Abstract
Background
Developmental changes in cognitive and affective processes contribute to adolescent risk-taking behavior, emotional intensification, and psychopathology. The current study examined adolescent development of cognitive control processes and their modulation by incentive, in health and psychopathology. Predictions include 1) better cognitive control in adults than adolescents, and in healthy adolescents than anxious and depressed adolescents, and 2) a stronger influence of incentives in adolescents than adults, and in healthy adolescents than their depressed and anxious counterparts.
Methods
Antisaccadic eye movement parameters, which provide a measure of cognitive control, were collected during a reward antisaccade task that included parameterized incentive levels. Participants were 20 healthy adults, 30 healthy adolescents, 16 adolescents with an anxiety disorder, and 11 adolescents with major depression. Performance accuracy and saccade latency were analyzed to test both developmental and psychopathology hypotheses.
Results
Development and psychopathology group differences in cognitive control were found. Specifically, adults performed better than healthy adolescents, and healthy adolescents than anxious and depressed adolescents. Incentive improved accuracy for all groups; however, incremental increases were not sufficiently large to further modulate performance. Incentives also affected saccade latencies, pushing healthy adolescent latencies to adult levels, while being less effective in adolescents with depression or anxiety. This latter effect was partially mediated by anxiety symptom severity.
Conclusions
Current findings evidence the modulation of cognitive control processes by incentives. While seen in both healthy adults and healthy adolescents, this modulatory effect was stronger in youth. While anxious and depressed adolescents exhibited improved cognitive control under incentives, this effect was smaller than that in healthy adolescents. These findings suggest differential incentive and/or cognitive control processing in anxiety and depression, and across development. Differences could result from disorder specific, or combined developmental and pathological mechanisms.
Keywords: Saccade, inhibition, motivation, valence, salience, eye movement, pediatric, adolescence, anxiety, cognition, depression, development
In adolescence, cognitive and affective processes mature to adult levels (Steinberg, 2004, 2005). Immaturity in these processes is believed to contribute to adolescent risk taking, intensification of emotional responses, and increased risk for onset of psychopathology (Ernst, Pine, & Hardin, 2006; Steinberg, 2005). Developmental changes have specifically been reported in cognitive control processes such as response inhibition (Luna, Garver, Urban, Lazar, & Sweeney, 2004), performance monitoring (Davies, Segalowitz, & Gavin, 2004; Ladouceur, Dahl, & Carter, 2004), and affective processes such as response to rewards (Bjork et al., 2004; Crone & van der Molen, 2004; Ernst et al., 2005; Overman et al., 2004).
The onset of psychopathology in adolescence signals alterations in cognitive/affective processes. Particularly, depression and anxiety manifest affective disturbances consistent with perturbations of motivational systems. Peak onset of these pathologies during adolescence may reflect vulnerability of these systems during this transition period. Study of motivational behavior from both a development and psychopathology perspective may help to clarify the nature of psychological processes that contribute to risk-taking behaviors and mood/anxiety disorders in adolescence.
Recent research has used oculomotor tasks to explore cognitive processes in both adults and adolescents (Luna & Sweeney, 2004; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). These tasks provide sensitive and specific assays of cognitive control processes, and they are particularly well suited to address cognitive neuroscience questions (Luna et al., 2004). Saccadic eye movements afford exquisite measures of response preparation and response execution, which serve as proxy measures of response inhibition and response monitoring. Traditional tasks monitor saccades of two types: prosaccades (i.e., reflexive prepotent eye movements in the direction of laterally appearing visual target stimuli) and antisaccades (i.e., voluntary eye movements in the opposite direction of laterally appearing visual target stimuli). The antisaccade is a useful cognitive neuroscience tool, because it requires inhibition of the exogenously stimulus-driven prepotent prosaccade that occurs with onset of a lateralized target stimulus, as well as the generation of an endogenously driven eye movement in the opposite direction as the target (Munoz & Everling, 2004). Latency to onset of an antisaccade following appearance of a target stimulus has been used as a metric of response inhibition (Jazbec, McClure, Hardin, Pine, & Ernst, 2005; Luna et al., 2004; Ridderinkhof et al., 2004).
