Abstract
Cancer treatment with chemotherapy or radiotherapy causes gonadal toxicity in male patients. The endpoint of most concern for future reproductive options is the induction of prolonged azoospermia, which may or may not be reversible. The immediate effects of therapy and its reversibility are most readily observed in post-pubertal patients, but the same antineoplastic regimens given to prepubertal males can induce permanent azoospermia. The probability of permanent azoospermia is related to the specific agents used and their doses. The most damaging are alkylating agents (particularly chlorambucil, procarbazine, cyclophosphamide, melphalan, and busulfan), cisplatin and radiation to the region of the testicles.
INTRODUCTION
For male children and young men who have cancer, the success of treatment with regimens that are toxic to testicular function has made infertility an important problem. After the cancer is controlled, the quality of life, which often includes the ability to have a normal child, becomes a major issue.
Chemotherapy and radiotherapy used in the treatment of cancer can cause long-term or permanent gonadal toxicity in male patients. Whereas endocrine dysfunction (e.g., testosterone reduction) only occurs in limited instances [1], the manifestation of the toxicity that is of most concern is the prolonged reduction in sperm count to the point of azoospermia. Damage to other aspects of sperm function, such as loss of motility or morphological abnormalities are less pronounced, as when and if spermatozoa are produced after therapy, their motility and the percentage that exhibit normal morphology are restored to pretreatment levels [2,3]. When sperm count recovers following cytotoxic therapy, fertility is generally restored. However, when the duration of azoospermia is long, sperm count may sometimes plateau in the severe oligospermic range, and the sperm may have morphological abnormalities [4] that are not compatible with fertility.
With current methods of assisted reproductive technologies, including in vitro fertilization, intracytoplasmic sperm injection, and testicular sperm extraction, the limitations on the numbers of sperm and their physical abilities to enter the oocyte can be bypassed. However, there may be an increased risk of passing genetic damage in the spermatozoa on to the children.
BASIC PRINCIPLES
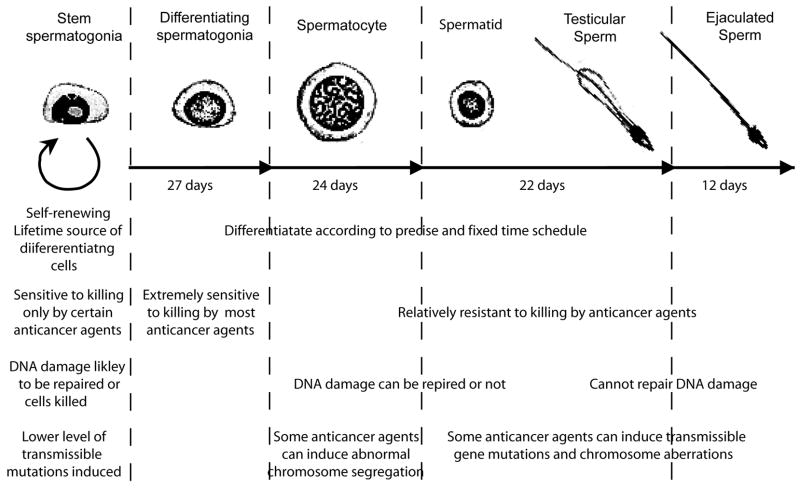
After a patient starts treatment with chemotherapy or radiotherapy, there may still be sperm produced for the first 2 months because of the relative resistance of the later stage germ cells (Figure 1). Even mild forms of chemotherapy and low gonadal doses of radiotherapy can cause transient reductions in sperm count lasting 2–3 months from the end of treatment because of killing of the very sensitive differentiating spermatogonia. However prolonged reductions in sperm count or azoospermia can occur after stronger chemotherapy regimens or after higher doses of radiation therapy. The eventual recovery of sperm production depends on the survival of the spermatogonial stem cells and their ability to differentiate. In mice the time interval before recovery of fertility is directly related to the degree of stem cell killing [5]; in rats the sterility is due to damage to the somatic environment that prevents surviving stem spermatogonia from differentiating [6]. In human males, both stem cell killing and a block in their differentiation appear to contribute to the duration of the azoospermic period after cytotoxic therapy.
Figure 1.
