Abstract
Sudden infant death syndrome (SIDS) is defined as the sudden and unexpected death of an infant less than 12 months of age that occurs during sleep and remains unexplained after a complete autopsy, death scene investigation, and review of the clinical history. It is the leading cause of postneonatal mortality in the developed world. The cause of SIDS is unknown, but is postulated to involve impairment of brainstem-mediated homeostatic control. Extensive evidence from animal studies indicates that serotonin (5-HT) neurons in the medulla oblongata play a role in the regulation of multiple aspects of respiratory and autonomic function. A subset of SIDS infants have several abnormalities in medullary markers of 5-HT function and genetic polymorphisms impacting the 5-HT system, informing the hypothesis that SIDS results from a defect in 5-HT brainstem-mediated control of respiratory (and autonomic) regulation. Here we review the evidence from postmortem human studies and animal studies to support this hypothesis and discuss how the pathogenesis of SIDS is likely to originate in utero during fetal development.
1. The Sudden Infant Death Syndrome
The sudden infant death syndrome (SIDS) is defined as the sudden death during sleep of an infant less than one year of age, that remains unexplained after a thorough investigation including performance of a complete autopsy, and review of the circumstances of death and the clinical history (Willinger et al. 1991). Despite significant reductions in SIDS rates in recent years due to successful risk-reduction campaigns, SIDS remains the leading cause of death for infants between 1 month and 1 year of age in developed countries (Mathews et al. 2002). The majority (90%) of SIDS deaths occur within the first 6 postnatal months, with the peak incidence observed at 2-4 months of age (Stewart 1975a; Stewart 1975b). Multiple studies have identified robust associations between SIDS and environmental risk factors, including prone or face down sleeping (Fleming et al. 1990; Fleming et al. 1996; Blair et al. 2006), bed sharing (Blair et al. 1999; Hauck et al. 2003; Tappin et al. 2004; Tappin et al. 2005; Pelayo et al. 2006; Mitchell 2007; O'Mara 2007; Vennemann et al. 2009), and over-bundling (Fleming et al. 1990; Fleming et al. 1996). Several risk factors for SIDS relate to the mother and pregnancy, including prematurity, low birth weight, prenatal and postnatal cigarette smoke exposure (Schoendorf et al. 1992; Haglund 1993; Blair et al. 1996; Fleming et al. 1996; MacDorman et al. 1997; Wisborg et al. 2000; Anderson et al. 2005; Mitchell et al. 2006) and prenatal alcohol ingestion (Scragg et al. 1993; Alm et al. 1999; Iyasu et al. 2002; Kinney et al. 2003; Klug et al. 2003; Duncan et al. 2008b). There is also a male bias in SIDS with twice as many boys than girls dying of SIDS (Froggatt et al. 1968; Beal 1972; Stewart 1975b; Arneil et al. 1985; Millar et al. 1993). Likewise, there is a noted ethnic disparity with significantly increased SIDS rates among African American infants and Native American infants (Mathews et al. 2002). The Triple Risk hypothesis of SIDS has proven useful in thinking about SIDS etiology (Filiano et al. 1994). It posits that SIDS occurs when three factors impinge on the infant simultaneously–an underlying vulnerability in the infant, a critical period in development (the first 6 postnatal months when 90% of SIDS occurs), and homeostatic stressors heightening the infant's vulnerability (e.g. at the time of infant death hypercapnia from rebreathing exhaled air as a result of sleeping prone in the face-down position). While the pathogenesis of SIDS remains unknown, consensus of opinion implicates impairment of brainstem-mediated respiratory and autonomic control, including reduced chemoreceptor sensitivity (Shannon et al. 1977; Hunt et al. 1981), respiratory rhythm abnormalities (Steinschneider 1977; Kelly et al. 1979), failure to initiate inspiration (Hunt 1981; Hunt et al. 1981), and gasping deficit (Hunt 1981) leading to the infant death. Extensive evidence from animal studies indicates that brainstem serotonin (5-HT) systems influence several aspects of respiratory function (Richerson 2004; Hodges et al. 2008a). Multiple abnormalities in markers of 5-HT function are present in the brainstem of approximately 70% of SIDS infants (Paterson et al. 2006b). This observation informs the hypothesis that a defect in brainstem 5-HT networks resulting in failure of protective respiratory (and autonomic) responses to potentially life-threatening, but normally occurring sleep-related events (e.g., face down position) with sequelae including hypoxia, hypercapnia might account for SIDS in a subset of cases. In this review we discuss the evidence from human and animal studies to support the hypothesis that defective 5-HT mediated regulation of respiratory and autonomic function contributes to the SIDS death and how specific features of 5-HT neuronal function and development are synergistic with the environmental and genetic risk factors associated with SIDS.
2. Distribution of 5-HT Neurons in the Brainstem
Neurons synthesizing 5-HT (and expressing tryptophan hydroxylase [TPH]), localized exclusively to the brainstem in distinct cell groups classically defined as B1-B9, differentially project to virtually all regions of the neuraxis (Dahlstrom et al. 1964; Steinbusch 1981; Steinbusch et al. 1981; Törk et al. 1990; Jacobs et al. 1992; Hornung 2003). These cell groups are divided into a rostral domain consisting of groups B4-B9 in the midbrain and a caudal domain consisting of groups B1-B3 in the medulla (Fig 1). The two domains of the brainstem 5-HT neurons are distinct in their developmental origins, functions, and connectivity (Dahlstrom et al. 1964; Steinbusch 1981; Steinbusch et al. 1981; Törk et al. 1990; Jacobs et al. 1992; Hornung 2003). The rostral domain, located in upper brainstem, projects “rostrally” and diffusely to the cerebral cortex, thalamus, hypothalamus, basal ganglia, hippocampus, and amygdala. It participates in the mediation of arousal, cognition, mood, motor activity, and cerebral blood flow. The caudal domain in the medulla projects “caudally” and diffusely to other brainstem sites (see below), cerebellum, and spinal cord (Dahlstrom et al. 1964; Steinbusch 1981; Steinbusch et al. 1981; Törk et al. 1990; Jacobs et al. 1992; Hornung 2003) and influences breathing, cardiovascular control, autonomic output, motor control, and pain processing.
Figure 1. Distribution of 5-HT Neurons in the Brainstem.
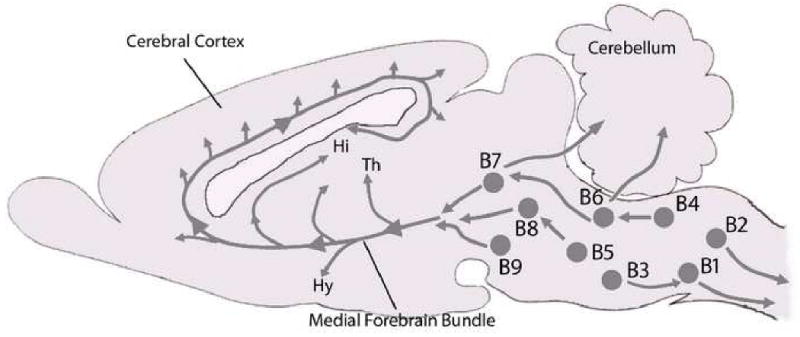
Neurons synthesizing 5-HT distribute in distinct cell groups in the brainstem classically defined as B1-B9. The rostral domain, consisting of groups B4-B9 projects “rostrally” and diffusely to the cerebral cortex, thalamus (Th), hypothalamus (Hy), basal ganglia, hippocampus (Hi), and amygdala and mediates arousal, cognition, mood, motor activity, and cerebral blood flow. The caudal domain consisting of groups B1-B3 in the medulla projects “caudally” and diffusely to other brainstem sites, cerebellum, and spinal cord and influences breathing, cardiovascular control, autonomic output, motor control, and pain processing.
3. Medullary 5-HT Neurons and Respiratory Function
In rodents, medullary 5-HT neurons are located in the midline raphé (including, the raphé pallidus, raphé magnus and raphé obscurus) and in the parapyramidal region at the ventrolateral medullary surface. These neurons project to the nucleus of the solitary tract (NTS), nucleus ambiguus (nAm) retrotrapezoid nucleus (RTN), preBötzinger Complex (preBötC), hypoglossal motor nucleus (HG) in the brainstem, and phrenic motor nucleus in the cervical cord (Steinbusch 1981; Steinbusch et al. 1981; Holtman et al. 1986; Holtman et al. 1987; Holtman 1988; Connelly et al. 1989; Smith et al. 1989; Zhan et al. 1989; Pilowsky et al. 1990; Voss et al. 1990; Jacobs et al. 1992; Manaker et al. 1993; Feldman et al. 2003) and modulate several aspects of respiratory function including respiratory rhythmogenesis, central chemosensitivity, and long-term changes in respiratory function (i.e., respiratory plasticity).
3.1 Respiratory Rhythm Generation
Medullary 5-HT neurons play an important role in the generation and modulation of respiratory rhythmogenesis. Raphé 5-HT neurons have reciprocal connections with neurons in the preBötC (the central pattern generator) and stimulation of these neurons releases 5-HT stimulating respiratory output-effects that are blocked by 5-HT receptor antagonists (Morin et al. 1990; Morin et al. 1991a; Morin et al. 1991b; Di Pasquale et al. 1992; Al-Zubaidy et al. 1996; Ptak et al. 2002; Schwarzacher et al. 2002; Ptak et al. 2009). While 5-HT is predominantly excitatory (Hodges et al. 2008a), specific aspects of preBötC function and respiratory rhythm are mediated by specific 5-HT receptor subtypes including 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B and 5-HT4A receptors (Morin et al. 1990; Morin et al. 1991a; Morin et al. 1991b; Di Pasquale et al. 1992; Onimaru et al. 1998; Manzke et al. 2003; Cao et al. 2006; Gunther et al. 2006; Qin et al. 2007; Manzke et al. 2008). Evidence suggests that the 5-HT2A receptor is particularly important in preBötC function and is necessary for the generation of normal respiration (Pena et al. 2002) and for gasping (Tryba et al. 2006; St-John et al. 2008).
