Abstract
Recent studies have shown that in the pedunculopontine tegmental nucleus (PPT), increased neuronal activity and kainate receptor-mediated activation of intracellular protein kinase A (PKA) are important physiological and molecular steps for the generation of REM sleep. In the present study performed on rats, phosphorylated cAMP response element-binding protein (pCREB) immunostaining was used as a marker for increased intracellular PKA activation and as a reflection of increased neuronal activity. To identify whether activated cells were either cholinergic or noncholinergic, the PPT and laterodorsal tegmental nucleus (LDT) cells were immunostained for choline acetyltransferase (ChAT) in combination with pCREB or c-Fos. The results demonstrated that during high REM sleep (HR, ~27%), significantly higher numbers of cells expressed pCREB and c-Fos in the PPT, of which 95% of pCREB-expressing cells were ChAT-positive. With high REM sleep, the numbers of pCREB-positive cells were also significantly higher in the medial pontine reticular formation (mPRF), pontine reticular nucleus oral (PnO), and dorsal subcoeruleus nucleus (SubCD) but very few in the locus coeruleus (LC) and dorsal raphe nucleus (DRN). Conversely, with low REM sleep (LR, ~2%), the numbers of pCREB expressing cells were very few in the PPT, mPRF, PnO, and SubCD but significantly higher in the LC and DRN. The results of regression analyses revealed significant positive relationships between the total percentages of REM sleep and numbers of ChAT+/pCREB+ (Rsqr = 0.98) cells in the PPT and pCREB+ cells in the mPRF (Rsqr = 0.88), PnO (Rsqr = 0.87), and SubCD (Rsqr = 0.84); whereas significantly negative relationships were associated with the pCREB+ cells in the LC (Rsqr = 0.70) and DRN (Rsqr = 0.60). These results provide evidence supporting the hypothesis that during REM sleep, the PPT cholinergic neurons are active, whereas the LC and DRN neurons are inactive. More importantly, the regression analysis indicated that pCREB activation in approximately 98% of PPT cholinergic neurons, was caused by REM sleep. Moreover the results indicate that during REM sleep, PPT intracellular PKA activation and a transcriptional cascade involving pCREB occurs exclusively in the cholinergic neurons.
Keywords: c-Fos, dorsal raphe, Locus coeruleus, neuronal activation, pedunculopontine tegmentum, rat
Early transection and lesion studies have shown that the neuronal substrates involved in the generation of REM sleep are located within the pontomesencephalic tegmentum region (Jouvet, 1972). Since then considerable progress has been made in identifying the specific brain regions and cell types whose activation and/or inactivation are critical for the generation of REM sleep (Vertes, 1984; Pace-Schott and Hobson, 2002; McCarley, 2004; Datta and MacLean, 2007). A combination of pharmacological, physiological, and anatomical studies have suggested that, in the absence of the aminergic influences of the locus coeruleus (LC) and dorsal raphe nuclei (DRN), activation of pedunculopontine tegmental (PPT) cholinergic cells elicits REM sleep (George et al., 1964; McCarley and Hobson, 1975; Lydic et al., 1983, 1991; Baghdoyan et al., 1987; Semba, 1993; Thakkar et al., 1998; Datta and MacLean, 2007). The role of PPT cholinergic cells is also supported by lesion studies (Jones and Webster, 1988; Shouse and Siegel, 1992).
Single cell activity in the PPT of behaving cats and rats have identified several different classes of cells whose firing rates correlate with both wakefulness (W) and REM sleep (El-Mansari et al., 1989; Steriade et al., 1990; Datta and Siwek, 2002). Some of these PPT neurons, called REM-On cells, progressively increase their firing rates as the animal moves from W to slow wave sleep (SWS) and then to REM sleep. Another cell type, Wake-REM-On cells, is tonically active during both wakefulness and REM sleep. Although REM sleep active neurons are suggested to be cholinergic, validation of this suggestion depends on chemical identification of the recorded cells. Previous studies have identified active cells by immunohistochemical examination of c-Fos, the product of the immediate early gene expressed in association with neuronal discharge (Shiromani et al., 1996; Yamuy et al., 1998; Maloney et al., 1999, 2000; Verret et al., 2005). The results of those studies are inconclusive because c-Fos is not a reliable indicator of neuronal activation (Luckman et al., 1994; Guo et al., 2007). In addition, c-Fos expression cannot ultimately discriminate between genomic versus discharge activity in neurons. This may explain why PPT neurons in the cat that are active during carbachol-induced REM sleep had been shown to be cholinergic by one group of researchers (Shiromani et al., 1996) and as noncholinergic by others (Yamuy et al., 1998). Similarly, in the rat, PPT neurons identified to be active during rebound REM sleep immediately after a long exposure to the Flowerpot method of REM sleep deprivation were shown to be cholinergic by one group (Maloney et al., 1999) and as noncholinergic by others (Verret et al., 2005).
The PPT makes up the major aggregation of cholinergic neurons in the brainstem (Mesulam et al. 1983; Jones and Beaudet, 1987; Rye et al. 1987; Vincent and Reiner, 1987). A number of neuroanatomical studies have demonstrated that the PPT also contains some GABAergic and glutamatergic cells (Mugnaini and Oertel. 1985; Kosaka et al. 1988; Clement and Grant, 1990; Lai et al. 1993; Ford et al. 1995; Liu et al. 1995; Torterolo et al. 2001; Wang and Morales, 2009). Over the last ten years, research has demonstrated that the excitatory neurotransmitter glutamate and its kainate-type of receptors are involved in the activation of PPT neurons and the induction of REM sleep (Datta and Siwek, 1997; Datta, 2002; Datta et al. 2002). Recent studies have demonstrated that activation of the cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) signal transduction pathway in the PPT is critical for generation of REM sleep (Datta and Prutzman, 2005; Bandyopadhya et al. 2006; Datta, 2007). Activation of PKA could activate the cAMP response element binding protein (CREB) transcription factor through phosphorylation at Serine 133. Activated CREB (pCREB) is an important molecular substrate regulating the expression of a number of downstream genes (Gonzalez and Montminy, 1989; Borrelli et al., 1992; Nestler and Greengard, 1994; Hagiwara et al., 1996; Saha and Datta, 2005). To date, the involvement of elevated pCREB activity within the PPT during REM sleep has not been evaluated, nor have potential increases in pCREB within specific PPT cholinergic and/or noncholinergic neurons. In the present study, we examined pCREB expression within immunohistochemically identified cholinergic and non-cholinergic PPT cells in association with three different REM sleep conditions (control, low, and high REM sleep) that are produced by our refined selective REM sleep deprivation technique (Datta et al., 2004; Bandyopadhya et al., 2006). Since expression of pCREB is thought of as a reliable indirect indicator of neuronal activation (Moore et al., 1996; Gammie and Nelson, 2001; Saha and Datta, 2005), we also identified REM-on and REM-off neurons in the other areas of the pons thought to be involved in the regulation of REM sleep.
EXPERIMENTAL PROCEDURES
Animals and surgery
Experiments were performed on 21 adult male Wister rats (Charles River, Wilmington, MA) weighing between 200 and 300 gm. The rats were housed individually at 24°C with free access to food and water. Lights were on from 7:00 to 19:00 (light cycle) and off from 19:00 to 7:00 h (dark cycle). The principles for the care and use of laboratory animals in research, as outlined by the National Institute of Health (1996), were strictly followed and approved by the institutional animal care and use committee (Protocol #: AN-14084). All surgical procedures were performed stereotaxically under pentobarbital anesthesia (40 mg/kg, i.p; Abbott Laboratories, Chicago, IL, USA). To record states of sleep and wakefulness, cortical electroencephalogram (EEG), dorsal neck muscle electromyogram (EMG), and hippocampal EEG (to record theta wave) recording electrodes were chronically implanted, as described elsewhere (Datta et al., 2002). All electrodes were secured to the skull with dental acrylic. Electrodes were crimped to mini-connector pins and brought together in a plastic connector. Following completion of the surgical procedure, potential post-operative pain was controlled with buprenorphine (0.05 mg/kg, s.c; Abbott Laboratories, Chicago, USA).
Adaptation recording session
After the post-surgical recovery period of 3-7 days, rats were habituated to the experimenter, the sound-attenuated recording cage (electrically shielded: 2.5 × 1.5 × 1.5 ft), and free-moving polygraphic recording conditions for 7-10 days (Grass model 15 Neurodata Amplifier System with 15A94 EEG/PSG quad amplifier, Grass Instrument Division, Astor-Med Inc., West Warwick, RI, USA). The last day of these adaptation recording sessions was determined when for 3 consecutive days, the day-to-day variation on the percentage of REM sleep was less than 5% of their total amount of REM sleep. All adaptation-recording sessions were performed between 9:00 and 16:00 h, when rats are normally sleeping. During recovery, habituation, and free moving recording conditions (adaptation recording sessions), all rats were housed under the same 12/12 hr light/dark cycle with free access to food and water.
Polygraphic recordings and REM sleep deprivation
To record cortical EEG, EMG and hippocampal EEG in freely moving conditions, each rat's head plug was mated to a male connector that was in turn connected to a commutator. Signals from this commutator were sent to a polygraphic recording system (located in the next room) via its electrode board (located inside the recording chamber). To allow rats to move freely inside the recording cage while maintaining the head plug connection, a counterbalanced connecting cable and a mechanical pulley system (attached to the roof of the recording chamber) were used. In a separate room, polygraphic signs of the rat were continuously observed on a computer monitor to identify ongoing behavioral stages, as described in our earlier publications (Datta et al., 2004; Bandyopadhya et al., 2006).