Sequence of spermatogenic cells showing the cell morphology, kinetics, relative sensitivity to killing by anticancer agents, ability to accumulate and repair DNA damage, and sensitivity to induction of transmissible mutations.
Many post-pubertal males become azoospermic at the completion of their cytotoxic therapy. If low doses of agents that kill stem spermatogonia or affect differentiation are used, recovery to normospermic levels can occur within 1 to 3 years, but at higher doses, azoospermia can be more prolonged or even permanent. Although the probability that spermatogenesis will recover decreases with the duration of azoospermia, in rare cases spermatogenesis has recovered in men after as long as 20 years of azoospermia [7].
Although in most individuals with iatrogenic azoospermia, the seminiferous tubules in testicular biopsies contain only Sertoli cells and no germ cells [8], occasionally a few tubules may contain isolated spermatogonia [9]. This would indicate that there is some potential for recovery but there is a block to spermatogonial development at that time.
SPECIFIC AGENTS
The duration and permanence of the induced azoospermia depends on the nature of the cytotoxic agent and dose [10]. It is primarily radiation, many of the alkylating chemotherapeutic agents (procarbazine, busulfan, cyclophosphamide, chlorambucil, and melphalan), and cisplatin, which like the alkylating agents produces adducts and cross-links in DNA, that can produce long-term azoospermia. The doses required to produce prolonged azoospermia with many of these agents in adults are known (Table I). At lower doses, recovery to normospermic levels can occur within 1 to 3 years, but at higher doses, azoospermia can be more prolonged or even permanent [11]. Some agents, including the anthracyclines (e.g., doxorubicin), microtubule inhibitors (e.g., vinblastine), and select antimetabolites (e.g., cytarabine) do not produce prolonged azoospermia if not given with more highly gonadotoxic agents listed above, but can have additive effects when given with these agents [12]. In addition there are many chemotherapeutic agents that do not produce prolonged azoospermia; these include antimetabolites (e.g., 6-mercaptopurine, methotrexate), topoisomerase inhibitors (e.g., etoposide, mitoxantrone), biologicals (e.g., interferon), corticosteroids (e.g., prednisone), and other agents such as bleomycin (Table II). If treatment is limited to the cytotoxic agents that do not kill stem spermatogonia or block their differentiation, normospermia can be restored at 3 months after the cytotoxic therapy [13]. Although individuals’ responses to these agents and combinations may vary, it is possible to predict the probability of prolonged azoospermia from the doses of each agent used in a combination, as the agents appear to have additive effects.
TABLE I.
Agents with Significant Long-Term Effects on Sperm Production in Men
| Agents (cumulative dose for effect) | Effect |
|---|---|
| Radiation (2.5 Gy to testis) [48] | Prolonged azoospermia |
| Busulfan (600 mg/m2) [49] | |
| Chlorambucil (1.4 g/m2) [50] | |
| Cyclophosphamide (19 g/m2) [51] | |
| Procarbazine (4 g/m2) [52] | |
| Melphalan (140 mg/m2) [53] | |
| Cisplatin (500 mg/m2) [54] | |
| BCNU (1 g/m2) [17] | Azoospermia in adulthood after treatment prior to puberty |
| CCNU (500 mg/m2) [17] | |
| Ifosfamide (42 g/m2) [55] | Likely, but always given with other highly sterilizing agents |
| BCNU (300 mg/m2) [53] | |
| Nitrogen mustard | |
| Actinomycin D |
This table was modified from Ref. [10]. Note that the dose of busulfan that contributes significantly to sterility was given incorrectly in that reference as 600 mg/kg; the correct value is 600 mg/m2. BCNU: Carmustine; CCNU: Lomustine
TABLE II.