3.2 Respiratory Chemosensitivity
Medullary 5-HT neurons, located in close proximity to large arteries entering the brainstem, are thought to detect arterial changes in PCO2 (see Corcoran et al., 2009 this issue), thereby impacting respiratory chemosensitivity. 5-HT neurons are intrinsically chemosensitive in vitro, and some increase their firing rate in vivo in response to hypercapnia (Richerson et al. 2001; Wang et al. 2001; Bradley et al. 2002; Richerson 2004; Hodges et al. 2008b). Raphé 5-HT neurons modulate the function of chemosensitive neurons in the retrotrapezoid nucleus (RTN) (Mulkey et al. 2007; Guyenet et al. 2008) (see Guyenet et al. and Onimaru et al., this issue) and application of 5-HT receptor agonists to the classic chemoreceptor zones on the ventral medullary surface stimulates respiration in anaesthetized rats and cats (Millhorn et al. 1986; Holtman et al. 1994; Lalley et al. 1994; Lalley et al. 1995; Richter et al. 1999; Valic et al. 2008).
3.3 Respiratory Plasticity
Medullary 5-HT neurons also play a significant role in several forms of respiratory plasticity (Feldman et al. 2003) including long term facilitation (LTF) of respiration following hypoxia. Long-term facilitation is an enhancement of ventilation or respiratory motor output that persists for hours after intermittent hypoxia (Mitchell et al. 2001). Activation of 5-HT2A receptors in the phrenic nerve nucleus is necessary to trigger a cascade of downstream events that ultimately result in a glutamatergic-mediated enhancement of respiratory drive to the diaphragm and accessory respiratory musculature (Baker-Herman et al. 2004; Mahamed et al. 2007; Mahamed et al. 2008). Similarly, 5-HT2A receptors are necessary for the induction of LTF in the hypoglossal nucleus following episodic hypoxia and evoke a persistent increase in genioglossus and hypoglossal nerve activity (Fuller et al. 2001; McKay et al. 2005). A recent study also suggests that 5-HT integrates cardio-respiratory responses to hypoxia as 5-HT3 receptor antagonists inhibit respiratory-related excitation of cardio-vagal neurons in the nAm during hypoxia (Dergacheva et al. 2009).
4. Lesion of Medullary 5-HT Neurons in Animals Results in Respiratory and Autonomic Dysfunction
Animals in which medullary 5-HT neurons have been lesioned or inhibited pharmacologically display respiratory and autonomic dysfunction. Permanent lesion of medullary 5-HT neurons via injection of 5-HT neuronal toxins (Nattie et al. 2004; Penatti et al. 2006) or focal acute inhibition of 5-HT neurons by dialysis of 8-hydroxy-2-[di-N-propylamino]-tetralin (8-OH-DPAT) (5-HT1A autoreceptor agonist) in the medullary raphé (Messier et al. 2004; Taylor et al. 2005) decreases the ventilatory response to CO2 in newborn piglets. Transgenic mice with near complete (Lm×1b knockout) and severe (Pet-1 knockout) loss of 5-HT neurons show distinct abnormalities in breathing during early postnatal life (Erickson et al. 2003), reduced ventilatory response to CO2 and an inability to maintain body temperature in cold stress (Hodges et al. 2008a; Hodges et al. 2008b). Notably, transgenic mice overexpressing the 5-HT1A auto-receptor (which arguably have reduced extracellular 5-HT levels) die suddenly and unexpectedly in postnatal life (Audero et al. 2008). These mice exhibit spontaneous episodes of bradycardia accompanied by a drop in body temperature, with some animals dying during these episodes (Audero et al. 2008). Animal models where 5-HT is in excess also display respiratory dysfunction. Injection of neonatal rats with the 5-HT precursor L-tryptophan increases brain 5-HT level and induces potentially fatal apneas (Hilaire et al. 1993). Similarly, mice lacking monoamine oxidase (MAO), the major enzyme for 5-HT breakdown, have elevated levels of 5-HT and display an increased frequency of respiratory pauses compared to wild-type mice, defects which are resolved by pharmacological blockade of 5-HT receptors or 5-HT biosynthesis (Real et al. 2007). In addition, 5-HTT knockout mice show a dramatic decrease in the ventilatory response to CO2 (Li et al. 2008); while mice lacking 5-HT2A protein display an increased frequency of respiratory pauses during non-REM sleep (Popa et al. 2005). The observations in these animal models support the idea that altering medullary 5-HT function is detrimental to physiological control systems and may contribute to sudden unexpected death. Taken together, these data provide conclusive evidence that medullary 5-HT neurons regulate multiple aspects of respiratory function including respiratory rhythm generation and chemosensitivity, and respiratory and autonomic plasticity.
5. The Medullary 5-HT System in Humans
In humans, medullary 5-HT neurons are located in regions homologous to the 5-HT neurons in the rodent brainstem, including the midline raphé (raphé obscurus and raphé pallidus), extra-raphé (gigantocellularis [GC], paragigantocellularis lateralis [PGCL] and intermediate reticular nucleus [IRZ]), and in the arcuate nucleus (Arc) at the ventral medullary surface (Kinney et al. 2004) (Fig 2). These cell groups constitute the Medullary 5-HT System as defined by Kinney et al., (2007). Evidence from neuron tract tracing studies with the lipophilic fluorescent dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) indicates that these 5-HT neurons send projections to one another as well as to nuclei with respiratory-related function including the NTS and HG (Zec et al. 1997; Zec et al. 2001; Zec et al. 2003); a hypothesis supported by the presence of serotonin transporter (5-HTT), 5-HT1A receptor, and 5-HT2A receptor binding sites in each of these regions (Paterson et al. 2004; Paterson et al. 2006b; Paterson et al. 2009). Comparative anatomy indicates that the Arc is homologous to the respiratory chemosensitive fields at the ventral medullary surface of rodents and cats (Filiano et al. 1990; Paterson et al. 2006a). It is proposed to play a similar role in respiratory CO2 sensitivity in humans as it expresses both 5-HT and glutamate neurons (Paterson et al. 2006a), recognized respiratory chemosensors (Mulkey et al. 2004; Richerson 2004; Weston et al. 2004). Similarly, the PGCL in the rostral ventrolateral medulla of the human is homologous to the medullary region in which the preBötC is located in rats (Kinney et al. 2007; Paterson et al. 2009). We have previously identified neurons in the PGCL that co-express immunoreactivity for neurokinin-1 (NK1) receptors, and somatostatin (SST), markers in combination for rodent preBötC neurons (Paterson et al., unpublished observations), raising the possibility that these neurons are homologues of rodent preBötC neurons and, likewise, play a role in respiratory rhythm generation. In addition, these neurons express immunoreactivity for both 5-HT1A and 5-HT2A receptors (Fig 3) suggesting that, as in rodents, respiratory rhythmogenesis in the human is modulated by 5-HT. These observations support the idea that 5-HT neurons in the human medulla play similar roles in the modulation of respiratory function as demonstrated for 5-HT neurons in rodents.
Figure 2. The Medullary 5-HT System in the Human Brainstem.
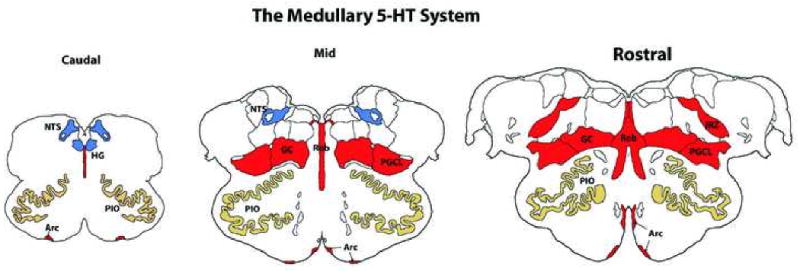
The Medullary 5-HT System consists of 5-HT neurons (areas in red) in the midline raphé (i.e., raphé obscurus (Rob), extra-raphé (i.e., (gigantocellularis [GC], paragigantocellularis lateralis [PGCL], intermediate reticular nucleus [IRZ], and at the ventral medullary surface (arcuate nucleus [Arc]) and sites to which they project (blue areas) that do not contain 5-HT neurons but mediate homeostatic functions (e.g., hypoglossal nucleus [HG], nucleus of the solitary tract [NTS]). Figure shows coronal sections at the level of the Caudal, Mid, and Rostral medulla.
Figure 3.
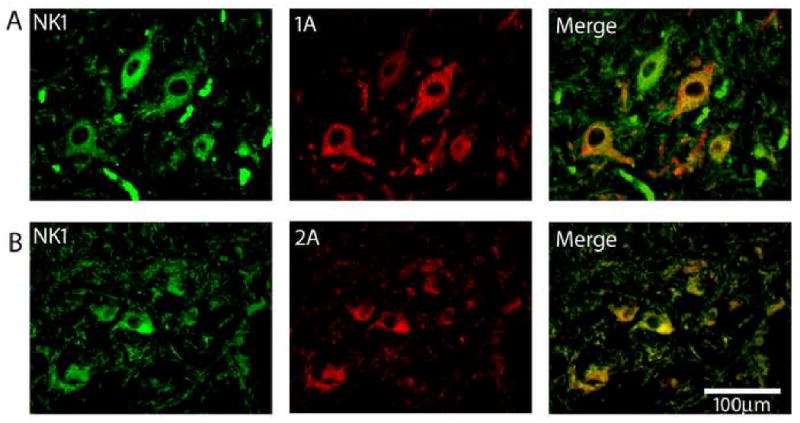
Double-label immunofluorescent staining of putative preBötC neurons in the human infant PGCL with immunoreactivity for multiple neurotransmitter receptors that modulate preBötC function in rats. NK1 receptor immunofluorescent neurons (red) in the PGCL co localize with A. 5-HT1A receptors, B. 5-HT2A receptors. Images at ×40.
