Abstract
To determine site and mechanism of suppression, regulatory T cell (Treg) migration and function were investigated in an islet allograft model. Treg first migrated from blood to the inflammed allografts, this depended on CCR2, CCR4, CCR5, and P- and E-selectin ligands, and was essential for suppression of alloimmunity. In the allograft, Treg were activated, upregulated effector molecules, migrated to the draining lymph nodes (dLN) in a CCR2, CCR5, and CCR7 fashion, and this movement was essential for optimal suppression. Treg inhibited dendritic cell migration in a TGFβ and IL-10 dependent fashion; and suppressed antigen specific T effector cell migration, accumulation, and proliferation in dLNs and allografts. These results showed that sequential migration from blood to the target tissue and then to dLNs were required for nTreg to differentiate and execute fully their suppressive function, by inhibiting DC in the peripheral tissue, and T effector cell responses in dLN and allografts.
Introduction
Treg are key for maintenance of immunological homeostasis and self-tolerance (Shevach, 2002). Trafficking and migration to tissues and secondary lymphoid organs are required for Treg function in vivo (Rudensky and Campbell, 2006). CD103 (αEβ7-integrin) has been used to distinguish Treg subsets. Naive CD103- Treg express L-selectin (CD62L) and CCR7 and recirculate through lymphoid tissues. Effector/memory CD103+ Treg express high levels of E- and P-selectin ligands and multiple adhesion molecules, including CD54, ICOS, β1-integrin, and LFA-1 (αLβ2), as well as the inflammatory chemokine receptors CCR2, CCR6 and CXCR3, allowing efficient migration into inflamed tissues (Huehn et al., 2004). Menning et al. found that naive CCR7-/- Treg had impaired migration into LN and reduced suppressive effects. In contrast, under inflammatory conditions, effector/memory CCR7-/- Treg accumulated in inflamed sites and demonstrated enhanced suppression of inflammation (Menning et al., 2007). Thus, by regulating Treg trafficking to both lymphoid and inflamed sites, CCR7 determined in vivo function, underlining the importance of appropriate Treg localization for physiologic suppression.
The question remains how important homing in lymphoid organs versus inflamed tissues is for Treg suppressive function. Our previous work showed that tolerance to cardiac allografts depended on CD62L-mediated LN homing, and generation of alloantigen specific Treg occurred only in the LN, but not in the spleen or grafts (Ochando et al., 2005). In an autoimmune diabetes model, Treg control T cell priming within the LN (Tang et al., 2006). Several recent studies identified Treg within inflamed tissues and transplanted grafts, suggesting these cells control effector T cells in peripheral tissues at sites of ongoing immune responses (Belkaid et al., 2002; Lee et al., 2005; Suffia et al., 2006). Such disparate results may be due to differences in the numbers, activation status and types of Treg. Notably, these studies did not assess Treg trafficking between tissues and dLNs; and it is uncertain whether Treg primarily exert their suppressive activity in lymphoid tissues, peripheral tissues or both, and if there is a relation between the Treg subsets in the two locations.
We investigated both natural Treg (nTreg) and TGFβ induced Treg (TGFβTreg) migration patterns and effector function in an islet allograft model. The results showed that Treg migrate to and display suppressive effector function in both the islet and dLN. Importantly, the results demonstrated a sequential and not a simultaneous migration pattern, such that Treg use P/E-selectin ligands and CCR2, CCR4 and CCR5 to migrate first into the inflamed allograft. Then, Treg use CCR2, CCR5, and CCR7 to migrate from the islet to the dLN. This pattern permitted Treg to differentiate and display optimal suppressive function to inhibit dendritic cell (DC) migration from the islet to the dLN, and then inhibit effector T cell responses in both the islets and the dLN.
Results
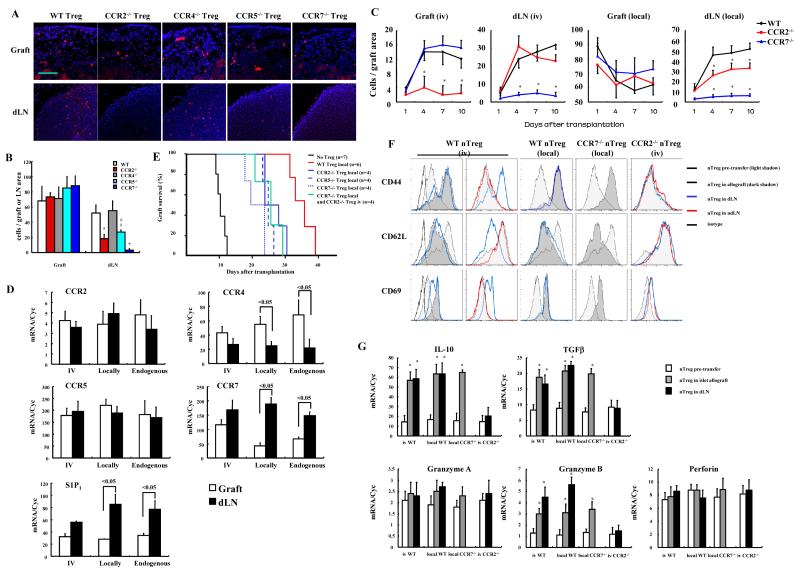
Treg migrate to both islet allografts and secondary lymphoid tissues
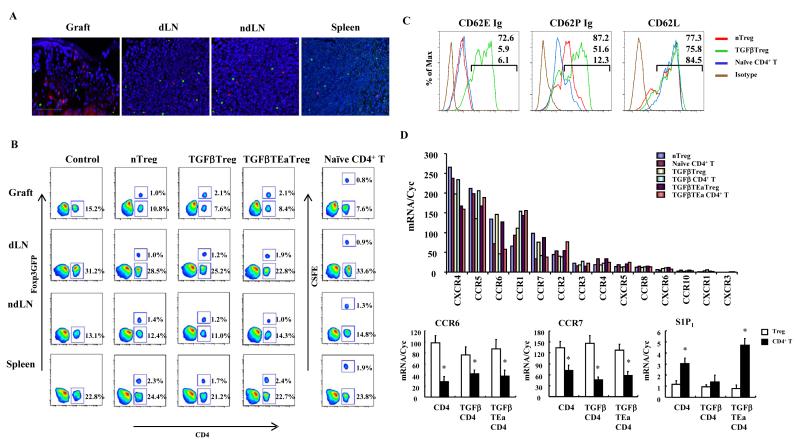
To determine if Treg preferentially migrated to the islet allograft or lymphoid tissues, T cell subsets were isolated, PKH26 labeled, and intravenously adoptively transferred to Foxp3GFP transgenic recipients following islet transplantation underneath the renal capsule. Transferred PKH labeled and endogenous GFP labeled T cells were monitored by fluorescence microscopy. Four days after islet transplantation, transferred nTreg, similar to endogenous nTreg, were widely distributed throughout the islet allograft, the single dLN to the kidney, non-draining LN (ndLN), and spleen (Figures 1A and 1B, Supplementary Figure 1). Natural Treg migrated into allografts at a level similar to naïve CD4+CD25- T cells, which represented 0.79% of islet allograft cells. TGFβTreg induced in vitro were also evaluated, since these are the Treg that are likely to be directly manipulated for therapeutic interventions in humans. The results showed that more adaptive or induced polyclonal TGFβTreg and alloantigen specific TGFβTEaTreg migrated to islet grafts (2.1% and 2.1% of graft cells) (Figure 1B). To investigate these differences in distribution, nTreg and TGFβTreg phenotypes were compared. Flow cytometric analysis showed that both nTreg and TGFβTreg expressed high levels of CD62L (Figure 1C, right). In contrast, E-selectin (CD62E) ligand was very low on naïve T cells and nTreg, while up-regulated on TGFβTreg (Figure 1C, left), likely as a consequence of TGFβ up regulation of fucosyltransferase (Wagers and Kansas, 2000). P-selectin (CD62P) ligand was also up regulated on TGFβTreg, was low on naïve T cells, and was expressed at intermediate levels on some nTreg (Figure 1C, middle). The data suggest that TGFβ induced CD62E/P ligand facilitated migration to the allograft. It should be noted that TGFβTreg were stimulated in vitro with anti-CD3 mAb so that this may also influence selectin expression or migration characteristics. Quantitative RT-PCR for chemokine receptor expression showed that nTreg and TGFβTreg otherwise expressed comparable levels (<2-fold difference) of chemokine receptor mRNA, with preferential transcription of CCR7 and CCR6 by Treg compared to non-Treg. Treg subsets also expressed comparable levels of the sphingosine 1-phosphate receptor 1 (S1P1), which is important for tissue and lymphatic migration 29, but less than non-Treg (Figure 1D). Taken together the data showed that nTreg and TGFβTreg were more similar than not for receptor expression, as least at the mRNA level, and in migration characteristics.