For the purpose of REM sleep deprivation, the beginning of each REM sleep episode was identified by observation of ongoing polygraphic records. From the room adjacent to the rat, the experimenter pressed a mechanical lever within 2-3 seconds of REM sleep onset, the animal's head was gently lifted (between 1.0 and 1.5 inch), and the animal was awakened. The detailed description and validation of this selective REM sleep deprivation method is described in our earlier publications (Datta et al., 2004; Bandyopadhya et al., 2006). One of the most important advantages of this REM sleep deprivation method is that this method successfully eliminates >75% of REM sleep without significantly reducing SWS (Datta et al., 2004; Bandyopadhya et al., 2006).
Determination of behavioral states
For the purpose of determining possible effects on sleep and wakefulness, polygraphic data were captured on-line in a computer using “Gamma” software (Grass product group, Astro-Med, Inc., West Warwick, RI, USA). From this captured data, three behavioral states (W, SWS, and REM sleep) were distinguished and scored visually using “Rodent Sleep Stager” software (Grass product group, Astro-Med, Inc., West Warwick, RI, USA). These three states were scored in successive 5-s epochs. The physiological criteria for the identification of these wake-sleep states were described in detail in our earlier publications (Datta et al., 2002, 2004). This epoch length allowed us to quantify the short periods of REM sleep (3-5 sec) in the rats where REM sleep was selectively terminated (Datta et al., 2004; Bandyopadhya et al., 2006).
Experimental design
On the day after the last adaptation recording session, when day-to-day variation on the total amount of REM sleep stabilized, rats underwent a session of baseline sleep-wakefulness recordings (between 9:00 and 14:00 h). The following day, the rats were subjected to an experimental recording session in which each animal was connected to the polygraphic recording system at 8:55 h. In the next room, polygraphic signs of the sleep-wake cycle were continuously observed on a computer monitor (between 9:00 and 14:00 h). At this point, the 21 rats were randomly divided into three groups. Group 1 (n = 7 rats): These rats were recorded for 5 hr (between 9:00 and 14:00 h) of undisturbed sleep-wakefulness (hereafter group 1 is labeled as “control/baseline REM sleep”; “CR”). Group 2 (n = 7 rats): The experimental protocol for these animals was almost identical to the protocol described above for “CR” group, except that for group 2 animals, from 12:00 to 14:00 h REM sleep episodes were selectively terminated at the beginning (within 3-5 sec) of each episode while the animals were connected to the polygraphic recording system (hereafter group 2 is labeled as “Low REM sleep”; “LR”). Group 3 (n = 7 rats): In this group of animals, REM sleep episodes were selectively terminated between 9:00 and 12:00 h and after which they were allowed to have undisturbed sleep-wake (hereafter group 3 is labeled as “High REM sleep”; “HR”). In summary, the main difference between these three groups of animals is the amount of REM sleep present during the last two hours of recording (between 12:00 and 14:00 h). The “CR” group had normal amounts of REM sleep. The “LR” group had about 82.8% less REM sleep than the “CR” group. The “HR” group had 130.2% more REM sleep then the “CR” group (Table 1). The experiments were conducted using three recording chambers and thus running on three animals at one time. At each time, three different rats were from three different groups (CR, LR, and HR).
Table 1.
The total percentage (mean ± SD) of time spent in wakefulness (W), slow-wave sleep (SWS), and rapid eye movement (REM) sleep during baseline and experimental recording sessions for control REM (CR), low REM (LR), and high REM (HR) groups (n = 7 animals/group)
| Day and State | CR (n = 7) | LR (n = 7) | HR (n = 7) | One-Way ANOVA |
|---|---|---|---|---|
| Baseline | ||||
| W | 25.2 ± 5.3 | 22.0 ± 3.9 | 29.2 ± 6.2 | F = 1.287; p = 0.15 |
| SWS | 62.6 ± 4.8 | 66.5 ± 5.1 | 57.4 ± 5.5 | F = 1.497; p = 0.11 |
| REM | 12.2 ± 2.2 | 11.5 ± 2.3 | 13.4 ± 3.4 | F = 0.296; p = 0.96 |
| Experimental | ||||
| W | 23.7 ± 4.0 | 22.8 ± 4.4 | 18.8 ± 4.4 | F = 2.633; p = 0.09 |
| SWS | 64.6 ± 3.4 | 75.4 ± 4.0*** | 54.5 ± 4.2***+ | F = 49.47; p<0.001 |
| REM | 11.6 ± 1.7 | 2.0 ± 1.0*** | 26.7 ± 4.1***+ | F = 155.6; p<0.001 |
Significant differences with respect to CR is indicated by
p<0.001
and with respect to LR by
p<0.001.
Perfusion and fixation
At the end of experimental recording session (at 14:00 h) all rats were transferred to a CO2 chamber and exposed to CO2 for 2 min to make them semi-conscious, as described earlier (Saha and Datta, 2005). These semi-conscious rats were deeply anaesthetized with sodium pentobarbital (70 mg/kg, i.p.) and perfused transcardially with heparinized cold phosphate buffer (0.1 M, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed and post-fixed in 4% buffered paraformaldehyde for 3-4 hours at 4°C. Post-fixed brains were then stored overnight in a solution of 20% sucrose in 0.1 M PBS (cryoprotectant) at 4 °C.
Immunocytochemistry
Coronal sections were cut through the selected portion of the brainstem (from interaural 1.5 mm to interaural -1.0 mm; based on the stereotaxic atlas of Paxinos and Watson, 1998) at 25-μm thickness on a cryostat (Bright Inst., Co., Huntington, UK). Four series of adjacent sections were collected and stored in 0.1 M PBS containing 0.005% sodium azide solution at 4 °C. In each series, adjacent sections were collected at interval of 100 μm. Next; three series of the tissue sections (first, third, and fourth) were processed on a rotary shaker inside a temperature-controlled see through refrigerator (GDM-3, Eastern Baker Supply, Boston, MA). The first series of sections was processed for pCREB expression as described elsewhere (Saha and Datta, 2005). In brief, free floating sections were first rinsed in three changes of PBS (10 min each) then immersed in 0.5% hydrogen peroxide for 30 min at 4 °C to remove endogenous peroxidase and to lyse red blood cells. Sections were then carried through rinses of PBS either three times until small gas bubbles were no longer present. Next, sections were treated with 0.5% Triton X-100 in PBS for 1 h to facilitate antibody penetration. Sections were then incubated for 30 min at 4 °C in a solution of PBS containing 10% normal goat serum and 0.2% Triton X-100 to block non-specific binding and to reduce background staining. The sections were then incubated in the presence of the monoclonal primary antiserum, phosphorylated CREB125-135 (1:5,000 dilution; Upstate Biotechnology, Lake Placid, NY) for 48 hours at 4°C. This primary antiserum solution was made in PBS containing 1.0% normal goat serum and 0.1% Triton X-100. Following incubation in the primary antiserum, sections were rinsed in three changes of PBS (10 min each) and then incubated in the secondary antibody (1:200, biotinylated goat anti-mouse-IgG; Vector Labs, Burlingame, CA) for 3 hours at 4 °C. The secondary antibody solution was made in PBS containing 1% normal goat serum and 0.1% Triton X-100. Following incubation with the secondary antibody, sections were washed in PBS (3 times, 10 min each) and then incubated in PBS containing avidin-biotin-peroxidase complex (1:100, Peroxidase Standard PK-4000, Vectastain ABC kit, Vector Labs, Burlingame, CA) for 1h at 4 °C. The tissue sections were then washed in PBS 3 times for 10 min each time. To visualize the immunoreaction product with light microscopy, free-floating sections were placed in 0.1 M PBS solution, containing 0.025% diaminobenzidine (DAB), 0.06% nickel ammonium sulfate, and 0.0027% hydrogen peroxide, for 4 min. This immunoreaction product is dark purple in color. In these sections, ongoing reaction was then stopped by three rinses in PBS containing 0.1% Triton X-100 and 0.1% sodium azide (PBST-Az). For double immunostaining experiments, some of these free-floating pCREB immunostained sections (between interaural 1.2 mm and 0.0 mm) were then incubated in rabbit anti-choline acetyltransferase (ChAT) antiserum (1:2000; Chemicon International, Temecula, CA) for 3 days at 4 °C. Following incubation in the primary antiserum, sections were rinsed in three changes of PBS (10 min each) and then incubated in the secondary antibody (1:200, biotinylated goat anti-rabbit-IgG; Vector Labs, Burlingame, CA) for 3 hours at 4 °C. Following incubation with the secondary antibody, sections were washed in PBS (3 times, 10 min each) and then incubated in PBS containing avidin-biotin-peroxidase complex (1:100, Peroxidase Standard PK-4000, Vectastain ABC kit, Vector Labs, Burlingame, CA) for 1h at 4 °C. The tissue sections were then washed in PBS 3 times for 10 min each time. To visualize the immunoreaction product with light microscopy, free-floating sections were placed in 0.1 M PBS solution, containing 0.025% diaminobenzidine (DAB), and 0.0027% hydrogen peroxide, for 15 min. This immunoreaction gives a brown color on the cholinergic cells. The sections were then mounted onto gelatin subbed glass slides and allowed to air dry. Slides were then sequenced through a series of graded ethanol followed by three washes in Histoclear (National Diagnostics, Atlanta, GA). From the final Histoclear wash, the sections were coverslipped using Permount (Fisher Scientific, Fair Lawn, NJ). Similarly, free-floating sections between interaural 1.2 mm and interaural 0.0 mm of the third series were double immunostained for c-Fos and ChAT using a protocol as described in another publication (Verret et al., 2005). In brief, free-floating sections were successively incubated in: (i) a rabbit antiserum to c-Fos (1:3000; Ab-5; Oncogene, CA) in PBST-Az for 72 h at 4 °C; (ii) a biotinylated goat antirabbit IgG (1:2000; Vector Laboratories, Burlingame, CA) for 90 min at room temperature; and (iii) an ABC-HRP solution (1:1000; Elite kit, Vector Laboratories) for 90 min at room temperature. The sections were immersed in a 0.05 M Tris-HCL buffer (pH 7.6) containing 0.025% DAB, 0.0027% hydrogen peroxide and 0.06% nickel ammonium sulfate for 4 min at room temperature. The reaction was stopped by three rinses in PBS containing 0.1% Triton X-100 and 0.1% sodium azide (PBST-Az). Free-floating sections were then immunoreacted for the ChAT, as described above. Immunoreaction product for c-Fos was dark purple and ChAT was brown, reflecting nuclear (purple) and cytoplasmic (brown) staining. To confirm the specificity of pCREB immunostaining, some sections of the fourth series were incubated with pCREB antibody, which was pre-absorbed overnight at 4°C with an excess (10 μM) of synthetic phosphopeptide corresponding to residues 125-135 (H-Arg-Glu-IIe-Leu-Ser-Arg-Arg-Pro-pSer-Tyr-Arg-OH) of CREB (Anaspec, San Jose, CA). Immunostaining of these sections was completely abolished when the pCREB antiserum was pre-absorbed with corresponding phosphopeptide. To ensure the absence of nonspecific single or dual immunostaining, controls in the absence of c-Fos and ChAT primary antibodies and in the presence of normal sera were routinely run on the fourth series of sections.
