions describing changes in contrast sensitivity versus mean luminance are due to age-related changes in neural mechanisms mediating the detection of the stimulus. Indeed, these authors found age-related increases in the slopes of functions describing contrast sensitivities to both the 0.5 and 2 cpd sinusoidal gratings flickered at 0.5 Hz (the spatial and temporal stimulus parameters that most closely resemble those of the current study) at a series of retinal illuminances. In our study, there were differences in the slopes relating contrast sensitivity to illuminance for the 0.3 and 1.2 cpd gratings, suggesting that different scotopic mechanisms were mediating detection of the stimulus. However, there were no age-related changes in the slopes associated with a particular stimulus condition. This result suggests that there may be a difference in the senescence of spatial mechanisms operating under photopic and scotopic conditions, or an age-related dependence on the spatio-temporal properties of the stimulus. If the latter is correct, then the differences between the results of these two studies may simply reflect differences in the stimulus conditions.
The combined results of this and our previous studies (Schefrin et al., 1998, 1999) suggest that age-related changes in spatial vision under scotopic conditions may be due to neuronal changes beyond the retina-geniculate level. Previous work has suggested that more than one spatial mechanism mediates human mesopic and scotopic vision (Greenlee, Magnussen, & Nordby, 1988; Hess & Howell, 1988; Peterzell et al., 2000) and that the mechanism responsible for detecting the 1.2 cpd grating at threshold also mediates the high spatial frequency cut-off in older observers under scotopic conditions (Schefrin et al., 1999). Our result demonstrating no change in the onset of Weber-like behavior for the 1.2 cpd grating across the age range of our subjects suggests that there is no accompanying change in the size of RFCs of ganglion cells subserving this particular spatial mechanism.
A possible explanation for our combined psychophysical results regarding the senescence of scotopic spatial vision is based upon reorganization of cortical cells following losses in ganglion cells. This change could be analogous to the enlargement of cortical cell receptive fields following the loss of their primary afferent inputs from a retinal lesion (Chino, Kass, Smith, Langston, & Cheng, 1992; Gilbert & Wiesel, 1992). One interpretation is that in both human aging and in retinal lesions, the loss of primary afferent inputs leads to an opening of otherwise subthreshold lateral connections in the visual cortex. This type of change at a cortical level is consistent with age-related losses in ganglion cell numbers (Curcio & Drucker, 1993; Harmon, Abrahams, Moore, & Hoskins, 2000), but no changes in receptive field center size of ganglion cells. Such a change may be responsible for the lowered high spatial frequency cut-off seen across adulthood under scotopic conditions and previously observed age-related increases in the area of complete spatial summation (Schefrin et al., 1998).
The results of this study show that there are no age-related changes in the onsets of Weber-like behavior for two low spatial frequency gratings under scotopic conditions. Based upon this result, we propose that despite cell losses at a retinal level there are no accompanying enlargements in RFCs of remaining ganglion cells that receive rod input. We hypothesize that age-related enlargements of the area of complete spatial summation are due, in part, to senescent neural reorganization of central mechanisms.
Acknowledgments
This research was supported by the National Institute on Aging (AG04058) and a Jules and Doris Stein Research to Prevent Blindness Professorship.
References
- Bedell HE. Eccentric regard, task, and optical blur as factors influencing visual acuity at low luminances. Night vision, current research and future directions, National Research Council symposium proceedings; Washington, DC: National Academy Press; 1987. pp. 141–161. [Google Scholar]
- Chino YM, Kass JH, Smith EL, Langston AL, Cheng H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vision Research. 1992;32:789–796. doi: 10.1016/0042-6989(92)90021-a. [DOI] [PubMed] [Google Scholar]
- Coletta NJ, Maggisano LA. Technical digest series: Vol. 1. Vision science and its applications. Washington, DC: Optical Society of America; 1998. Tolerance to defocus at low spatial frequencies: Effect of luminance; pp. 144–147. [Google Scholar]
- Crawford BH. The Stiles–Crawford effects and their significance in vision. In: Jameson D, Hurvich LM, editors. Visual psychophysics: Vol. VII/4. Handbook of sensory physiology. New York: Springer-Verlag; 1972. pp. 470–483. [Google Scholar]
- Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Annals of Neurology. 1993;33:248–257. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- Daitch JM, Green DG. Contrast sensitivity of the human peripheral retina. Vision Research. 1969;9:947–952. doi: 10.1016/0042-6989(69)90100-x. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Shapley RM. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. Journal of Physiology (London) 1973;233:311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive filed dynamics in adult primary visual cortex. Nature (London) 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Greenlee MW, Magnussen S, Nordby K. Spatial vision of the achromat: Spatial frequency and orientation-specific adaptation. Journal of Physiology (London) 1988;395:661–678. doi: 10.1113/jphysiol.1988.sp016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A, Abrahams B, Moore S, Hoskins R. Neuronal density in the human retinal ganglion cell layer from 16–77 years. Anatomical Record. 2000;260:124–131. doi: 10.1002/1097-0185(20001001)260:2<124::AID-AR20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Harvey LO., Jr Efficient estimation of sensory thresholds. Behavior Research Methods and Instrumentation Computers. 1986;18:623–632. [Google Scholar]
- Harvey LO., Jr Efficient estimation of sensory thresholds with ML-PEST. Spatial Vision. 1997;11:121–128. doi: 10.1163/156856897x00159. [DOI] [PubMed] [Google Scholar]
- Hennelly ML, Barbur JL, Edgar DF, Woodward EG. The effect of age on the scattering characteristics of the eye. Ophthalmic and Physiological Optics. 1998;18:197–203. [PubMed] [Google Scholar]
- Hess RF, Howell ER. Detection of low spatial frequencies: A single filter or multiple filters? Ophthalmic and Physiological Optics. 1988;8:378–385. doi: 10.1111/j.1475-1313.1988.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Howell ER, Hess RF. The functional area for summation to threshold for sinusoidal gratings. Vision Research. 1978;18:369–374. doi: 10.1016/0042-6989(78)90045-7. [DOI] [PubMed] [Google Scholar]
- IJspeert JK, de Waard PWT, van den Berg TJTP, de Jong PTVM. The intraocular straylight function in 129 healthy volunteers; dependence on angle, age and pigmentation. Vision Research. 1990;30:699–707. doi: 10.1016/0042-6989(90)90096-4. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. Pupillary changes related to age. In: Thompson HS, Daro R, Frisen L, Glaser JS, Sanders MD, editors. Topics in neuro-ophthalmology. Baltimore, MD: Williams and Wilkens; 1979. [Google Scholar]
- Pelli DG, Zhang L. Accurate control of contrast on microcomputer displays. Vision Research. 1991;31:1337–1350. doi: 10.1016/0042-6989(91)90055-a. [DOI] [PubMed] [Google Scholar]
- Peterzell DH, Schefrin BE, Tregear SJ, Werner JS. Optical society of America technical digest series: Vol. 3. Vision science and its applications. Washington, DC: Optical Society of America; 2000. Spatial frequency tuned covariance channels underlying scotopic contrast sensitivity; pp. 39–42. [Google Scholar]
- Savage GL, Banks MS. Scotopic visual efficiency: Constraints by optics, receptor properties and rod pooling. Vision Research. 1992;32:645–656. doi: 10.1016/0042-6989(92)90181-h. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Bieber ML, McLean R, Werner JS. The area of complete scotopic spatial summation enlarges with age. Journal of the Optical Society of America A. 1998;15:340–348. doi: 10.1364/josaa.15.000340. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Tregear SJ, Harvey LO, Werner JS. Senescent changes in scotopic contrast sensitivity. Vision Research. 1999;39:3728–3736. doi: 10.1016/s0042-6989(99)00072-3. [DOI] [PubMed] [Google Scholar]
- Sloane ME, Owsley C, Jackson CA. Aging and luminance-adaptation effects on spatial contrast sensitivity. Journal of the Optical Society of America A. 1988;5:2181–2190. doi: 10.1364/josaa.5.002181. [DOI] [PubMed] [Google Scholar]
- Smith RA. Luminance-dependent changes in mesopic visual contrast sensitivity. Journal of Physiology (London) 1973;230:115– 135. doi: 10.1113/jphysiol.1973.sp010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness FL, Bouman MA. Spatial modulation transfer in the human eye. Journal of the Optical Society of America. 1967;57:401–406. doi: 10.1364/josa.57.001082. [DOI] [PubMed] [Google Scholar]
- Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human eye. Journal of the Optical Society of America. 1982;72:247–258. doi: 10.1364/josa.72.000247. [DOI] [PubMed] [Google Scholar]
- Westheimer G, Liang J. Influence of ocular light scatter on the eye’s optical performance. Journal of the Optical Society of America A. 1995;12:1417–1424. doi: 10.1364/josaa.12.001417. [DOI] [PubMed] [Google Scholar]
- Wyszecki G, Stiles WS. Color science: Concepts and methods, quantitative data and formulae. 2. John Wiley & Sons; New York: 1982. [Google Scholar]