Figure 1. Treg migration to islet allografts and lymphoid tissues.
(A) 1×106 nTreg labeled with PKH26 and intravenously transferred to Foxp3GFP islet allograft recipients at the time of transplantation. Grafts, dLN, peripheral axillary LN, and spleens harvested and sectioned 4 days later. Adoptively transferred Treg (red) and endogenous Treg (green) shown by fluorescent microscopy. Pictures representative of 10 sections/sample from 3 mice. Magnification x100. (B) 1×106 nTreg, TGFβTreg or TGFβTEaTreg from Foxp3GFP mice or CSFE labeled naïve CD4+CD25- T cells intravenously transferred to recipients on the day of transplantation. Four days after transplantation, grafts, dLN, ndLN and spleen were isolated, and T cell migration with pooled samples from 2 mice/group analyzed with flow cytometry. Results are gated on CD3+ cells and representative of 3 independent experiments. (C) Comparison of CD62E ligand, CD62P ligand and CD62L expression on T cell subsets (gated on CD4+Foxp3GFP+ cells). Results representative of 3 independent experiments. Gating on CD4+GFP+or CD4+GFP- population. (D) qRT-PCR analysis of chemokine receptor and S1P1 expression in T cell subsets from Foxp3-GFP mice. Freshly isolated nTreg or naïve CD4+ T cells, or cells stimulated in culture and Treg and non-Treg CD4+ subsets separated by GFP expression. Results representative of 3 independent experiments. (*p<0.05 compared to counterpart Treg group).
Treg migration to islet allografts is required for suppressive effector function
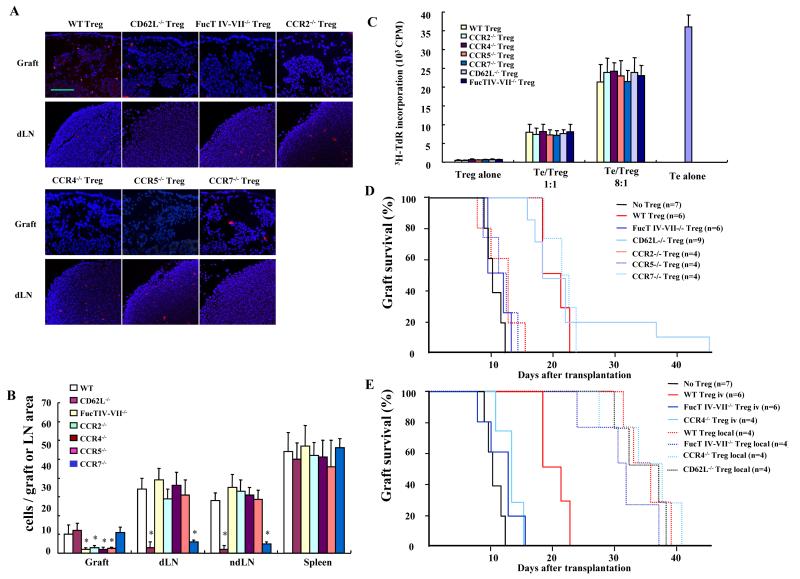
We next determined if migration to islet allografts and/or secondary lymphoid tissues was important for Treg effector function. Natural Treg were isolated from wild type, CD62L-/-, FucT IV-VII-/-, CCR2-/-, CCR4-/-, CCR5-/-, and CCR7-/- mice to compare migration and ability to prolong graft survival. FucT IV-VII deficiency results in a lack of E- and P-selectin ligands on leukocytes (Huang et al., 2000; Maly et al., 1996), required for migration into inflamed tissue. CCR2, CCR4 and CCR5 are inflammatory chemokine receptors (Sallusto et al., 2000) required for migration into inflamed tissues. CD62L and CCR7 are required for migration through HEV into LNs (Forster et al., 1999; Hemmerich et al., 1994). The results showed that intravenously transferred nTreg from FucT IV-VII-/-, CCR2-/-, CCR4-/-or CCR5-/- mice migrated normally to lymphoid tissues but poorly to the inflamed grafts. Conversely, CD62L-/- and CCR7-/- Treg migrated normally to grafts, but poorly to LN (Figures 2A and B).
Figure 2. Treg migration to islet allografts is important for their effector function.
1×106 wild type, CD62L-/-, FucT IV-VII-/-, CCR2-/-, CCR4-/-, CCR5-/- or CCR7-/- nTreg intravenously transferred at the time of transplantation. (A) Treg labeled with PKH26, and grafts and dLN harvested at day 4 and sectioned for fluorescence microscopy. Pictures representative of 12 sections/sample from 3 mice/group. Magnification x100. (B) PKH26 stained Treg were counted in the whole graft area or dLN area in 12 consecutive sections/sample from 3 mice/group (* p<0.05 compared to wild type control). (C) In vitro suppressive activity of nTreg from the various mouse strains (p>0.05 CCR2-/-, CCR4-/-, CCR5-/-, CCR7-/-, CD62L-/-, and FucT IV-VII-/- vs. wild type Treg). (D) Graft survival after intravenous transfer of 1×106 nTreg from the indicted mouse strains (p>0.05 for FucT IV-VII-/-, CCR2-/-, CCR4-/-, and CCR5-/- groups vs. no Treg group, p<0.05 for wild type, CD62L-/- and CCR7-/- groups vs. no Treg group). (E) 1×106 wild type, CCR4-/-, CD62L-/- or FucT IV-VII-/- nTreg transferred intravenously, or mixed with islets and then transplanted under the kidney capsule, and graft survival determined (p<0.05 for wild type Treg local vs. iv, FucT IV-VII-/- Treg local vs. iv, and CCR4-/- local vs. iv groups). D and E compared the same no, WT, and Fuct IV-VII-/- Treg iv groups. Results are representative of 3 separate preparations of islets and Treg.
Intravenous transfer of 1 × 106 wild type nTreg prolonged graft survival from 11.6 ± 1.1 days to 20.2 ± 1.81 days (p<0.05). Although there was no difference in suppressive activity in vitro among the various gene deficient nTreg (Figure 2C), adoptive transfer of CCR2-/-, CCR5-/- , CCR4-/-, or FucT IV-VII-/- nTreg did not prolong survival (13.8 ± 1.7 days, 12.2 ± 1.6 days, 13.4 ± 1.9 days and 12.6 ± 1.4 days, respectively, p>0.05 vs. control) (Figure 2D). On the other hand, CD62L-/- or CCR7-/- nTreg prolonged survival as well as wild type Treg (24.3 ± 4.6 days, 21.5 ± 2.4 days, respectively, p>0.05 vs. wild type Treg). Furthermore, when wild type nTreg were transferred directly with the islets, graft survival was even greater, suggesting that Treg were required within the allograft for maximal suppressive effect and graft protection. This was supported by the observation that locally transferred nTreg from FucT IV-VII-/- and CCR4-/- prolonged survival as well as wild type Treg (32.6 ± 5.9 days, 36.7 ± 2.4 days vs. 35.6 ± 4.6 days, respectively, p>0.05) (Figure 2E). Together, these findings showed that migration to the islet was essential to prolong graft survival, while migration only from the blood to the LN via the HEV was not required.