We recently developed a saccadic eye movement task to examine the interactions between cognitive control and reward-related processes (Jazbec et al., 2006; Jazbec et al., 2005). Findings from our previous work revealed performance differences between healthy adolescents and healthy adults (Jazbec et al., 2006), and between healthy adolescents and adolescents with anxiety and/or mood disorders (Jazbec et al., 2005). While the task required performance of both prosaccades and antisaccades, previous group differences were exhibited primarily during antisaccade performance. Specifically, healthy adults were better than healthy adolescents at inhibiting reflexive prosaccades and generating antisaccadic eye movements. Additionally, incentives facilitated antisaccade performance across all groups. Incentives had a particularly strong influence in healthy adolescents, as their performance was enhanced to adult-like levels in the context of potential incentive. These findings support the notion that adolescence is a period of high sensitivity to incentives, and they suggest that adolescent reward sensitivity modulates cognitive control processes. In addition to developmental effects, healthy adolescents showed better antisaccade performance than adolescents with an anxiety and/or mood disorder.
Three main questions emerged from previous findings. First, would it be possible to enhance the sensitivity of our task design to detect incentive type (reward or punishment) and incentive magnitude differences by using different values of potential reward and punishment? Such a modification would provide dose effect functions showing group differences in sensitivity to reward or punishment separately.
Second, group differences in previous incentive modulation occurred almost exclusively during antisaccades. Moreover, they emerged for measures of latency but not for overall accuracy, the most frequently used index of saccade quality. Thus, would a more simplistic task design better identify unique patterns of incentive influence on cognitive function in normative development and psychopathology? By using only antisaccades and excluding prosaccades, we would facilitate interpretation of findings, while simultaneously doubling trial numbers and increasing power to detect significant effects, particularly for saccade accuracy. This approach would avoid the additional confounding effects of rule switching (switch from prosaccades to antisaccades and vice versa) and working memory (remember categorical meaning of the cue) and provide a design more directly focused on response inhibition, and the initiation and execution of internally generated actions.
Third, would we improve our ability to characterize performance by enhancing the analytical method? Our previous work identified saccades based on the time and distance separating gazes. The use of a more conventional velocity-based method of saccade identification would more accurately characterize performance than our previous method of extrapolating from gazes.
The present work addresses these 3 questions. To these aims, we modified the original task design by parameterizing incentive levels and employing only antisaccades. Additionally, we employed a velocity-based saccade identification method. From previous findings, we hypothesized: 1) performance would be better for healthy adults compared to healthy adolescents, and healthy adolescents compared to adolescents with anxiety or depression; 2) performance for all participants would be enhanced by incentives; 3) however, incentives would influence performance of healthy adolescents more than that of healthy adults; 4) incentives would influence performance of anxious and depressed adolescents less than that of healthy adolescents; and 5) performance would show a parametric enhancement with increasing levels of potential incentives, but with differing dose-effect curves as a function of group membership.
Methods
Participants
Participants were 20 adults (M = 30.5 years, SD = 8.65 years), 30 healthy adolescents (M = 13.85 years, SD = 2.51 years) 16 adolescents diagnosed with an anxiety disorder (M = 12.02 years, SD = 2.3 years), and 11 adolescents diagnosed with major depressive disorder (MDD) (M = 14.3 years, SD = 2.11 years). Of the adolescents with MDD, five had a secondary comorbid anxiety disorder. None of these data have been presented previously.
Participants were recruited through local newspaper advertisements and word of mouth. The study was approved by the National Institute of Mental Health Institutional Review Board. Adult participants and parents of adolescent participants gave informed consent, while adolescents provided informed assent.
For adolescent depression and anxiety participants, inclusion criteria included: 1) primary diagnosis of an anxiety or major depressive disorder by semi-structured diagnostic interview (K-SADS; Kaufman et al., 1997); 2) Children’s Global Assessment Scale’s score < 60 (CGAS; Shaffer et al., 1983); 3) age between 9 and 17 years; 4) desire for outpatient treatment. Additionally, participants with an anxiety disorder required a Pediatric Anxiety Rating Scale score > 9 (PARS; Walkup & Davies, 1999), and participants with depression required a Children’s Depression Rating Scale score > 39 (CDRS-R; Poznanski et al., 1984). Exclusion criteria included: 1) current use of any psychoactive substance; 2) current Tourette’s syndrome, obsessive-compulsive disorder, PTSD, conduct disorder, exposure to extreme trauma, or suicidal ideation; 3) lifetime history of mania, psychosis, or pervasive developmental disorder; or 4) IQ < 70.