Agents with Limited, No, or Unknown Long-Term Effects on Sperm Production in Men
| Agents (cumulative dose for effect) | Effect |
|---|---|
| Carboplatin (2 g/m2) [56] | Contributes less to prolonged azoospermia than does cisplatin. |
| Adriamycin (770 mg/m2) [2] Thiotepa (400 mg/m2) [4] Cytosine arabinoside (1 g/m2) [19] Vinca alkaloids [56]* |
Can be additive with highly gonadotoxic agents (see Table I) in causing prolonged azoospermia, but cause only temporary reductions in sperm count when not combined with those agents |
| Amsacrine, bleomycin, dacarbazine, daunorubicin, epirubicin, etoposide, fludarabine, 5-fluorouracil, 6-mercaptopurine, methotrexate, mitoxantrone, thioguanine [13,57–59] | Only temporary reductions in sperm count at doses used in conventional regimens, but additive effects are possible |
| Prednisone [11] Interferon-α [11] |
No effects on sperm production |
| Taxanes Tyrosine kinase inhibitors Monoclonal antibodies Nanoparticles |
No published data available |
This table was modified from Ref. [10]. Note that the doses of vinblastine and vincristine that appear to have additive effects were given incorrectly in that reference as 50 g/m2 and 8 g/m2, respectively; the correct values are 50 mg/m2 and 300 mg/m2.
Patients treated with vinblastine and vincristine were grouped in the analysis. Average vinblastine doses were 50 mg/m2 and vincristine doses were 300 mg/m2.
COMPARISON BETWEEN POST- AND PRE-PUBERTAL MALES
In the prepubertal testis, the seminiferous tubules contain only immature Sertoli cells and spermatogonia, both of which show very low proliferative rates. There are no recognizable Leydig cells in the interstitium but there are mesenchymal precursors that will proliferate and form the adult Leydig cells, which produce testosterone.
It had been incorrectly assumed that the prepubertal testis would be more resistant to cytotoxic agents because of the low proliferation rates and this assumption was apparently supported by erroneously comparing sterilizing doses of chemotherapy to boys and adult men on a dose per kg basis [14]. However by appropriately expressing chemotherapy doses to boys on a per meter squared basis and calculating radiation doses to the gonad, the doses of a variety of chemotherapy [15] and radiotherapy [16] regimens to produce permanent azoospermia in survivors of childhood and adolescent cancer after they reach puberty appear to be the same as those for adults.
As in adults, radiation, alkylating agents, such as procarbazine, cyclophosphamide, chlorambucil, BCNU, and CCNU, and cisplatin are the most sterilizing and produce prolonged and sometimes permanent azoospermia in boys [17,18]. In addition, high doses of cytosine arabinoside have an additive effect with the above agents in producing germ cell damage [19]. Regimens lacking alkylating agents, such as those used for treatment of acute lymphocytic leukemia do not affect subsequent sperm counts or fertility [20,21].
The only aspect in which prepubertal males and adults display different gonadal sensitivity is with respect to damage to eventual Leydig cell formation and testosterone production, with boys showing greater sensitivity to high doses of radiation [1]. Chemotherapy regimens do not have any marked effect on Leydig cell function, either in pre- or postpubertal males [22].
GENETIC CONSIDERATIONS
Since anticancer agents damage DNA and interfere with chromosome segregation there is concern that these agents may induce both single gene and chromosomal mutations in germ cells, both of which can cause genetic disease in offspring [23]. It is important to consider the induction of mutations in differentiating germ cells separately from induction of mutations in stem spermatogonia. Animal studies indicate that the differentiating germ cells are more sensitive to induction and transmission of mutations than are stem spermatogonia [24]. Whereas mutations induced in later stages of spermatogenesis will only result in production of mutation-carrying sperm for about 3 months, those induced in stem spermatogonia will continue to produce mutation-carrying sperm for the lifetime of the male (Figure 1). Attempts to assess the risks of paternal transmission of treatment-induced genetic disease to the offspring of male cancer patients have been done using actual data on pregnancy outcomes, measurement of mutations in spermatozoa, which will be transmitted to any resulting fertilizations, and measurement of DNA damage in spermatozoa, which may or may not pose a risk to offspring conceived.
Clinical reports of outcomes of pregnancies in which conception occurred while the father was undergoing cytotoxic therapy, and hence the germ cells were exposed while differentiating, are too limited to evaluate the risks [24]. There are some large studies of genetic disease in offspring, nearly all of which were conceived long after the end of therapy, which is especially true in the studies of long-term survivors of childhood and adolescent cancer. Thus the spermatozoa must be derived from cells that were exposed to the potentially genotoxic agents as stem spermatogonia. The large studies all indicated no significant increase, above the background in the general population of about 4%, in birth defects or genetic disease in offspring conceived naturally after cytotoxic treatment [25–28]. In addition, the atomic-bomb studies in Japan also showed no significant increase in genetic damage in offspring born to radiation-exposed parents [29]. These observations should reassure those who wish to have children following treatment for cancer. However, the power of these studies can only rule out ≥2-fold increases in abnormalities over background; the possibility of a small genetic risk remains. Also these studies do not include many patients receiving the newer chemotherapeutic agents or pregnancies from spermatozoa obtained after treatment and achieved using assisted reproductive technologies.