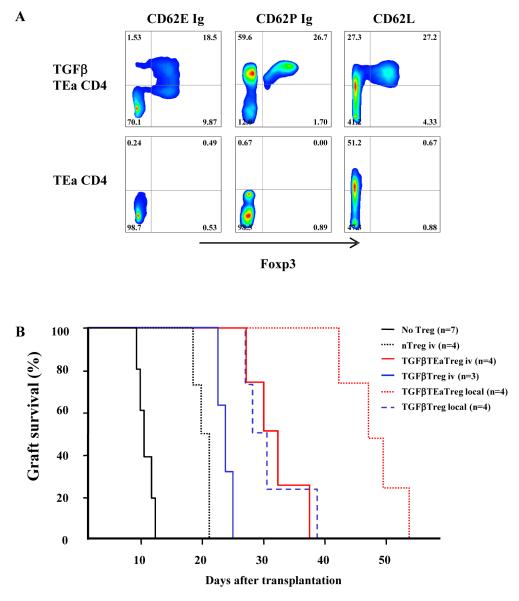
To further pinpoint the anatomic specificity of Treg we took advantage of the observation above that TGFβTreg migrated preferentially to the graft (Figure 1B, Supplementary Figure 1). To pinpoint the antigenic specificity of Treg, I-Ed alloantigen specific TEa TCR transgenic mice were crossed with Foxp3GFP mice. Since the TEa TCR transgene results in the development of extremely small numbers of nTreg (Ochando et al., 2006), TGFβTEaTreg were generated in culture and sorted for Foxp3GFP+ cells. As expected, TGFβTEaTreg had higher CD62E and CD62P ligands than CD4+Foxp3GFP- T cells stimulated without TGFβ (Figure 3A). Intravenously transferred polyclonal TGFβTreg prolonged graft survival slightly longer than wild type nTreg (p<0.05), which correlated with up-regulation of CD62E ligand and increased migration to the allograft (Figures 1B and 1C, Supplementary Figure 1). The TGFβTEaTreg induced even more prolonged survival (36.5 ±7.6 days) (p<0.05 vs. TGFβTreg groups), and local transfer induced even longer prolongation (48 ± 5.2 days) (Figure 3B). Together, these results confirmed that nTreg, polyclonal adaptive TGFβTreg, and alloantigen specific adaptive TGFβTEaTreg all were required within the allograft to display in vivo suppression and increased delivery to the graft correlated with better graft survival.
Figure 3. TGFβTEaTreg express CD62E ligand and are more potent suppressors for islet allograft survival.
(A) CD4+ T cells from TEaFoxp3GFP double transgenic mice cultured with or without TGFβ for 5 days, then stained for CD62E ligand, CD62P ligand and CD62L. Results are representative of 4 independent experiments. Gating on CD4+ cells. (B) 1×106 TGFβTreg from Foxp3GFP or TEaFoxp3GFP transgenic mice transferred intravenously into recipients, or mixed with islets and then transplanted under the kidney capsule, and graft survival determined (p<0.05 for TGFβTEaTreg iv vs. TGFβTreg iv group, and TGFβTEaTreg local vs. TGFβTreg local group). Results are representative of 3 separate preparations of islets and Treg.
Treg migration from islet graft to dLN is required for optimal Treg function
During these studies, we observed that locally and intravenously transferred wild type nTreg both migrated to the cortex of the dLN, with increased numbers of Treg in the dLN after local transfer (8.5%) compared to intravenous transfer (1.9%) (vide infra, Figure 5B). This indicated that locally transferred Treg migrated from the graft to the afferent lymphatics of the renal subcasular space and then into the dLN. Thus, in addition to suppressing immunity in the inflamed graft, another potential mechanism for increased graft survival was the suppressive effects of Treg migrating to dLN. To explore this further, nTreg from wild type, CCR2-/-, CCR4-/-, CCR5-/- or CCR7-/- mice were labeled with PKH26 and locally transferred with islets. Figures 4A and B show that CCR7-/- nTreg were not detected in the dLN, confirming that Treg entry into afferent lymphatic vessels and migration to the dLN was CCR7 dependent (Bromley et al., 2005; Debes et al., 2005). There were moderately reduced numbers of CCR2-/- and CCR5-/- nTreg in the dLN, suggesting these receptors regulated entry into afferent lymphatics and migration to the dLN. CCR4-/- Treg migrated normally from the islet to the dLN, showing that this receptor did not regulate afferent lymphatic migration. Importantly, kinetic analysis of Treg distribution was most consistent with migration from the graft to the dLN, and these migration characteristics remained stable over time (Figure 4C). The kinetic analysis also demonstrates that there is prompt lymphatic migration from the graft to the dLN, showing that afferent lymphatics are immediately functional after the transplant procedure. These data also show that the Treg are unlikely to be proliferating rapidly since there is not a loss of PKH labeled cells in the graft or dLN between 4-7 days.
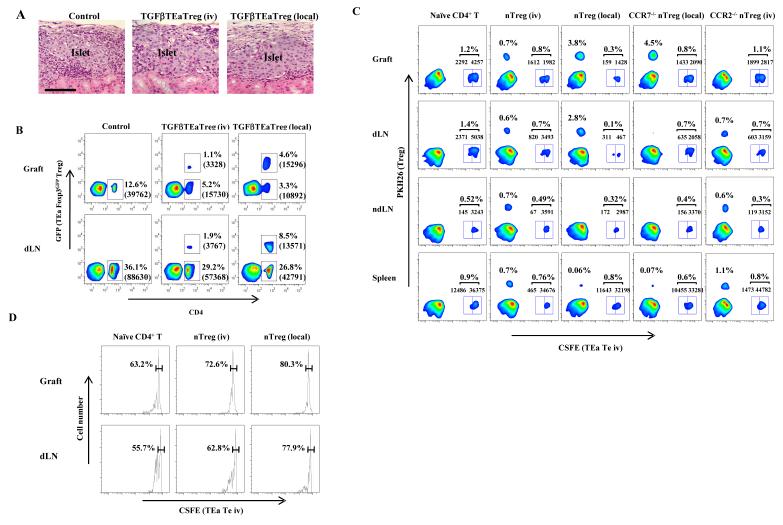
Figure 5. TGFβTEaβTreg preserve islet structure and inhibit effector T cell infiltration of islet allografts.
(A and B) 1×106 TGFβTEaTreg transferred intravenously or locally to grafts. (A) Grafts harvested 7 days after transplantation, processed for H&E staining. Pictures representative of 10 sections/graft from 3 mice/group. Magnification x100. (B) Allografts and dLN harvested 7 days after transplantation dispersed into single cell suspensions and stained with anti-CD4 mAb for flow cytometry. (C and D) 1×106 CSFE labeled naive TEa CD4+ T cells intravenously injected, and 1×106 PKH26 labeled nTreg transferred intravenously or locally into recipients at the time of transplantation. (C) Grafts, dLN, ndLN, and spleens harvested 4 days after transplantation and analyzed with flow cytometry. Cell percentages and numbers shown. (D) Proliferation of CSFE labeled TEa CD4+ T cells analyzed by CSFE dilution; percent undivided cells shown. Samples from 2 mice/group per experiment pooled for analysis. Results representative of 3 independent experiments in (B-D).
Figure 4. Treg migration from islet allografts to dLN is required for optimal Treg function.
1×106 wild type, CCR2-/-, CCR4-/-, CCR5-/- or CCR7-/- nTreg transferred with grafts under the kidney capsule. (A) Treg labeled with PKH26, and grafts and dLN harvested at day 4 and sectioned for fluorescence microscopy. Pictures representative of 12 sections/sample from 3 mice/group. Magnification x100. (B) PKH26 stained Treg counted in whole graft area or dLN area in 12 consecutive sections/sample from 3 mice/group (* p<0.01, # p<0.05 vs wild type). (C) nTreg from wild type, CCR2-/- and CCR7-/- Treg were transferred either intravenously or locally to grafts; and migration to grafts and dLN determined 1, 4, 7 and 10 days after transfer. Treg were counted in the whole graft area or dLN area in 12 consecutive sections/sample from 3 mice/group (* p<0.05 compared to wild type control). (D) 1×106 nTreg transferred intravenously or locally with grafts, and then sorted 4 days after transplantation from 3 pooled grafts or dLNs per experiment. Chemokine receptor and S1P1 expression analyzed by qRT-PCR. Representative of 3 experiments. (E) Graft survival determined after local or combined local plus intravenous transfer of 1×106 nTreg from the indicated mouse strains (p>0.05 for CCR2-/-, CCR5-/-, or combined groups vs. CCR7-/- group, and p<0.05 vs. wild type group). Results representative of 3 separate preparations of islets and Treg. (F and G) Wild type, CCR2-/-, or CCR7-/- nTreg were labeled with PKH26, transferred as indicated into islet allograft recipients, isolated 4 days after transplantation, and analyzed by (F) fluorescent flow cytometry and (G) qRT-PCR (pooled samples from 4 mice/group and gated on CD4+PKH26+ population, * p<0.05 vs nTreg before transfer). Results representative of 2 independent experiments in (E and F).