6. Medullary 5-HT System Abnormalities, Respiratory and Autonomic Dysregulation, and SIDS
Abnormalities in markers of 5-HT function have been observed in the medullary 5-HT system (i.e., raphé obscurus, PGCL, IRZ, Arc, HG, NTS) in SIDS infants, including an increased number of 5-HT neurons, many of which are immature (Paterson et al. 2006b), reduced 5-HT1A and 5-HT2A receptor expression (Panigrahy et al. 2000; Ozawa et al. 2002a; Ozawa et al. 2002b; Kinney et al. 2003; Paterson et al. 2006b), reduced 5-HTT binding (Paterson et al. 2006b), abnormal TPH expression (Sawaguchi et al. 2003; Machaalani et al. 2008), reduced brain 5-HT levels (Sparks et al. 1991), altered 5-HT turnover (Cann-Moisan et al. 1999) and altered 5-HT breakdown (Sparks et al. 1991). These observations inform the idea that multiple elements of respiratory and autonomic regulation, mediated by the 5-HT System, are defective in SIDS, including but not restricted, to respiratory rhythmogenesis and respiratory responses to hypercapnic and/or hypoxic challenge. This idea is supported by a SIDS infant who was observed to have subtle respiratory and cardiac dysfunction at birth and 5-HT receptor binding abnormalities at autopsy 2 weeks later (Kinney et al. 2005). Taken together, the above observations provide evidence to support the idea that medullary 5-HT abnormalities cause respiratory dysfunction that potentially contributes to the death of the infant in SIDS, i.e., in terms of the Triple Risk Model of SIDS, the medullary 5-HT defect is, or is part of, the underlying abnormality that predisposes the infant to sleep related death particularly when combined with an environmental stressor, such as prone sleeping, during the critical developmental period.
7. Medullary 5-HT System Abnormalities in SIDS Originate During Fetal Development
Several observations indicate that SIDS is a developmental disorder that originates during fetal life: the incidence of SIDS is greater in preterm and growth restricted infants; the peak incidence of SIDS is related to a critical and finite early developmental period (2-4 postnatal months); and prenatal exposure to environmental toxins including cigarette smoke (Schoendorf et al. 1992; Haglund 1993; Blair et al. 1996; Fleming et al. 1996; MacDorman et al. 1997; Wisborg et al. 2000; Anderson et al. 2005; Mitchell et al. 2006) and alcohol (Scragg et al. 1993; Alm et al. 1999; Iyasu et al. 2002; Kinney et al. 2003; Klug et al. 2003; Duncan et al. 2008b; Lavezzi et al. 2009) are major risk factors for SIDS. Evidence from post-mortem human studies suggests that the development of the medullary 5-HT System is abnormal in SIDS, including an increased number of 5-HT neurons with immature morphology (Paterson et al. 2006b), abnormal/immature synapse formation (Paterson et al. 2006b), and differential age-related changes in 5-HT receptor binding (i.e., binding decreases significantly with postnatal age in SIDS cases but not controls) in the medulla of SIDS cases compared to controls (Panigrahy et al. 2000; Kinney et al. 2003). These observations offer a possible explanation for the low incidence of SIDS during the first postnatal month, followed by the period of peak incidence at 2-4 months: at birth 5-HT function is relatively normal, but becomes progressively defective during the first postnatal month as 5-HT receptor binding decreases; by 2-4 months 5-HT dysfunction reaches the “threshold” whereby the infant is unable to respond appropriately to an environmental stressor (e.g., hypoxia), ultimately leading to the sudden death of the infant.
7.1 Altering Fetal 5-HT Levels Results in Postnatal Respiratory Dysfunction in Animals
In both rodents (Gaspar et al. 2003; Nakamura et al. 2007) and humans (Kinney et al. 2007) 5-HT neurons are among the first to be expressed during embryogenesis: they develop from embryonic days 10-12 in mice and are present as early as 7 gestational weeks in humans (Kinney et al. 2007). During development 5-HT has neurotrophic actions and plays a role in regulating cell division, migration, differentiation and synaptogenesis (Lauder 1988; Lauder 1990). Thus, alterations in 5-HT levels during fetal life may adversely affect the intrauterine and postnatal development of the 5-HT and other related neuronal systems. Indeed, both depletion and elevation of 5-HT during gestation adversely affect respiratory neuronal network development and function in animals in the postnatal period. MAOA-deficient mice, who have endogenous levels of 5-HT that are 5 to 10-fold higher than wild type mice during the fetal and neonatal periods (Cases et al. 1995; Lajard et al. 1999), display abnormal expression of 5-HT1A and 5-HT1B receptors (Bou-Flores et al. 2000a; Bou-Flores et al. 2000b; Bras et al. 2008), abnormal phrenic nerve nucleus morphology, are unable to generate stable respiration in the postnatal period (Bou-Flores et al. 2000a; Bou-Flores et al. 2000b) and have attenuated respiratory responses to hypoxia (Burnet et al. 2001). Increasing 5-HT levels in wild type mice by pharmacological blockade of MAOA increases the number of sleep apneas (Real et al. 2007) and mice with absence of the 5-HTT protein show a dramatic decrease in the ventilatory response to CO2 (Li et al. 2008). Similarly, transgenic mice with significant deficits in 5-HT during gestation (i.e., lm×1b and Pet-1 knockout mice) display abnormal respiratory rhythm and attenuated respiratory responses to CO2 (Erickson et al. 2003; Hodges et al. 2008b). In addition, depletion of maternal 5-HT by injection of pregnant rats with para-chlorophenylalanine (pCPA) reduces 5-HT levels in the raphé nuclei and delays 5-HT neuronal development in the progeny (Nakajima et al. 1998; Butkevich et al. 2003).
7.2 Prenatal Exposure to Cigarette Smoke and Alcohol Adversely Affects Brainstem 5-HT System Development and Function
Exposure of the developing fetus to cigarette smoke and alcohol through the maternal circulation are major risk factors for SIDS. The mechanisms through which these toxins adversely affect the infant to predispose the baby to succumbing to SIDS are unknown, but may involve, at least partly, disruption of the developing 5-HT system as described above. Indeed, maternal cigarette smoking 3 months before or during pregnancy results in lower 5-HT receptor binding in the infant postnatally (Fig 4) (Kinney et al. 2003). In addition, a recent study identified an association between prenatal exposure to cigarette smoke and hypoplasia of the medullary 5-HT system in SIDS (Lavezzi et al. 2009). Similarly, in experimental animals, prenatal exposure to nicotine and cigarette smoke results in altered 5-HT neuron firing, 5-HT receptor expression (Kenny et al. 2001; Slotkin et al. 2006a; Slotkin et al. 2006b; Slotkin et al. 2006c; Slotkin et al. 2007b), 5-HTT expression (Muneoka et al. 2001; Xu et al. 2001; Slotkin et al. 2007a; Slotkin et al. 2007b), 5-HT turnover and depletion of brain 5-HT in the postnatal period (King et al. 1991; Muneoka et al. 1997). These adverse effects appear to result from the binding of nicotine to nicotinic and/or 5-HT receptors on 5-HT neurons (Bitner et al. 2002; Cucchiaro et al. 2003; Aznar et al. 2005). In the developing human medulla, nicotinic receptors are expressed by 5-HT neurons throughout the medullary 5-HT system, including in the raphé obscurus and arcuate nucleus (Duncan et al. 2008a), thus nicotine may act directly on medullary 5-HT neurons to alter their development and/or function. Indeed, a recent study identified that prenatal nicotine exposure abolishes 5-HT mediated activation of cardio-vagal neurons in response to hypoxia/hypercapnia (Kamendi et al. 2009). Prenatal exposure to alcohol in the maternal circulation is also associated with reduced 5-HT receptor binding (Fig 4) (Kinney et al. 2003). In animal models of prenatal alcohol exposure, various abnormalities of the 5-HT system have been observed, including reduced 5-HT levels and reduced 5-HT receptor binding (Druse et al. 1988), retarded process outgrowth and migration of 5-HT neurons, reduced density of 5-HT fibers in the medial forebrain bundle, and reduced 5-HT neurons in the median and dorsal raphé (Sari et al. 2001; Zhou et al. 2002) and lower brainstem (Druse et al. 2004). Prenatal alcohol also adversely affects signaling molecules and transcription factors necessary for 5-HT development, e.g., sonic hedgehog which is involved in the early specification of 5-HT precursors (Ahlgren et al. 1999; Ahlgren et al. 2002), and results in defective neurogenesis, cell migration, synaptogenesis, and dendritic organization (Haydon et al. 1987; Lauder 1990; Ivgy-May et al. 1994; Mazer et al. 1997; Werner et al. 1998; Faber et al. 1999; Luo et al. 2003; Kondoh et al. 2004). Notably, the delivery of the 5-HT1A agonists buspirone and ipsapirone in maternal rats prevents the alcohol-induced loss of brainstem 5-HT neurons in the pups, indicating a critical role for the 5-HT1A receptor in neuronal development and alcohol neurotoxicity (Kim et al. 1996; Druse et al. 2004). These observations indicate that prenatal exposure to cigarette smoke and/or alcohol adversely affects the development and function of brainstem 5-HT systems in the postnatal period. Pre and/or perinatal exposure to these (and other toxins) may, therefore, disrupt the development of the medullary 5-HT system and account, at least in part, for the increased SIDS risk associated with maternal smoking and alcohol ingestion during pregnancy. Observations in our laboratory indicate that the human medullary 5-HT system is not fully developed at birth but continues to mature at least through the end of the first year of life (Kinney et al. 2007). This observation suggests an extended period from gestation through infancy where the medullary 5-HT system is potentially vulnerable to environmental toxins and pharmacologically active agents that may disrupt its development and function.
Figure 4. 5-HT receptor binding is significantly lower in the arcuate nucleus of infants exposed to cigarette smoke prenatally.
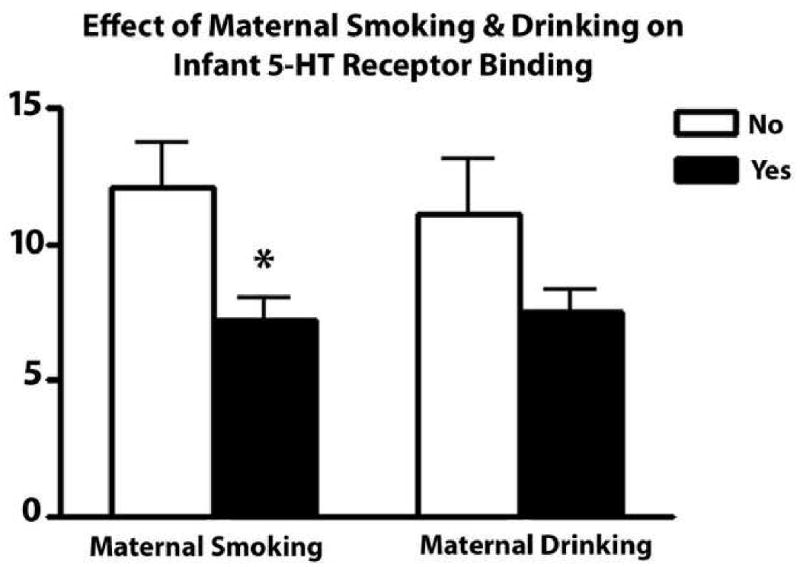
Graphs comparing the effects of prenatal smoking and prenatal alcohol on the effects of 5-HT receptor binding measured with 3H LSD autoradiography in the infant postnatally. Maternal smoking during pregnancy is associated with a 40% reduction (*p=0.011) and maternal alcohol ingestions during pregnancy is associated with a 32% reduction (p=0.075) in 5-HT receptor binding postnatally in infants.