Nissl staining
To delineate architectonic borders, a second series of sections were processed for Nissl staining using thionin as described earlier (Saha and Datta, 2005). Sections mounted on gelatin-coated slides were airdried then defatted by dipping them in a solution containing 100% ethanol and chloroform (in a ratio of 1:1) for one hour. Sections were then re-hydrated through a series of graded alcohols and distilled deionized water, stained in 0.05% thionin for 1.5 min, differentiated through graded alcohols, cleared in Histoclear, and coverslipped using Permount.
Analysis of immunohistochemical data
The sections were microscopically examined (Eclipse E800, Nikon Corp., Tokyo, Japan) and images were captured using a color CCD camera (Optronics, Goleta, CA) using Image-Pro Plus 5.1 imaging software (Media Cybernatics, Inc. Silver Spring, MD). The experimenter, while performing immunohistochemical staining and cell counting, did not have knowledge of the experimental group to which the individual brains belonged. ChAT-immunoreactive cells and c-Fos-immunoreactive nuclei in the PPT and LDT and pCREB-immunoreactive (p-CREB-IR) nuclei in seven other areas of the brain were counted in a box (300 × 300 μm) centrally placed on those selected areas on both sides of the brain in three consecutive sections 100 μm apart (Fig. 1). The results of these six counts per animal were then averaged. For the PPT, ventrolateral periaqueductal gray (vlPAG), lateral pontine tegmentum (LPT), dorsal raphe nucleus (DRN), pontine reticular nucleus oral (PnO), cells were counted at levels corresponding to about ~1.00, ~1.10, and ~1.20 mm anterior to interaural zero in the atlas of Paxinos and Watson (1998). For the laterodorsal tegmental nucleus (LDT) and medial pontine reticular formation (mPRF), cells were counted at levels ~0.10, ~0.20, and ~0.30 mm anterior to interaural zero. For the locus coeruleus nucleus (LC) and dorsal subcoeruleus nucleus (SubCD), cells were counted at levels ~0.6, ~0.7, and ~0.8 mm posterior to interaural zero.
Figure 1.
Photomicrographs of thionin stained coronal sections through the brainstem showing the counting boxes (300 × 300 μm) within areas of interest for counting ChAT-positive, p-CREB-positive, and c-Fos-positive cells. Sampled areas in the boxes are shown at a level corresponding to about 1.20 mm anterior to interaural zero (corresponding to the atlas of Paxinos and Watson, 1998) are: dorsal raphe nucleus (DRN); ventrolateral periaquiductal grey (vlPAG), lateral pontine tegmentum (LPT), pedunculopontine tegmental nucleus (PPT), pontine reticular nucleus oral (PnO). Boxes at 0.26 mm anterior to interaural zero are: laterodorsal tegmental nucleus (LDT), medial pontine reticular formation (mPRF). Boxes at 0.48 mm posterior to interaural zero are: locus coeruleus nucleus (LC) and dorsal part of the subcoeruleus nucleus (SubCD).
Statistical analysis
To determine group effect and individual subject variation, the counts of pCREB-positive (pCREB+), c-Fos+, ChAT+, both pCREB+ and ChAT+ (pCREB+/ChAT+), and both c-Fos+ and ChAT+ (c-Fos+/ChAT+) in each area of interest were first compared across the three different groups (CR, LR, and HR) using one-way ANOVAs. Finally, to determine the individual group difference (CR vs. LR, CR vs HR, and LR vs HR), post hoc comparisons were made using Bonferroni's multiple comparison tests. All of these statistical analyses were performed using Graphpad Prism statistical software (Version 5.0; GraphPad Software, Inc., La Jolla, CA). To determine the effects of REM sleep manipulations on changes in the sleep-wake data, the polygraphic measures of the rats (n = 21) were analyzed to calculate the total percentages of time spent in W, SWS, and REM sleep in three different groups: during the last two hours of the last adaptation recording session (between 12:00 h and 14:00 h) and during the last two hours of the experimental recording session (between 12:00 h and 14:00 h), before they were sacrificed to collect brain tissues. These sleep-wake data were subjected to one-way ANOVAs and post-hoc Bonferroni's multiple comparison tests to compare the total percentages of time spent in W, SWS, and REM sleep among the CR, LR, and HR groups using Graphpad Prism statistical software.
RESULTS
Wake-sleep states
During the last two hours (between 12:00 h and 14:00 h) of baseline recording, the total percentages of time spent in W, SWS, and REM sleep were not significantly different (one-way ANOVA) among CR, LR, and HR groups (Table 1). Thus, during this period of baseline recording, CR, LR, and HR groups of animals were equal in terms of time spent in W, SWS, and REM sleep. The total percentage of REM sleep in the CR group during the last two hours of experimental recording session was comparable with the values of total percentages of REM sleep in the last two hours of baseline recording in the CR, LR, and HR groups (Table 1). Therefore, for the experimental recording session, the total amount of REM sleep in the CR group during the experimental recording session was considered the baseline REM sleep value. A comparison of the total percentages of REM sleep during the last two hours of the experimental recording session was significantly different among the CR, LR, and HR groups (one-way ANOVA). Subsequent post-hoc comparisons between the CR and LR groups on the total percentages of REM sleep during the last two hours of the experimental recording session indicated that the LR group of rats spent significantly less time in REM sleep (82.76% less than in CR; t = 6.84; p<0.001). Thus, the selective REM sleep deprivation protocol in the LR group was very effective in producing the low REM sleep condition (Table 1). Similar post-hoc comparison between the CR and HR groups indicated that during the same period of the experimental recording session, the HR group of rats spent 130.17% more time in REM sleep (Table 1). Therefore, on the experimental recording day, during the last two hours prior to perfusion, three different groups of rats (CR, LR, and HR) had three distinctly different levels of REM sleep, as reported previously (Datta et al., 2004; Bandyopadhya et al., 2006). Importantly, during the experimental recording session, the selective REM sleep deprivation protocol did not change the total percentages of W among the CR, LR, and HR groups (Table 1). During the experimental recording session, the total percentages of SWS were decreased in the HR group and increased in the LR group (Table 1). In summary, the CR group of rats displayed the baseline amount of REM sleep, with the LR group of rats demonstrating a very low amount of REM sleep, and the HR group of rats demonstrating a very high amount of REM sleep.
pCREB and c-Fos expression in the PPT and LDT
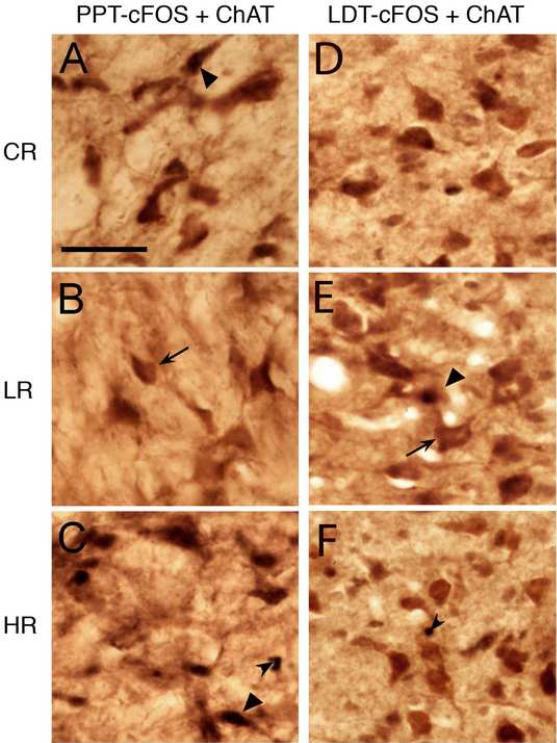
pCREB and c-Fos immunoreactivity was observed in the cholinergic cell compartment of the PPT and LDT and these immunoreactivities were restricted to neuronal nuclei (Figs. 2 and 3). The numbers of both pCREB and c-Fos immunopositive (+) cells in the PPT varied significantly as a function of REM sleep and were significantly greater in the HR group than in the CR and LR groups (Table 2). Compared to the CR group, the number of PPT pCREB+ cells was 77.9% more in the HR group and 72.63% less in the LR group. Similarly, compared to the CR group, the number of c-Fos+ cells was 427.18% more in the HR group and 52.97% less in the LR group (Table 2). These results indicate that the expression of pCREB and c-Fos in the PPT increased with high REM sleep and decreased with low REM sleep. On the contrary, the numbers of pCREB+ and c-Fos+ cells in the LDT were not significantly different among the CR, LR, and HR groups (Table 2), indicating increased or decreased REM sleep did not change the expressions of pCREB or c-Fos in the LDT.
