Abstract
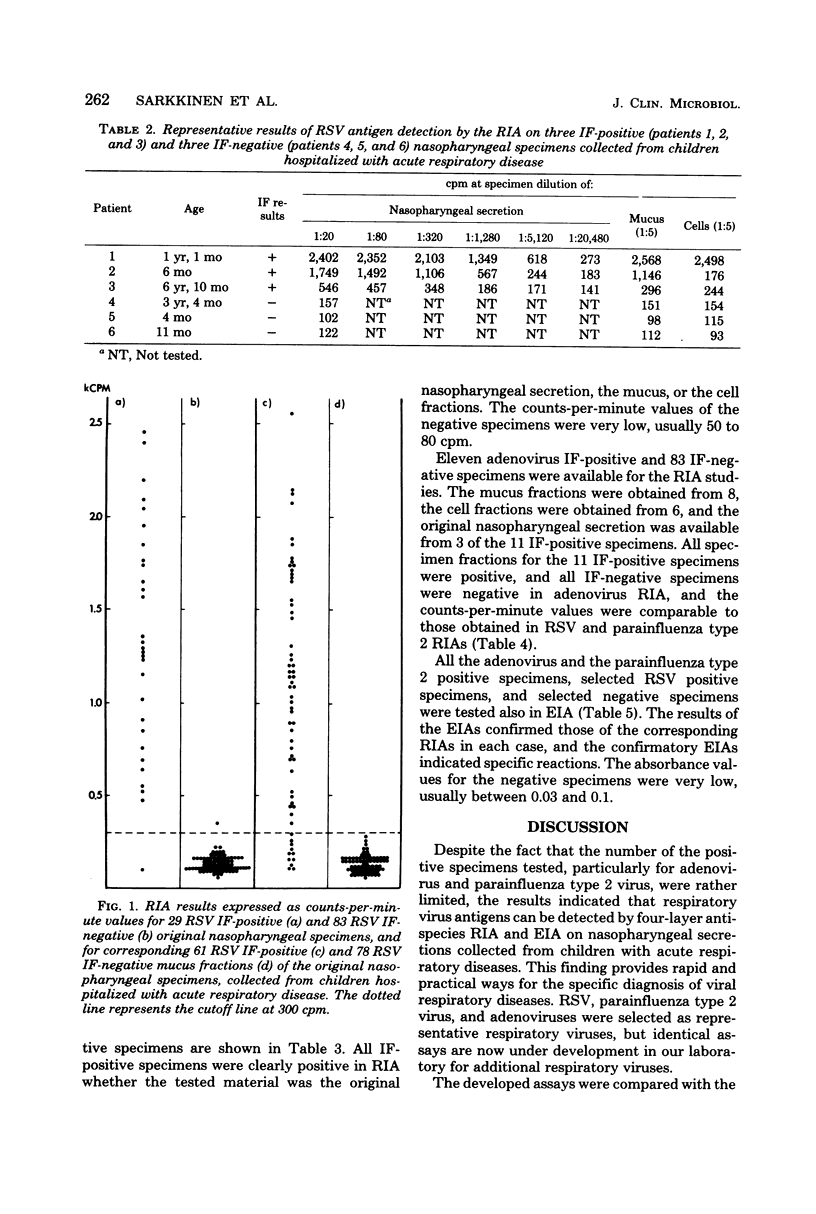
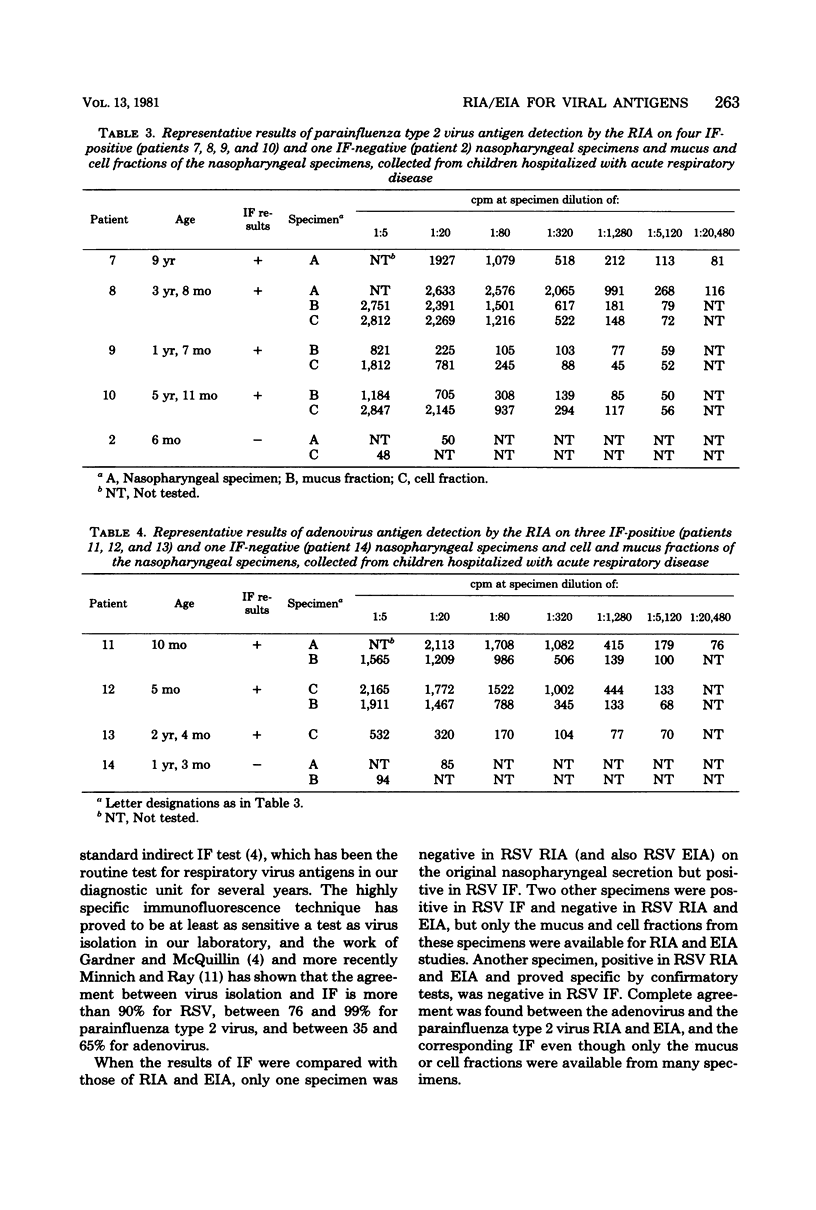
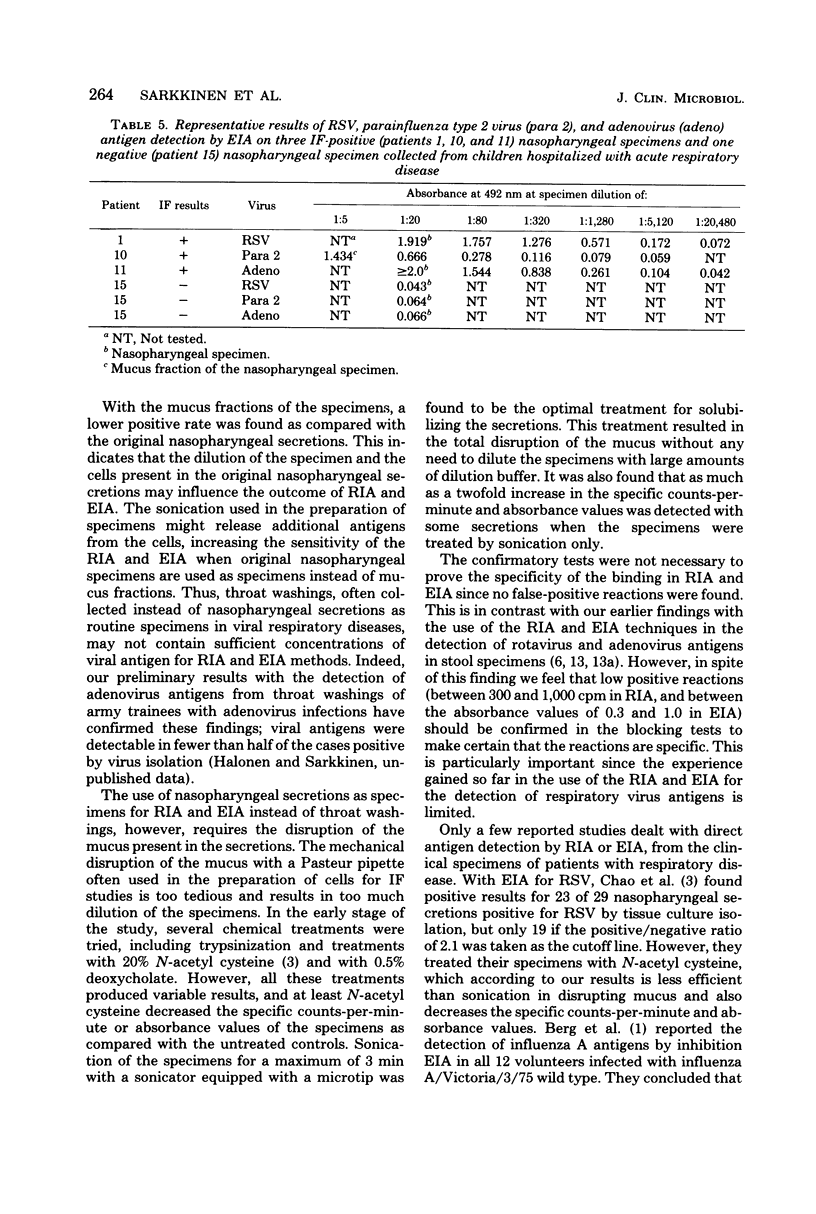
Four-layer antispecies radioimmunoassay (RIA) and enzyme immunoassay (EIA) procedures were developed for the detection of respiratory syncytial virus (RSV), parainfluenza type 2 virus, and adenovirus antigens in nasopharyngeal specimens from children hospitalized for acute respiratory disease. Polystyrene beads (RIA) or flat-bottomed polystyrene microtiter plates (EIA) were used as the solid phases, guinea pig anti-virus immunoglobulins were used as the captive antibodies, rabbit anti-virus immunoglobulins were used as the secondary antibodies, and 125I-labeled sheep anti-rabbit (RIA) or horseradish peroxidase-labeled swine anti-rabbit (EIA) immunoglobulins were used as the indicator antibodies. A comparison of the EIAs and RIAs with routinely used immunofluorescence (IF) techniques was made with 164 nasopharyngeal specimens collected from children with acute respiratory disease. Only 3 of 66 RSV IF-positive specimens were negative in RSV RIA, and of 83 RSV, parainfluenza type 2 virus, and adenovirus IF-negative specimens, 1 was positive in RSV RIA. Of 4 parainfluenza type 2 virus IF-positive and 11 adenovirus IF-positive specimens, each was positive in corresponding RIAs, and all 83 IF-negative specimens were negative in parainfluenza type 2 virus and adenovirus RIAs. The results of the RSV, parainfluenza type 2, and adenovirus EIAs confirmed the results of corresponding RIAs in each selected case tested. The RIAs and EIAs were found to be as specific and sensitive as IF