Abstract
Infection of rabbits with aerosolized rabbitpox virus (RPXV) produces a disease similar to monkeypox and smallpox in humans and provides a valuable, informative model system to test medical countermeasures against orthopoxviruses. Due to the eradication of smallpox, the evaluation of the efficacy of new-generation smallpox vaccines depends on relevant well-developed animal studies for vaccine licensure. In this study, we tested the efficacy of IMVAMUNE® [modified vaccinia Ankara-Bavarian Nordic (MVA-BN®)] for protecting rabbits against aerosolized RPXV. Rabbits were vaccinated with either phosphate-buffered saline (PBS), Dryvax®, a single low dose of IMVAMUNE®, a single high dose of IMVAMUNE®, or twice with a high dose of IMVAMUNE®. Aerosol challenge with a lethal dose of RPXV was performed 4 weeks after the last vaccination. All PBS control animals succumbed to the disease or were euthanized because of the disease within 7 days postexposure. The rabbits vaccinated with Dryvax®, a low dose of IMVAMUNE®, or a single high dose of IMVAMUNE® showed minimal to moderate clinical signs of the disease, but all survived the challenge. The only clinical sign displayed by rabbits that had been vaccinated twice with a high dose of IMVAMUNE® was mild transient anorexia in just two out of eight rabbits. This study shows that IMVAMUNE® can be a very effective vaccine against aerosolized RPXV.
Keywords: Rabbitpox virus, Aerosol, IMVAMUNE®, MVA, Smallpox vaccine
1. Introduction
Smallpox was a devastating human disease caused by variola virus, a member of the genus Orthopoxvirus in the family Poxviridae. Other orthopoxviruses that can infect humans include monkeypox virus, cowpox virus, and vaccinia virus. Infection by vaccinia virus usually produces a mild and limited disease in immunocompetent individuals and provides cross-protection from infection by the other orthopoxviruses. Therefore, Dryvax® vaccine (Wyeth Pharmaceuticals, Marietta, PA) containing the live vaccinia virus strain New York City Board of Health was one of the vaccines used during the worldwide smallpox eradication program of the 1960s and 1970s.
Rabbitpox is a severe and frequently lethal disease of domestic rabbits caused by rabbitpox virus (RPXV), which is also an Orthopoxvirus. Rabbitpox was first reported in the 1930s among rabbits in a research colony at the Rockefeller Institute in New York [1]. Over the next few decades, several other outbreaks of rabbitpox occurred in research institutes in Europe and the United States [2]. Whether through natural transmission or experimental studies, the disease was shown to be easily transmitted by aerosolized virus [3], [4].
RPXV infection in rabbits exposed by aerosol is very similar to smallpox disease seen in humans [5]. A fever ensues followed by the rapid onset of anorexia, weakness, rapid weight loss, and depression. Subsequently, facial edema and purulent discharges, both ocular and nasal, often develop. Lethargy and hypothermia leads to death, which generally occurs between day 6 and 10 after aerosol exposure of RPXV. Aerosolized RPXV infection in rabbits is a good animal model of human smallpox and is one of the very few models to convey animal-to-animal transmission of aerosolized Orthopoxvirus virions [3], [4].
Due to the eradication of smallpox and the side effects of Dryvax®, vaccination was discontinued in 1972 for the general population in the United States. However because of concerns over the potential use of variola virus and/or other orthopoxviruses as agents of bioterrorism, Dryvax® vaccination was restarted in 2002 for high-risk individuals, such as healthcare workers. In conjunction, potentially life-threatening complications, including eczema vaccinatum and progressive vaccinia in individuals such as eczema sufferers and the immunocompromised, caused concerns about the vaccine and prompted the search for a safer alternative vaccine.
Modified vaccinia Ankara (MVA) is a highly attenuated replication-deficient strain of vaccinia virus, which was developed in the 1960s [6]. It was derived from serial passages of the chorioallantois vaccinia virus Ankara (CVA) strain in chicken embryo fibroblast (CEF) cultures and has six large genomic deletions compared to the Dryvax® vaccine virus [7], [8]. MVA was used to vaccinate more than 100,000 individuals in parts of West Germany in the 1970s and was well tolerated [6], [9]. However, MVA has never been evaluated in an area where smallpox was endemic and thus its protective efficacy in humans remains unknown.
The MVA-based vaccine IMVAMUNE®, manufactured by Bavarian Nordic (Martinsried, Germany), is a third-generation vaccine against smallpox that has been shown to be safe and well tolerated in healthy and immune compromised individuals [10]. Due to the inability of IMVAMUNE® to replicate in human cell lines, it provides a safer alternative for vaccinating immunocompromised individuals [11].
Because smallpox has been eradicated, clinical trials to determine the ability of IMVAMUNE® to protect humans from variola virus infection cannot be conducted. Therefore, it is important to evaluate the efficacy of this new vaccine in animal models to meet United States Food and Drug Administration's (FDA) requirement for licensing under; the “Animal Rule” that requires that efficacy of medical countermeasures against smallpox be tested in well-defined animal models [12]. Thus, the purpose of this study was to test the efficacy of IMVAMUNE® for protecting rabbits against a lethal dose of aerosolized RPXV.
2. Materials and methods
2.1. Cells and virus
CV-1 African green monkey kidney fibroblast cells (ATCC, Manassas, VA) were maintained in Eagle's minimal essential medium (EMEM) supplemented with 1% nonessential amino acids (NEAA), 1% 200 nanomolar (nM) l-glutamine, 7.5% fetal bovine serum (FBS), and 0.5% penicillin/streptomycin.