To further confirm the importance of specific receptors for migration, PKH26 labeled wild type nTreg were transferred intravenously or locally to Foxp3GFP recipients. Both endogenous and adoptively transferred nTreg migrating to allograft and dLN were sorted and compared for chemokine receptor and S1P1 expression by quantitative RT-PCR (Figure 4D). nTreg migrating to islets and dLN expressed similar levels of CCR2 and CCR5, regardless of their site of transfer or origin. CCR4 was expressed preferentially in islet migrating nTreg. CCR7 was expressed preferentially in dLN migrating Treg. These results corroborated the findings in Figures 2 and 4 that CCR2 and CCR5 were important for both islet and dLN migration, CCR4 for islet migration, and CCR7 for dLN migration. S1P1 was preferentially expressed in dLN migrating Treg, consistent with the importance of S1P1 in lymphocyte afferent lymphatic migration and LN retention (Ledegerwood et al., 2008). While it is clear that in our experimental model the adoptive transfer provides more nTreg than are normally endogenously mobilized immediately post-transplant, the results showed that endogenous and adoptively transferred Treg expressed almost identical patterns of receptors and migration, which supports the physiologic validity of our transplant and adoptive transfer model, and argues against the notion that the events observed were artifacts. These observations also support the concept that the therapeutic value of Treg can be harnessed through adoptive transfer.
To test the functional importance of nTreg migration from allograft to dLN, nTreg were transferred locally with islets. Local transfer of wild type nTreg further prolonged islet allograft survival to 35.6 ± 4.6 days (p<0.05 vs. intravenous wild type group). Local transfer of CCR4-/-, FucT IV-VII-/- and CD62L-/- nTreg, which could migrate to dLN, prolonged survival as effectively as wild type cells (Figures 2D and 2E). However, local transfer of CCR2-/-, CCR5-/- or CCR7-/- nTreg did not further prolong allograft survival (Figures 2D and 4E), suggesting that Treg migration from allograft to dLN was important for suppression. To test if sequential versus simultaneous migration of nTreg to the allograft and dLN were required for optimal suppression, CCR7-/- nTreg were transferred locally (and thus were excluded from the dLN) and CCR2-/- nTreg were transferred intravenously (and thus were excluded from the grafts) to the same recipients, so that nTreg would migrate simultaneously but not sequentially to both allograft and dLN. Graft survival was not prolonged beyond CCR7-/- or CCR2-/- transfer alone, and was not as great as local transfer of wild type nTreg alone (Figure 4E), demonstrating that sequential nTreg migration from the allograft to the dLN was required for optimal suppression.
We next investigated why nTreg that migrated from blood to graft and then into the dLN were functionally different from nTreg that entered the dLN directly. Wild type, CCR7-/- and CCR2-/- nTreg were transferred intravenously or locally, so that nTreg that migrated from the allograft via afferent lymphatics to dLN, or from blood across HEV to dLN, could be distinguished. These separate nTreg were compared by flow cytometry and RT-PCR for relevant markers. The results (Figures 4F and 4G) showed that wild type or CCR7-/- nTreg became activated in the allograft, with increased expression of CD44 and CD69, and decreased expression of CD62L. Wild type nTreg that migrated by afferent lymphatics to the dLN had the same activation pattern. In contrast, wild type nTreg that migrated to ndLN or CCR2-/- nTreg that migrated to dLN by HEV were not activated. Activated nTreg up regulated the expression of the effector molecules IL-10, TGFβ, and granzyme B, but not perforin or granzyme A. These results demonstrate that the sequential migration of nTreg from blood to tissue to dLN resulted in differentiation to an activated effector phenotype, correlating with more effective suppression and prolongation of graft survival.
Treg inhibit T cell accumulation in the islet and dLN
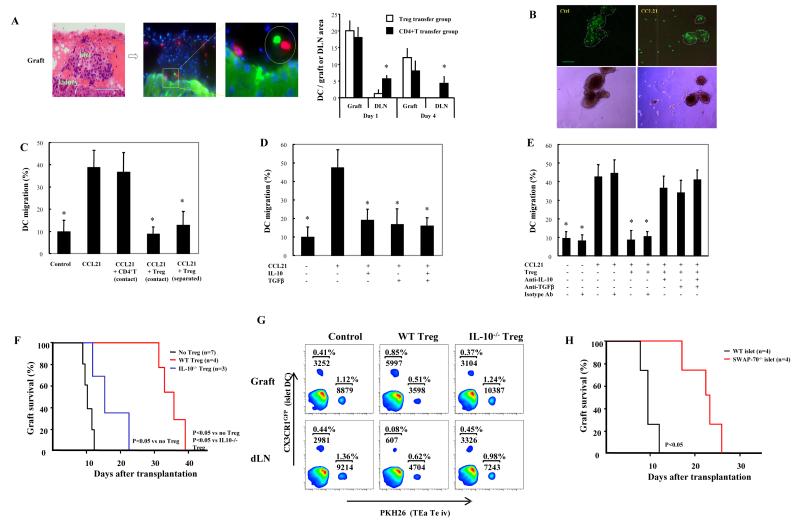
To explore why Treg were required in two sites and how the differentiation and activation of Treg manifested as optimal suppression, histochemical analysis was performed. Islet allografts showed less leukocyte infiltration after transfer of TGFβTEaTreg compared to controls, and local transfer preserved islet structure better than intravenous transfer (Figure 5A). Consistent with the morphological findings, flow cytometric analysis showed that 12.6% of islet cells (39,762 cells) were CD4+ in the control group, 5.2% (15,730 CD4+ T cells) in the TGFβTEaTreg intravenous transfer group, and 3.3% (10,892 CD4+ T cells) in TGFβTEaTreg local transfer group (Figures 1B and 5B, Supplementary Figures 1 and 2). As expected, there was a greater percentage of Treg in the islets of the local transfer group (4.6% or 15,296 cells) compared to the intravenous transfer group (1.1% or 3328 cells).
Draining LN also showed decreased hypertrophy and accumulation of recipient CD4+ T cells after transfer of TGFβTEaTreg (Figures 1B and 5B, Supplementary Figures 1 and 2). Thus, recipient CD4+ T cells in the dLN were 36.1% of the total LN cells (88,630 cells) in controls, 29.2% (57,268 cells) in the intravenous Treg transfer group, and 26.8% (42,791 cells) in the local Treg transfer group (Figure 5B). Treg did not change CD4+ T cell content at distant sites, such as ndLN or spleen (Figure 1B). Together, these results showed that Treg inhibited inflammation at both sites, and that suppressing inflammation at the graft inhibited downstream dLN hypertrophy, likely at least partially by suppressing cytokine and chemokine production in the graft (Chen et al., 2006).
To explore the effect of Treg on antigen specific T cells in the islets and dLNs, wild type nTreg labeled with PKH26 were co-transferred with alloantigen-specific, naïve, TEa CD4+CD25- T cells labeled with CSFE. Intravenously transferred nTreg reduced accumulation of TEa T cells in both allografts (from 1.2% or 6549 islet cells, to 0.8% or 3594 islet cells), and dLN (from 1.4% or 7410 dLN cells, to 0.7% or 4313 dLN cells) (Figure 5C, Supplementary Figure 3). Local transfer of wild type nTreg further reduced the accumulation of TEa CD4+ T cells in both allografts (1587 cells or 0.3% of islet cells) and dLN (778 cells or 0.1% of dLN cells) (Figure 5C, Supplementary Figure 3). Transferred nTreg also reduced the proliferation of TEa CD4+ T cells in both allografts and dLN (Figure 5D). Together, these data showed that Treg inhibited accumulation of specific effector T cells in islets and the dLN, likely through effects on migration, proliferation, and/or survival.
To explore immunosuppressive effects at specific sites, receptor deficient nTreg were transferred. Local transfer of CCR7-/- nTreg resulted in large numbers of Treg retained within the graft, but none detected in the dLN (Figures 4A and 5C, Supplementary Figure 3). Even though CCR7-/- nTreg were retained in the graft, they were far less effective than wild type Treg in inhibiting TEa CD4+ T cell migration into both islets and dLN (Figure 5C, Supplementary Figure 3) or at prolonging survival (Figure 4E), although the CCR7+ nTreg were intrinsically suppressive (Figures 2D and 4E). This shows that large numbers of nTreg within the graft were not sufficient for optimal immunosuppression and survival, and in particular their presence did not fully inhibit effector T cell migration into the graft. Intravenously transferred CCR2-/- nTreg, which migrated to dLN but not to islets (Figure 2B, Supplementary Figure 3), were also less effective than wild type nTreg in inhibiting effector T cell migration into islets (Figure 5C, Supplementary Figure 3). This showed that Treg located only in grafts or only in dLN were not sufficient for full suppressive effects for prolonging survival or for inhibiting effector T cell migration into grafts or dLN. Together with the results showing activation and up regulated effector molecules (Figures 4F and 4G), these findings demonstrate that Treg must not only be in both sites, but also have migrated sequentially from tissue to dLN to become activated for full suppressive function.