8. 5-HT Gene Polymorphisms and SIDS
Several studies have identified significant associations between SIDS and gene polymorphisms resulting in alterations in 5-HT neuronal function and development. These polymorphisms include two polymorphisms in the 5-HTT gene: an insertion-deletion polymorphism in the promoter region (5-HTTLPR) (Heils et al. 1997; Lesch et al. 1998) and variable number tandem repeat (VNTR) polymorphism in the second intron (Ogilvie et al. 1996; Narita et al. 2001; Weese-Mayer et al. 2003a; Weese-Mayer et al. 2003b; Maher et al. 2006; Nonnis Marzano et al. 2008; Opdal et al. 2008; Lavezzi et al. 2009), both of which are associated with increased 5-HTT expression. Similarly, a VNTR polymorphism in the promoter region of the MAOA gene resulting in increased transcription and protein expression has also recently been associated with SIDS (Filonzi et al. 2008). Individuals with these polymorphisms are postulated to have a relative reduction in synaptic 5-HT, as a result of increased 5-HT uptake and breakdown of 5-HT associated with elevated 5-HTT and MAO protein expression, respectively (Heils et al. 1997; Lesch et al. 1998; Greenberg et al. 1999; van Dyck et al. 2004). Thus, these polymorphisms may predispose an infant to increased SIDS risk by contributing to the development of, or exacerbating existing, medullary 5-HT dysfunction. Moreover, they may reduce the resilience of the infant to environmental toxins that disrupt the development and/or function of 5-HT neurons as described above. Indeed, this idea is supported by a recent study identifying an association between the LL genotype of the 5-HTT promoter polymorphism and hypoplasia in the medullary raphé and arcuate nucleus in stillborns and in SIDS cases (Lavezzi et al. 2009). Similarly, a rare mutation (IVS2 191_190insA) upstream of the third exon of the human fifth Ewing variant (FEV) gene may contribute to the development of medullary 5-HT abnormalities in a subset of SIDS (Rand et al. 2007). FEV is the human homologue of the ETS domain transcription factor Pet1 that is necessary for differentiation and development of 5-HT neurons (Hendricks et al. 1999) including regulation of TPH, 5-HTT and 5-HT1A receptor gene expression (Hendricks et al. 1999; Pfaar et al. 2002; Hendricks et al. 2003; Maurer et al. 2004; Iyo et al. 2005). Loss of the Pet1 gene in mice results in failure of approximately 70% ofl 5-HT neurons to differentiate (Hendricks et al. 1999) and deficient expression of genes required for 5-HT synthesis, uptake, and vesicular storage in the remaining 5-HT neurons (Hendricks et al. 2003). The FEV gene mutation may, therefore, result in or predispose an infant to medullary 5-HT dysfunction and, thus, SIDS. Interestingly, both the 5-HTT intron 2 gene polymorphism and the FEV gene mutation were significantly associated with SIDS in African American but not Caucasian populations (Weese-Mayer et al. 2003b; Rand et al. 2007), while the 5-HTT promoter polymorphism is present in greater frequency in Caucasian compared to African American SIDS cases (Weese-Mayer et al. 2003a). These differences among ethnic-specific gene polymorphisms/mutations may pre-dispose African American infants to greater SIDS risk than Caucasian infants and may, therefore, help explain the ethnic disparity in SIDS rates. However, despite these encouraging findings, several reports have observed no significant association between SIDS and polymorphisms in other genes pertinent to the 5-HT system, including the 5-HT1A receptor (Morley et al. 2008), 5-HT2A receptor, (Rand et al. 2009), and tryptophan hydroxylase 2 (Nonnis Marzano et al. 2008) genes and a polymorphism in the untranslated region downstream of the 5-HTT gene (Maher et al. 2006). These observations support the idea that the medullary 5-HT abnormalities in SIDS results from a combination of environmental and genetic factors and involves exposure of an infant with a predisposing genetic background to environmental toxins during a critical period in development, which in humans may extend from the preconceptional period through the first postnatal year.
9. Sexual Dimorphism in 5-HT Function may Predispose Male Infants to Increased SIDS risk
Twice as many male infants die of SIDS as female infants (Hoffman et al. 1992; Brooke et al. 1997; Vennemann et al. 2005). The reason for this is unknown, but identification of a significantly lower density of 5-HT1A receptor binding in male compared to female SIDS infants (Fig 5) (Paterson et al. 2006b) suggests that sexual dimorphism in 5-HT function may play a role in predisposing male infants to SIDS. Indeed, significant differences in TPH, 5-HT, 5-HT metabolites, and 5-HT receptor expression, including a lower level of 5-HT1A receptors, normally exist between males and females in several brain regions (Dillon et al. 1991; Arango et al. 1995; Ferrari et al. 1999; Bethea et al. 2002; Parsey et al. 2002). Interestingly, a recent study also observed that variations in the coding sequence of the 5-HT1A receptor gene occur more frequently in males compared to females (Morley et al. 2008). Evidence from studies in animals with 5-HT lesions have reported male gender-specific abnormalities in respiration, chemosensitivity and thermoregulation (Penatti et al. 2006; Hodges et al. 2008b; Li et al. 2008)-responses that are modulated in part by 5-HT1A receptors in the medullary raphé and extra-raphé (Messier et al. 2004; Darnall et al. 2005; Hoffman et al. 2007; Brown et al. 2008). These observations raise the possibility that male human infants may similarly have reduced sensitivity to CO2 and temperature and that loss of medullary 5-HT1A receptors, as observed in SIDS, may attenuate protective homeostatic responses to a greater extent in male compared to female infants, thus placing them at greater risk for SIDS. Evidence from animal studies also suggests reduced plasticity in 5-HT neuron function in males compared to females. Deficits in postnatal brain levels of 5-HT1A receptor expression following prenatal cocaine and cigarette smoke exposure persist for a greater length of time in male compared to female rats (Johns et al. 2002; Slotkin et al. 2007a; Slotkin et al. 2007b), suggesting that the neonatal male infant brain is less resilient to exposure to at least some pharmacologically active toxins affecting 5-HT function in the maternal circulation than the neonatal female brain. Testosterone and estrogen also influence the 5-HT system and its control of respiration (Matsumoto et al. 1985; Pickett et al. 1989; Bayliss et al. 1990; Regensteiner et al. 1990; Bayliss et al. 1992; Emery et al. 1994; Fogel et al. 2001; Liu et al. 2003; Zhou et al. 2003). Elevated levels of serum testosterone have been observed in both male and female SIDS infants compared to controls, with the highest level of testosterone observed in male SIDS infants (Emery et al. 2005). The cause(s) of elevated serum testosterone in SIDS is unknown, although prenatal exposure to nicotine increases fetal plasma testosterone in rats via inhibition of cytochrome p450 aromatase, the enzyme which converts testosterone to estradiol (Lephart et al. 2001; Stoffel-Wagner 2001). Also noteworthy is the overlap in peak SIDS incidence between 2-4 months of age and peak postnatal increase in gonadal steroids (Winter et al. 1976; Peterson et al. 1979; Forest et al. 1980). Likewise, preterm infants, a group at heightened SIDS risk, have significantly higher adrenal-derived androgens in the first year of life compared to term infants (Tapanainen et al. 1981). Thus, the normal higher levels of testosterone in male infants compared to female infants may be responsible for blunted respiratory responses to homeostatic challenges such as hypercapnia, thereby contributing to their greater SIDS risk. Taking these observations together, intrinsic differences in baseline brain 5-HT function, 5-HT neuronal plasticity, and CO2 sensitivity between males and females provide evidence that may explain, at least in part, the greater risk of SIDS in male infants.
Figure 5. 5-HT1A receptor binding is lower in male SIDS cases.
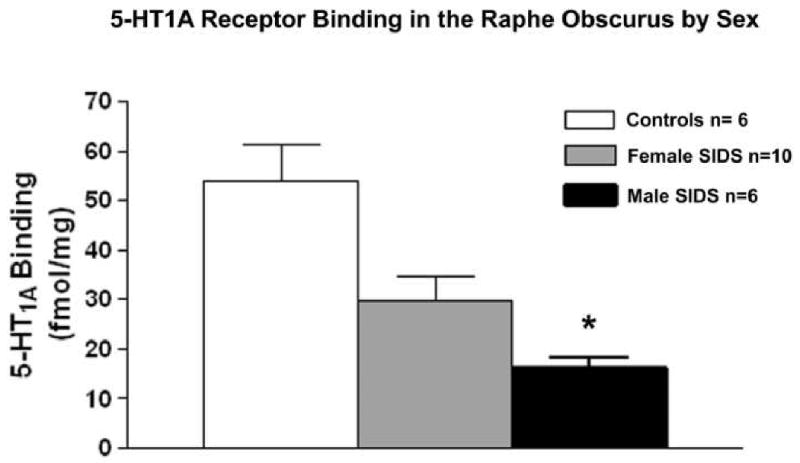
Graph comparing 5-HT1A receptor binding density measured with 3H 8-OH DPAT autoradiography in the raphé obscurus in male and female SIDS cases compared to controls infants. 5-HT1A receptor binding density in male SIDS infants is significantly lower compared to female SIDS infants (*p=0.04). 5-HT1A receptor binding in both male (p=0.02) and female (p=0.05) SIDS infants is significantly lower compared to controls.