RPXV was obtained from ATCC (Manassas, VA) and propagated in CV-1 cells. Briefly, confluent monolayers of CV-1 cells were infected with RPXV in T-150 flasks containing 5 ml of complete medium and incubated at 37 °C + 5% CO2 incubator for 1 h by rocking every 15 min. After 1 h, medium containing EMEM supplemented with 1% NEAA, 1% 200 nM l-glutamine, and 7.5% FBS was added to flasks. Flasks were incubated at 37 °C + 5% CO2 incubator then observed daily for the evidence of cytopathic effects (CPE). When 4 + CPE (>90% CPE) was observed, cells were harvested. The harvest mixture was centrifuged at 2000 RPM for 10 min, supernatant was separated and the pellet was resuspended with 5 ml of medium and stored at −80 °C overnight. The next day, this suspension was thawed and sonicated 20 sec and then again stored at −80 °C. This procedure was repeated three times. At the end, viral stock was distributed in 1-ml volumes and stored at −80 °C for future use.
2.2. Animals
Forty (20 male, 20 female) “Specific Pathogen Free” (SPF) New Zealand white (NZW) rabbits (Oryctolagus cuniculus) weighing approximately 2.5–3.0 kg were purchased from Charles River Laboratories (Wilmington, MA). The rabbits were maintained on a 12 h light/12 h dark cycle and fed standard rabbit food supplemented with fresh leafy vegetables and water ad libitum.
Each animal was implanted subcutaneously between its scapulae with a programmable temperature transponder chip (Bio Medic Data Systems, Seaford, DE) to determine rabbit identification and subcutaneous body temperature.
2.3. Vaccines and vaccinations
IMVAMUNE® (lot no: 0031105) was provided by Bavarian Nordic. The vaccine had a nominal titer of 2 × 108 TCID50 per ml and the administration of 0.5 ml subcutaneously (s.c.) achieved the high dose of IMVAMUNE® (1 × 108 TCID50). To evaluate a low dose (1 × 107 TCID50), IMVAMUNE® was diluted in sterile PBS prior to subcutaneous administration (0.5 ml). The final amounts of IMVAMUNE® present in each low and high dose of vaccines were 1 × 107 TCID50 and 1 × 108 TCID50, respectively.
Dryvax® (Wyeth Laboratories, Marietta, PA (lot no: 4020075) was provided by the Centers for Disease Control and Prevention in Atlanta, GA.
Vaccinations were given to five groups of eight rabbits and all vaccinations were administered in a shaved area of skin over the left flank of each rabbit. Six weeks before aerosol exposure to a lethal dose of RPXV, each rabbit in group 5 was vaccinated with the first high dose of 1 × 108 TCID50 IMVAMUNE® s.c.; 2 weeks later, these animals received a second high dose of the vaccine. On the same day that the group 5 animals were vaccinated the second time (i.e., 4 weeks before aerosol challenge), the rabbits in the other groups were also vaccinated. Group 1 was sham-vaccinated by s.c. injection with PBS (0.5 ml per animal) and served as the negative control. Each animal in group 2 was vaccinated with 2 × 105 plaque-forming units (pfu) of Dryvax® on its left flank by scarification using a bifurcated needle; this group served as the positive control. Group 3 was vaccinated s.c. with 1 × 107 TCID50 in a 0.5 ml volume of IMVAMUNE® (low dose) and group 4 was vaccinated s.c. with 1 × 108 TCID50 in a volume of 0.5 ml of IMVAMUNE® (high dose).
2.4. Biosamples
A blood sample was collected from each rabbit in group 5 for serum antibody testing 43 days before the viral challenge (day −43); blood samples for antibody testing were collected from all of the rabbits on days −29, −15, and −1. Rabbits were anesthetized with an intramuscular ketamine–xylazine mixture (20 mg/kg and 4 mg/kg, respectively) and blood samples were collected from a peripheral ear vein. In addition, blood samples for determination of viral titers were collected the day before exposure and then day 2 postexposure and every other day after that through day 14 postexposure.
2.5. Aerosol challenge
Rabbits were challenged with a lethal dose of aerosolized RPXV under conditions as previously described [13]. Briefly, the respiratory function of each rabbit was first measured using whole-body plethysmography (Buxco Systems, Sharon, CT) immediately prior to exposure. Rabbits were exposed to aerosolized RPXV using a dynamic muzzle-only (nose and mouth) inhalation chamber, operated within a class III biosafety cabinet maintained under negative pressure as described previously [4]. In order to calculate the presented dose for each animal, the atmosphere within the inhalation chamber was continuously sampled during each exposure for aerosol concentration using an all-glass impinger (AGI: Ace Glass, Vineland, NJ). The collection fluid and starting concentrations for each exposure were assayed by plaque assay using CV-1 cells for determination of aerosol concentration. Determination of presented dose to each rabbit was calculated using respiratory minute volume (V m) estimates derived from the respiratory function measurements performed before the exposures. The presented aerosol dose was then calculated by multiplying the total volume (V t) of experimental atmosphere inhaled by each animal (V t = V m × length of exposure) by the empirically determined exposure concentration from chamber sampling (C e) (‘presented dose’ = C e × V t) [14].
2.6. Plaque assay
Viral contents of starting concentrations and AGIs were determined by performing a plaque assays in CV-1 cell lines as described previously [4]. Plaques were visualized on a light box and counted.
2.7. Clinical observations and euthanasia
Rabbits were observed at least twice per day by study personnel for 21 days, starting 24 h after challenge; more frequent observations were made if warranted due to clinical illness or changes in behavior. The body weight and temperature of each rabbit were recorded daily, starting the day before aerosol exposure, and continuing 15 days after exposure. When signs of severe disease were observed, such as marked lethargy, dyspnea, severe anorexia, and/or open mouth breathing, rabbits were anesthetized with the ketamine/xylazine mixture mentioned above and then euthanized with an administration of intravenous pentobarbital solution.