All adolescent diagnoses were based on semi-structured interviews using the K-SADS-PL. Adult participants were evaluated using the Structured Clinical Interview for DSM-IV (SCID; Spitzer, Williams, Gibbon, & First, 1992). Interviews were conducted by experienced clinicians who demonstrated excellent inter-rater reliability (κ > .75). Additional self-report anxiety data was collected with the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al., 1997), and depression data with the Children’s Depression Inventory (CDI; Kovacs, 1982; Helsel & Matson, 1984). Adults completed the Beck Depression Inventory (BDI; Beck, Steer, & Garbin, 1988; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), and the State Trait Anxiety Inventory (STAI-Y; Spielberg, 1983).
Reward Saccade Task
We used a modified design of the Reward Saccade Task (RST; Figure 1), which differed from the original design (Jazbec et al., 2006; Jazbec et al., 2005) in two important ways: 1) a salience factor was added by parameterizing money amounts that could be won or lost at each trial; 2) participants made only antisaccadic eye movements.
Figure 1.
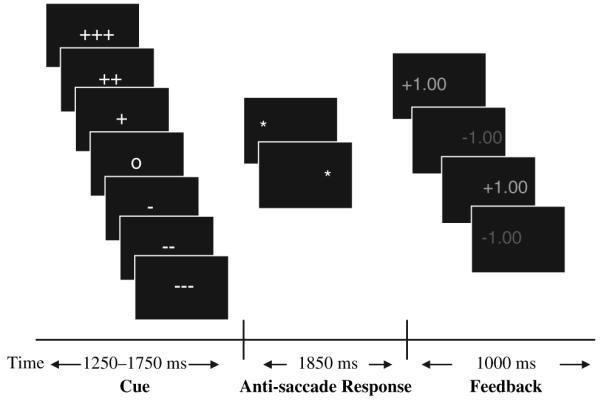
Reward Saccade Task (RST)
The antisaccade RST allows for assessment of voluntary eye movements in three valence contexts: 1) potential monetary gain (Reward); 2) potential monetary loss (Punishment); and 3) no monetary incentive (Neutral). Salience was manipulated during Reward and Punishment conditions. Participants could win or lose a High ($4), Medium ($2), or Low ($1) amount of money. Each trial was comprised of three phases: 1) initial cue phase (1250-1750 ms), which informed participants of valence (Reward, Punishment, or Neutral) and salience (High, Medium, or Low); 2) target phase (1850 ms) when participants made an eye movement response; and 3) feedback phase (1000 ms) when participants were informed of the accuracy of their response.
Each trial began with presentation of one of seven cues displayed in the center of a black computer screen, subtending approximately .5° visual angle. Valence of trials was indicated by cues: ‘+’ for Reward, ‘-’ for Punishment, and ‘o’ for Neutral. Salience was indicated by the number of repeated cues (e.g., for Reward: ‘+’ = $1, ‘++’ = $2, and ‘+++’ = $4). After a variable period of 1250-1750 ms, the cue was replaced by a lateral target stimulus. The target, a white asterisk, subtended .5° visual angle and appeared at approximately 6.15° eccentricity on the horizontal meridian to the left or the right of the cue. To succeed on trials, participants had to fixate for at least 100 ms in an area of 1° radius around the correct target location. Feedback (1000 ms) was presented 1850 ms after target onset, and consisted of dollar amounts (e.g., +$1.00, -$1.00, $0.00) subtending 1.8° visual angle. Feedback was presented in green font for a correct response and red font for an incorrect response.
The task consisted of three runs of four minutes each. Each run contained 56 trials. Participants began with $0.00 and could win up to $56 per run. Participants were told they would receive the dollar amount they won.
Eye tracking procedures
Eye movements were recorded with an ASL Model 504 eye tracking system (Applied Science Laboratories, Boston, MA) with 60 Hz temporal resolution and .25° spatial resolution. A magnetic head tracker and auto focusing lens were used to minimize head movement artifact. Raw eye movement data was analyzed off-line with in-house created software. Gaze fixations were defined as occurring when 6 or more consecutive data sample points were within a .5° range of one another. Saccades were defined as occurring when the velocity between sample points was greater than 30°/s for a minimum of 3 consecutive data sample points (≈50 ms). Saccade latency was the elapsed time between onset of the target and the start of a saccade. Direction errors occurred when the first saccade following onset of the lateralized target was in the direction of the target (see Figure 2). Accuracy was indexed by percent of direction errors. To ensure only task-relevant saccades were used, analyses were conducted selectively with saccades occurring 80-500 ms of target onset.
Figure 2.