Two types of mutations have been analyzed in spermatozoa of patients treated with cytotoxic agents for cancer. Minisatellite repeat number mutations represent a change in the number of tandem repeats in a type of DNA sequence called a minisatellite. Indeed, such mutations were reported to be increased in offspring of men exposed to radiation from Chernobyl [30]. However, the lack of any increases in these mutations in spermatozoa from nearly all men analyzed after treatment with radiotherapy or chemotherapy indicates that they are not a significant concern [31,32]. Chromosomal abnormalities have been measured by sperm karyotyping after fusion with hamster eggs or by fluorescence in situ hybridization (FISH). Structural chromosomal aberrations were present in sperm more than 5 years after the end of MOPP chemotherapy combined with radiation therapy indicating they are induced in stem cells [33]. Abnormal numbers of chromosomes (aneuploidy) can be present during and within a defined period after chemotherapy (up to 4 or 18 months, depending on the study) and then return to baseline [34–36]. These results demonstrate that there can be significant genetic risks if conception or storage of sperm occurs during or shortly after cytotoxic therapy, but that this risk declines after the end of such therapy (Figure 1).
Recently, there have been a few studies of DNA damage in spermatozoa after cancer therapy. The presence of high levels of DNA damage in spermatozoa has been correlated with reduced fertilizing capacity. In addition DNA damage sites are considered to be potentially premutational lesions. If they are repaired correctly in the oocyte or zygote, they may not have any consequence. However, if they are not repaired or repaired incorrectly, they may lead to a mutation in DNA sequence or chromosome content. One study showed a transient increase in DNA damage just at the completion of chemotherapy [37]. Several other studies have failed to show any persistent increase in DNA damage in spermatozoa obtained after chemotherapy, but one of these studies did show that there may be a persistent increase in DNA damage for up to 2 years after radiation therapy [38–40]. Since these techniques only measure what might be premutational damage and knowledge of repair of such damage within the oocyte is incomplete, no estimates of mutational risk to the offspring are yet possible from these data.
In summary, mutational risks will be highest when a pregnancy occurs during or within several months after the male is exposed to the damaging agent. After this time, the incidence of mutations declines to a lower level, which so far has not been detectible as adverse pregnancy outcomes.
FUTURE DIRECTIONS
Monitoring and minimizing the gonadal toxicity of cancer treatments is an ongoing need. New treatment regimens are being developed that may have unexpected gonadal toxicity. Although it is less likely that new specifically targeted therapies, as opposed to the DNA-damaging therapies that constitute the bulk of antineoplastic agents, will display prolonged gonadal toxicities, they still must be monitored, especially for possible synergistic effects when used in combination with existing therapies.
A better understanding of the mechanisms causing prolonged azoospermia following anticancer therapies is needed. As stated above, the eventual recovery of sperm production depends on the survival of the spermatogonial stem cells and their ability to differentiate. It is generally thought that the prolonged period of azoospermia is caused by killing many of the stem spermatogonia by antineoplastic agents and, until they gradually recover their number, they will not produce differentiating cells and spermatozoa. However in rats exposed to certain antineoplastic agents, the stem spermatogonia that survive and recover their number still are blocked from differentiating [41] as a result of damage to the somatic environment within the testis, not to the spermatogonia [6,42]. It is important to determine whether such damage to the somatic environment of the testis occurs in human males as well and the relative roles of germ cell and somatic cell damage in the gonadal toxicity from anticancer therapy.