2.8. DNA isolation and real-time PCR
Starting the day before exposure and continuing every second day after exposure until day 14, blood was collected from all surviving rabbits for viral load analysis in whole blood. In addition, the rabbits that succumbed to disease were necropsied and selected tissue samples were collected for viral load determination by real-time PCR assay. DNA was isolated from blood and tissue samples using Biorobot M48 (Qiagen, Valencia, CA) in accordance with the manufacturer's instructions. A pan-orthopox hemagglutinin (HA) assay was used to measure viral load in whole blood and tissues [15]. The limit of detection (LOD) for this assay was 5000 genomes/ml. Data were analyzed by using the Roche LightCycler data analysis software (version 4.0).
2.9. Vaccinia-specific total immunoglobulin (IgG) ELISA (enzyme-linked immunosorbent assay)
Vaccinia specific serum IgG antibodies were measured using a direct ELISA on days −1, −15, −29 (all groups), and −43 (only group 5), as previously described [16]. Briefly, 96-well transparent Fluoronunc Maxisorp plates (Nunc, Wiesbaden, Germany) were coated overnight at 4 °C with 100 μl (1 μg/ml) of MVA antigen (crude extract of MVA-BN® infected CEF cells) in coating buffer (200 mM Na2CO3, pH 9.6). Plates were washed and immediately blocked with 200 μl of PBS containing 5% FCS and 0.05% Tween 20 for 30 min at room temperature. Plates were washed and heat-inactivated (56 °C for 30 min). Test sera were titrated in duplicate using twofold serial dilutions starting at 1:100. The plates were incubated for 1 h at room temperature, washed and incubated for 1 h with 100 μl of the detection antibody (1:70,000 dilution), sheep anti-rabbit IgG-HRP, before washing and developing with 50 μl of 3,3′5,5′-tetramethyl-benzidine for 30 min at room temperature in the dark. The reaction was stopped with 50 μl of 1 M H2SO4 and read out at 450 nm. The antibody titers were calculated by linear regression and defined as the serum dilution that resulted in an optical density of 0.30.
2.10. Plaque reduction neutralization assay (PRNT)
Vaccinia virus-specific, serum-neutralizing antibody levels were measured by a PRNT. Sera were heat-inactivated and serially diluted (start dilution 1 in 10) in DMEM medium (Invitrogen) containing 7% FCS (PAA Laboratories) and incubated with a 1:250,000 dilution of 3.16 × 108 TCID50/ml vaccinia virus-Western Reserve (Advanced Biotechnologies Inc.) for 20 h at 36 °C and 5% CO2. As a 100% virus control, virus was incubated in medium only. After incubation, the serum/virus mixtures and controls were added to pre-seeded Vero cells on 48-well plates and were allowed to adsorb for 70 min at 36 °C and under 5% CO2. After the adsorption, 250 μl of pre-warmed DMEM medium containing 7% FCS, 100 U/ml penicillin/streptomycin (Sigma–Aldrich) was added to each well and incubated for 24 h at 36 °C and under 5% CO2. Plates were stained for 10–60 min at room temperature with a crystal violet solution (Sigma–Aldrich), which visually identified the plaques. The staining solution was removed and plates were dried. The 100% virus control contained 126 TCID50 of vaccinia virus per well and therefore a range of 80–180 plaques in that control was accepted. The neutralizing titer of each sample was determined as the serum dilution which was able to neutralize 50% of the mature virus, using the plaque count in the 100% virus control as 100% value.
2.11. Post mortem examination
The carcasses from all rabbits that succumbed to the disease or were euthanized due to the severity of the clinical signs were submitted for a complete gross necropsy in biosafety level-3 containment. Samples of the following organs were aseptically collected and stored at −80 °C for real-time PCR assay: mandibular lymph node, liver, spleen, left adrenal gland, left gonad, left kidney, lungs, and brain. The following tissues from each rabbit were sampled for histology and fixed by immersion in 10% neutral buffered formalin: mandibular lymph node, mesenteric lymph node, tongue, larynx, trachea, thyroid gland, lungs, esophagus, mediastinum (including thymus, aorta, and mediastinal lymph nodes), heart, liver, spleen, right adrenal gland, kidneys, gonad, uterus or penis, upper lip and nares, skin, soft palate, brain, and rostral skull with nasal passages.
At the end of this study (≥day 25 after aerosol exposure), the surviving rabbits were anesthetized and then euthanized with an intravenous administration of pentobarbital solution as described above. Although a complete gross necropsy was performed on each of these animals, no tissue samples were collected for the PCR assay. For rabbits that had displayed clinical signs and/or had gross lesions at necropsy, samples of the following tissues were collected and fixed for histology: mandibular lymph node, respiratory tract (including nasal passages), thyroid gland, tongue, esophagus, and mediastinum.
2.12. Histology
The set of formalin-fixed tissue samples from each rabbit was held for a minimum of 21 days under biosafety level-3 containment and then was decontaminated and transferred to the USAMRIID histology laboratory. Samples of bone were decalcified for 48–72 h in 26% formic acid solution. All tissue samples were then trimmed, routinely processed, and embedded in paraffin. Sections of the paraffin-embedded tissues 5 μm thick were cut and placed on glass histology slides. The histology slides were deparaffined, stained with hematoxylin and eosin (H&E), and coverslips were added.
2.13. Statistical analysis
Repeated measures analysis of variance (RM-ANOVA) was used to compare temperature and weight over time between groups. Post-hoc Tukey's test was used for pairwise comparisons at each time point. RM-ANOVA of log10 transformed antibody titers with step-down Bonferroni adjustment for multiple pairwise comparisons and ANOVA of log10 transformed antibody titers with post-hoc Tukey's test for pairwise comparisons at each time point were utilized to determine differences in antibody response across groups and time points. All analyses were two tailed and conducted using SAS Version 9.1.3.