Figure 2.
Photomicrographs showing representative examples of ChAT-positive (ChAT+, arrows), pCREB-positive (pCREB+, small arrow head), and ChAT+ and pCREB+ (ChAT+/pCREB+, large arrow head) cells in the pedunculopontine tegmental nucleus (PPT, A-C) and laterodorsal tegmental nucleus (LDT, D-F) in animal sacrificed after control/baseline REM sleep (CR, A and D), low REM sleep (LR, B and E), and high REM sleep (HR, C and F) conditions. Scale bar in A for A-F = 100 μm.
Figure 3.
Photomicrographs showing representative examples of ChAT-positive (ChAT+, arrows), c-Fos-positive (c-Fos+, small arrow head), and ChAT+ and c-Fos+ (ChAT+/c-Fos+, large arrow head) cells in the pedunculopontine tegmental nucleus (PPT, A-C) and laterodorsal tegmental nucleus (LDT, D-F) in animals sacrificed after control/baseline REM sleep (CR, A and D), low REM sleep (LR, B and E), and high REM sleep (HR, C and F) conditions. Scale bar in A for A-F = 100 μm.
Table 2.
The number (mean ± SEM) of ChAT+, pCREB+, c-Fos+, ChAT+/pCREB+, and ChAT+/c-Fos+ cells in the pedunculopontine tegmentum nucleus (PPT) and laterodorsal tegmental nucleus (LDT) in control REM sleep (CR), low REM sleep (LR), and high REM sleep (HR) groups (n = 7 animals/group)
| Brain Area & I. R. | CR (n =7) | LR (n =7) | HR (n =7) | One-Way ANOVA |
|---|---|---|---|---|
| PPT | ||||
| ChAT+ | 97.86 ± 6.62 | 97.14 ± 5.7 | 103.0 ± 5.9 | F = 0.297; p = 0.76 |
| pCREB+ | 54.29 ± 3.99 | 14.86 ± 1.91*** | 96.57 ± 5.19*** + | F = 107.7; p<0.001 |
| ChAT+/pCREB+ | 38.43 ± 3.02 | 8.43 ± 1.73*** | 92.14 ± 5.22*** + | F = 137.2; p<0.001 |
| PPT | ||||
| ChAT+ | 101.6 ± 5.10 | 104.0 ± 7.50 | 103.9 ± 6.40 | F = 0.045; p = 0.95 |
| c-Fos+ | 12.14 ± 1.24 | 5.71 ± 1.19* | 64.0 ± 3.91*** + | F = 167.9; p<0.001 |
| ChAT+/c-Fos+ | 8.57 ± 1.27 | 2.43 ± 0.43*** | 48.86 ± 4.03*** + | F = 106.0; p<0.001 |
| LDT | ||||
| ChAT+ | 114.1 ± 4.94 | 118.7 ± 6.69 | 119.9 ± 5.36 | F = 0.280; p = 0.76 |
| pCREB+ | 19.43 ± 2.27 | 25.57 ± 3.10 | 22.86 ± 3.35 | F = 0.261; p<0.65 |
| ChAT+/pCREB+ | 10.29 ± 1.17 | 15.43 ± 1.88 | 14.43 ± 1.69 | F = 2.880; p<0.85 |
| LDT | ||||
| ChAT+ | 117.7 ± 5.55 | 118.9 ± 6.25 | 118.3 ± 5.40 | F = 0.009; p = 0.99 |
| c-Fos+ | 10.57 ± 1.13 | 7.25 ± 1.35 | 8.50 ± 0.76 | F = 0.108; p = 0.75 |
| ChAT+/c-Fos+ | 5.29 ± 0.61 | 4.43 ± 0.53 | 4.29 ± 0.52 | F = 0.010; p = 0.81 |
Bilateral cell counts for each nucleus were taken from three adjacent series of sections cut at 25 μm and collected at 100 μm intervals. The total number of labeled (+, immunoreacted, I.R.) cells in each nucleus of each rat was counted in six boxes (300 × 300 μm) placed on the center of the nucleus. Significant differences with respect to CR is indicated by
p<0.05
p<0.001
and with respect to LR by
p<0.001
To determine whether pCREB+ or c-Fos+ cells in the PPT and LDT were of a cholinergic or noncholinergic phenotype, sections containing PPT and LDT were double-immunostained for ChAT (ChAT+/pCREB+ or ChAT+/c-Fos+). The results show that the numbers of ChAT+/pCREB+ cells in the PPT were significantly different (one-way ANOVA; Table 2) among the CR, LR, and HR groups. The results also show that in the CR group, 39.27% of those PPT ChAT+ cells were pCREB+ (ChAT+/pCREB+). Similarly, in the LR and HR groups 8.68% and 89.46% cells of their ChAT+ cells were ChAT+/pCREB+ (Table 2). Despite these group differences in the number of ChAT+/pCREB+ cells, the numbers of ChAT+ cells in the PPT and LDT were not significantly different among the CR, LR, and HR groups (one-way ANOVA; Table 2). Another interesting aspect of these results was that in the HR group, 95.41% of pCREB+ cells were ChAT+, but in the CR and LR groups, 70.79% and 56.73% of pCREB+ cells were ChAT+. These results indicated that in the PPT, almost all of those cells that expressed pCREB with increased REM sleep were ChAT+. Similar to ChAT+/pCREB+ cells, ChAT+/c-Fos+ cells in the PPT varied significantly among the CR, LR, and HR groups (one-way ANOVA; Table 2). The results show that compared to the CR condition, the number of ChAT+/c-Fos+ cells in the PPT was much higher in the HR condition and lower in the LR condition (Table 2). These results also show that in the HR condition, 76.34% of the c-Fos+ PPT cells are ChAT+. This result indicated that the majority of those PPT cells that expresses c-Fos with increased REM sleep are of a cholinergic phenotype. To our surprise, unlike in the PPT, the numbers of ChAT+/pCREB+ or ChAT+/c-Fos+ cells in the LDT were not significantly different among the CR, LR, and HR groups (Table 2).
pCREB in the LC, DRN, vlPAG, and LPT
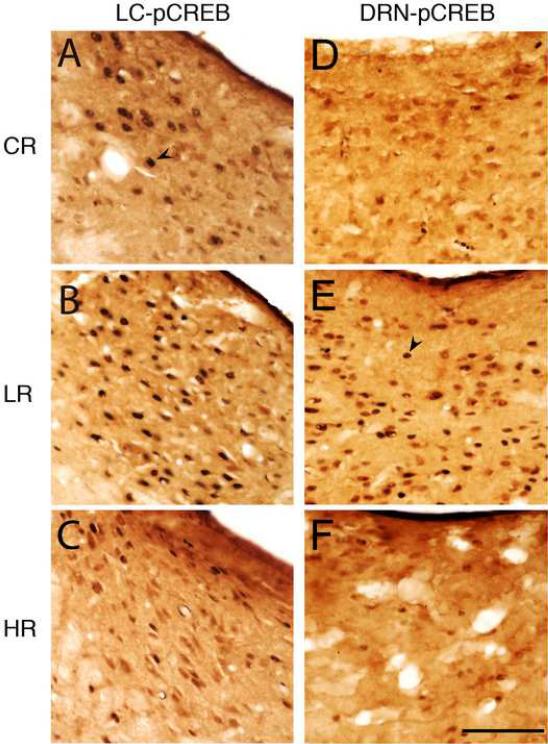
In the past, all single cell-recording studies have demonstrated that REM-off cells are located within the LC and DRN, but some recent immunohistochemical studies have offered contrasting results by suggesting REM-off cells are located mainly within the vlPAG and LPT. As a consequence of this discrepancy, in addition to the LC and DRN, we also examined the changes in pCREB expression in the vlPAG, and LPT as a function of three different REM sleep conditions. The numbers of pCREB+ cells, both in the LC and DRN varied significantly as a function of REM sleep and were significantly greater in the LR group compared to the CR and HR groups (Fig. 4 and Table 3). In the LC, the number of pCREB+ cells in the LR group was 1543.32% more than in the CR group and 2011.67% more than in the HR group (Table 3). Similarly, in the DRN, the numbers of pCREB+ cells in the LR group was 437.40% more than in the CR group and 2176.08% more than in the HR group (Table 3). These results demonstrated that in the LC and DRN, pCREB expression increases with low REM sleep. In contrast, in the vlPAG and LPT, the numbers of pCREB+ cells were not significantly different from the CR and HR groups (Fig. 5 and Table 3). These results indicated that changes in the total percentages of REM sleep does not significantly change the numbers of pCREB+ cells in the vlPAG and LPT.
Figure 4.
Photomicrographs showing representative examples of pCREB-positive (arrow head) cells in the locus coeruleus nucleus (LC, A-C) and dorsal raphe nucleus (DRN, D-F) in animals sacrificed after control/baseline REM sleep (CR, A and D), low REM sleep (LR, B and E), and high REM sleep (HR, C and F) conditions. Scale bar in F for A-F = 100 μm.
Table 3.