Methods to prevent these adverse effects on sperm production and to restore gonadal function after the toxic treatment are of great importance to young male patients. A variety of biochemical and biological approaches have been tested in experimental animal models to protect the testes against radiation and chemotherapy (reviewed in [43]). However, the greatest research interest and all clinical trials have involved hormonal modulation in attempts to prevent or reverse damage to the germline from radiotherapy and chemotherapy [44]. Gonadotropin and testosterone suppression in rats before or after cytotoxic therapy dramatically enhances the recovery of spermatogenesis and fertility [41]. Although the mechanism of this phenomenon is not fully known, it is known that it is not a consequence of the induction of quiescence as originally speculated but rather involves the reversal, by suppression of testosterone, of the block in differentiation of surviving spermatogonia caused by damage to the somatic environment [6]. However, in men, only one of eight clinical trials using hormonal suppression was successful in protecting or restoring spermatogenesis after cytotoxic therapy [45]. It is therefore important to identify the specific beneficial effects of hormonal suppression in the rat model that may be applicable to human
Since many chemotherapy and radiotherapy regimens may result in complete killing of the stem spermatogonia, cryopreservation of spermatogonia from prepubertal males and autologous transplantation after therapy is considered a potential method for restoring spermatogenesis and possibly rescuing fertility [46]. However, possible damage to the somatic cells that might render the testis unable to support differentiation of transplanted cells may have to be considered [6]. Methods such as gonadotropin and testosterone suppression, which promotes the survival and differentiation of spermatogonia that were transplanted into testes of rodents depleted of endogenous stem cells, might restore a favorable somatic environment [6,47].
Finally and most importantly the information available must be regularly and proactively used by health care professionals to inform the cancer patients about the probability of sterility and the genetic risk from their disease and its treatment. Options for preservation of fertility, including choice of less gonadotoxic chemotherapy regimens, gonadal shielding during radiation therapy, semen cryopreservation, and the investigational approach of testicular tissue cryopreservation for later use, should be discussed with the patient.
Acknowledgments
Some of the research performed in Dr. Meistrich’s laboratory referred to here was supported by NIH Grant ES-08075 from the National Institute of Environmental Health Sciences and the Florence M. Thomas Professorship in Cancer Research. The editorial assistance of Walter Pagel is greatly appreciated.
References
- 1.Shalet SM, Tsatsoulis A, Whitehead E, et al. Vulnerability of the human Leydig cell to radiation damage is dependent upon age. J Endocrinol. 1989;120:161–165. doi: 10.1677/joe.0.1200161. [DOI] [PubMed] [Google Scholar]
- 2.Meistrich ML, Wilson G, Brown BW, et al. Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing’s and soft tissue sarcomas. Cancer. 1992;70:2703–2712. doi: 10.1002/1097-0142(19921201)70:11<2703::aid-cncr2820701123>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Gandini L, Sgro P, Lombardo F, et al. Effect of chemo- or radiotherapy on sperm parameters of testicular cancer patients. Hum Reprod. 2006;21:2882–2889. doi: 10.1093/humrep/del167. [DOI] [PubMed] [Google Scholar]
- 4.Anserini P, Chiodi S, Spinelli S, et al. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30:447–451. doi: 10.1038/sj.bmt.1703651. [DOI] [PubMed] [Google Scholar]
- 5.Meistrich ML. Quantitative correlation between testicular stem cell survival, sperm production, and fertility in the mouse after treatment with different cytotoxic agents. J Androl. 1982;3:58–68. [Google Scholar]