Treg inhibit DC migration
To explore the possibility that Treg also inhibited DC function, CX3CR1GFP mice, in which islet DCs are internally labeled with GFP (Jung et al., 2000), were used as islet donors. After PKH26 labeled TGFβTreg were intravenously transferred, Treg-DC juxtaposition could be visualized in the graft (Figure 6A, left side panels), with decreased numbers of DC found in dLN (Figure 6A, right graph), suggesting direct interactions of Treg and DC inhibited DC migration from the graft to the dLN. An in vitro model was developed to explore further this interaction. Freshly isolated islets from CX3CR1GFP mice were cultured for 24 hours, and migration of the GFP+ DC out of the islets in response to CCL21 was monitored. Thirty-five to 50% of islet DC migrated in response to CCL21, and addition of Treg to the culture inhibited this response (Figures 6B, 6C). If a permeable membrane separated the islets and Treg, DC migration was still inhibited (Figure 6C). As shown above, Treg produce TGFβ and IL-10. Blocking TGFβ and/or IL-10 with specific mAbs restored DC migration; and adding recombinant purified TGFβ or IL-10, without Treg, inhibited DC migration (Figures 6D and 6E). Together, these findings demonstrated that Treg inhibited islet DC migration by secreting TGFβ and IL-10. To prove the significance of IL-10 for Treg function in vivo, nTreg from wild type or IL-10-/- mice were locally transferred to grafts. Local transfer of wild type nTreg prolonged survival, however, IL-10-/- nTreg were significantly impaired in their ability to prolong survival (Figure 6F). To further prove the role of Treg derived IL-10, BALB/c CX3CR1GFP islets were transplanted to C57BL/6 recipients, along with wild type or IL-10-/- nTreg transferred locally, and PKH26 labeled TEa CD4+CD25- effector T cells transferred intravenously. Flow cytometric analysis of grafts and dLN showed that wild type nTreg inhibited graft derived DC migration to dLN, along with inhibiting TEa effector T cell infiltration into both graft and dLN. IL-10-/- nTreg failed to inhibit islet derived DC migration to the dLN, and were less inhibitory for TEa effector T cell migration to both the graft and the dLN (Figure 6G, Supplementary Figure 4). Together, these data showed that Treg secretion of IL-10 in the graft inhibited both donor DC egress from, and recipient T cell entry into, the islets. To further determine the significance of donor-derived DC migration to dLN for graft survival, SWAP-70-/- islets were transplanted. SWAP-70 is involved in actin rearrangement and cell migration (Shinohara et al., 2002; Sivalenka and Jessberger, 2004), and SWAP-70 deficiency results in a profound reduction in DC migration to dLN (Ochando et al., 2006). SWAP-70-/- grafts survived longer than wild type islets (23.6 ± 3.5 days vs 10.6 ± 1.6 days, p<0.05), suggesting that impaired donor-derived DC migration to dLN favored graft survival (Figure 6H).
Figure 6. Treg inhibit the migration of islet DC by secreting TGFβ and IL-10.
(A) PKH26 labeled TGFβTreg were intravenously transferred along with CX3CR1GFP BALB/c islets to C57BL/6 recipient mice. Transferred TGFβaTreg and DC in were monitored 1 and 4 days later by microscopy and cell counting. Treg, red; DC, green. Pictures representative of 12 sections/graft from 2 mice. Magnification x100, left panel; x450, middle and right panels. GFP+ DC in islet grafts or dLN were counted in the whole graft or dLN areas in 12 consecutive sections/sample from 3 mice/group (* p<0.05 compared to CD4+ control group). (B) Islets from CX3CR1GFP mice were cultured with or without CCL21 (0.5 μg/ml) for 24 hours and GFP+ DC were visualized by fluorescent (upper panel) and light microscopy (lower panel). Pictures representative of observations in 9 independent experiments. Magnification x50. (C) CX3CR1GFP islets incubated with the indicated chemokine and T cells (* p<0.05 vs. CCL21 group). (D) CX3CR1GFP islets incubated with the indicated chemokine and cytokines (* p<0.05 vs. CCL21 group). (E) CX3CR1GFP islets incubated with the indicated chemokine, Treg, and mAbs (* p<0.05 vs. CCL21 group). Calculations based on 100 islets/group per experiment, and results representative of 3 independent experiments in (C-E). (F) Graft survival after local transfer of 1 × 106 wild type or IL-10-/- nTreg with islets. Results are representative of 3 separate preparations of islets and Treg. (G) C57BL/6 mice received BALB/c CX3CR1GFP islets, local transfer of wild type or IL-10-/- nTreg, and intravenous transfer of PKH26 labeled naïve TEa CD4+ T cells. Islet DC and TEa CD4+ T cell migration were analyzed by flow cytometry after 4 days. Samples from 2 mice/group per experiment pooled for flow cytometry analysis. Results representative of 3 independent experiments. (H) SWAP-70-/- 129/Sv EMS or 129/Sv EMS islets transplanted to BALB/c recipients. Results are representative of 2 separate preparations of islets and Treg.
Discussion
Natural Treg and TGFβTreg express a panel of trafficking molecules and chemokine receptors orienting their migration to both the inflammatory bed of grafts and dLNs to prolong islet allograft survival. Treg migration to grafts was essential for their in vivo suppressive function. Treg protective effects for graft survival were abrogated if they failed to migrate to the graft due to CCR2, CCR4, CCR5 or P- and E-selectin ligand deficiency, and protection was enhanced when Treg were delivered locally into the grafts. TGFβ induced Treg up-regulated E-selectin ligand expression, and more efficiently migrated to and protected grafts. Strikingly, Treg migration from the graft to the dLN was also required for optimal suppression, and dLN migration depended on CCR2, CCR5, and CCR7. The recent demonstration (Huehn et al., 2004; Kocks et al., 2007) that CCR7 and LN migration were necessary for Treg function are consonant with our findings. In addition, our data showed the important finding that CCR7 was required for afferent lymphatic rather HEV transmigration of Treg into the dLN, in order to prolong graft survival. Some important limitations to our interpretations relate to the model employed here. Adoptive transfer of Treg results in greater numbers than could be mobilized from endogenous sources so that the results may not be entirely reflective of endogenous Treg physiology, although our comparisons of transferred and endogenous Treg demonstrated congruent receptor expression and migration characteristics. Transplantation to the renal subcapsular space exposes a unique set of blood microvascular and lymphatic vessels, so that the choice of chemokines and receptors may vary according to the intrinsic tissue characteristics. Lastly, measurements of chemokine receptors relied on mRNA rather than functional protein, although RT-PCR results were commensurate with the gene deficiency migration studies in vivo.
Our data also demonstrated that nTreg that sequentially migrated from the blood to the islets and then to the dLN acquired an activated phenotype, while those that entered the dLN via HEV did not. This suggests a functionally important differentiative and activation step that accompanies the migration of Treg. This also suggests that nTreg may be efficiently primed or activated at the site of tissue inflammation. In contrast conventional effector T cells are expected to be primed and activated in the dLN. Similarly, our previous results in a cardiac allograft model suggested that adaptive or peripherally induced TGFβTreg are also primed and activated in dLN (Ochando et al., 2005). In the present study, Treg directly suppressed effector T cell migration, proliferation, and accumulation in both the dLN and in the graft; Treg had to migrate to the dLN to optimally suppress effector T cell infiltration of the graft; and inhibition of both dLN and graft infiltration were dependent on Treg production of IL-10. Treg targeted donor-derived islet DC, inhibiting their migration by secreting TGFβ and IL-10. Together, these results suggested that migration to the site of inflammation and then to dLNs were both necessary for Treg to differentiate and execute fully their suppressor function, so that Treg interact with DC in the peripheral tissue, and then target effector T cells in dLN.