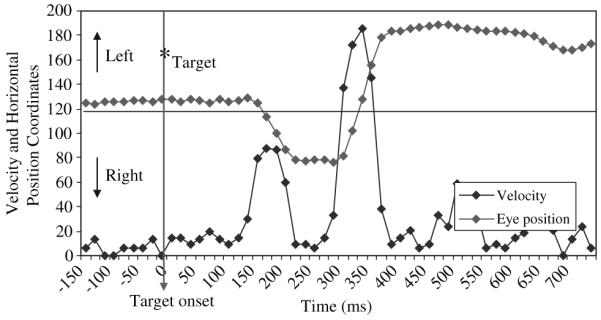
Data registration graph for a single task trial demonstrating velocity and eye position change during the production of an antisaccade direction error
Analysis of the results
Analyses were conducted to address 1) developmental changes comparing healthy adolescents and healthy adults, and 2) psychopathological deviances comparing healthy, anxious, and depressed adolescents. For each question, valence (reward, punishment, and neutral) and salience (high, moderate, and low $ amounts) were analyzed in two separate sets of repeated measures analyses of variance (r-ANOVAs). Dependent variables included performance accuracy, and latency of correct antisaccades and direction errors. The first set of r-ANOVAs used valence as the within-subjects factor and group as the between-subjects factor. Since the Neutral condition was not parameterized, the second set of r-ANOVAs used salience and valence without the Neutral condition as the two within-subjects factors, and group as the between-subjects factor.
Because of our a priori hypotheses, post-hoc analyses followed either significant main effects or interactions. Significance for tests with clear directional a priori hypotheses (e.g., better accuracy performance in adults than adolescents) used a one-tailed alpha level of .05. Otherwise, a two-tailed alpha level of .05 was used. Partial η2 is presented as an estimate of effect size for all significant ANOVAs.
Because adolescents with an anxiety disorder were significantly younger than healthy and depressed adolescents (Table 1), psychopathology analyses used age as a nuisance covariate. Additional regression analyses were conducted with age, SCARED, and CDI scores to evaluate the contribution of age and anxiety/depression symptomatology to each dependent measure.
Table 1.
Group demographic information
| Age* |
Sex (M/F) |
Tanner Score |
IQ |
CDI+***/BDI† |
SCARED+**/STAI-Y† |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Adult | 30.5 | 8.65 | 11/9 | N/A | N/A | 118.42 | 13.71 | 2 | 3 | 33.28 | 8.26 |
| Healthy Adolescent | 13.85 | 2.51 | 15/15 | 3.29 | 1.15 | 110.30 | 13.74 | 41.00 | 4.88 | 10.77 | 7.71 |
| Anxiety | 12.02 | 2.30 | 9/7 | 2.91 | 1.14 | 109.94 | 18.04 | 51.93 | 13.20 | 27.27 | 14.94 |
| Depression | 14.31 | 2.11 | 5/6 | 3.67 | 1.22 | 106.55 | 20.03 | 62.89 | 10.42 | 34.45 | 16.29 |
F(2,54) = 4.01, p < .025: Anxiety < Healthy Adolescents, p = .05; Healthy Adolescents < Depression, p = .05.
F(2,52) = 17.84, p < .001; Healthy Adolescent < Anxiety, p < .001; Healthy Adolescent < Depression, p < .001.
F(2,49) = 22.31, p < .001; Healthy Adolescent < Anxiety, p = .01; Healthy Adolescent < Depression, p = .001.
Administered to healthy adolescents only.
Administered to adults only.
Results
Our primary results included 1) significant effects of incentive valence (reward vs. punishment vs. neutral) on all dependent variables (accuracy, latency), and 2) significant effects of group and group by incentive valence interactions on accuracy and latency of direction errors. These findings are presented in greater detail below.
Contrary to expectations, no significant effects of incentive salience ($4 vs. $2 vs. $1) alone or in interaction with group or valence were found. Additionally, there was no significant group or group by incentive valence effect on latency data. For the sake of parsimony, these results will not be presented, but are available upon request.
Accuracy
Accuracy was indexed as the percent of antisaccade trials that resulted in direction errors. Development relevant group (adult, adolescent) by incentive valence (Reward, Punishment, Neutral) analysis revealed a main effect of valence, F(2,96) = 9.28, p < .001, , and a main effect of group, F(1,48) = 3.9, p = .04, with adolescents performing below adults (Figure 3). No group by valence interaction was present.
Figure 3.