- 6.Zhang Z, Shao S, Meistrich M. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol. 2007;211:149–158. doi: 10.1002/jcp.20910. [DOI] [PubMed] [Google Scholar]
- 7.Marmor D, Grob-Menendez F, Duyck F, et al. Very late return of spermatogenesis after chlorambucil therapy: Case reports. Fertil Steril. 1992;58:845–846. doi: 10.1016/s0015-0282(16)55342-4. [DOI] [PubMed] [Google Scholar]
- 8.Van Thiel DH, Sherins RJ, Myers GH, et al. Evidence for a specific seminiferous tubular factor affecting follicle-stimulating hormone secretion in man. J Clin Invest. 1972;51:1009–1019. doi: 10.1172/JCI106861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreuser ED, Kurrle E, Hetzel WD, et al. Reversible germ cell toxicity after aggressive chemotherapy in patients with testicular cancer: Results of a prospective study. Klin Wochenschr. 1989;67:367–378. doi: 10.1007/BF01711264. [DOI] [PubMed] [Google Scholar]
- 10.Meistrich ML, Vassilopoulou-Sellin R, Lipshultz LI. Gonadal dysfunction. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 7. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 2560–2574. [Google Scholar]
- 11.Pryzant RM, Meistrich ML, Wilson E, et al. Long-term reduction in sperm count after chemotherapy with and without radiation therapy for non-Hodgkin’s lymphomas. J Clin Oncol. 1993;11:239–247. doi: 10.1200/JCO.1993.11.2.239. [DOI] [PubMed] [Google Scholar]
- 12.Meistrich ML, Chawla SP, da Cunha MF, et al. Recovery of sperm production after chemotherapy for osteosarcoma. Cancer. 1989;63:2115–2123. doi: 10.1002/1097-0142(19890601)63:11<2115::aid-cncr2820631108>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Meistrich ML, Wilson G, Mathur K, et al. Rapid recovery of spermatogenesis after mitoxantrone, vincristine, vinblastine, and prednisone chemotherapy for Hodgkin’s disease. J Clin Oncol. 1997;15:3488–3495. doi: 10.1200/JCO.1997.15.12.3488. [DOI] [PubMed] [Google Scholar]
- 14.Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA. 1988;259:2123–2125. [PubMed] [Google Scholar]
- 15.Jaffe N, Sullivan MP, Ried H, et al. Male reproductive function in long-term survivors of childhood cancer. Med Pediatr Oncol. 1988;16:241–247. doi: 10.1002/mpo.2950160404. [DOI] [PubMed] [Google Scholar]
- 16.Sy Ortin TT, Shostak CA, Donaldson SS. Gonadal status and reproductive function following treatment for Hodgkin’s disease in childhood: The Stanford experience. Int J Radiat Oncol Biol Phys. 1990;19:873–880. doi: 10.1016/0360-3016(90)90007-7. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed SR, Shalet SM, Campbell RHA, et al. Primary gonadal damage following treatment of brain tumors in childhood. J Pediatr. 1983;103:562–565. doi: 10.1016/s0022-3476(83)80584-8. [DOI] [PubMed] [Google Scholar]
- 18.Wallace WHB, Shalet SM, Crowne EC, et al. Gonadal dysfunction due to cisplatinum. Med Pediatr Oncol. 1989;17:409–413. doi: 10.1002/mpo.2950170510. [DOI] [PubMed] [Google Scholar]
- 19.Lendon M, Hann IM, Palmer MK, et al. Testicular histology after combination chemotherapy in childhood for acute lymphoblastic leukaemia. Lancet. 1978;2:439–441. doi: 10.1016/s0140-6736(78)91442-3. [DOI] [PubMed] [Google Scholar]
- 20.Muller J, Skakkebaek NE, Hertz H. Initiation of spermatogenesis during chemotherapy for leukemia. Acta Paediatr Scand. 1985;74:956–960. doi: 10.1111/j.1651-2227.1985.tb10064.x. [DOI] [PubMed] [Google Scholar]
- 21.Byrne J, Mulvihill JJ, Myers MH, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. 1987;317:1315–1321. doi: 10.1056/NEJM198711193172104. [DOI] [PubMed] [Google Scholar]
- 22.Gerres L, Bramswig JH, Schlegel W, et al. The effects of etoposide on testicular function in boys treated for Hodgkin’s disease. Cancer. 1998;83:2217–2222. doi: 10.1002/(sici)1097-0142(19981115)83:10<2217::aid-cncr22>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Witt KL, Bishop JB. Mutagenicity of anticancer drugs in mammalian germ cells. Mutat Res. 1996;355:209–234. doi: 10.1016/0027-5107(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 24.Meistrich ML. Potential genetic risks of using semen collected during chemotherapy. Hum Reprod. 1993;8:8–10. doi: 10.1093/oxfordjournals.humrep.a137880. [DOI] [PubMed] [Google Scholar]