The number (mean ± SEM) of pCREB+ cells in the locus coeruleus nucleus (LC), dorsal raphe nucleus (DRN), ventrolateral periaqueductal gray area (vlPAG), lateral pontine tegmentum (LPT), medial pontine reticular formation (mPRF), pontine reticular nucleus oral (PnO), and dorsal subcoeruleus nucleus (SubCD) in control REM sleep (CR), low REM sleep (LR), and high REM sleep (HR) groups (n = 7 rats/group)
| Brain Area | CR (n = 7) | LR (n = 7) | HR (n = 7) | One-Way ANOVA |
|---|---|---|---|---|
| LC | 7.71 ± 1.29 | 126.7 ± 5.39*** | 6.0 ± 1.0+ | F = 727.2; p<0.001 |
| DRN | 33.29 ± 2.94 | 178.9 ± 5.0*** | 7.86 ± 1.26***+ | F = 452.8; p<0.001 |
| VLPAG | 135.6 ± 5.81 | 115.0 ± 8.93 | 133.9 ± 4.84 | F = 0.362; p = 0.70 |
| LPT | 43.14 ± 3.61 | 30.0 ± 4.4 | 36.0 ± 2.50 | F = 2.377; p = 0.12 |
| mPRF | 68.71 ± 2.50 | 18.71 ± 2.0*** | 196.7 ± 6.24***+ | F = 515.4; p<0.001 |
| PnO | 26.29 ± 1.21 | 3.43 ± 0.78*** | 136.9 ± 3.74***+ | F = 949.1; p<0.001 |
| SubCD | 36.19 ± 2.53 | 20.29 ± 1.49** | 150.6 ± 3.62***+ | F = 696.6; p<0.001 |
Bilateral cell counts for each nucleus were taken from three adjacent series of sections cut at 25 μm and collected at 100 μm intervals. The total number of labeled (+, immunoreacted, I.R.) cells in each nucleus of each rat was counted in six boxes (300 × 300 μm) placed on the center of the nucleus. Significant differences with respect to CR is indicated by
p<0.001
and with respect to LR by
p<0.001.
Figure 5.
Photomicrographs showing representative examples of pCREB-positive (arrow head) cells in the lateral pontine tegmental nucleus (LPT) and ventro lateral periaquiductal grey (vlPAG) in animals sacrificed after control/baseline REM sleep (CR, A), low REM sleep (LR, B), and high REM sleep (HR, C). On the right panel, magnified view of pCREB labeled cells in the vlPAG in CR (D), LR (E), and HR (F) conditions. Scale bar in A: for A-C = 500 μm and for D-F = 50 μm.
pCREB expression in the mPRF, PnO, and SubCD
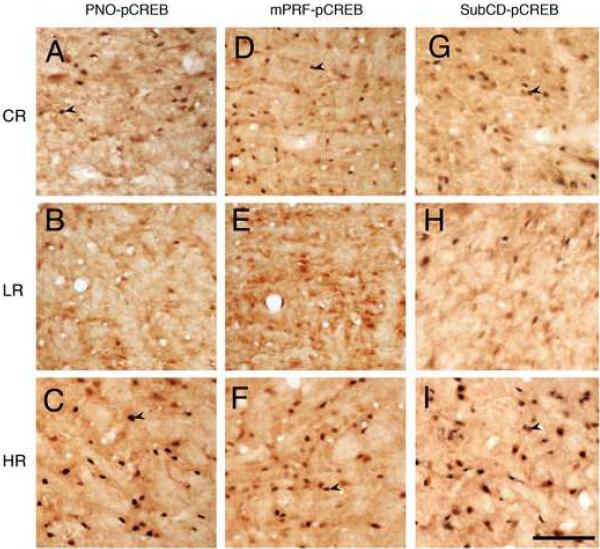
A number of studies using single cell recordings, localized lesion, and pharmacological manipulations have shown that the mPRF, PnO, and SubCD participate positively in the regulation of REM sleep. In this study, we also examined the changes in pCREB expression in the mPRF, PnO, and SubCD as a function of three different conditions of REM sleep (Fig. 6). The results show that the numbers of pCREB+ cells in these regions varied significantly (one-way ANOVAs) as a function of REM sleep conditions (Table 3). The results of individual comparisons with the CR group show that the number of pCREB+ cells in the mPRF was 186.28% greater in the HR group and 72.77% less in the LR group. Similarly, the numbers of pCREB+ cells in the PnO and SubCD, were significantly more in the HR group and less in the LR group compared to the CR group (Table 3). These results suggest that the expression of pCREB in the mPRF, PnO, and SubCD, increased with increased REM sleep and decreased with the reduced REM sleep.
Figure 6.
Photomicrographs showing representative examples of pCREB-positive (arrow head) cells in the pontine reticular nucleus oral (PnO, A-C), medial pontine reticular formation (mPRF, D-F) and dorsal subcoeruleus nucleus (SubCD, G-I) in animals sacrificed after control/baseline REM sleep (CR, A, D and G), low REM sleep (LR, B, E and H), and high REM sleep (HR, C, F and I) conditions. Scale bar in I for A-I = 100 μm.
Relationship between the number of pCREB expressing neurons and amount of REM sleep
The results of this study documented that the number of pCREB+ cells in the HR group increased in the PPT, mPRF, PnO, and SubCD and decreased in the LC and DRN. However, in the LR group, the number of pCREB+ cells decreased in the PPT, mPRF, PnO, and SubCD and increased in the LC and DRN. Based on these results, we expected to see a precise relationship between individual animals total percentages of REM sleep and number of pCREB+ cells in the individual nuclei. The results of these regression analyses revealed significant positive relationships between the total percentages of REM sleep and number of ChAT+/pCREB+ cells in the PPT (DF = 19; Rsqr = 0.980, F = 955.8, p<0.001) but not in the LDT (DF = 19; Rsqr = 0.00003, F = 0.0006, p = 0.980). These results suggest that the increased number of ChAT+/pCREB+ in the PPT was the result of increased REM sleep. Linear regression analysis between total percentages of REM sleep and the number of pCREB+ cells also revealed similar significant positive relationships in the mPRF (DF = 19, Rsqr = 0.879, F = 139.135, p<0.001), PnO (DF = 19, Rsqr = 0.867, F = 123.787, p<0.001), and SubCD (DF = 19, Rsqr = 0.838, F = 97.938, p<0.001). These results indicated that in the mPRF, PnO, and SubCD, increased numbers of pCREB+ cells (88%, 87%, and 84% respectively) were the result of increased REM sleep. Linear regression analysis revealed a significant negative relationship between individual animals total percentage of REM sleep and number of pCREB+ cells in the LC (DF = 19, Rsqr = 0.703, F = 44.995, p<0.001) and DRN (DF = 19, Rsqr = 0.600, F = 28.54, p<0.001). Similar analysis did not reveal any significant relationship between total percentages of REM sleep and number of pCREB+ cells in the vlPAG (DF = 19, Rsqr = 0.029, F = 0.577, p = 0.457) or LPT (DF = 19, Rsqr = 0.036, F = 0.707, p = 0.411). These results suggest that the number of pCREB+ cells in the vlPAG and LPT do not vary with the variation of total percentages of REM sleep.
DISCUSSION
The results of this study showed that high REM sleep increased the number of neurons expressing pCREB in the PPT, mPRF, PnO, and SubCD, whereas the numbers of neurons expressing pCREB decreased in the LC and DRN. In the PPT, 95% of those neurons expressing pCREB in the high REM sleep condition were cholinergic. Low REM sleep decreased the number of neurons expressing pCREB in the PPT, mPRF, PnO, and SubCD but increased pCREB expression in the LC and DRN. Additionally, the numbers of pCREB expressing neurons in the PPT, mPRF, PnO, and SubCD of individual animals were positively correlated with their total percentages of REM sleep. Similarly, the numbers of pCREB expressing neurons in the LC and DRN of individual animals were negatively correlated with their total percentages of REM sleep. The results of this study also demonstrated that the numbers of pCREB expressing neurons in the LDT, vlPAG, and LPT did not vary as a function of REM sleep. These results suggest that for the generation of REM sleep, neuronal activities increase within the PPT, mPRF, PnO, and SubCD, while there is a concomitant decrease within the LC and DRN. These results also suggest that the neuronal activation and/or inactivation in the LDT, vlPAG, and LPT might not be directly involved in the regulation of REM sleep.