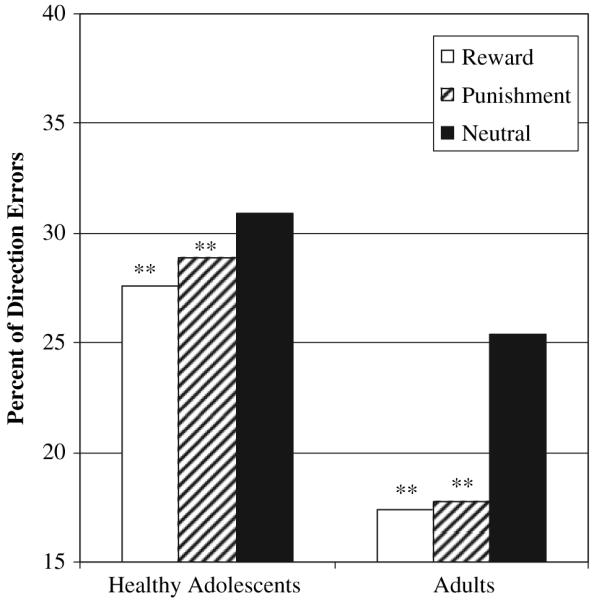
Mean percent of direction errors for developmental group (Healthy Adult, Adolescent) by valence (Reward, Punishment, Neutral) **Reward < Neutral, p = .003; Punishment < Neutral, p = .008
Psychopathology relevant group (healthy adolescent, anxiety, depression) by incentive valence (Reward, Punishment, Neutral) analysis revealed a main effect of valence, F(2,108) = 7.13, p = .001, , and group, F(2,54) = 5.02, p = .01, , but no interaction (Figure 4).
Figure 4.
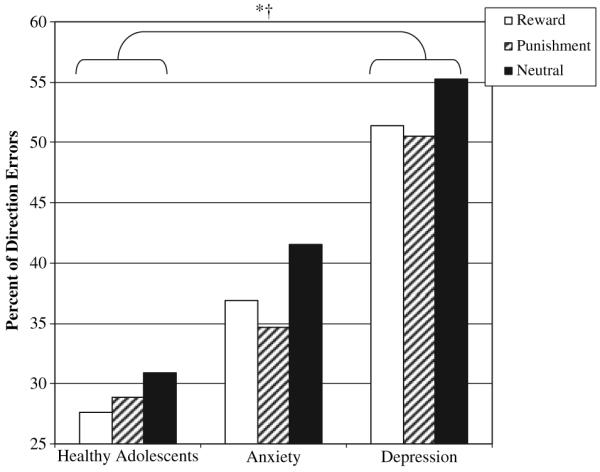
Mean percent of direction errors for psychopathology group (Healthy Adolescent, Anxiety, Depression) by valence (Reward, Punishment, Neutral) *†Main effect Group, p < .01; Depressed>Healthy Adolescents, p = .005. **Main effect Valence, p < .001
A hierarchical regression was used to further explore the relationship between age, psychopathology, and resulting percentage of direction errors (Table 3). Participant age was entered in the first step of the analysis, with a resulting R2 of .076. Total SCARED score was entered in the second step, with a resulting R2 of .172. The addition of SCARED scores in step 2 resulted in an increment in R2 of .096, F(2,45) = 4.67, p = .014. Higher scores on the SCARED, indicative of more anxiety, predicted worse performance, as evidenced by more direction errors. Addition of CDI scores in step 3 resulted in no significant addition to R2. This result suggested that anxiety severity accounted for the greatest amount of variability in performance accuracy in this sample.
Table 3.
Summary of regression analysis variables predicting percentage of direction errors in the Healthy Adolescent, Anxiety, and Depression groups (N = 57); and latency to direction errors in Healthy Adolescent, Anxiety, and Depression groups (N = 57)
| Variable | B | SE B | β | R | R2 |
|---|---|---|---|---|---|
| Percent direction errors | |||||
| Step 1 | |||||
| Age | -.30 | .016 | -.276 | .265* | .076 |
| Step 2 | |||||
| Age | -.28 | .015 | -.257 | ||
| SCARED | .005 | .002 | .310 | .414* | .172 |
| Step 3 | |||||
| Age | -.28 | .015 | -.258 | ||
| SCARED | .005 | .004 | .294 | ||
| CDI | .000 | .005 | .021 | .415 | .172 |
| Direction error latency | |||||
| Step 1 | |||||
| Age | 1.22 | 1.7 | .105 | .105 | .011 |
| Step 2 | |||||
| Age | .976 | 1.62 | .084 | ||
| SCARED | -.578 | .240 | -.337 | .352* | .124 |
| Step 3 | |||||
| Age | .961 | 1.651 | .083 | ||
| SCARED | -.607 | .389 | -.353 | ||
| CDI | .050 | .526 | .021 | .352 | .124 |
p < .05.