3. Results
3.1. Vaccine-induced antibody responses measured by ELISA and PRNT
Antibody response was tested in the serum obtained from rabbits on day −43 (only group 5) and on days −29, −15 and −1 (all groups) by ELISA and PRNT and geometric mean titers (GMT) of the results are shown in Fig. 1 . As expected, no antibody titers were detected by ELISA or PRNT for the rabbits that had been sham-vaccinated with PBS. By ELISA, the rabbits vaccinated with two high doses of IMVAMUNE® showed a rapid increase in antibody response 2 weeks after the first vaccination, and a robust boost in titer was observed 2 weeks after the second vaccination (day −15). Four weeks after the second vaccination (1 day before challenge), there was a drop in antibody levels for group 5 (Fig. 1a). In contrast, while we observed a similar trend with PRNT through day −15, the antibody levels continued to increase a day before challenge with PRNT (Fig. 1b). Similarly, ELISA showed that the group vaccinated once with a high dose of IMVAMUNE® had increased antibody titers in 2 weeks and then the titer slightly dropped just before challenge (Fig. 1a). Again with this group, we observed increased neutralizing antibody titers through day −1 (Fig. 1b). For the group vaccinated once with a low dose of IMVAMUNE®, low antibody titers were detected 2 weeks after vaccination by ELISA and this slightly increased 2 weeks thereafter (Fig. 1a). This group showed neutralizing antibody titers only 4 weeks after vaccination (day −1) (Fig. 1b). Surprisingly, only low levels of antibody titers were detected in Dryvax®-vaccinated rabbits’ serum by ELISA and they were undetectable by PRNT. ANOVA analysis from pre exposure days −1, −15, −29, and −43 showed that groups 3, 4 and 5 were significantly different from each other and the control group and Dryvax® group (p < 0.0001). One possible explanation for this would be that not all Dryvax® vaccinated rabbits had a “take” reaction – which is series of events at the vaccination side after primary vaccination [17]. While some papules and minimal pustule formation were observed at the Dryvax® vaccination site of some rabbits, the majority of the rabbits developed only redness at the vaccination site. A similar phenomenon was reported in humans; those individuals who had “take” reactions had significantly higher ELISA and neutralizing antibody responses compared to no ‘take’ responders [18].
Fig. 1.
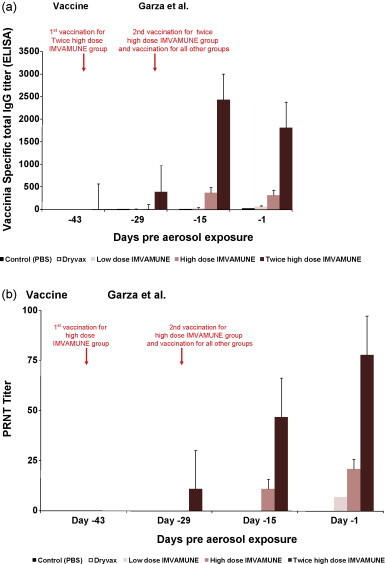
(a) Geometric mean titers (GMT) of antibodies by ELISA for rabbits in all groups on days −43, −29, −15, and −1 pre-aerosol exposure. Antibody titers were calculated by linear regression and defined as the serum dilution that resulted in an optical density of 0.30. Sera with OD value below 0.3 set to titer of 1 (negative). (b) Geometric mean of neutralizing antibody titers by PRNT of all groups pre-aerosol exposure. Individual titers were determined as serum dilution able to neutralize 50% of the virus. The group mean is shown [+standard error of mean (SEM)].
3.2. Clinical signs of disease
All rabbits were exposed to aerosolized RPXV on day 0. The average calculated dose that was presented to the rabbits was 10,100 pfu/rabbit which corresponds to ∼500 LD50 of aerosolized RPXV. ANOVA analysis showed that there were no significant differences in mean challenge dose for any of the groups compared. In previous studies, we found that aerosolized RPXV at this dose caused 100% mortality in exposed animals (Nalca, unpublished data). To test the robustness of the vaccines, a high viral challenge dose was preferred for this study.
After the challenge, the rabbits were observed daily for temperature increase, weight loss, and other clinical signs of rabbitpox. Rabbits vaccinated with PBS showed the most severe clinical signs. These included a high fever, severe anorexia and weight loss, copious amounts of ocular and nasal discharge, depression, dyspnea and open mouth breathing, facial and cervical edema, dehydration, and diarrhea. All of the group 1 rabbits either succumbed or were euthanized because of the severity of the disease between days 5 and 7 postexposure.
In contrast, all of the rabbits that were vaccinated with either Dryvax® or IMVAMUNE® survived the viral challenge. A few of the rabbits vaccinated with Dryvax®, low-dose IMVAMUNE®, and single high-dose IMVAMUNE® experienced some clinical signs such as a slight increase in body temperature, mild anorexia, mild ocular and nasal discharge, and mild depression. Only minimal anorexia occurred in two out of eight rabbits that had been vaccinated twice with a high dose of IMVAMUNE®.
The PBS-vaccinated control group developed weight loss beginning on day 4 (Fig. 2a). In contrast, all other rabbits were protected from significant weight loss. ANOVA analysis comparing the percentage of weight loss from postexposure day 1 to 7 (control group included) showed a significant difference in percent change in weight between the control group and other vaccinated groups (p < 0.0001). ANOVA analysis comparing the percentage of weight loss from postexposure day 8 to 15 (control group not included) showed no significant difference in percent change in weight between the vaccinated groups.