10. Conclusions and Remaining Questions
The data reviewed here provide evidence that the medullary 5-HT neuronal system assumes varied roles in mediating respiratory regulation and that disruption of medullary 5-HT neurotransmission by pharmacological, chemical, or genetic means produces defects in baseline respiratory and autonomic regulation as well as respiratory responses to perturbation such as hypoxic and hypercapnic challenges. These observations support the hypothesis that the medullary 5-HT abnormalities identified in SIDS cases result in respiratory and autonomic dysfunction that heightens the vulnerability of the infant. The data reviewed here also support the idea that the pathogenesis of the medullary 5-HT defect(s) in SIDS originates in utero and involves a combination of environmental and genetic factors. Moreover, evidence suggests that intrinsic differences in 5-HT function between males and females contribute to the increased incidence of SIDS in boys. However, the specific nature of the 5-HT dysfunction in SIDS is still unclear, i.e., is there an excess or a deficit of available 5-HT? The abnormalities in markers of 5-HT function observed in the medulla of SIDS infants may be interpreted as evidence of either 1) an increased number of 5-HT neurons leading to an excess of extracellular 5-HT and a compensatory downregulation of 5-HT receptors, or 2) 5-HT synthesis and/or release may be dysfunctional in the 5-HT neurons (which are overabundant in compensation) resulting in a deficiency of extracellular 5-HT. Indeed, both an excess and a deficit in 5-HT levels during development and in the postnatal period produce respiratory dysfunction in animal models. Determination of the level of available 5-HT in the medulla under “normal” and pathological states is therefore critical in determining the specific nature and pathogenesis of 5-HT dysfunction in SIDS. Such studies are currently underway in our laboratories. Is 5-HT the only abnormal neurotransmitter system in SIDS? It seems unlikely that this is the case. An important potential consequence of the 5-HT neuronal abnormalities in SIDS is the possibility of associated defects in GABA and substance P, known co-transmitters with 5-HT, that also regulate respiratory function. In addition, multiple other neurotransmitter systems in the medulla regulate respiratory and autonomic function in conjunction with 5-HT, including glutamate, acetylcholine, norepinephrine, somatostatin and glycine (Liu et al. 2005; Wong-Riley et al. 2005). Therefore, we propose that SIDS results from a complex interaction of multiple dysfunctional neurotransmitter systems in the brainstem of which the abnormalities in 5-HT markers are the most widely identified thus far. A systematic analysis of multiple transmitters/modulators that interface with the medullary 5-HT system is needed to establish the precise neurochemical pathology in all or subsets of SIDS cases. Without such information, the pathogenesis of the disorder cannot be established, and optimal interventions cannot be determined and implemented.
Acknowledgments
The authors would like to thank the “Naitre et Vivre” Association for their financial support to the organization of the International Symposium on Respiratory Control (Saint Maximin, France) and the Scottish Cot Death Trust, Evelyn Deborah Barrett Fellowship, CJ Foundation for SIDS, First Candle/SIDS Alliance, CJ Murphy Foundation, National Institute of Child Health and Human Development, and Children's Hospital Mental Retardation Core Grant for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlgren SC, Bronner-Fraser M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr Biol. 1999;9:1304–1314. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- Ahlgren SC, Thakur V, Bronner-Fraser M. Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proc Natl Acad Sci U S A. 2002;99:10476–10481. doi: 10.1073/pnas.162356199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflugers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Alm B, Wennergren G, Norvenius G, Skjaerven R, Oyen N, Helweg-Larsen K, Lagercrantz H, Irgens LM. Caffeine and alcohol as risk factors for sudden infant death syndrome. Nordic Epidemiological SIDS Study. Arch Dis Child. 1999;81:107–111. doi: 10.1136/adc.81.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME, Johnson DC, Batal HA. Sudden Infant Death Syndrome and prenatal maternal smoking: rising attributed risk in the Back to Sleep era. BMC Med. 2005;3:4. doi: 10.1186/1741-7015-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arneil GC, Brooke H, Gibson AA, Harvie A, McIntosh H, Patrick WJ. National Post-Perinatal Infant Mortality and Cot Death Study, Scotland 1981-82. Lancet. 1985;1:740–743. doi: 10.1016/s0140-6736(85)91273-5. [DOI] [PubMed] [Google Scholar]
- Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science. 2008;321:130–133. doi: 10.1126/science.1157871. [DOI] [PubMed] [Google Scholar]
- Aznar S, Kostova V, Christiansen SH, Knudsen GM. Alpha 7 nicotinic receptor subunit is present on serotonin neurons projecting to hippocampus and septum. Synapse. 2005;55:196–200. doi: 10.1002/syn.20108. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Cidlowski JA, Millhorn DE. The stimulation of respiration by progesterone in ovariectomized cat is mediated by an estrogen-dependent hypothalamic mechanism requiring gene expression. Endocrinology. 1990;126:519–527. doi: 10.1210/endo-126-1-519. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Millhorn DE. Central neural mechanisms of progesterone action: application to the respiratory system. J Appl Physiol. 1992;73:393–404. doi: 10.1152/jappl.1992.73.2.393. [DOI] [PubMed] [Google Scholar]
- Beal S. Sudden infant death syndrome. Med J Aust. 1972;2:1223–1229. [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Nikkel AL. Alpha-7 nicotinic receptor expression by two distinct cell types in the dorsal raphe nucleus and locus coeruleus of rat. Brain Res. 2002;938:45–54. doi: 10.1016/s0006-8993(02)02485-x. [DOI] [PubMed] [Google Scholar]
- Blair PS, Fleming PJ, Bensley D, Smith I, Bacon C, Taylor E, Berry J, Golding J, Tripp J. Smoking and the sudden infant death syndrome: results from 1993-5 case-control study for confidential inquiry into stillbirths and deaths in infancy. Confidential Enquiry into Stillbirths and Deaths Regional Coordinators and Researchers. Bmj. 1996;313:195–198. doi: 10.1136/bmj.313.7051.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PS, Fleming PJ, Smith IJ, Platt MW, Young J, Nadin P, Berry PJ, Golding J. Babies sleeping with parents: case-control study of factors influencing the risk of the sudden infant death syndrome. CESDI SUDI research group. Bmj. 1999;319:1457–1461. doi: 10.1136/bmj.319.7223.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PS, Platt MW, Smith IJ, Fleming PJ. Sudden infant death syndrome and sleeping position in pre-term and low birth weight infants: an opportunity for targeted intervention. Arch Dis Child. 2006;91:101–106. doi: 10.1136/adc.2004.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Flores C, Hilaire G. 5-Hydroxytryptamine(2A) and 5-hydroxytryptamine(1B) receptors are differently affected by the monoamine oxidase A-deficiency in the Tg8 transgenic mouse. Neurosci Lett. 2000a;296:141–144. doi: 10.1016/s0304-3940(00)01653-0. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci. 2000b;20:4646–4656. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Bras H, Gaytan SP, Portalier P, Zanella S, Pasaro R, Coulon P, Hilaire G. Prenatal activation of 5-HT2A receptor induces expression of 5-HT1B receptor in phrenic motoneurons and alters the organization of their premotor network in newborn mice. Eur J Neurosci. 2008;28:1097–1107. doi: 10.1111/j.1460-9568.2008.06407.x. [DOI] [PubMed] [Google Scholar]
- Brooke H, Gibson A, Tappin D, Brown H. Case-control study of sudden infant death syndrome in Scotland, 1992-5. Bmj. 1997;314:1516–1520. doi: 10.1136/bmj.314.7093.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphe disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2008;294:R884–894. doi: 10.1152/ajpregu.00655.2007. [DOI] [PubMed] [Google Scholar]
- Burnet H, Bevengut M, Chakri F, Bou-Flores C, Coulon P, Gaytan S, Pasaro R, Hilaire G. Altered respiratory activity and respiratory regulations in adult monoamine oxidase A-deficient mice. J Neurosci. 2001;21:5212–5221. doi: 10.1523/JNEUROSCI.21-14-05212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkevich IP, Khozhai LI, Mikhailenko VA, Otellin VA. Decreased serotonin level during pregnancy alters morphological and functional characteristics of tonic nociceptive system in juvenile offspring of the rat. Reprod Biol Endocrinol. 2003;1:96. doi: 10.1186/1477-7827-1-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann-Moisan C, Girin E, Giroux JD, Le Bras P, Caroff J. Changes in cerebrospinal fluid monoamine metabolites, tryptophan, and gamma-aminobutyric acid during the 1st year of life in normal infants. comparison with victims of sudden infant death syndrome. Biology of the Neonate. 1999;75:152–159. doi: 10.1159/000014091. [DOI] [PubMed] [Google Scholar]
- Cao Y, Matsuyama K, Fujito Y, Aoki M. Involvement of medullary GABAergic and serotonergic raphe neurons in respiratory control: electrophysiological and immunohistochemical studies in rats. Neurosci Res. 2006;56:322–331. doi: 10.1016/j.neures.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Commons KG. Alpha 4 nicotinic acetylcholine receptor subunit links cholinergic to brainstem monoaminergic neurotransmission. Synapse. 2003;49:195–205. doi: 10.1002/syn.10218. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Harris MB, Gill WH, Hoffman JM, Brown JW, Niblock MM. Inhibition of serotonergic neurons in the nucleus paragigantocellularis lateralis fragments sleep and decreases rapid eye movement sleep in the piglet: implications for sudden infant death syndrome. J Neurosci. 2005;25:8322–8332. doi: 10.1523/JNEUROSCI.1770-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Kamendi H, Wang X, Pinol RM, Frank J, Jameson H, Gorini C, Mendelowitz D. The Role of 5-HT3 and Other Excitatory Receptors in Central Cardiorespiratory Responses to Hypoxia: Implications for Sudden Infant Death Syndrome. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181a16e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Research. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Paul LH. Effects of in utero ethanol exposure on serotonin uptake in cortical regions. Alcohol. 1988;5:455–459. doi: 10.1016/0741-8329(88)90082-1. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin NF, Gillespie RA, Dickson E, Atieh M, Pietrzak CA, Le PT. The serotonin-1A agonist ipsapirone prevents ethanol-associated death of total rhombencephalic neurons and prevents the reduction of fetal serotonin neurons. Brain Res Dev Brain Res. 2004;150:79–88. doi: 10.1016/j.devbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Kinney HC. The development of nicotinic receptors in the human medulla oblongata: inter-relationship with the serotonergic system. Auton Neurosci. 2008a;144:61–75. doi: 10.1016/j.autneu.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Randall LL, Belliveau RA, Trachtenberg FL, Randall B, Habbe D, Mandell F, Welty TK, Iyasu S, Kinney HC. The effect of maternal smoking and drinking during pregnancy upon (3)H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high risk population. Brain Pathol. 2008b;18:21–31. doi: 10.1111/j.1750-3639.2007.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery MJ, Hlastala MP, Matsumoto AM. Depression of hypercapnic ventilatory drive by testosterone in the sleeping infant primate. J Appl Physiol. 1994;76:1786–1793. doi: 10.1152/jappl.1994.76.4.1786. [DOI] [PubMed] [Google Scholar]