Similar to our findings, in a colitis model Treg co-transferred with pathogenic T cells migrated to mesenteric LNs and the inflamed colon, and were located between clusters of CD11c+ cells and pathogenic T cells (Mottet et al., 2003), however, the relationship of the Treg at the two sites to each other was not defined. Evidence from autoimmune and transplantation models suggested that CD62Lhigh and CCR7+ Treg with lymphoid tissue tropism are superior to CD62Llow or CCR7- Treg in preventing autoimmunity or suppressing graft-versus-host disease (Ermann et al., 2005; Szanya et al., 2002; Taylor et al., 2004). Tang et al. demonstrated that antigen specific Treg inhibit effector T cell and DC priming interactions in the cortex of the LN in the NOD model of autoimmune diabetes (Tang et al., 2006). Our study in a cardiac allograft model showed that alloantigen-specific Foxp3+ Treg accumulate in the LNs of tolerant animals, and inhibition of T cell LN migration prevents tolerance (Ochando et al., 2005). Alterative conclusions hinted at the importance of other trafficking events in the function of Treg. Chen et al. showed that islet autoantigen-specific Treg prevent diabetes by inhibiting effector T cell function in the islet (Chen et al., 2005). In the cardiac transplantation model, Lee et al. reported CCR4 dependent recruitment of Treg to the allograft (Lee et al., 2005). The islet transplant model here provided the opportunity to examine specificity, site of action, and changes in the transplanted tissue and dLN arising from the migration and suppressive activity of Treg. Our findings reconcile these other reports by showing that nTreg migrated first to the grafts, and then migrated from the graft to dLN. Importantly, as summarized in Table 1, these two activities were functionally tightly linked, since preventing Treg migration to grafts but not to LNs (as in intravenous transfer of FucT VI-VII-/-, CCR2-/-, CCR4-/- or CCR5-/- Treg) or restraining Treg solely to the graft (as in intravenous transfer of CCR7-/- Treg, or local transfer of CCR2-/-, CCR5-/-, or CCR7-/- Treg) all limited the suppressive capabilities of Treg to prolong survival. Treg migration to the graft alone afforded significant survival advantage, while migration to the dLN via the HEV alone provided no protection. Importantly, the co-transfer of CCR7-/- Treg locally and CCR2-/- Treg intravenously, in which the Treg subsets were constrained to the graft and LN, respectively, demonstrated that simultaneous migration to islets and dLNs was not as effective as sequential migration to promote suppression. Rather it was necessary that Treg first enter the graft and then migrate to the dLN in order to differentiate and acquire the activated phenotype.
Table 1.
Summary of Treg localization after transfer or migration and effect on graft survival
| Treg | Transfer Site |
Migrate to |
Entry to dLN |
Inhibit Infiltration of CD4 T cells |
Graft survival |
|||
|---|---|---|---|---|---|---|---|---|
| Islets | dLN | HEV | Afferent Lymphatic s |
Islets | dLN | |||
| nTreg | iv | + | + | + | + | + | + | + |
| TGFβTreg | iv | ++ | + | + | + | + | + | ++ |
| TGFβTEaTreg | iv | ++ | + | + | + | ++ | ++ | +++ |
| CCR7-/- | iv | + | - | - | - | + | ||
| CD62L-/- | iv | + | - | - | + | + | ||
| CCR4-/- | iv | - | + | + | - | - | ||
| CCR2-/- | iv | - | + | + | - | - | + | - |
| CCR5-/- | iv | - | + | + | - | - | ||
| FucT IV-VII-/- | iv | - | + | + | - | - | ||
| nTreg | islet | ++ | + | - | + | ++ | ++ | +++ |
| TGFβTEaTreg | islet | ++ | + | - | + | ++ | ++ | ++++ |
| FucT IV-VII-/- | islet | ++ | + | - | + | +++ | ||
| CD62L-/- | islet | ++ | + | - | + | +++ | ||
| CCR4-/- | islet | ++ | + | - | + | +++ | ||
| CCR7-/- | islet | ++ | - | - | - | + | + | + |
| CCR2-/- | islet | ++ | ± | - | ± | +/++ | ||
| CCR5-/- | islet | ++ | ± | - | ± | ++ | ||
| CCR7-/- | islet | |||||||
| + | ++ | + | + | - | + | |||
| CCR2-/- | iv | |||||||
It is not certain if Treg have unique migration patterns or mechanisms that differentiate them from non-Treg or conventional T cells. Our study, as well as others on human Treg (Lim et al., 2006), showed that Treg and non-Treg CD4 T cells shared similar chemokine receptor profiles and had similar capacities to migrate to tissues and to LN. In the present study, the data demonstrated that graft infiltrating Treg migrated into dLN, and CCR7 not only regulated Treg migration from blood across HEV and into LN, but also regulated Treg exit from the graft through afferent lymphatics into dLN, in agreement with reports on the role of CCR7 in afferent lymphatic migration of naïve and inflammatory T cells (Bromley et al., 2005; Ledegerwood et al., 2008). CCR2 and CCR5 are major inflammatory chemokine receptors (Schroppel et al., 2004). We found that Treg utilized CCR2 and CCR5 to migrate to grafts, and to migrate from grafts into dLN. This is reminiscent of reports for antigen presenting cells whereby homing into dLN is dependent on CCR2, CCR7, CCR8 and CXCR3 (MartIn-Fontecha et al., 2003; Palframan et al., 2001; Peters et al., 2000; Qu et al., 2004). The grafts produced CCR2 and CCR5 ligands and it is likely these were carried by afferent lymphatics into dLN, creating the gradients necessary for Treg migration (Gretz et al., 2000; Schroppel et al., 2004). Together, these results showed that Treg shared similar chemokine receptors and migration potential with conventional T effector cells, but that Treg utilized migration mechanisms in a unique fashion to traffic from inflamed tissue to dLN, and that some of the same chemokine receptors were involved in both steps of this trafficking pattern. One difference among T cell subsets was higher S1P1 expression by conventional T cells. We recently demonstrated that S1P1 stimulation by S1P, generated in acutely inflamed sites, caused tissue retention of T cells (Ledgerwood at al., 2008). Perhaps the differences in S1P1 expression permit Treg to escape S1P-induced retention and then to egress from peripheral tissue to enter dLN to fulfill their regulatory functions (Sawicka et al., 2005). These findings also suggest that nTreg may be initially stimulated at sites of tissue inflammation, while conventional effector T cells and adaptive TGFβTreg may be initially stimulated in lymphoid tissues.
The cellular and molecular mechanisms by which Treg inhibit immunity and protect tissues are due to many discrete functions. Treg inhibit IFNγ production and CXCR3 expression by diabetogenic T cells (Sarween et al., 2004), preventing a number of downstream mechanisms that contribute to islet destruction (Frigerio et al., 2002; Hill et al., 2003; Schroppel et al., 2005; Schroppel et al., 2004; Zhang et al., 2003). Treg also affect many DC functions, including preventing stable formation of long-lasting conjugates of DC and effector T cells (Tadokoro et al., 2006; Tang et al., 2006), inducing expression of indolamine 2,3-dioxygenase through CTLA4-CD86 interactions (Fallarino et al., 2003; Puccetti and Grohmann, 2007; Waldmann et al., 2006), inducing expression of IL-10 as well as the inhibitory molecule B7-H3 (Mahnke et al., 2007), and decreasing MHC class II-antigen peptide formation and DC maturation (Houot et al., 2006; Mahnke et al., 2007). Our data demonstrated that Treg prevented donor DC migration to the dLN in a TGFβ and IL-10 dependent fashion. The inhibition of donor-derived DC migration to the dLN likely attenuated priming of effector T cells (Fiorina et al., 2007; Tadokoro et al., 2006; Tang et al., 2006). An independent model, the SWAP-70 deficient mouse in which DC migration is also impaired (Ochando et al., 2006), further supports the role of DC migration for graft rejection. It was also possible that retaining DC in the islet facilitated Treg-DC interactions and the generation or activation of additional antigen specific Treg inside the graft (Ochando et al., 2006).
Studies on effector T cell development demonstrated that Treg prevented clonal expansion and differentiation by limiting access to DCs (Fiorina et al., 2007; Tadokoro et al., 2006; Tang et al., 2006). The results here demonstrated that Treg limited effector T cell migration, proliferation, and accumulation within the dLN. This effect was likely multifactorial due to the suppressive effects of Treg on islet chemokine expression, islet donor derived DC migration, and direct effects of Treg on dLN DC antigen presentation and effector T cell responses. An important finding was that locally transferred Treg optimally inhibited effector T cell infiltration of grafts only when the Treg migrated to the dLN. The presence of Treg in the graft alone, such as with CCR7-/- Treg, was not sufficient to prevent effector T cell migration into the graft. This indicated that Treg migration from the graft to the dLN was of functional significance for suppressive effects in the graft. These results suggested that effector T cells are primed in the dLN, exit through efferent lymphatics, and enter the inflammatory islet bed via the microvasular circulation; and that Treg inhibit effector T cell priming or exit from the dLN (Sarween et al., 2004).