Fig. 2.
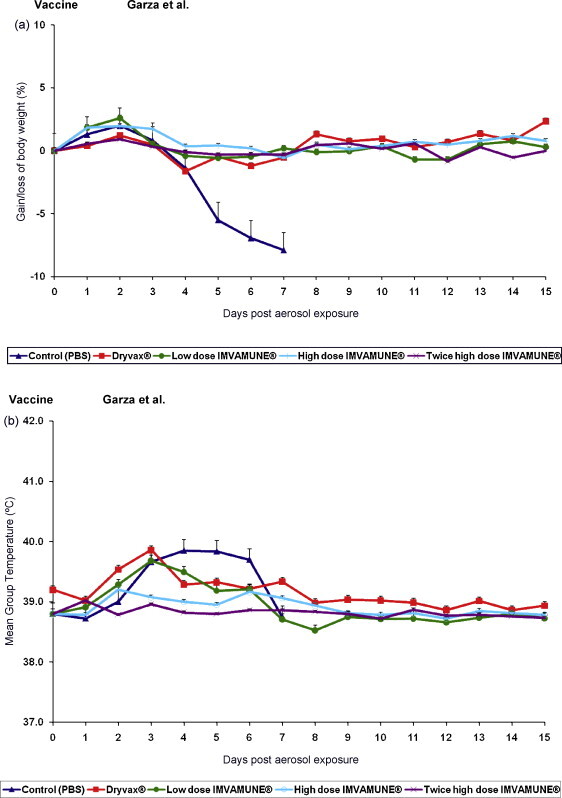
The changes on weight and temperature of rabbits challenged with aerosolized RPXV. (a) Mean percent of body weight gained or lost for rabbits in all groups. Rabbits were weighed daily from the day of aerosol exposure and continued through day 15 post-aerosol exposure. (b) Mean group temperature changes of rabbits in all groups. Temperatures were taken daily beginning on the day of aerosol exposure and continued through day 15 post-aerosol exposure.
PBS control rabbits showed elevated body temperatures from day 3 to 6 (Fig. 2b). The Dryvax® vaccinated group demonstrated elevated mean body temperature on days 2 and 3 which decreased back to normal range on day 4 postexposure. Similarly, the rabbits that had received a single low dose of IMVAMUNE® had an elevated mean body temperature between days 2 and 4. In contrast, animals vaccinated with a single high dose or two high doses of IMVAMUNE® experienced no temperature increase. Indeed, ANOVA analysis from postexposure day 1 to 7 (control group included) showed overall significant temperature change between groups (p < 0.0014).
Control group rabbits as well as those in the vaccinated groups did not show any cutaneous pox lesions. Our previous studies indicated that exposing rabbits to a similar high dose of aerosolized RPXV produces an overwhelming and uniformly lethal infection with high viral titers in the blood and these rabbits succumbed to disease before the skin lesions appeared. In contrast, rabbits exposed to much lower viral doses have a more prolonged clinical course and develop skin lesions but ultimately succumb to disease (Nichols and Nalca, unpublished data).
3.3. Whole blood and tissue viral load of rabbits exposed to aerosolized RPXV
Fig. 3a shows the geometric means of whole blood viral load from each group of rabbits and Table 1 shows the number of rabbits with viral loads for all groups. Rabbits vaccinated with PBS had a measurable viral load in their blood (>5 × 103 genomes/ml) on days 4 and 6 postexposure (Fig. 3a, Table 1). Three rabbits vaccinated with Dryvax® had a measurable viral load peaking on days 4 and 6 postexposure. Three of the low dose IMVAMUNE®-vaccinated rabbits had a detectable viral load between days 4 and 8 postexposure. High dose IMVAMUNE®-vaccinated rabbits had no detectable viral load, indicating that complete viral clearance and thereby protection was achieved by this dose of IMVAMUNE®. However, the twice-vaccinated high dose IMVAMUNE® had a single rabbit with a detectable viral load on days 6 and 8 postexposure. Due to an insufficient number of samples having a measurable viral load, comparative statistical analyses could not be completed for these groups.
Fig. 3.
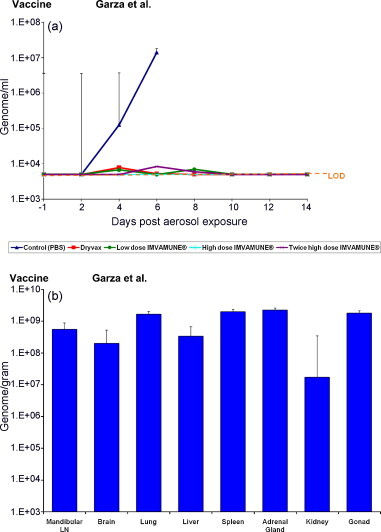
The changes in whole blood viral load and tissue viral load of rabbits challenged with aerosolized RPXV. (a) Blood samples were collected on day −1, day 2 post-aerosol exposure and every other day through day 14. DNA was isolated from whole blood and used for real-time PCR assay. The graph shows the average whole blood viral load from each group of rabbits, LOD: limit of detection. (b) Necropsies were performed on all of the rabbits that had succumbed to the disease. Selected tissues were collected, processed, and DNA was isolated and used for real-time PCR assay. The graph shows the average tissue viral load from the control group (group 1).
Table 1.