techniques, and more practical in the rapid detection of respiratory viruses in nasopharyngeal secretions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Yolken R. H., Rennard S. I., Dolin R., Murphy B. R., Straus S. E. New enzyme immunoassays for measurement of influenza A/Victoria/3/75 virus in nasal washes. Lancet. 1980 Apr 19;1(8173):851–853. doi: 10.1016/s0140-6736(80)91356-2. [DOI] [PubMed] [Google Scholar]
- Bishai F. R., Spence L., Goodwin D., Petro R. Use of antisera against bovine (NCDV) and simian (SA11) rotaviruses in ELISA to detect different types of human rotavirus. Can J Microbiol. 1979 Sep;25(9):1118–1124. doi: 10.1139/m79-174. [DOI] [PubMed] [Google Scholar]
- Chao R. K., Fishaut M., Schwartzman J. D., McIntosh K. Detection of respiratory syncytial virus in nasal secretions from infants by enzyme-linked immunosorbent assay. J Infect Dis. 1979 Apr;139(4):483–486. doi: 10.1093/infdis/139.4.483. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Halonen P., Bennich H., Torfason E., Karlsson T., Ziola B., Matikainen M. T., Hjertsson E., Wesslen T. Solid-phase radioimmunoassay of serum immunoglobulin A antibodies to respiratory syncytial virus and adenovirus. J Clin Microbiol. 1979 Aug;10(2):192–197. doi: 10.1128/jcm.10.2.192-197.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen P., Sarkkinen H., Arstila P., Hjertsson E., Torfason E. Four-layer radioimmunoassay for detection of adenovirus in stool. J Clin Microbiol. 1980 Jun;11(6):614–617. doi: 10.1128/jcm.11.6.614-617.