- Emery MJ, Krous HF, Nadeau-Manning JM, Marck BT, Matsumoto AM. Serum testosterone and estradiol in sudden infant death. J Pediatr. 2005;147:586–591. doi: 10.1016/j.jpeds.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Hendricks TJ, Wegman LF, Deneris ES. Serotonin-deficient Pet-1 null mice display increased mortality, depressed ventilation, and an increased incidence of hypoxia-induced apnea during the early postnatal period. Soc Neurosci Abst. 2003 vol. 609.13. [Google Scholar]
- Faber KM, Haring JH. Synaptogenesis in the postnatal rat fascia dentata is influenced by 5-HT1a receptor activation. Brain Res Dev Brain Res. 1999;114:245–252. doi: 10.1016/s0165-3806(99)00036-x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Lowther S, Tidbury H, Greengrass P, Wilson CA, Horton RW. The influence of gender and age on neonatal rat hypothalamic 5-HT1A and 5-HT2A receptors. Cell Mol Neurobiol. 1999;19:775–784. doi: 10.1023/A:1006909207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano JJ, Choi JC, Kinney HC. Candidate cell populations for respiratory chemosensitive fields in the human infant medulla. J Comp Neurol. 1990;293:448–465. doi: 10.1002/cne.902930308. [DOI] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biology of the Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Filonzi L, Magnani C, Lavezzi AM, Rindi G, Parmigiani S, Bevilacqua G, Matturri L, Nonnis Marzano F. Association of dopamine transporter and monoamine oxidase molecular polymorphisms with sudden infant death syndrome and stillbirth: new insights into the serotonin hypothesis. Neurogenetics. 2008 doi: 10.1007/s10048-008-0149-x. [DOI] [PubMed] [Google Scholar]
- Fleming PJ, Blair PS, Bacon C, Bensley D, Smith I, Taylor E, Berry J, Golding J, Tripp J. Environment of infants during sleep and risk of the sudden infant death syndrome: results of 1993-5 case-control study for confidential inquiry into stillbirths and deaths in infancy. Confidential Enquiry into Stillbirths and Deaths Regional Coordinators and Researchers. Bmj. 1996;313:191–195. doi: 10.1136/bmj.313.7051.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming PJ, Gilbert R, Azaz Y, Berry PJ, Rudd PT, Stewart A, Hall E. Interaction between bedding and sleeping position in the sudden infant death syndrome: a population based case-control study [see comments] Bmj. 1990;301:85–89. doi: 10.1136/bmj.301.6743.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, White DP. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- Forest MG, de Peretti E, Bertrand J. Testicular and adrenal androgens and their binding to plasma proteins in the perinatal period: developmental patterns of plasma testosterone, 4-androstenedione, dehydroepiandrosterone and its sulfate in premature and small for date infants as compared with that of full-term infants. J Steroid Biochem. 1980;12:25–36. doi: 10.1016/0022-4731(80)90247-2. [DOI] [PubMed] [Google Scholar]
- Froggatt P, Lynas MA, Marshall TK. Sudden death in babies: epidemiology. Am J Cardiol. 1968;22:457–468. doi: 10.1016/0002-9149(68)90152-5. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. discussion 2000. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–87. [PubMed] [Google Scholar]
- Gunther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol. 2006;66:949–961. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Mulkey DK, Stornetta RL, Moreira TS, Takakura AT. The retrotrapezoid nucleus and central chemoreception. Adv Exp Med Biol. 2008;605:327–332. doi: 10.1007/978-0-387-73693-8_57. [DOI] [PubMed] [Google Scholar]
- Haglund B. Cigarette smoking and sudden infant death syndrome: some salient points in the debate. Acta Paediatr Suppl. 1993;82 389:37–39. doi: 10.1111/j.1651-2227.1993.tb12872.x. [DOI] [PubMed] [Google Scholar]
- Hauck FR, Herman SM, Donovan M, Iyasu S, Merrick Moore C, Donoghue E, Kirschner RH, Willinger M. Sleep environment and the risk of sudden infant death syndrome in an urban population: the Chicago Infant Mortality Study. Pediatrics. 2003;111:1207–1214. [PubMed] [Google Scholar]
- Haydon PG, McCobb DP, Kater SB. The regulation of neurite outgrowth, growth cone motility, and electrical synaptogenesis by serotonin. J Neurobiol. 1987;18:197–215. doi: 10.1002/neu.480180206. [DOI] [PubMed] [Google Scholar]
- Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism--basic research and clinical implications. J Neural Transm. 1997;104:1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Morin D, Lajard AM, Monteau R. Changes in serotonin metabolism may elicit obstructive apnoea in the newborn rat. J Physiol. 1993;466:367–381. [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008a;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008b;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HJ, Hillman LS. Epidemiology of the sudden infant death syndrome: maternal, neonatal, and postneonatal risk factors. Clin Perinatol. 1992;19:717–737. [PubMed] [Google Scholar]
- Hoffman JM, Brown JW, Sirlin EA, Benoit AM, Gill WH, Harris MB, Darnall RA. Activation of 5-HT1A receptors in the paragigantocellularis lateralis decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R518–527. doi: 10.1152/ajpregu.00816.2006. [DOI] [PubMed] [Google Scholar]
- Holtman JR., Jr Immunohistochemical localization of serotonin- and substance P-containing fibers around respiratory muscle motoneurons in the nucleus ambiguus of the cat. Neuroscience. 1988;26:169–178. doi: 10.1016/0306-4522(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Dick TE, Berger AJ. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. Journal of Neuroscience. 1986;6:1185–1193. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR, Jr, Dick TE, Berger AJ. Serotonin-mediated excitation of recurrent laryngeal and phrenic motoneurons evoked by stimulation of the raphe obscurus. Brain Res. 1987;417:12–20. doi: 10.1016/0006-8993(87)90174-0. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, King KA. Effect of activation of 5-HT1A receptors at the ventral medulla on phrenic nerve activity. European Journal of Pharmacology. 1994;253:307–310. doi: 10.1016/0014-2999(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hunt CE. Abnormal hypercarbic and hypoxic sleep arousal responses in Near-Miss SIDS infants. Pediatr Res. 1981;15:1462–1464. doi: 10.1203/00006450-198111000-00015. [DOI] [PubMed] [Google Scholar]
- Hunt CE, McCulloch K, Brouillette RT. Diminished hypoxic ventilatory responses in near-miss sudden infant death syndrome. Journal of Applied Physiology: Respiratory, Environmental & Exercise Physiology. 1981;50:1313–1317. doi: 10.1152/jappl.1981.50.6.1313. [DOI] [PubMed] [Google Scholar]
- Ivgy-May N, Tamir H, Gershon MD. Synaptic properties of serotonergic growth cones in developing rat brain. J Neurosci. 1994;14:1011–1029. doi: 10.1523/JNEUROSCI.14-03-01011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyasu S, Randall LL, Welty TK, Hsia J, Kinney HC, Mandell F, McClain M, Randall B, Habbe D, Wilson H, Willinger M. Risk factors for sudden infant death syndrome among northern plains Indians. Jama. 2002;288:2717–2723. doi: 10.1001/jama.288.21.2717. [DOI] [PubMed] [Google Scholar]
- Iyo AH, Porter B, Deneris ES, Austin MC. Regional distribution and cellular localization of the ETS-domain transcription factor, FEV, mRNA in the human postmortem brain. Synapse. 2005;57:223–228. doi: 10.1002/syn.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Lieberman JA, Lauder JM. Developmental effects of prenatal cocaine exposure on 5-HT1A receptors in male and female rat offspring. Dev Neurosci. 2002;24:522–530. doi: 10.1159/000069363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamendi HW, Cheng Q, Dergacheva O, Gorini C, Jameson HS, Wang X, McIntosh JM, Mendelowitz D. Abolishment of serotonergic neurotransmission to cardiac vagal neurons during and after hypoxia and hypercapnia with prenatal nicotine exposure. J Neurophysiol. 2009;101:1141–1150. doi: 10.1152/jn.90680.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DH, Shannon DC. Periodic breathing in infants with near-miss sudden infant death syndrome. Pediatrics. 1979;63:355–360. [PubMed] [Google Scholar]
- Kenny PJ, File SE, Rattray M. Nicotine regulates 5-HT(1A) receptor gene expression in the cerebral cortex and dorsal hippocampus. Eur J Neurosci. 2001;13:1267–1271. doi: 10.1046/j.0953-816x.2001.01501.x. [DOI] [PubMed] [Google Scholar]
- Kim JA, Druse MJ. Protective effects of maternal buspirone treatment on serotonin reuptake sites in ethanol-exposed offspring. Brain Res Dev Brain Res. 1996;92:190–198. doi: 10.1016/0165-3806(96)00015-6. [DOI] [PubMed] [Google Scholar]
- King JA, Davila-Garcia M, Azmitia EC, Strand FL. Differential effects of prenatal and postnatal ACTH or nicotine exposure on 5-HT high affinity uptake in the neonatal rat brain. Int J Dev Neurosci. 1991;9:281–286. doi: 10.1016/0736-5748(91)90048-q. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Belliveau RA, Trachtenberg FL, Rava LA, Paterson DS. The development of the medullary serotonergic system in early human life. Auton Neurosci. 2007;132:81–102. doi: 10.1016/j.autneu.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Myers MM, Belliveau RA, Randall LL, Trachtenberg FL, Fingers ST, Youngman M, Habbe D, Fifer WP. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: a case report. J Neuropathol Exp Neurol. 2005;64:689–694. doi: 10.1097/01.jnen.0000174334.27708.43. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Paterson DS. Sudden Infant Death Syndrome. In: Golden J, Harding B, editors. Pathology and Genetics: Developmental Neuropathology. Neuropath Press; Basel: 2004. vol. [Google Scholar]
- Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK. Serotonergic Brainstem Abnormalities in Northern Plains Indians with the Sudden Infant Death Syndrome. Journal of Neuropathology and Experimental Neurology. 2003;62:1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- Klug MG, Burd L, Kerbeshian J, Benz B, Martsolf JT. A comparison of the effects of parental risk markers on pre- and perinatal variables in multiple patient cohorts with fetal alcohol syndrome, autism, Tourette syndrome, and sudden infant death syndrome: an enviromic analysis. Neurotoxicol Teratol. 2003;25:707–717. doi: 10.1016/j.ntt.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Kondoh M, Shiga T, Okado N. Regulation of dendrite formation of Purkinje cells by serotonin through serotonin1A and serotonin2A receptors in culture. Neurosci Res. 2004;48:101–109. doi: 10.1016/j.neures.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Lajard AM, Bou C, Monteau R, Hilaire G. Serotonin levels are abnormally elevated in the fetus of the monoamine oxidase-A-deficient transgenic mouse. Neurosci Lett. 1999;261:41–44. doi: 10.1016/s0304-3940(98)01012-x. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol. 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW. 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. J Physiol. 1995;487(Pt 3):653–661. doi: 10.1113/jphysiol.1995.sp020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as morphogens. Progress in Brain Research. 1988;73:365–387. doi: 10.1016/S0079-6123(08)60516-6. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. discussion 314. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Casale V, Oneda R, Weese-Mayer DE, Matturri L. Sudden Infant Death Syndrome and Sudden Intrauterine Unexplained Death: Correlation Between Hypoplasia of Raphe Nuclei and Serotonin Transporter Gene Promoter Polymorphism. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181a7bb73. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: biosynthesis, distribution and function. Brain Res Brain Res Rev. 2001;37:25–37. doi: 10.1016/s0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Mossner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol Psychiatry. 1998;44:179–192. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol. 2008;586:2321–2329. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Yee B, Wishart SM, Jimenez M, Jung DG, Grunstein RR, Handelsman DJ. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003;88:3605–3613. doi: 10.1210/jc.2003-030236. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Luo X, Persico AM, Lauder JM. Serotonergic regulation of somatosensory cortical development: lessons from genetic mouse models. Dev Neurosci. 2003;25:173–183. doi: 10.1159/000072266. [DOI] [PubMed] [Google Scholar]
- MacDorman MF, Cnattingius S, Hoffman HJ, Kramer MS, Haglund B. Sudden infant death syndrome and smoking in the United States and Sweden. Am J Epidemiol. 1997;146:249–257. doi: 10.1093/oxfordjournals.aje.a009260. [DOI] [PubMed] [Google Scholar]
- Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol. 2008 doi: 10.1007/s00401-008-0468-x. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol. 2008;586:2171–2181. doi: 10.1113/jphysiol.2007.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BS, Marazita ML, Rand C, Zhou L, Berry-Kravis EM, Weese-Mayer DE. 3′ UTR polymorphism of the serotonin transporter gene and sudden infant death syndrome: haplotype analysis. Am J Med Genet A. 2006;140:1453–1457. doi: 10.1002/ajmg.a.31261. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Manzke T, Preusse S, Hulsmann S, Richter DW. Developmental changes of serotonin 4(a) receptor expression in the rat pre-Botzinger complex. J Comp Neurol. 2008;506:775–790. doi: 10.1002/cne.21581. [DOI] [PubMed] [Google Scholar]
- Mathews TJ, MacDorman MF, Menaker F. Infant mortality statistics form the 1999 period linked birth/infant death data set. National Center for Health Statistics; Hyattsville, MD: 2002. vol. [PubMed] [Google Scholar]
- Matsumoto AM, Sandblom RE, Schoene RB, Lee KA, Giblin EC, Pierson DJ, Bremner WJ. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin Endocrinol (Oxf) 1985;22:713–721. doi: 10.1111/j.1365-2265.1985.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Maurer P, Rorive S, de Kerchove d'Exaerde A, Schiffmann SN, Salmon I, de Launoit Y. The Ets transcription factor Fev is specifically expressed in the human central serotonergic neurons. Neurosci Lett. 2004;357:215–218. doi: 10.1016/j.neulet.2003.12.086. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier ML, Li A, Nattie EE. Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol. 2004;96:1909–1919. doi: 10.1152/japplphysiol.00805.2003. [DOI] [PubMed] [Google Scholar]
- Millar WJ, Hill GB. Prevalence of and risk factors for sudden infant death syndrome in Canada. Cmaj. 1993;149:629–635. [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL. Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems. J Appl Physiol. 1986;61:1249–1263. doi: 10.1152/jappl.1986.61.4.1249. [DOI] [PubMed] [Google Scholar]
- Mitchell EA. Sudden infant death syndrome: should bed sharing be discouraged? Arch Pediatr Adolesc Med. 2007;161:305–306. doi: 10.1001/archpedi.161.3.305. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Milerad J. Smoking and the sudden infant death syndrome. Rev Environ Health. 2006;21:81–103. doi: 10.1515/reveh.2006.21.2.81. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Morin D, Hennequin S, Monteau R, Hilaire G. Depressant effect of raphe stimulation on inspiratory activity of the hypoglossal nerve: in vitro study in the newborn rat. Neurosci Lett. 1990;116:299–303. doi: 10.1016/0304-3940(90)90090-v. [DOI] [PubMed] [Google Scholar]
- Morin D, Monteau R, Hilaire G. 5-Hydroxytryptamine modulates central respiratory activity in the newborn rat: an in vitro study. Eur J Pharmacol. 1991a;192:89–95. doi: 10.1016/0014-2999(91)90073-y. [DOI] [PubMed] [Google Scholar]
- Morin D, Monteau R, Hilaire G. Serotonin and cervical respiratory motoneurones: intracellular study in the newborn rat brainstem-spinal cord preparation. Exp Brain Res. 1991b;84:229–232. doi: 10.1007/BF00231779. [DOI] [PubMed] [Google Scholar]
- Morley ME, Rand CM, Berry-Kravis EM, Zhou L, Fan W, Weese-Mayer DE. Genetic variation in the HTR1A gene and sudden infant death syndrome. Am J Med Genet A. 2008;146:930–933. doi: 10.1002/ajmg.a.32112. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Mimura Y, Kato H, Takigawa M. Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur J Pharmacol. 2001;411:279–282. doi: 10.1016/s0014-2999(00)00925-0. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, Kato H, Suzuki MR, Takigawa M. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Brain Res Dev Brain Res. 1997;102:117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Matsuyama K, Mori S. Prenatal administration of para-chlorophenylalanine results in suppression of serotonergic system and disturbance of swimming movements in newborn rats. Neurosci Res. 1998;31:155–169. doi: 10.1016/s0168-0102(98)00034-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hasegawa H. Developmental role of tryptophan hydroxylase in the nervous system. Mol Neurobiol. 2007;35:45–54. doi: 10.1007/BF02700623. [DOI] [PubMed] [Google Scholar]
- Narita N, Narita M, Takashima S, Nakayama M, Nagai T, Okado N. Serotonin transporter gene variation is a risk factor for sudden infant death syndrome in the Japanese population. Pediatrics. 2001;107:690–692. doi: 10.1542/peds.107.4.690. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnis Marzano F, Maldini M, Filonzi L, Lavezzi AM, Parmigiani S, Magnani C, Bevilacqua G, Matturri L. Genes regulating the serotonin metabolic pathway in the brain stem and their role in the etiopathogenesis of the sudden infant death syndrome. Genomics. 2008;91:485–491. doi: 10.1016/j.ygeno.2008.01.010. [DOI] [PubMed] [Google Scholar]
- O'Mara L. Review: bed sharing between parents and infants exposed to smoke may increase the risk of sudden infant death syndrome. Evid Based Nurs. 2007;10:119. doi: 10.1136/ebn.10.4.119. [DOI] [PubMed] [Google Scholar]
- Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwim GM, Smith CA. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch. 1998;435:485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- Opdal SH, Vege A, Rognum TO. Serotonin transporter gene variation in sudden infant death syndrome. Acta Paediatr. 2008;97:861–865. doi: 10.1111/j.1651-2227.2008.00813.x. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002a;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Takashima S. Developmental neurotransmitter pathology in the brainstem of sudden infant death syndrome: a review and sleep position. Forensic Sci Int. 2002b;130(Suppl):S53–59. doi: 10.1016/s0379-0738(02)00139-1. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Belliveau RA, Trachtenberg F, Kinney HC. Differential development of 5-HT receptor and the serotonin transporter binding in the human infant medulla. J Comp Neurol. 2004;472:221–231. doi: 10.1002/cne.20105. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Darnall R. 5-HT(2A) receptors are concentrated in regions of the human infant medulla involved in respiratory and autonomic control. Auton Neurosci. 2009 doi: 10.1016/j.autneu.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Thompson EG, Kinney HC. Serotonergic and glutamatergic neurons at the ventral medullary surface of the human infant: Observations relevant to central chemosensitivity in early human life. Auton Neurosci. 2006a;124:112–124. doi: 10.1016/j.autneu.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. Jama. 2006b;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Pelayo R, Owens J, Mindell J, Sheldon S. Bed sharing with unimpaired parents is not an important risk for sudden infant death syndrome: to the editor. Pediatrics. 2006;117:993–994. doi: 10.1542/peds.2005-2748. author reply 994-996. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol. 2006 doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- Peterson BA, Kennedy BJ. Aging and cancer management. Part I: Clinical observations. CA Cancer J Clin. 1979;29:322–332. doi: 10.3322/canjclin.29.6.322. [DOI] [PubMed] [Google Scholar]
- Pfaar H, von Holst A, Vogt Weisenhorn DM, Brodski C, Guimera J, Wurst W. mPet-1, a mouse ETS-domain transcription factor, is expressed in central serotonergic neurons. Dev Genes Evol. 2002;212:43–46. doi: 10.1007/s00427-001-0208-x. [DOI] [PubMed] [Google Scholar]