Number of rabbits with viral load in whole blood for each group.
| Group (n = 8) | # of rabbits with viral load | Highest viral load |
|---|---|---|
| Control (PBS) | 8 | 3.2 × 109 genome/ml |
| Dryvax® | 2 | 6.6 × 104 genome/ml |
| Low dose IMVAMUNE® | 3 | 1 × 105 genome/ml |
| High dose IMVAMUNE® | 0 | 0 |
| Twice high dose IMVAMUNE® | 1 | 3 × 105 genome/ml |
Rabbits vaccinated with PBS were the only animals that succumbed to the disease and that had tissue samples collected for PCR analysis. Therefore, only control animal tissues were processed for viral load determination (Fig. 3b). The lungs, spleen, adrenal gland, and gonads had the highest average viral load content at approximately 2 × 109 genomes/g.
3.4. Post mortem examination
All of the PBS control rabbits succumbed or were euthanized due to rabbitpox on day 5, 6, or 7 postexposure. The gross lesions present in these rabbits were typical of those that occur due to aerosolized RPXV infection and included moderate to marked subcutaneous edema along the ventral aspect of the neck, congestion and edema of the mandibular lymph nodes, and pulmonary edema with consolidated foci of pneumonia (Fig. 4a). Histologically, the most severe lesions were located in the respiratory tract, primarily in the lungs and mucosa lining the nasal passages (Fig. 4b and c), and in the mandibular and mediastinal lymph nodes, which receive lymphatic drainage from the respiratory tract. However, the presence of foci of necrosis and/or inflammation in multiple other organs indicates that widely disseminated viral infection had occurred before the deaths of these rabbits.
Fig. 4.
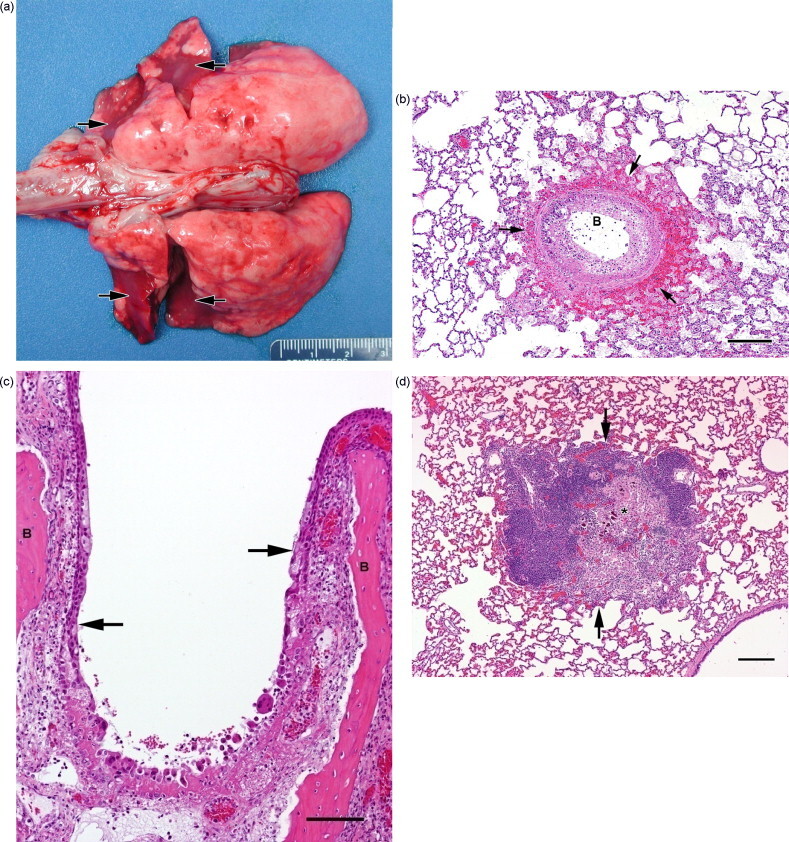
Lesions in rabbits caused by aerosolized RPXV. Parts (a)–(c) are from group 1 rabbits (PBS control) that expired due to rabbitpox. (a) Dorsal view of lungs with diffuse edema and multiple red consolidated foci of pneumonia (arrows). Scale units are mm; numbers on scale are cm. (b) Histologic section of lung showing a large bronchiole (B) with diffuse degeneration and necrosis of the lining epithelium, accompanied by hemorrhage, edema, and fibrin exudation into surrounding alveoli (arrows). Magnification 4×; bar = 20 μm. (c) Histologic section of a nasal passage showing diffuse congestion, edema, and subacute inflammation of the nasal mucosa overlying turbinate bones (B), with a large focus of epithelial degeneration and necrosis (area extending from one arrow to the other arrow). Magnification 10×; bar = 10 μm. (d) Histologic section of lung from a group 2 rabbit (Dryvax® vaccinated) that had displayed clinical signs but survived. There is a large focus of granulomatous inflammation (between arrows); the necrotic and mineralized materials in the center of this lesion (asterisk) are probably remnants of a bronchiole. Magnification 4×; bar = 20 μm.
Three of the rabbits that had been vaccinated with Dryvax® displayed clinical signs of rabbitpox. Although no gross lesions were seen during the necropsies of these animals, histology revealed that two of them had areas of chronic inflammation in the lungs and nasal mucosa indicating poxvirus infection (Fig. 4d); however, there was no evidence of an on-going viral infection at the time these rabbits were euthanized. In addition, one of the group 2 rabbits that had not shown any clinical signs was found at necropsy to have multiple foci of granulomatous pneumonia as a result of the previous viral insult. The other four group 2 rabbits never displayed clinical signs and did not have any gross lesions at necropsy.
Seven of the eight animals that were vaccinated with the low dose of IMVAMUNE® developed clinical signs of rabbitpox. Although all of these recovered and survived to the end of the study, five of them were found to have gross and/or histologic lesions in the respiratory tract – similar to those described above in the group 2 rabbits. At necropsy, the one group 3 rabbit that had not displayed clinical signs was also found to have residual foci of chronic granulomatous inflammation in the lungs.