Methodological considerations
The major objective of this study was to identify the neurotransmitter phenotype(s) of PPT neurons that increased neuronal activity with naturally increased REM sleep in the freely moving rat. In recent years, a number of studies have used pCREB expression as an immunohistochemical marker for the identification of increased neuronal activity (Gonzalez and Montminy, 1989; Borrelli et al., 1992; Nestler and Greengard, 1994; Hagiwara et al., 1996; Shaywitz and Greenberg, 1999; Lonze and Ginty, 2002; Saha and Datta, 2005; Han et al., 2006; Mamiya et al., 2009). Thus, the present results are interpreted according to the assumption that pCREB expression reflects increased neuronal activity. Another method for studying increased neuronal activity, which permits immunohistochemical identification of the active cells, is by examination of c-Fos expression. Indeed, c-Fos expression has been successfully used to study sleep-dependent neuronal activity changes (Shiromani et al., 1992; Yamuy et al., 1993, 1998; Maloney et al., 1999, 2000; Alam et al., 2005). These studies have also demonstrated that immunohistochemical identification of c-Fos expression combined with immunohistochemical staining for cholinergic, monoaminergic, and GABAergic neurons could be used to identify the neurotransmitter phenotype of neurons that are active during SWS or REM sleep. More importantly, c-Fos activation has been used as a marker for the identification of activated PPT and LDT cells during carbachol microinjection-induced REM sleep in the cat (Shiromani et al., 1992; Yamuy et al., 1998) and in the rebound REM sleep following the flowerpot method of REM sleep deprivation in the rat (Maloney et al., 1999, 2000; Verret et al., 2005). In order to compare our results showing cell groups activated during physiological REM sleep to previous results from carbachol-induced REM sleep and rebound REM sleep following the flowerpot method of REM sleep deprivation, we have also used c-Fos as a marker of neuronal activation for the identification of REM sleep-active PPT and LDT cells. Although c-Fos has been used as a marker of neuronal activation, we acknowledge that there is also a limitation, especially when this marker is used in behaving animals to identify sleep-active cells. It is a well-known fact that depending on the cell types and the duration of excitation, after a critical period, by the process of auto-regulation, c-fos expression disappears even if the cell remains active (Sassone-Corsi et al., 1988; Schonthal et al., 1989). Thus, there is a chance that c-Fos will not be present in some REM sleep-active cells, even if those cells remain active. In addition to auto-regulation, it requires more time to activate c-Fos than to activate pCREB. On the contrary, phosphorylation of CREB is a direct result of calcium signaling which couples membrane depolarization with c-Fos expression (Sheng et al., 1988; Sohm et al., 1999; Grewal et al., 2000). In this respect, CREB activity lies upstream of c-Fos expression, and in this position within the molecular cascade, CREB is closer to the events occurring at the membrane. Thus, it is a potentially more reliable marker of neuronal activity, as well as genomic activity related to the CRE promoter. Therefore, we have used only pCREB immunohistochemistry to identify activated neurons in the mPRF, PnO, SubCD, DRN, LC, vlPAG, and LPT.
In this study, to produce high and low REM sleep conditions, we have used a novel and efficacious selective REM sleep deprivation technique (Datta et al., 2004; Bandyopadhya et al., 2006). Compared with the CR group, the total percentage of REM sleep during the last 2 h of experimental recording session was 82.76% less in the LR group and 130.17% more in the HR group. We believe that the differences observed in the number of neurons expressing pCREB between CR, LR, and HR groups are a result of the differences in the total amount of time spent in REM sleep, based on the following reasons. First, the data showed that the total amounts of W during the last 2 h of each experimental recording session were not significantly different. Second, like our earlier studies (Datta et al., 2004; Bandyopadhya et al., 2006), use of this selective REM sleep-deprivation protocol for a 2 h period did not cause significant changes in motor behavior or decreases in SWS. Finally, diurnal factors seem unlikely to have played a major role in the different levels of pCREB expression in different groups of rats because all 21 rats were sacrificed at a fixed time of the day (2:00 P.M.).
Typically, for this type of immunohistochemical study, two other methods have been used to produce high and low REM sleep conditions. To identify REM sleep-active and/or inactive cells in the brainstem, some studies have used the flowerpot method to deprive REM sleep (Maloney et al., 1999, 2000; Verret et al., 2005). To be exact, adult rats (280-320 gm body weight) were placed on a 6.5 cm (about 2.5 inch) diameter platform, surrounded by cold water, for 48-72 hours. At the end of this exposure to the flowerpot method of REM sleep deprivation, some rats were allowed to rest for 3 hours before they were killed. Those publications reported that during this 3 h period, rats spent 28% - 49% of the time in REM sleep and thus this epoch was considered to be the REM sleep rebound period (Maloney et al., 1999, 2000; Verret et al., 2005). Brainstem sections from their REM sleep deprived and REM sleep rebound rats were then double labeled for c-Fos and ChAT immunoreactivity. Those publications reported that brainstem neurons that expressed c-Fos during the REM sleep rebound period were REM sleep-active neurons. In other rats that were sacrificed immediately at the end of the flowerpot exposure, the c-Fos labeled cells in the brainstem sections were considered as REM-off cells. Since the results of those studies also showed that the use of this flowerpot method for the selective REM sleep deprivation also reduced SWS, it is our opinion that those cells should have been considered as sleep-off cells. Another major interpretative limitation of the above-mentioned studies is that while the rats were on the flowerpot, they were in an immobilized and socially isolated condition for 72 hours (Maloney et al., 1999, 2000; Verret et al., 2005). This is a classic condition of long-term exposure to uncontrollable stress. Indeed, the flowerpot method of REM sleep deprivation has been shown to induce high stress, and the resultant sleep deficit has been shown to be caused by overwhelming nonspecific stress rather than the selective REM sleep deprivation (Vogel, 1975; Rechtschaffen et al., 1999). In the past, REM sleep deprivation for 48-72 hours using the flowerpot method of REM sleep deprivation has also been used successfully as an animal model of post-traumatic stress disorder and anxiety (Silva et al., 2004). It has also been shown that after 48-72 hours of flowerpot exposure, the anxiety effect reaches its maximum level within 3 hours and this effect lasts for at least 24 hours. During this first 3-hours, animals spend most of their time in quiet wakefulness. Since frontal cortical EEG wave amplitudes and frequencies are very similar during quiet wakefulness and REM sleep, and EMG measures of muscle tone in the rat are also very low during quiet wakefulness, it is possible that those studies might have misidentified some quiet wake episodes as REM sleep episodes (Maloney et al., 1999, 2000; Verret et al., 2005). Moreover, the intended REM sleep rebound animals in those studies demonstrated c-Fos labeled cell groups in similar anatomical locations to animals exposed to anxiety provoking stressors (Liu et al., 2003). Based on the studies discussed above, we believe that the changes in c-Fos expression reported in those earlier studies (Maloney et al., 1999, 2000; Verret et al., 2005) were more likely caused by the overwhelming nonspecific stress of the flowerpot method rather than by the specific effects of REM sleep deprivation and/or rebound REM sleep. Verret and colleagues (2005) also reported that in their REM sleep rebound rats, the maximum number of c-Fos labeled cells were located in the periaqueductal grey (PAG). Unlike the observation of Verret and colleagues (2005), in the present study we did not observe any changes in pCREB activation as a function of REM sleep in the PAG. This is not a surprising difference because it is a well-known fact that PAG neurons are activated in response to stress-induced pain (Dostrovsky et al., 1983; Auerbach et al., 1984; Fardin et al., 1984; Bandler and Shipley, 1994; Giola et al., 2005). Thus, this difference further supports our suggestion that the use of flowerpot method for this type of study is problematic and is not appropriate. To identify REM sleep-active cells in the brainstem, some other studies have used the carbachol microinjection-induced high REM sleep condition in the cat (Shiromani et al., 1992; Yamuy et al., 1993). The use of this technique for producing high REM sleep-like condition is better than the use of the flowerpot method and this method has been used successfully to understand the mechanisms of REM sleep regulation (Lydic et al., 1991). However, because carbachol acts directly on target neurons of the cholinergic and aminergic neurons (Greene and Carpenter, 1985; Shiromani and McGinty, 1986; Baghdoyan et al., 1987; Yamamoto et al., 1990a,b), its pharmacological effect would not always depend on increased or decreased activities by the cholinergic or aminergic cells. Another limitation for the use of the carbachol-induced REM sleep method is that this method may not be sensitive enough for the identification of REM-off cells. Therefore, in the current study, for the identification of REM-on and REM-off cells, the use of our selective REM sleep deprivation method was relatively more appropriate than the flowerpot and/or carbachol-induced REM sleep method.
REM sleep and cholinergic cells in the PPT and LDT
The high REM sleep condition resulted in increased numbers of neurons expressing pCREB in the PPT. Of those pCREB expressing PPT neurons, 95.4% were cholinergic. More importantly, numbers of pCREB expressing cholinergic neurons in the PPT of individual animals were positively correlated with their total percentages of REM sleep. Since this is the first study that used pCREB immunohistochemistry to identify sleep-wake active/inactive neurons, the results of this study could not be compared with any other published study. However, these immunohistochemical results are consistent with the results of PPT single cell activity recordings study, where PPT neurons were recorded across the multiple sleep-wake cycles in the freely moving rat (Datta and Siwek, 2002). The result of that single cell recording study has shown that 72.86% of cells within the cholinergic cell compartment of the PPT change their activity patterns consistently in a state-specific manner across multiple sleep-wake cycles. Interestingly, in 100% of those sleep-wake state-dependent cells, the firing rates were very high during REM sleep compared to those firing rates during SWS. Single cell recording studies in the cat also showed that a majority of the PPT cells are more active during REM sleep (Steriade et al., 1990; Thakkar et al., 1998). Collectively, the results of present study and the single cell activity recording study support the hypothesis that the activation of PPT cholinergic cells is involved in REM sleep generation. These results also shed some light on the results of our earlier pharmacological studies (Datta and Prutzman, 2005; Bandyopadhya et al., 2006; Datta, 2007), in which we have shown that activation of the cAMP-PKA signal transduction pathway in the PPT increases REM sleep. Since the downstream transcription pathway for the cAMP-PKA is CREB, it is extremely reasonable to suggest that the cellular target of our earlier pharmacological studies was the cholinergic neurons of the PPT.