Our findings resolve diverse data from various studies addressing the location of Treg for suppression in vivo. Treg have dynamic trafficking properties, homing first to the islet allograft from blood in a selectin and chemokine dependent fashion, and then migrating from the graft to dLN in a chemokine and perhaps S1P1 dependent fashion, acquiring an activated phenotype along the way. Critical targets of Treg included DC, effector T cells, and islet parenchymal cells producing chemokines. The findings suggest that nTreg may be initially stimulated at sites of tissue inflammation, while adaptive TGFβTreg may be initially stimulated in lymphoid tissues. Manipulation of Treg differentiation and dynamic trafficking may be therapeutically beneficial for immunotherapy designed to engage their suppressive function.
Materials and Methods
Animals
C57BL/6 (wild type, CCR2-/-, CCR5-/-, CD62L-/- and IL-10-/-) and BALB/c mice (8-10 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME). CX3CR1GFP (Jung et al., 2000) mice on BALB/c backgrounds were from Dr. D. Littman (New York University). Foxp3GFP (Fontenot et al., 2005) and TEa TCR transgenic (Grubin et al., 1997) mice on a C57BL/6 background were from Dr. A. Rudensky (University of Washington). The TEa TCR transgene recognizes I-Ed peptide presented bu I-Ab. CCR7-/- (Forster et al., 1999), fucosyltransferase (FucT) IV—VII-/- (Maly et al., 1996) (Dr. J. Lowe, Cleveland Clinic), and CCR4-/- (Schuh et al., 2002) mice (Dr. C. Hogaboam, University of Michigan) were all on a C57BL/6 background. SWAP-70-/- 129/Sv EMS and congenic 129/Sv EMS mice have been described (Ochando et al., 2006; Shinohara et al., 2002). All experiments were performed with age- and sex-matched mice in accordance with Institutional Animal Care and Utilization Committee approved protocols.
Reagents
Collagenase P was from Roche Diagnostics (Mannheim, GermanyP. PE-CD3ε (clone 145-2C11), PerCP-Cy5.5-CD4 (clone RM4-5), FITC-CD25 (clone 7D4), PE-Foxp3 (clone 3G3), APC-CD11c (clone HL3), PE-Cy7-CD62L (clone MEL-14), anti-IL-10 (JES5-2A5), and anti-TGFβ (clone 1D11) were from BD Pharmingen (San Diego, CA). A chimeric E-selectin Ig (Huang et al., 2000) was from Dr. J. Lowe. A chimeric P-selectin Ig was from Dr. P.S. Frenette (Mount Sinai). CFSE was from Molecular Probes (Eugene, OR). PKH26 dye was from Sigma-Aldrich (St. Louis, MO). Recombinant murine CCL21 was from R&D Systems (Minneapolis, MN).
Islet isolation and transplantation
BALB/c (H-2d) islets were isolated as previously reported (Zhang et al., 2004). Pancreata were perfused with collagenase P, harvested, digested, purified over a discontinuous Ficoll gradient. 400 freshly isolated islets were transplanted to streptozocin induced diabetic (blood glucose >300 mg/dl) C57BL/6 (H-2b) recipients. Blood glucose < 150 mg/dl after transplantation was considered engraftment, and > 300 mg/dl was considered islet graft rejection.
Islet graft single cell preparations
Recipient mice were sacrificed, islet grafts exposed by partially peeling the capsule from the kidney surface, harvested, digested with collagenase P (1.5mg/ml) at 37°C water bath for 5 minutes, and then gently made into a single cell suspension through a nylon mesh.
Cell staining and flow cytometric analysis
Staining was performed with the specific antibody (1 μg/106 cells) at 4°C for 30 minutes. Cells were analyzed using the FACSCaliber flow cytometer using CELLQuestTM software (BD Biosciences, Mountain View, CA). Data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). To compare Treg in islet grafts, LN or spleen, all single cell suspension were stained and gated on CD3+ cells for further analysis.
Cell purification and primary cell culture
nTreg and TGFβTreg were stimulated, purified, and in vitro suppressive function assayed with MLR as previously described (Fu et al., 2004). To sort nTreg or naïve CD4+ T cells, spleens were harvested from the indicated strains of mice; and red blood cells removed using hypotonic lysis buffer. Splenocytes were enriched for CD4+ T cells using CD4+ negative selection kit (R&D Systems, Minneapolis, MN). Cells were stained with APC anti-mouse CD4, FITC anti-mouse CD25, and PE anti-mouse CD8 for 30 minutes on ice. CD4+CD25+ and CD4+CD25- cells were sorted using FACS Vantage DiVA (BD Bioscience) or MoFlo (DakoCytomation, Fort Collins, CO). Flow cytometry confirmed the purity of sorted cells was greater than 99%, and greater than 98% of sorted CD4+CD25+ cells were CD4+Foxp3+. In experiments with TGFβTreg or TGFβTEaTreg, Foxp3GFP transgenic and TEa/Foxp3GFP double transgenic mouse were used. CD4+Foxp3GFP+ T cells and CD4+Foxp3GFP- T cells were sorted with above procedure, but gated on Foxp3GFP rather than FITC anti-mouse CD25. T cell depleted splenocytes were used as stimulator antigen presenting cells (APC). For stimulation cultures to generate activated T cells or TGFβTreg, purified CD4+Foxp3GFP- wild type or TEa transgenic cells (5×104 cells/well) were cultured with gamma-irradiated (800 Rad) syngeneic APC (5×104 cells/well) in the presence of IL-2 (10 ng/ml), anti-CD3ε mAb (1 μg/ml), with or without TGFβ (5 ng/ml) in a final volume of 200 μl of complete RPMI medium (RPMI-1640 supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin and non-essential amino acids) in U-bottomed 96 well plates (Corning, Inc, Lowell, MA) for 5 days. Cultured cells were then stained with CD4 and re-sorted for CD4+Foxp3GFP+ and CD4+Foxp3GFP- cells.
T cell suppression assay
Subsets of Tregs and CD4+ T cells were sorted as described above and used in a suppression assay. Freshly sorted responder CD4+ CD25- T cells (5×104 cells/well) were cultured in the absence or presence of Treg (responder: suppressor ratio of 1:1 and 8:1) with irradiated (800 Rad) syngeneic T cell depleted splenocytes (5×104 cells/well) and anti-CD3ε mAb (1 μg/ml) for 3 days. Cells were pulsed with 1 μCi[3H]TdR for the last 18 hours of culture. The incorporated [3H]TdR was measured with a Wallac Betaplate Counter (PerkinElmer, Boston, MA).
Treg adoptive transfers
Treg or control cell suspensions were differentially labeled with PKH26 or CFSE, and 1×106 labeled Treg cells in 300 μl of PBS were injected intravenously, or 1×106 Treg were mixed and pelleted with freshly isolated islets, and then transplanted in 20 μl. At the indicated times after adoptive transfer, islet grafts, draining LNs, spleen, and peripheral axillary LN were harvested and single cell suspensions or tissue sections prepared.
Quantitative Real Time-PCR (qRT-PCR)
RNA isolation, reverse transcription to cDNA, PCR conditions, and chemokine receptor and S1P1 primer sequences were previously published (Ledegerwood et al., 2008), with additional primer sequences for IL-10 forward 5′-AGTGCCTGGTTGCTGCTTTACC-3′ and reverse 5′-AGCCGTAGGGTCTTTAGATTTTCAC-3′; TGFβ forward 5′-CTGCTGAGGCTCAAGTTAAAAGTG-3′ and reverse 5′-CAGCCGGTTGCTGAGGTAG-3′; perforin forward 5′-GAGAAGACCTATCAGGACCA-3′ and reverse 5′-AGCCTGTGGTAAGCATG-3′; granzyme A forward 5′-TTTCATCCTGTAATTGGACTAA-3′ and reverse 5′-GCGATCTCCACACTTCTC-3′; granzyme B forward 5′-CCTCCTGCTACTGCTGAC-3′ and reverse 5′-GTCAGCACAAAGTCCTCTC-3′. mRNA expression levels were quantified by real-time PCR using SYBR Green PCR kit (Qiagen) with the LightCycler 2.0 (Roche Diagnostic Corp, Indianapolis, IN). PCR consisted of a 15-minute 95C denaturation step followed by 45 cycles of 15s at 94C, 20s at 56C, 20s at 72C. Normalized values for specific gene mRNA expression were calculated as: 2(Ct of Cyclophilin A — Ct specific gene). Relative fold expression was calculated by dividing the specific gene mRNA expression with mRNA expression in control T cells. Each RNA sample was run in duplicate, and each experimental group consisted of 2-3 individual samples to generate values for statistical analysis.