Only one of the rabbits that had been given a single high dose of IMVAMUNE® became clinically ill. Necropsy revealed that the lungs of this rabbit, as well as two other group 4 animals that had not shown clinical signs, contained multiple foci of granulomatous inflammation. Chronic residual inflammation was also present in the nasal mucosa of the rabbit that had shown clinical signs. The other five group 4 animals were never clinically ill and had no lesions at necropsy.
None of the rabbits that were vaccinated twice with a high dose of IMVAMUNE® displayed clinical signs and none of them were found to have any residual lesions at necropsy. Table 2 shows the summary of clinical signs and pathology results.
Table 2.
Number of rabbits in each group with clinical signs and/or respiratory lesions.
| Group (n = 8) | Clinical signs and lesions | Clinical signs w/o lesions | No clinical signs/lesions present | No clinical signs/no lesions |
|---|---|---|---|---|
| Control (PBS) | 8 | 0 | 0 | 0 |
| Dryvax® | 2 | 1 | 1 | 4 |
| Low dose IMVAMUNE® | 5 | 2 | 1 | 0 |
| High dose IMVAMUNE® | 1 | 0 | 2 | 5 |
| Twice high dose IMVAMUNE® | 0 | 0 | 0 | 8 |
4. Discussion
All of the PBS control animals developed severe clinical signs of rabbitpox and none of these animals survived past day 7 after aerosol exposure. PCR results revealed high viral loads in the blood and various organs of these rabbits, and pathology studies demonstrated virus-induced lesions in multiple tissues with the respiratory tracts most severely affected. Thus, the dose of aerosolized RPXV used in this study caused a widely disseminated viral infection leading to 100% morbidity and mortality in naïve rabbits.
In contrast, all of the rabbits that received either Dryvax® or any of the different doses of IMVAMUNE® survived the viral challenge. Although both vaccines fully protected the rabbits from dying of rabbitpox, when given as a single dose, they were less than 100% effective in preventing virus-induced clinical signs and/or lesions. Three of the rabbits that were vaccinated with Dryvax® became clinically ill and chronic virus-induced lesions were also present in the respiratory tract of one of the rabbits in this group that had not been clinically ill. Therefore, 50% (4/8) of the group 2 animals were not fully protected from the viral infection.
A single low dose of IMVAMUNE® was less effective than Dryvax® against the viral challenge; all eight (100%) of the rabbits in group 3 developed clinical signs and/or had virus-induced respiratory lesions. However, a single high dose of IMVAMUNE® provided slightly better protection than Dryvax®; only one out of eight animals in group 4 became clinically ill and this rabbit plus two others in this group that had not shown clinical signs were found to have respiratory lesions. Thus, 37.5% (3/8) of the group 4 rabbits were not fully protected from viral infection.
The only clinical sign that was observed in the rabbits that received two high doses of IMVAMUNE® was very mild anorexia in two animals. However, these rabbits did not lose weight and none of the group 5 animals was found to have lesions at necropsy. Thus, two high doses of IMVAMUNE® provided protection from significant virus-induced disease in 100% of the twice-vaccinated rabbits.
The results from this study have confirmed that a prime boost regime using the high dose of IMVAMUNE® was the optimal effective dose in the rabbit aerosolized rabbitpox model, while a single IMVAMUNE® vaccination at this dose was equally protective as Dryvax®. Similar findings have also been reported using mice or nonhuman primates challenged with lethal doses of either ectromelia virus (mousepox) or monkeypox virus, respectively [19], [20]. Moreover, this has also been shown to be the optimal human dose both in terms of safety and immunogenicity induced by IMVAMUNE® [16], [21].
ELISA and PRNT data showed that IMVAMUNE® induced a humoral immune response in rabbits within 2 weeks after vaccination, and that a second vaccination (for group 5 only) gave a robust increase in the antibody levels. The increase in antibody levels was dose and frequency dependent (Fig. 1). Surprisingly, the antibody levels in the rabbits vaccinated with Dryvax® were very low by ELISA and undetectable by PRNT, even though all of these rabbits survived the viral challenge. While MVA has been shown to induce faster protective response in animals, the disparity in the immune responses generated in rabbits was unexpected because MVA has been shown to induce similar peak immune responses as Dryvax® in mice, nonhuman primates and people [16], [19], [20], [21]. The fact that our ELISA utilized MVA as antigen seems to be an unlikely explanation for this disparity; viral proteins of MVA and other vaccinia strains are 97–99% identical [22] and in other species, such as mice and non-human primates, MVA and Dryvax® have been shown to induce comparable ELISA titers using various strains of vaccinia virus as antigens [23], [24], [25]. In fact, optimized ELISA using either Dryvax® or MVA-BN® as the antigen have shown similar titers in people vaccinated with MVA or traditional smallpox vaccines (Volkmann and Chaplin, unpublished observation). Moreover, vaccinia virus-Western Reserve was used in our PRNT, yet, no measurable PRNT titer was induced by Dryvax® vaccination. Therefore, one possible explanation is that the administration of Dryvax® by scarification of the flank in rabbits was inefficient and only successfully primed the animals albeit, this appeared to be protective. This explanation is also supported with the absence of a “take” reaction in the majority of rabbits as explained previously in Section 3.1.
Overall, this study demonstrated that a single high dose of IMVAMUNE® provides rabbits the same degree of protection from rabbitpox as vaccination with Dryvax® does. We were also able to determine that rabbits given two high doses of IMVAMUNE® were fully protected from the disease. This study has shown that IMVAMUNE® can be an efficacious alternative to Dryvax® as a vaccine against orthropoxviruses.