Latency of direction errors
Development relevant group (adult, adolescent) by incentive valence (Reward, Punishment, Neutral) analysis revealed an effect of valence, F(2, 90) = 63.71, p < .001, , but no group or interaction effects (Table 2).
Table 2.
Mean (SD) percentage of correct and direction error responses by valence and group, and Mean (SD) latency (ms) of correct and direction error responses by valence and group
| Correct |
Direction Error*† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Reward | Punish | No Incentive | Overall | Reward | Punish | No Incentive | |
| Accuracy | ||||||||
| Adults | 65.55 (25.32) | 66.60 (26.08) | 65.80 (25.59) | 61.67 (26.62) | 19.82 (17.01) | 17.43 (18.63) | 17.78 (17.18) | 25.42 (18.77) |
| Healthy Adolescent | 52.94 (21.70) | 53.26 (23.51) | 53.39 (21.29) | 50.63 (22.51) | 29.24 (19.54) | 27.62 (20.84) | 28.88 (18.90) | 30.90 (21.13) |
| Anxiety | 45.46 (24.64) | 46.61 (24.84) | 45.09 (26.15) | 42.06 (25.23) | 37.38 (21.44) | 36.89 (23.07) | 34.67 (20.93) | 41.54 (22.52) |
| Depression | 35.34 (33.27) | 39.49 (34.67) | 35.61 (34.67) | 31.06 (27.40) | 52.16 (32.79) | 51.39 (34.90) | 50.51 (32.18) | 55.30 (28.69) |
| Correct+ |
Direction Error**†† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Latency | ||||||||
| Adults | 266.10 (47.68) | 233.95 (39.82) | 237.30 (40.66) | 271.29 (56.97) | 191.05 (25.59) | 221.11 (38.76) | 225.94 (37.56) | 185.17 (24.34) |
| Healthy Adolescent | 269.79 (37.67) | 228.18 (41.25) | 228.74 (35.44) | 270.88 (46.34) | 176.21 (28.81) | 215.50 (40.81) | 214.06 (35.37) | 167.03 (25.39) |
| Anxiety | 261.78 (38.16) | 206.02 (41.75) | 213.04 (41.84) | 267.03 (36.30) | 171.47 (21.25) | 192.64 (38.50) | 199.88 (38.09) | 181.57 (51.93) |
| Depression | 283.42 (38.85) | 207.42 (43.49) | 212.14 (46.86) | 282.48 (47.61) | 164.21 (23.60) | 184.16 (48.91) | 184.66 (51.90) | 165.31 (29.25) |
Developmental Analysis: main effect group, p < .05; main effect valence, p < .001.
Psychopathology Analysis: main effect group, p < .01, main effect valence, p < .01.
Developmental Analysis: main effect of valence, p < .001.
Psychopathology Analysis: group x valence interaction, p = .005.
Main effect valence: Developmental Analysis, p < .001; Psychopathology Analysis, p < .001.
Psychopathology relevant group (healthy adolescent, anxiety, depression) by incentive valence (Reward, Punishment, Neutral) analysis revealed a group by valence interaction, F(4, 102) = 3.99, p = .005, , but no main effects of group or valence. To better understand the interaction, we calculated Reward minus Neutral difference scores and Punishment minus Neutral difference scores. Analysis of the [Reward - Neutral] differences revealed a group effect, F(2,52) = 5.185, p = .009, , with a significant post-hoc contrast between anxious and healthy adolescents (p = .013), and the depressed adolescents showing no difference with either of the other two groups. The greatest difference scores (indicating a greater latency to Reward compared to Neutral) occurred for healthy adolescents, followed by depressed and then anxious adolescents. Similarly, a group effect was present for the [Punishment-Neutral] difference scores, F(2,52) = 3.85, p = .028, . Here again, the mean difference score was significantly higher for healthy adolescents than for adolescents with anxiety (p < .05). Depressed adolescents scored between the healthy and the anxiety adolescent groups.
A hierarchical regression was used to explore the relationship between age, psychopathology, and latency to direction errors (Table 3). Participant age was entered in the first step of the analysis, which resulted in a R2 of only .011 and a non-significant change. However, total SCARED score was entered in the second step, with a resulting R2 of .124. Addition of SCARED scores in step 2 resulted in a increment in R2 of .113, F(2,45) = 3.18, p = .05. Addition of CDI scores in step 3 resulted in no significant addition to R2. Therefore, similar to the effect on accuracy, anxiety severity was the greatest modulator of latency to direction errors in this sample.