- 25.Senturia YD, Peckham CS, Peckham MJ. Children fathered by men treated for testicular cancer. Lancet. 1985;2:766–769. doi: 10.1016/s0140-6736(85)90640-3. [DOI] [PubMed] [Google Scholar]
- 26.Dodds L, Marrett LD, Tomkins DJ, et al. Case-control study of congenital anomalies in children of cancer patients. Br Med J. 1993;307:164–168. doi: 10.1136/bmj.307.6897.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blatt J. Pregnancy outcome in long-term survivors of childhood cancer. Med Pediatr Oncol. 1999;33:29–33. doi: 10.1002/(sici)1096-911x(199907)33:1<29::aid-mpo6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Meistrich ML, Byrne J. Genetic disease in offspring of long-term survivors of childhood and adolescent cancer treated with potentially mutagenic therapies. Am J Hum Genet. 2002;70:1069–1071. doi: 10.1086/339466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neel JV, Schull WJ, Awa AA, et al. The children of parents exposed to atomic bombs: Estimates of the genetic doubling dose of radiation for humans. Am J Hum Genet. 1990;46:1053–1072. [PMC free article] [PubMed] [Google Scholar]
- 30.Dubrova YE, Grant GR, Chumak AA, et al. Elevated minisatellite mutation rate in the post-Chernobyl families from Ukraine. Am J Hum Genet. 2002;71:801–809. doi: 10.1086/342729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May CA, Tamaki K, Neumann R, et al. Minisatellite mutation frequency in human sperm following radiotherapy. Mutat Res. 2000;453:67–75. doi: 10.1016/s0027-5107(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 32.Zheng N, Monckton DG, Wilson G, et al. Frequency of minisatellite repeat number changes at the MS205 locus in human sperm before and after cancer chemotherapy. Environ Mol Mutagen. 2000;36:134–145. doi: 10.1002/1098-2280(2000)36:2<134::aid-em8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Brandriff BF, Meistrich ML, Gordon LA, et al. Chromosomal damage in sperm of patients surviving Hodgkin’s disease following MOPP therapy with and without radiotherapy. Hum Genet. 1994;93:295–299. doi: 10.1007/BF00212026. [DOI] [PubMed] [Google Scholar]
- 34.Robbins WA, Meistrich ML, Moore D, et al. Chemotherapy induces transient sex chromosomal and autosomal aneuploidy in human sperm. Nat Genet. 1997;16:74–78. doi: 10.1038/ng0597-74. [DOI] [PubMed] [Google Scholar]
- 35.Frias S, Van Hummelen P, Meistrich ML, et al. NOVP chemotherapy for Hodgkin’s disease transiently induces sperm aneuploidies associated with the major clinical aneuploidy syndromes involving chromosomes X, Y, and 18 and 21. Cancer Res. 2003;63:44–51. [PubMed] [Google Scholar]
- 36.Tempest HG, Ko E, Chan P, et al. Sperm aneuploidy frequencies analysed before and after chemotherapy in testicular cancer and Hodgkin’s lymphoma patients. Hum Reprod. 2008;23:251–258. doi: 10.1093/humrep/dem389. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee R, Haines GA, Perera DM, et al. Testicular and sperm DNA damage after treatment with fludarabine for chronic lymphocytic leukaemia. Hum Reprod. 2000;15:762–766. doi: 10.1093/humrep/15.4.762. [DOI] [PubMed] [Google Scholar]
- 38.Thomson AB, Critchley HO, Kelnar CJ, et al. Late reproductive sequelae following treatment of childhood cancer and options for fertility preservation. Best Pract Res Clin Endocrinol Metab. 2002;16:311–334. doi: 10.1053/beem.2002.0200. [DOI] [PubMed] [Google Scholar]
- 39.Stahl O, Eberhard J, Jepson K, et al. Sperm DNA integrity in testicular cancer patients. Hum Reprod. 2006;21:3199–3205. doi: 10.1093/humrep/del292. [DOI] [PubMed] [Google Scholar]
- 40.Spermon JR, Ramos L, Wetzels AM, et al. Sperm integrity pre- and post-chemotherapy in men with testicular germ cell cancer. Hum Reprod. 2006;21:1781–1786. doi: 10.1093/humrep/del084. [DOI] [PubMed] [Google Scholar]