The results of this present study also demonstrated that the high REM sleep condition increased the numbers of neurons expressing c-Fos in the PPT, and 76.3% of those c-Fos expressing cells were cholinergic. Previous studies examining c-Fos+ cells reported increases in number of c-Fos+ cells within the PPT in association with the REM sleep recovery period of 1-3 hours after 48-72 hours of sleep deprivation protocol using flowerpot method (Merchant-Nancy et al., 1995; Maloney et al., 1999; Verret et al., 2005). Two of these studies have also examined dual immunostaining for c-Fos and ChAT (Maloney et al., 1999; Verret et al., 2005). The results of these dual immunostaining have shown that those flowerpot method of REM sleep deprivation exposure recovery periods' associated c-Fos expressing cells were both cholinergic and noncholinergic, but the majority of those cells were noncholinergic. As outlined above, we believe that those flowerpot method of REM sleep deprivation exposure recovery periods' associated c-Fos expression in the noncholinergic cells was mainly due to the non-specific stress of the flowerpot method of REM sleep deprivation. Indeed, the results of those studies showed that the numbers of c-Fos expressing cells associated with the period of exposure to flowerpot method of REM sleep deprivation were much higher than those numbers associated with the period of normal REM sleep (Merchant-Nancy et al., 1995; Maloney et al., 1999; Verret et al., 2005), Furthermore, the number of c-Fos expressing cell further increases with increased duration of exposure time to flowerpot method of REM sleep deprivation (Verret et al., 2005). The results of the current REM sleep associated c-Fos expression in PPT cholinergic cells could also be compared with two other studies in the cat (Shiromani et al., 1992; Yamuy et al., 1993). In these two studies, REM sleep-like behavior was induced by microinjection of carbachol into the mPRF. The results of these two studies have shown increases in the number of cholinergic neurons expressing c-Fos during carbachol-induced REM sleep-like behavior. However, the numbers of cholinergic cells in the PPT expressing c-Fos was only a small proportion of the total number of cholinergic cells. It is not surprising that a small proportion of PPT cholinergic cells express c-Fos during the carbachol-induced REM sleep-like period, because carbachol acts directly on target neurons of the PPT cholinergic cells (Greene and Carpenter, 1985; Shiromani and McGinty, 1986; Baghdoyan et al., 1987; Yamamoto et al., 1990a,b), and its pharmacological effect would not depend on increased activity by the PPT cholinergic cells.
In the present study, unlike in PPT cholinergic cells, both in the cholinergic and noncholinergic cells of the LDT, the numbers of cells expressing pCREB or c-Fos did not change with the change in percentages of REM sleep. Contrary to the results of present study, earlier studies have shown that the number of c-Fos expressing cells in the LDT of the rat increase during 48-72 hours of flowerpot exposure and its recovery period, compared to during their normal sleep-wake period (Merchant-Nancy et al., 1995; Maloney et al., 1999; Verret et al., 2005). This increase in c-Fos expressing cells in the LDT is not surprising, because as noted above, the flowerpot method of REM sleep deprivation produces intense stress (Vogel, 1975; Rechtschaffen et al., 1999), and earlier studies have shown that the exposure to an anxiety provoking stressor increases the number of cells expressing c-Fos in the LDT (Liu et al., 2003). Thus, it seems likely that the LDT neurons are not directly involved in the regulation of REM sleep.
REM sleep and pCREB expression in the DRN and LC
In the DRN and LC, low REM sleep resulted in increased numbers of neurons expressing pCREB. The numbers of pCREB expressing neurons in the DRN in the high REM sleep condition was very low compared to control and low REM sleep conditions. These results are consistent with single cell recording studies in the cat and rat. For example, a number of studies have recorded single cell activity patterns of DRN neurons in the cat across sleep-wake cycle and all of those studies have shown DRN neurons remain active throughout the W and SWS period and stop firing as soon as animal enters into REM sleep (Hobson et al., 1975; McCarley and Hobson, 1975; McGinty and Harper, 1976; Trulson and Jacobs, 1979; Lydic et al., 1983). Similarly, a number of single cell recording studies in the rat and cat have shown that most of the neurons of the LC are active during W and SWS but remain silent during REM sleep (Chu and Bloom, 1973; Aston-Jones and Bloom, 1981; Foote et al., 1983). The results of the present study also demonstrated that the numbers of pCREB expressing neurons in the LC and DRN of individual animals are negatively correlated with their total percentages of REM sleep. Collectively, the results of present study and earlier single cell recoding studies could be interpreted as activation of DRN and LC neurons promoting the reduction of REM sleep. Indeed, the local application of a selective 5-HT1A receptor agonist, which suppresses neuronal activity of the DRN neurons (Sprouse and Aghajanian, 1987), increases REM sleep in the cat and rat (Portas et al., 1996). In another study, it has been shown that the localized cooling of the LC induces REM sleep in the behaving cats (Cespuglio et al., 1982). Collectively, these results suggest that the withdrawal of LC and DRN neuronal activity facilitate REM sleep.
REM sleep and pCREB expression in the vlPAG and LPT
One recent study has suggested that the vlPAG and LPT neurons in the rat are of the REM-on type by showing rebound REM sleep increases number of cells expressing c-Fos in the vlPAG and LPT (Verret et al., 2005). One single cell recording study in the PAG of cat had encountered few cells in the vlPAG that showed bursting type of activity during REM sleep, an indication of REM-on type of activity (Thakkar et al., 2002). Consistent with the results of this single cell recording study, during the low REM sleep condition, the numbers of pCREB labeled cells in the vlPAG and LPT were slightly less (see Table 3). On the contrary, another study had shown that lesions of the vlPAG and LPT increase REM sleep in the rat (Lu et al., 2006). Based on the lesion results, it has been proposed that vlPAG and LPT neurons in the rat are REM-off type (Lu et al., 2006). To our surprise, results of the present study did not reveal any significant changes in the number of pCREB expressing cells in the vlPAG or LPT. Given that the vlPAG is involved in the regulation of pain (Reynolds, 1969; Dostrovsky et al., 1983; Fardin et al., 1984; Auerbach et al., 1985), the increased c-Fos activation in rebound REM sleep after flowerpot exposure might have been the effect of a homeostatic phenomenon to attenuate stress and pain caused by the flowerpot method. Since the anticipation of stress and pain reduces REM sleep and since vlPAG and LPT neurons are involved in controlling stress and pain, the decreased REM sleep after vlPAG and LPT lesion might have been caused by the absence of such pain attenuating systems of our brain. Thus, at this time, it is reasonable to suggest that the vlPAG and LPT may not be involved in the regulation of REM sleep.
REM sleep and pCREB expressing cells in the mPRF, PnO, and SubCD
Our results demonstrated that the high REM sleep condition resulted in increased numbers of neurons expressing pCREB in the mPRF, PnO, and SubCD. The results also showed that the numbers of neurons expressing pCREB in the control REM sleep condition are much higher than those numbers in the low REM sleep condition. In addition, numbers of pCREB expressing neurons in the mPRF, PnO, and SubCD of individual animals are positively correlated with their total percentages of REM sleep. These results suggest that the neurons of these areas of the brain are actively involved in the generation of REM sleep and/or REM sleep signs. This suggestion is also supported by a number of other studies in cats and rats. Since there are no clear anatomical or functional demarcation between mPRF and PnO, most of those early sleep studies in cats and rats have rarely differentiated between mPRF and PnO. Therefore, in those publications, some studies have labeled this part of the brain as mPRF and some others as PnO. However, all of those studies have shown that depending on the location within the mPRF/PnO, application of carbachol elicits some or all signs of REM sleep, including cortical activation, muscle atonia, hippocampal theta, rapid eye movements, and ponto-geniculo-occipital waves (George et al., 1964; Mitler and Dement, 1974; Gnadt and Pegram, 1986; Baghdoyan et al., 1987; Yamamoto et al., 1990a,b; Vertes et al., 1993; Garzon et al., 1998; Horner and Kubin, 1999). Single cell recording studies have shown that application of carbachol and acetylcholine excites mPRF/PnO cells (Greene and Carpenter, 1985; Shiromani and McGinty, 1986; Yamamoto et al., 1990b). Single cell recording studies have also shown that the majority of mPRF/PnO cells are more active during natural REM sleep than during SWS and W (Hobson et al., 1975; Shiromani and McGinty, 1986; Ito et al., 2002). Based on the results of these microinjection and single cell recording studies, it is reasonable to suggest that the carbachol microinjection-induced REM sleep-like behaviors were caused by the cholinoceptive activation of those mPRF/PnO cells. Multiple lines of evidence indicate that one of the sources of acetylcholine for these carbachol-induced REM sleep sign generating areas in the mPRF/PnO is the PPT (Shiromani et al., 1988; Quattrochi et al., 1989; Lydic and Baghdoyan, 1993; Semba, 1993; Garcia-Rill et al., 2001). Like the involvement of mPRF and PnO, several other physiological and anatomical studies have demonstrated that the SubCD is also actively involved in REM sleep sign generation (Datta et al., 1998, 1999, 2004, 2008; Mavanji and Datta, 2003; Heister et al., 2007). These studies have shown carbachol microinjection into the SubCD generates the Pontine-wave (P-wave), a cardinal sign of REM sleep in the rat (Datta et al., 1998; Mavanji and Datta, 2003). In the past, we have demonstrated that the localized neurotoxic lesion in the SubCD suppresses REM sleep P-wave activity (Datta et al., 2004, 2008). We have also shown that the P-wave generator in the SubCD receives cholinergic inputs from the PPT (Datta et al., 1999). Collectively, results of the present study and earlier studies, discussed above, suggest that SubCD neurons are actively involved in the REM sleep P-wave sign generation.
CONCLUSION
The current findings provide evidence, for the first time, that the combination of our selective REM sleep deprivation method and examination of pCREB expression is an excellent method for the identification of neurochemical phenotypes of physiological REM sleep associated neurons in the brain. The results of this study suggest that the activation of PPT cholinergic cells and cells in the mPRF, PnO, SubCD are involved in the generation of REM sleep. The present results also indicate that the REM-off characteristics of DRN and LC neurons play an integral role in the generation of REM sleep. These data are also consistent with the view that the simultaneous activation of PPT cholinergic cells and inactivation of DRN and LC cells is the primary cellular mechanism for the generation of REM sleep.
ACKNOWLEDGEMENTS
This study was supported by NIH research grants MH 59839 and NS 34004.