Discussion
The current study examined the modulation of cognitive control by incentives from a development (adolescents vs. adults) and a pediatric psychopathology (anxiety and depression) perspective. Using a reward antisaccade task, we predicted that performance indexing cognitive control would be superior in adulthood than in adolescence. Moreover, performance of anxious and depressed adolescents was predicted to be impaired compared to healthy adolescents. In addition, incentives were hypothesized to influence performance differentially as a function of development and psychopathology. Likewise, we predicted a parametric enhancement of performance with increased levels of potential incentive.
In line with these hypotheses, differences in performance accuracy were found between adults and healthy adolescents, and between healthy adolescents and adolescents with anxiety or depression. Adolescence is a period of considerable changes, particularly with respect to cognitive control and affective information processing (Casey, Galvan, & Hare, 2005; Ernst et al., 2006; Munoz, Broughton, Goldring, & Armstrong, 1998). Such functional changes, in addition to their role in the health consequences of typical adolescent risk-taking behavior (Steinberg, 2004), can portend vulnerability for mood/anxiety disorders. Findings of impaired inhibition of unwanted prosaccades in healthy adolescents relative to adults supports the notion of immature inhibitory control, and is consistent with previous work using antisaccade tasks (Luna & Sweeney, 2004; Munoz et al., 1998). The even greater decrease in accuracy for adolescents with a mood/anxiety disorder suggests additional deficits in cognitive control for these pathologies. The association of inhibitory deficits with mood and anxiety disorders has been proposed as a possible mechanism in the maintenance of negative affect and attention bias towards negative stimuli (Derryberry & Reed, 2002; Levesque et al., 2003; Mogg, Philippot, & Bradley, 2004).
With respect to reward manipulation and contrary to our hypotheses, no parametrically observed salience effects were found. This could have occurred for a number of reasons. While our reward values were within the range of those that have shown parametric reward effects in other studies (i.e., Knutson et al., 2001), they may not have been discrepant enough to elicit cognitive control differences. Alternatively, as evidenced by the lack of differences between reward and punishment incentives, the modulation of saccadic responses may operate to some extent in an ‘all-or-nothing’ manner. That is, a minimal amount of incentive, regardless of valence or salience level, is sufficient for modulation.
Generally, the modulation of cognitive control by incentives was robust, found across all performance measures for both correct and incorrect responses. Consistent with our previous work (Jazbec et al., 2006; Jazbec et al., 2005), this reward-related modulation differed as a function of group during direction errors. These differences reflected greater incentive influence in healthy adolescents than in adults, and weaker incentive influence in adolescents with an anxiety or depressive disorder than in healthy adolescents. The effect of anxiety/mood disorder seemed to have been at least partly mediated through anxiety symptoms (indexed by SCARED ratings), which were significantly correlated with accuracy (greater anxiety ratings and lower accuracy) and with latency of direction errors (greater anxiety ratings and shorter latency).
The selectivity of these reward-related modulation findings to direction errors is consistent with previous work, and indicates that successful antisaccades are stereotypical and under very tight controls. However, the deficits identified were restricted to latency and were found only in patients. Moreover, the current design evidenced psychopathology-related differences in overall antisaccade accuracy. This is in contrast to findings with the mixed pro-antisaccade design showing no group differences in accuracy but consistent group differences in latency and peak velocity, which affected both adolescents vs. adults and patients. This suggests that the antisaccade-only design is more sensitive to differences in accuracy, but less sensitive at detecting group differences in latency than the mixed pro-antisaccade design. Two reasons may account for this reduced sensitivity to latency. First, the additional deficits in the mixed design may reflect alterations in specific cognitive processes more prominent in this design than in the antisaccade-only design. These specific cognitive processes include greater conflict due to a greater prepotency drive, working memory, and cognitive shift. Alternatively, the discrepancy between designs may be unspecific, reflecting the higher degree of overall difficulty of the mixed design.