- 41.Meistrich ML, Shetty G. Inhibition of spermatogonial differentiation by testosterone. J Androl. 2003;24:135–148. doi: 10.1002/j.1939-4640.2003.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Shao S, Meistrich ML. Irradiated mouse testes efficiently support spermatogenesis derived from donor germ cells of mice and rats. J Androl. 2006;27:365–375. doi: 10.2164/jandrol.05179. [DOI] [PubMed] [Google Scholar]
- 43.Meistrich ML, Zhang Z, Porter KL, et al. Prevention of adverse effects of cancer treatment on the germline. In: Anderson D, Brinkworth MH, editors. Male-Mediated Developmental Toxicity. Cambridge: Royal Society of Chemistry; 2007. pp. 114–123. [Google Scholar]
- 44.Meistrich M, Shetty G. Hormonal suppression for fertility preservation. Reproduction. 2008 doi: 10.1530/REP-08-0096. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shetty G, Meistrich ML. Hormonal approaches to preservation and restoration of male fertility after cancer treatment. J Natl Cancer Inst Monogr. 2005;34:36–39. doi: 10.1093/jncimonographs/lgi002. [DOI] [PubMed] [Google Scholar]
- 46.Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005;34:51–56. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- 47.Dobrinski I, Ogawa T, Avarbock MR, et al. Effect of the GnRH-agonist leuprolide on colonization of recipient testes by donor spermatogonial stem cells after transplantation in mice. Tissue Cell. 2001;33:200–207. doi: 10.1054/tice.2001.0177. [DOI] [PubMed] [Google Scholar]
- 48.Gordon W, Siegmund K, Stanisic TH, et al. A study of reproductive function in patients with seminoma treated with radiotherapy and orchidectomy: (SWOG-8711) Int J Radiat Oncol Biol Phys. 1997;38:83–94. doi: 10.1016/s0360-3016(97)00235-6. [DOI] [PubMed] [Google Scholar]
- 49.Sanders JE, Hawley J, Levy W, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–3052. [PubMed] [Google Scholar]
- 50.Marina S, Barcelo P. Permanent sterility after immunosuppressive therapy. Int J Androl. 1979;2:6–13. [Google Scholar]
- 51.Buchanan JD, Fairley KF, Barrie JV. Return of spermatogenesis after stopping cyclophosphamide therapy. Lancet. 1975;2:156–157. doi: 10.1016/s0140-6736(75)90059-8. [DOI] [PubMed] [Google Scholar]
- 52.da Cunha MF, Meistrich ML, Fuller LM, et al. Recovery of spermatogenesis after treatment for Hodgkin’s disease: Limiting dose of MOPP chemotherapy. J Clin Oncol. 1984;2:571–577. doi: 10.1200/JCO.1984.2.6.571. [DOI] [PubMed] [Google Scholar]
- 53.Jacob A, Barker H, Goodman A, et al. Recovery of spermatogenesis following bone marrow transplantation. Bone Marrow Transplant. 1998;22:277–279. doi: 10.1038/sj.bmt.1701332. [DOI] [PubMed] [Google Scholar]
- 54.Hansen PV, Trykker H, Helkjaer PE, et al. Testicular function in patients with testicular cancer treated with orchiectomy alone or orchiectomy plus cisplatin-based chemotherapy. J Natl Cancer Inst. 1989;81:1246–1250. doi: 10.1093/jnci/81.16.1246. [DOI] [PubMed] [Google Scholar]
- 55.Longhi A, Macchiagodena M, Vitali G, et al. Fertility in male patients treated with neoadjuvant chemotherapy for osteosarcoma. J Pediatr Hematol Oncol. 2003;25:292–296. doi: 10.1097/00043426-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Lampe H, Horwich A, Norman A, et al. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol. 1997;15:239–245. doi: 10.1200/JCO.1997.15.1.239. [DOI] [PubMed] [Google Scholar]
- 57.Evenson DP, Arlin Z, Welt S, et al. Male reproductive capacity may recover following drug treatment with the L-10 protocol for acute lymphocytic leukemia. Cancer. 1984;53:30–36. doi: 10.1002/1097-0142(19840101)53:1<30::aid-cncr2820530108>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 58.Viviani S, Santoro A, Ragni G, et al. Gonadal toxicity after combination chemotherapy for Hodgkin’s disease. Comparative results of MOPP vs. ABVD. Eur J Cancer Clin Oncol. 1985;21:601–605. doi: 10.1016/0277-5379(85)90088-4. [DOI] [PubMed] [Google Scholar]
- 59.Kreuser ED, Hetzel WD, Heit W, et al. Reproductive and endocrine gonadal functions in adults following multidrug chemotherapy for acute lymphoblastic or undifferentiated leukemia. J Clin Oncol. 1988;6:588–595. doi: 10.1200/JCO.1988.6.4.588. [DOI] [PubMed] [Google Scholar]