- Pickett CK, Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Moore LG. Progestin and estrogen reduce sleep-disordered breathing in postmenopausal women. J Appl Physiol. 1989;66:1656–1661. doi: 10.1152/jappl.1989.66.4.1656. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, de Castro D, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990;10:1091–1098. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Lena C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M, Adrien J. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25:11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Burnet H, Blanchi B, Sieweke M, De Felipe C, Hunt SP, Monteau R, Hilaire G. The murine neurokinin NK1 receptor gene contributes to the adult hypoxic facilitation of ventilation. Eur J Neurosci. 2002;16:2245–2252. doi: 10.1046/j.1460-9568.2002.02305.x. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Wu ZH, Wang XF. 5-HT(1A) receptors are involved in the modulation of respiratory rhythmical discharge activity in the medulla oblongata slice preparation of neonatal rats. Sheng Li Xue Bao. 2007;59:293–298. [PubMed] [Google Scholar]
- Rand CM, Berry-Kravis EM, Fan W, Weese-Mayer DE. HTR2A variation and sudden infant death syndrome: a case-control analysis. Acta Paediatr. 2009;98:58–61. doi: 10.1111/j.1651-2227.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- Rand CM, Berry-Kravis EM, Zhou L, Fan W, Weese-Mayer DE. Sudden infant death syndrome: rare mutation in the serotonin system FEV gene. Pediatr Res. 2007;62:180–182. doi: 10.1203/PDR.0b013e3180a725a0. [DOI] [PubMed] [Google Scholar]
- Real C, Popa D, Seif I, Callebert J, Launay JM, Adrien J, Escourrou P. Sleep apneas are increased in mice lacking monoamine oxidase A. Sleep. 2007;30:1295–1302. doi: 10.1093/sleep/30.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner JG, McCullough RG, McCullough RE, Pickett CK, Moore LG. Combined effects of female hormones and exercise on hypoxic ventilatory response. Respir Physiol. 1990;82:107–114. doi: 10.1016/0034-5687(90)90027-v. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats [see comments] Journal of Physiology. 1999;514:567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Powrozek T, Zhou FC. Alcohol deters the outgrowth of serotonergic neurons at midgestation. J Biomed Sci. 2001;8:119–125. doi: 10.1007/BF02255980. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Patricia F, Kadhim H, Groswasser J, Sottiaux M, Nishida H, Kahn A. The correlation between serotonergic neurons in the brainstem and sleep apnea in SIDS victims. Early Hum Dev. 2003;75(Suppl):S31–40. doi: 10.1016/j.earlhumdev.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Schoendorf KC, Kiely JL. Relationship of sudden infant death syndrome to maternal smoking during and after pregnancy. Pediatrics. 1992;90:905–908. [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Gunther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Scragg R, Mitchell EA, Taylor BJ, Stewart AW, Ford RP, Thompson JM, Allen EM, Becroft DM. Bed sharing, smoking, and alcohol in the sudden infant death syndrome. New Zealand Cot Death Study Group. Bmj. 1993;307:1312–1318. doi: 10.1136/bmj.307.6915.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon DC, Kelly DH, O'Connell K. Abnormal regulation of ventilation in infants at risk for sudden-infant-death syndrome. New England Journal of Medicine. 1977;297:747–750. doi: 10.1056/NEJM197710062971403. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007a;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Seidler FJ. Perinatal environmental tobacco smoke exposure in rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ Health Perspect. 2006a;114:34–39. doi: 10.1289/ehp.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Tate CA, Seidler FJ. Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: separate or sequential exposure during fetal development and adulthood. Brain Res Bull. 2007b;73:259–272. doi: 10.1016/j.brainresbull.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Cholinergic receptor subtypes in the olfactory bulbectomy model of depression. Brain Res Bull. 2006b;68:341–345. doi: 10.1016/j.brainresbull.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: Serotonin receptors and cell signaling. Neuropsychopharmacology. 2006c;31:2462–2475. doi: 10.1038/sj.npp.1300988. [DOI] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. Journal of Comparative Neurology. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hunsaker JCd. Sudden infant death syndrome: altered aminergic-cholinergic synaptic markers in hypothalamus. Journal of Child Neurology. 1991;6:335–339. doi: 10.1177/088307389100600409. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of alpha1-adrenergic receptors and serotonin 5-HT2 receptors. J Appl Physiol. 2008;104:665–673. doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Verhofstad AA, Penke B, Varga J, Joosten HW. Immunohistochemical characterization of monoamine-containing neurons in the central nervous system by antibodies to serotonin and noradrenalin. A study in the rat and the lamprey (Lampetra fluviatilis) Acta Histochem Suppl. 1981;24:107–122. [PubMed] [Google Scholar]
- Steinschneider A. Prolonged sleep apnea and respiratory instability: a discriminative study. Pediatrics. 1977;59(Suppl):962–970. [PubMed] [Google Scholar]
- Stewart A. Infant leukaemias and cot deaths. Br Med J. 1975a;2:605–607. doi: 10.1136/bmj.2.5971.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM. Cot deaths. Nurs Mirror Midwives J. 1975b;141:57–58. [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid metabolism in the human brain. Eur J Endocrinol. 2001;145:669–679. doi: 10.1530/eje.0.1450669. [DOI] [PubMed] [Google Scholar]
- Tapanainen J, Kellokumpu-Lehtinen P, Pelliniemi L, Huhtaniemi I. Age-related changes in endogenous steroids of human fetal testis during early and midpregnancy. J Clin Endocrinol Metab. 1981;52:98–102. doi: 10.1210/jcem-52-1-98. [DOI] [PubMed] [Google Scholar]
- Tappin D, Brooke H, Ecob R. Bedsharing and sudden infant death syndrome (SIDS) in Scotland, UK. Lancet. 2004;363:994. doi: 10.1016/S0140-6736(04)15806-6. [DOI] [PubMed] [Google Scholar]
- Tappin D, Ecob R, Brooke H. Bedsharing, roomsharing, and sudden infant death syndrome in Scotland: a case-control study. J Pediatr. 2005;147:32–37. doi: 10.1016/j.jpeds.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törk I, Hornung JP. Raphe Nuclei and the Serotonergic System. In: Paxinos G, editor. The Human Nervous System. Academic Press; San Diego, CA: 1990. pp. 1001–1022. vol. [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valic M, Pecotic R, Dogas Z. Phrenic nerve activity is enhanced by 5-HT1A receptor agonist 8-OH-DPAT in spontaneously breathing anesthetized rats. J Physiol Pharmacol. 2008;59:17–25. [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Central serotonin transporter availability measured with [123I]beta-CIT SPECT in relation to serotonin transporter genotype. Am J Psychiatry. 2004;161:525–531. doi: 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]
- Vennemann MM, Bajanowski T, Brinkmann B, Jorch G, Sauerland C, Mitchell EA. Sleep environment risk factors for sudden infant death syndrome: the German sudden infant death syndrome study. Pediatrics. 2009;123:1162–1170. doi: 10.1542/peds.2008-0505. [DOI] [PubMed] [Google Scholar]
- Vennemann MM, Findeisen M, Butterfass-Bahloul T, Jorch G, Brinkmann B, Kopcke W, Bajanowski T, Mitchell EA. Modifiable risk factors for SIDS in Germany: results of GeSID. Acta Paediatr. 2005;94:655–660. doi: 10.1111/j.1651-2227.2005.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Voss MD, De Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Maher BS, Silvestri JM, Curran ME, Marazita ML. Sudden infant death syndrome: association with a promoter polymorphism of the serotonin transporter gene. Am J Med Genet. 2003a;117A:268–274. doi: 10.1002/ajmg.a.20005. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Zhou L, Berry-Kravis EM, Maher BS, Silvestri JM, Marazita ML. Association of the serotonin transporter gene with sudden infant death syndrome: a haplotype analysis. Am J Med Genet. 2003b;122A:238–245. doi: 10.1002/ajmg.a.20427. [DOI] [PubMed] [Google Scholar]
- Werner M, Hatt H, Gottmann K. Synapse formation and morphological differentiation of neuron types in embryonic rat dentate gyrus explants in vitro. Brain Res Dev Brain Res. 1998;105:9–23. [PubMed] [Google Scholar]
- Weston MC, Stornetta RL, Guyenet PG. Glutamatergic neuronal projections from the marginal layer of the rostral ventral medulla to the respiratory centers in rats. J Comp Neurol. 2004;473:73–85. doi: 10.1002/cne.20076. [DOI] [PubMed] [Google Scholar]
- Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol. 1991;11:677–684. doi: 10.3109/15513819109065465. [DOI] [PubMed] [Google Scholar]
- Winter JS, Hughes IA, Reyes FI, Faiman C. Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. J Clin Endocrinol Metab. 1976;42:679–686. doi: 10.1210/jcem-42-4-679. [DOI] [PubMed] [Google Scholar]
- Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. A prospective study of smoking during pregnancy and SIDS. Arch Dis Child. 2000;83:203–206. doi: 10.1136/adc.83.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- Zec N, Filiano JJ, Kinney HC. Anatomic relationships of the human arcuate nucleus of the medulla: a DiI-labeling study. J Neuropathol Exp Neurol. 1997 1997 Sep;56(9):1070. doi: 10.1097/00005072-199705000-00007. [DOI] [PubMed] [Google Scholar]; Journal of Neuropathology & Experimental Neurology. 56:509–522. doi: 10.1097/00005072-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Zec N, Kinney HC. Anatomic relationships of the human nucleus paragigantocellularis lateralis: a DiI labeling study. Auton Neurosci. 2001;89:110–124. doi: 10.1016/S1566-0702(01)00258-2. [DOI] [PubMed] [Google Scholar]
- Zec N, Kinney HC. Anatomic relationships of the human nucleus of the solitary tract in the medulla oblongata: a DiI labeling study. Auton Neurosci. 2003;105:131–144. doi: 10.1016/S1566-0702(03)00027-4. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Ellenberger HH, Feldman JL. Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neuroscience. 1989;31:105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Li TK, Goodlett C, Azmitia EC. Deviations in brain early serotonergic development as a result of fetal alcohol exposure. Neurotox Res. 2002;4:337–342. doi: 10.1080/10298420290030532. [DOI] [PubMed] [Google Scholar]
- Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol. 2003;94:101–107. doi: 10.1152/japplphysiol.00264.2002. [DOI] [PubMed] [Google Scholar]