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mathiesen L. R., Feinstone S. M., Wong D. C., Skinhoej P., Purcell R. H. Enzyme-linked immunosorbent assay for detection of hepatitis A antigen in stool and antibody to hepatitis A antigen in sera: comparison with solid-phase radioimmunoassay, immune electron microscopy, and immune adherence hemagglutination assay. J Clin Microbiol. 1978 Feb;7(2):184–193. doi: 10.1128/jcm.7.2.184-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P. J., Holdaway M. D., Petric M., Szymanski M. T., Tam J. S. Solid-phase radioimmunoassay for the detection of rotavirus. Infect Immun. 1977 May;16(2):439–444. doi: 10.1128/iai.16.2.439-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich L., Ray C. G. Comparison of direct immunofluorescent staining of clinical specimens for respiratory virus antigens with conventional isolation techniques. J Clin Microbiol. 1980 Sep;12(3):391–394. doi: 10.1128/jcm.12.3.391-394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell R. H., Wong D. C., Alter H. J., Holland P. V. Microtiter solid-phase radioimmunoassay for hepatitis B antigen. Appl Microbiol. 1973 Oct;26(4):478–484. doi: 10.1128/am.26.4.478-484.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkkinen H. K., Halonen P. E., Arstila P. P. Comparison of four-layer radioimmunoassay and electron microscopy for detection of human rotavirus. J Med Virol. 1979;4(4):255–260. doi: 10.1002/jmv.1890040403. [DOI] [PubMed] [Google Scholar]
- Sarkkinen H. K., Tuokko H., Halonen P. E. Comparison of enzyme-immunoassay and radioimmunoassay for detection of human rotaviruses and adenoviruses from stool specimens. J Virol Methods. 1980;1(6):331–341. doi: 10.1016/0166-0934(80)90050-6. [DOI] [PubMed] [Google Scholar]