Findings suggest that cognitive specificity, rather than a nonspecific overall difficulty, may account for these discrepancies. Whereas both present and previous studies found a significant effect of incentives on direction error latency, this effect differed qualitatively between studies. Jazbec et al. (2005) reported shorter latency with incentives, whereas we report longer latency with incentives, suggesting that different mechanisms were at play. Two opposing processes need to occur during the motor preparation period: inhibition of a prepotent prosaccadic response, and initiation of an internally driven, goal-directed action (Everling & Fischer, 1998; Munoz & Everling, 2004). The latency period can be construed as the time during which resolution of these processes is achieved. Various interpretations for alterations of this period are possible. Longer preparation time to direction errors may reflect the greater capacity to maintain inhibition of a prepotent action (albeit not sufficient to completely suppress the prepotent response). Alternatively, longer preparation time could reflect the reduced ability to initiate a goal-directed action in the face of a normal or even insufficient capacity to maintain inhibition of a prepotent response. We submit that task architectures may have influenced which processes were predominantly modulated by incentives, and the stronger prepotency drive in the mixed paradigm could be a critical factor.
Another aspect of the incentive effect is important to note. Similarly to previous work, no differences in health, development, or psychopathology emerged between the relative influence of potential gains and potential losses. This is noteworthy as it suggests that positive and negative incentives modulate cognitive control by similar neural systems, and thus behave similarly in varying diatheses. Here again, more work is warranted to further explore this possibility, particularly in psychopathology affecting reward systems.
Use of a velocity-based saccades identification method proved to be useful. This analyses method was sensitive enough to detect group differences and provided measures that were consistent with those found in the saccadic eye movement literature. Because the velocity-based method is more conventional, the current approach provides advancement over our previous method. This said, the sampling rate of 60 Hz, although sufficient for accuracy measures, should be upgraded to provide greater sampling precision for recordings of dynamic parameters like latency.
A number of limitations need be considered. As mentioned above, the range of incentives may have been too narrow to influence performance. Second, while there was sufficient power to detect meaningful group differences, samples were relatively small, particularly with respect to the depression/anxiety groups. Increasing sample sizes might reveal additional effects. Third, a high degree of comorbid anxiety existed in the depressed sample. While preliminary analyses showed no differences between adolescents with comorbid anxiety and depression and those with pure depression, the distinct contribution of pure depression to cognitive control modulation should be studied further. Of note, no association was found between our measure of depression severity and performance scores, in contrast to correlations found between performance and anxiety ratings. Additionally, since our assessment of dimensional psychopathology was primarily limited to anxiety and depression, we cannot rule out the possibility that the current findings were partially influenced by non-internalizing symptoms.
Despite these limitations, we found the reward saccade task useful for 1) quantifying incentive influences on cognitive control processes, and 2) detecting developmental and psychopathological differences in cognitive control and modulation of cognitive control by incentives. Findings suggest 1) healthy adolescents are more sensitive to incentives relative to adults, and 2) anxious and depressed adolescents demonstrate impaired cognitive control and reduced sensitivity to incentives compared to healthy adolescents. Further study using more sensitive eye-tracking equipment, larger samples, and more powerful incentives may provide precise and consistent patterns of neuropsychological function in health and pathology. Findings also suggest a mixed pro-antisaccade design may be more sensitive detecting differences in saccadic metrics among groups compared to an antisaccade-only design. Neural correlates of such distinct functional patterns could then be assayed in functional neuroimaging studies, and eventually serve as endophenotypes for genetic and other future research.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health.
Footnotes
Conflict of interest statement: No conflicts declared
References
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Developmental changes in real life decision making: Performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Developmental Neuropsychology. 2004;25:251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error-monitoring event-related potentials in adolescents. Annals of the New York Academy of Sciences. 2004;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: A review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Helsel WJ, Matson JL. The assessment of depression in children: The internal structure of the Child Depression Inventory (CDI) Behaviour Research and Therapy. 1984;22:289–298. doi: 10.1016/0005-7967(84)90009-3. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age related influence of contingencies on a saccade task. Experimental Brain Research. 2006 doi: 10.1007/s00221-006-0520-9. online early publication, May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: An antisaccade task. Biological Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory: A self-rating depression scale for school-aged youngsters. 1982. Unpublished manuscript. [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. ERP correlates of action monitoring in adolescence. Annals of the New York Academy of Sciences. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: The antisaccade task and the voluntary control of eye movement. Nature Reviews. Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42:1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A children’s global assessment scale (CGAS) Archives of General Psychiatry. 1983;40:1228–31. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: What changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Science. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Walkup J, Davies M. The Pediatric Anxiety Rating Scale (PARS): A reliability study; Annual Meeting of the American Academy of Child and Adolescent Psychiatry; 1999.Oct 19-24, [Google Scholar]