Acknowledgements
The authors acknowledge the efforts of the personnel in the Aerosol Services branch of the Center for Aerobiological Sciences for conducting the aerosol study, and the personnel of the Veterinary Medicine Division for the care and handling of the animals used in these studies. All animal research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals, and adheres to principles stated in the “Guide for the Care and Use of Laboratory Animals”, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, and National Academy Press, Washington, DC, 1996. The USAMRIID facility, where the animal research was conducted, is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. We also thank to Robert Johnson and Blair Osborn (NIAID) for excellent discussions during study and also for critically reviewing the manuscript.
This study was supported by an interagency agreement between Office of Biodefense Research Affairs (OBRA)/National Institute of Allergy and Infectious Diseases (NIAID) and USAMRIID.
Footnotes
Disclaimer: Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army or the Department of Defense.
References
- 1.Greene H.S.N. A pandemic of rabbit-pox. Proc Soc Exp Biol Med. 1933;30:892–894. [Google Scholar]
- 2.Fenner F. In: Virus infections of rodents and lagomorphs. Osterhaus A.D., editor. Elsevier; New York: 1994. Rabbitpox virus; pp. 51–57. [Google Scholar]
- 3.Adams M.M., Rice A.D., Moyer R.W. Rabbitpox virus and vaccinia virus infection of rabbits as a model for human smallpox. J Virol. 2007;81:11084–11095. doi: 10.1128/JVI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalca A., Hatkin J.M., Garza N.L., Nichols D.K., Hurby D.E., Jordan R. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antiviral Res. 2008;79:121–127. doi: 10.1016/j.antiviral.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Henderson D.A. Countering the posteradication threat of smallpox and polio. Clin Infect Dis. 2002;34:79–83. doi: 10.1086/323897. [DOI] [PubMed] [Google Scholar]
- 6.Mayr A., Hochstein-Mintzel V., Stickl H. Abstammung, eigenschaften und verwendung des attenuierten vaccinia-stammes MVA (passage history, properties, and applicability of the attenuated vaccinia virus strain MVA) Infection. 1975;3:6–14. [Google Scholar]
- 7.Meyer H., Sutter G., Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 8.Meisinger-Henschel C., Schmidt M., Lukassen S., Linke B., Krause L., Konietzny S., et al. Genomic sequence of chorioallantois vaccinia virus Ankara, the ancestor of modified vaccinia virus Ankara. J Gen Virol. 2007;88:3249–3259. doi: 10.1099/vir.0.83156-0. [DOI] [PubMed] [Google Scholar]
- 9.Stickl H., Hochstein-Mintzel V., Mayr A., Huber H.C., Schafer H., Holzner A. MVA—stufenimpfung gegen pocken. Kleinische erprobung des attenuierten pocken-lebendimpfstoffes, stamm MVA (MVA vaccination against smallpox:clinical tests with an attenuated live vaccinia virus strain (MVA)) Dtsch Med Wschr. 1974;99:2386–2392. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy J.S., Greenberg R.N. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines. 2009;8:13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones T. IMVAMUNE, an attenuated modified vaccinia Ankara virus vaccine for smallpox infection. Curr Opin Mol Ther. 2008;10:407–417. [PubMed] [Google Scholar]
- 12.21 CFR Parts 314 and 601. New Drug and Biological Drug Products; Evidence Needed to Demonstrate Effectiveness of New Drugs When Human Efficacy Studies Are Not Ethical or Feasible; 2002. Food and Drug Administration, HHS. [PubMed]
- 13.Pitt M.L., Little S.F., Ivins B.E., Fellows P., Barth J., Heweston J., et al. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine. 2001;19:4768–4773. doi: 10.1016/s0264-410x(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 14.Roy C.J., Pitt L.M.L. In: Biodefense: research methodology and animal models. Swearengen J.L., editor. CRC Press; Boca Raton, FL: 2006. Infectious disease aerobiology: aerosol challenge methods; pp. 61–76. [Google Scholar]
- 15.Kulesh D.A., Baker R.O., Loveless B.M., Norwood D., Zwiers S.H., Mucker E., et al. Smallpox and pan-orthopox virus detection by real-time 3′-minor groove binder TaqMan assays on the Roche lightcycler and the Cepheid smart cycler platforms. J Clin Microbiol. 2004;42:601–609. doi: 10.1128/JCM.42.2.601-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vollmar J., Arndtz N., Eckl K.M., Thomsen T., Petzold B., Mateo L., et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24:2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;1803:1–14. doi: 10.1016/s0166-3542(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 18.McClain D.J., Harrison S., Yeager C.L., Cruz J., Ennis F.A., Gibbs P., et al. Immunologic responses to vaccinia vaccines administrated by different parenteral routes. J Infect Dis. 1997;175:756–763. doi: 10.1086/513968. [DOI] [PubMed] [Google Scholar]
- 19.Samuelsson C., Hausmann J., Lauterbach H., Schmidt M., Akira S., Wagner H., et al. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J Clin Invest. 2008;118:1776–1784. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frey S.E., Newman F.K., Kennedy J.S., Sobek V., Ennis F.A., Hill H., et al. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax® challenge. Vaccine. 2007;25:8562–8573. doi: 10.1016/j.vaccine.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies D.H., Wyatt L.S., Newman F.K., Earl P.L., Chun S., Hernandez J.E., et al. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus Ankara is comparable to that of Dryvax. J Virol. 2008;82:652–663. doi: 10.1128/JVI.01706-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meseda C.A., Garcia A.D., Kumar A., Mayer A.E., Manischewitz J., King L.R., et al. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology. 2005;339:164–175. doi: 10.1016/j.virol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 25.Wyatt L.S., Earl P.L., Eller L.A., Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. PNAS. 2004;March (101):4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]