In vitro islet DC migration assays
CX3CR1GFP islets were incubated in complete RPMI-1640 medium containing 10% FBS (Sigma-Aldrich, St. Louis, MO) in 24 well plates and exposed to CCL21 (0.5 μg/ml) for 24 hours. The numbers of CX3CR1GFP cells that migrated out of islets (mDC) and that were retained inside the islets (rDC) were counted under the fluorescence microscope. Percent migration was calculated as mDC/(mDC+rDC) × 100%.
Immunofluorescent microscopy
Fresh islet grafts, LNs, and spleens were harvested, frozen directly in Optimal Cutting Temperature (OCT) (Sacura Finetek, CA), and stored at -80°C. Sections of 5 μm were cut with a Leica 1900CM cryomicrotome, slides were mounted with Vectashield mounting solution (Vector Laboratories, Burlingame, CA), with or without DAPI. Images were acquired using a Leica DMRA2 fluorescence microscope and a digital Hamamatsu close circuit device camera. Separate images were collected on the CY3, GFP, and DAPI channels, overlaid, and analyzed with Openlab software (Improvision, Lexington, MA).
In vitro transwell migration assays
In vitro migration assays were performed as previously described (Ledegerwood et al., 2008). A total of 3 × 105 CSFE labeled CD4+CD25- T cells in RPMI 1640 containing 0.5% fatty acid-free BSA (Sigma-Aldrich) mixed with PKH26 labeled Treg at different ratios were added in a volume of 100 μl to the upper wells of a 24-well transwell plate with a 5 mm insert (Corning International, Corning, NY). Lower wells contained various concentrations of CCL19 in 600 μl of RPMI 1640/0.5% fatty acid-free BSA. The number of T cells that migrated to the lower well following 4 hours incubation were counted under a fluorescent microscope using a hemocytometer.
Statistics
In vivo migration results represent pooled samples from two to three mice per group per experiment. In vitro migration results represent mean values of triplicate samples. All experiments were performed two to five times. Data are presented as mean ± standard deviation. Comparisons between groups were done by independent sample t-tests or analysis of variance (ANOVA) between groups with Microsoft Excel software. Differences between graft survival times were assessed by Kaplan-Meier survival analysis with StatView software. P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 AI-44929, NIH R01 AI-62765, JDRF 1-2005-16, and the Emerald Foundation (all to JSB); NIH K08 AI071038 (to BS); and the Ministerio de Educación y Ciencia, Spain SAF2007-63579 (to JCO).
Abbreviations
- CCR
CC chemokine receptor
- DC
dendritic cell
- dLN
draining lymph node
- Foxp3
forkhead box P3
- FucT
fucosyltransferase
- GFP
green fluorescent protein
- HEV
high endothelial venule
- ndLN
non-draining lymph node
- nTreg
natural Treg
- qRT-PCR
quantitative RT-PCR
- S1P1
sphingosine 1-phosphate receptor 1
- TCR
T cell receptor
- TGFβ
transforming growth factor β
- TGFβTreg
TGFβ induced Treg
- Treg
regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhang N, Fu S, Schroppel B, Guo Q, Garin A, Lira SA, Bromberg JS. CD4+ CD25+ regulatory T-cells inhibit the islet innate immune response and promote islet engraftment. Diabetes. 2006;55:1011–1021. doi: 10.2337/diabetes.55.04.06.db05-1048. [DOI] [PubMed] [Google Scholar]
- Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, Negrin RS, Fathman CG, Strober S. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Fiorina P, Jurewicz M, Tanaka K, Behazin N, Augello A, Vergani A, Von Adrian U, Smith NR, Sayegh MH, Abdi R. Characterization of donor dendritic cells and enhancement of dendritic cell efflux with CC-chemokine ligand 21: a novel strategy to prolong islet allograft survival. Diabetes. 2007;56:912–920. doi: 10.2337/db06-1445. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, Piali L. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- Fu S, Zhang N, Yopp AC, Chen D, Mao M, Zhang H, Ding Y, Bromberg JS. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and adhesion-blocking monoclonal antibody. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NJ, Van Gunst K, Sarvetnick N. Th1 and Th2 pancreatic inflammation differentially affects homing of islet-reactive CD4 cells in nonobese diabetic mice. J Immunol. 2003;170:1649–1658. doi: 10.4049/jimmunol.170.4.1649. [DOI] [PubMed] [Google Scholar]
- Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–5298. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- Huang MC, Zollner O, Moll T, Maly P, Thall AD, Lowe JB, Vestweber D. P-selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases Fuc-TIV and Fuc-TVII in mouse neutrophils. J Biol Chem. 2000;275:31353–31360. doi: 10.1074/jbc.M005449200. [DOI] [PubMed] [Google Scholar]
- Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007;204:723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledegerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, Peche H, Lira SA, Ding Y, Yang Y, et al. Peripheral tissue T lymphocyte entry into afferent lymphatics is inhibited by sphingosine 1-phosphate receptor S1P1. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol. 2006;177:840–851. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- Mahnke K, Ring S, Johnson TS, Schallenberg S, Schonfeld K, Storn V, Bedke T, Enk AH. Induction of immunosuppressive functions of dendritic cells in vivo by CD4(+)CD25(+) regulatory T cells: Role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37:2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menning A, Hopken UE, Siegmund K, Lipp M, Hamann A, Huehn J. Distinctive role of CCR7 in migration and functional activity of naive- and effector/memory-like Treg subsets. Eur J Immunol. 2007;37:1575–1583. doi: 10.1002/eji.200737201. [DOI] [PubMed] [Google Scholar]
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, Llodra J, Ding Y, Lira SA, Krieger NR, Bromberg JS. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174:6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W, Dupuis M, Charo IF. A mechanism for the impaired IFN-gamma production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J Immunol. 2000;165:7072–7077. doi: 10.4049/jimmunol.165.12.7072. [DOI] [PubMed] [Google Scholar]
- Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J Exp Med. 2006;203:489–492. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- Sawicka E, Dubois G, Jarai G, Edwards M, Thomas M, Nicholls A, Albert R, Newson C, Brinkmann V, Walker C. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J Immunol. 2005;175:7973–7980. doi: 10.4049/jimmunol.175.12.7973. [DOI] [PubMed] [Google Scholar]
- Schroppel B, Zhang N, Chen P, Chen D, Bromberg JS, Murphy B. Role of donor-derived monocyte chemoattractant protein-1 in murine islet transplantation. J Am Soc Nephrol. 2005;16:444–451. doi: 10.1681/ASN.2004090743. [DOI] [PubMed] [Google Scholar]
- Schroppel B, Zhang N, Chen P, Zang W, Chen D, Hudkins KL, Kuziel WA, Sung R, Bromberg JS, Murphy B. Differential expression of chemokines and chemokine receptors in murine islet allografts: the role of CCR2 and CCR5 signaling pathways. J Am Soc Nephrol. 2004;15:1853–1861. doi: 10.1097/01.asn.0000130622.48066.d9. [DOI] [PubMed] [Google Scholar]
- Schuh JM, Power CA, Proudfoot AE, Kunkel SL, Lukacs NW, Hogaboam CM. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4-/- mice. Faseb J. 2002;16:1313–1315. doi: 10.1096/fj.02-0193fje. [DOI] [PubMed] [Google Scholar]
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–763. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- Sivalenka RR, Jessberger R. SWAP-70 regulates c-kit-induced mast cell activation, cell-cell adhesion, and migration. Mol Cell Biol. 2004;24:10277–10288. doi: 10.1128/MCB.24.23.10277-10288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. Journal of Experimental Medicine. 2006;203:505–511. doi: 10.1084/jem.20050783. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, June CH, Serody JS, Blazar BR. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–3812. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Kansas GS. Potent induction of alpha(1,3)-fucosyltransferase VII in activated CD4+ T cells by TGF-beta 1 through a p38 mitogen-activated protein kinase-dependent pathway. J Immunol. 2000;165:5011–5016. doi: 10.4049/jimmunol.165.9.5011. [DOI] [PubMed] [Google Scholar]
- Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006;212:301–313. doi: 10.1111/j.0105-2896.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, Zhang H, Song K, Meseck M, Bromberg J, Dong H. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963–970. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- Zhang N, Schroppel B, Chen D, Fu S, Hudkins KL, Zhang H, Murphy BM, Sung RS, Bromberg JS. Adenovirus transduction induces expression of multiple chemokines and chemokine receptors in murine beta cells and pancreatic islets. Am J Transplant. 2003;3:1230–1241. doi: 10.1046/j.1600-6143.2003.00215.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.