ABBREVIATIONS
- ChAT
choline acetyltransferase
- CR
control/baseline REM sleep condition/group
- DRN
dorsal raphe nucleus
- EEG
electroencephalogram
- EMG
electromyogram
- HR
high REM sleep condition/group
- LC
locus coeruleus nucleus
- LDT
laterodorsal tegmental nucleus
- LPT
lateral pontine tegmentum
- LR
low REM sleep condition/group
- mPRF
medial pontine reticular formation
- PBS
phosphate-buffered saline
- pCREB
phosphorylated cAMP response element-binding protein
- PKA
protein kinase A
- PnO
pontine reticular nucleus oral
- PPT
pedunculopontine tegmental nucleus
- REM
rapid eye movement
- SubCD
dorsal subcoeruleus nucleus
- SWS
slow-wave sleep
- vlPAG
ventrolateral periaqueductal gray
- W
wakefulness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alam MN, Kumar S, Bashir T, Suntsova S, Methippara M, Szymusiak R, McGinty D. Evidence for GABA mediated suppression of c-Fos protein in hypocretin and not MCH neurons during sleep in rats. J Physiol (London) 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach S, Fornal C, Jacobs BL. Response to serotonin-containing neurons in nucleus raphe magnus to morphine, noxious stimuli, and periaqueductal gray stimulation in freely moving cats. Exp Neurol. 1985;88:609–628. doi: 10.1016/0014-4886(85)90075-5. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columner organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bandyopadhya RS, Datta S, Saha S. Activation of pedunculopontine tegmental protein kinase A: a mechanism for rapid eye movement sleep generation in the freely moving rat. J Neurosci. 2006;26:8931–8942. doi: 10.1523/JNEUROSCI.2173-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E, Montmayeur JP, Foulkes NS, Sassone-Corsi P. Signal transduction and gene control: The cAMP pathway. Crit Rev Oncog. 1992;3:321–338. [PubMed] [Google Scholar]
- Cespuglio R, Gomez ME, Faradji H, Jouvet M. Alterations in the sleep-waking cycle induced by cooling of the locus coeruleus area. Electroenceph Clinical Neurophysiol. 1982;54:570–578. doi: 10.1016/0013-4694(82)90042-6. [DOI] [PubMed] [Google Scholar]
- Chu NS, Bloom FE. Norepinephrine-containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science. 1973;179:908–910. doi: 10.1126/science.179.4076.908. [DOI] [PubMed] [Google Scholar]
- Clements JR, Grant S. Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci Lett. 1990;120:70–73. doi: 10.1016/0304-3940(90)90170-e. [DOI] [PubMed] [Google Scholar]
- Datta S. Evidence that REM sleep is controlle d by the activation of brainstem pedunculopontine tegmental kainate receptors. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- Datta S. Activation of pedunculopontine tegmental PKA prevents GABA-B receptor activation-mediated rapid eye movement sleep suppression in the freely moving rat. J Neurophysiol. 2007;97:3841–3850. doi: 10.1152/jn.00263.2007. [DOI] [PubMed] [Google Scholar]
- Datta S, MacLean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Prutzman SL. Novel role of brain stem pedunculopontine tegmental adenylyl cyclase in the regulation of spontaneous REM sleep in the freely moving rat. J Neurophysiol. 2005;94:1928–1937. doi: 10.1152/jn.00272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Excitation of the brainstem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–423. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Siwek DF. Brainstem afferents of the cholinoceptive pontine wave generation sites in the rat. Sleep Res Online. 1999;2:79–82. [PubMed] [Google Scholar]
- Datta S, Spoley EE, Mavanji VK, Patterson EH. A novel action of pedunculopontine tegmental kainate receptors: a mechanism of REM sleep generation in the rat. Neurosci. 2002;114:157–164. doi: 10.1016/s0306-4522(02)00250-6. [DOI] [PubMed] [Google Scholar]
- Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–1427. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Li G, Auerbach S. Activation of phasic pontine-wave generator in the rat: a mechanism for expression of plasticity-related genes and proteins in the dorsal hippocampus and amygdala. Europ J Neurosci. 2008;27:1876–1892. doi: 10.1111/j.1460-9568.2008.06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky JO, Shah Y, Gray BG. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. II. Effects on medullary dorsal horn nociceptive and nonnociceptive neurons. J Neurophysiol. 1983;49:948–960. doi: 10.1152/jn.1983.49.4.948. [DOI] [PubMed] [Google Scholar]
- El-Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqeductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 1984;306:125–139. doi: 10.1016/0006-8993(84)90361-5. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001;898:232–241. doi: 10.1016/s0006-8993(01)02189-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Miyazato H, Homma Y. Pedunculopontine stimulation induces prolonged activation of pontine reticular neurons. Neurosci. 2001;104:455–465. doi: 10.1016/s0306-4522(01)00094-x. [DOI] [PubMed] [Google Scholar]
- Garzon M, deAndres I, Reinoso-Suarez F. Sleep patterns after carbachol delivery in the ventral oral pontine tegmentum of the cat. Neurosci. 1998;83:1137–1144. doi: 10.1016/s0306-4522(97)00494-6. [DOI] [PubMed] [Google Scholar]
- George R, Haslett WL, Jenden DJ. A Cholinergic Mechanism in the Brainstem Reticular Formation: Induction of Paradoxical Sleep. Int J Neuropharmacol. 1964;72:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- Giola M, Moscheni C, Gagliano N. Distribution of extracellular signal-regulated kinase 1- and 2-activated neurons in the rat periaqueductal gray matter after noxious stimulation. The Anatomical Record. 2005;284A:460–465. doi: 10.1002/ar.a.20188. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Pegram GV. Cholinergic brainstem mechanism of REM sleep in the rat. Brain Res. 1986;384:29–41. doi: 10.1016/0006-8993(86)91216-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Greene RW, Carpenter DO. Actions of neurotransmitters on pontine medial reticular formation neurons of the cat. J Neurophys. 1985;54:520–531. doi: 10.1152/jn.1985.54.3.520. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Fass DM, Yao H, Ellig CL, Goodman RH, Stork PJ. Calcium and cAMP signals differentially regulate cAMP-responsive element-binding protein function via a Rap1-extracellular signal-regulated kinase pathway. J Biol Chem. 2000;44:34433–34441. doi: 10.1074/jbc.M004728200. [DOI] [PubMed] [Google Scholar]
- Guo YP, Sun X, Li C, Wang NQ, Chan YS, He J. Corticothalamic synchronization leads to c-fos expression in the auditory thalamus. Proc Natl Acad Sci U S A. 2007;104:11802–11807. doi: 10.1073/pnas.0701302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Shimomura A, Yoshida K, Imaki J. Gene expression and CREB phosphorylation induced by cAMP and Ca2+ in neuronal cells. Adv Pharmacol. 1996;36:277–285. doi: 10.1016/s1054-3589(08)60586-4. [DOI] [PubMed] [Google Scholar]
- Han M-H, Bolanos CA, Green TA, Olson VG, Neve RL, Liu R-J, Aghajanian GK, Nestler EJ. Role of cAMP response element-binding protein in the rat locus coeruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heister DS, Hayar A, Charlesworth A, Yates C, Zhou Y-H, Garcia-Rill E. Evidence for electrical coupling in the subCoeruleus (SubC) nucleus. J Neurophysiol. 2007;97:3142–3147. doi: 10.1152/jn.01316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillations: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Horner RL, Kubin L. Pontine carbachol elicits multiple rapid eye movement sleep-like neural events in urethane-anesthetized rats. Neurosci. 1999;93:215–226. doi: 10.1016/s0306-4522(99)00126-8. [DOI] [PubMed] [Google Scholar]
- Ito K, Yanagihara M, Imon H, Dauphin L, McCarley RW. Intracellular recordings of pontine medial gigantocellular tegmental field neurons in the naturally sleeping cat: behavioral state-related activity and soma size difference in order of recruitment. Neurosci. 2002;114:23–37. doi: 10.1016/s0306-4522(02)00253-1. [DOI] [PubMed] [Google Scholar]
- Jones BE, Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brain stem studied by choline acetyltransferase and tyrosine hydroxylase immunohistochemistry. J Comp Neurol. 1987;261:15–32. doi: 10.1002/cne.902610103. [DOI] [PubMed] [Google Scholar]
- Jones BE, Webster HH. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentumcholinergic cell area in the cat. I. Effects upon the cholinergic innervation of the brain. Brain Res. 1988;451:13–32. doi: 10.1016/0006-8993(88)90745-7. [DOI] [PubMed] [Google Scholar]
- Jouvet M. The role of monoamines and acetylcho waking cycle. Ergeb Physiol. 1972;64:165–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Tauchi M, Dahl JL. Cholinergi decarboxylase-like immunoreactivities in various brain line-containing neurons in the regulation of sleep-c neurons containing GABA-like and/or glutamic acid regions of the rat. Exp Brain Res. 1988;70:605–617. doi: 10.1007/BF00247609. [DOI] [PubMed] [Google Scholar]
- Lai YY, Clements JR, Siegel JM. Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J Comp Neurol. 1993;336:321–330. doi: 10.1002/cne.903360302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RH, Fung SJ, Reddy VK, Barnes CD. Localization of glutamatergic neurons in the dorsolateral pontine tegmentum projecting to the spinal cord of the cat with a proposed role of glutamate on lumbar motoneuron activity. Neurosci. 1995;64:193–208. doi: 10.1016/0306-4522(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Tang X, Sanford LD. Fear-conditioned suppression of REM sleep: relationship to Fos expression patterns in limbic and brainstem regions in BALB/cJ mice. Brain Res. 2003;991:1–17. doi: 10.1016/j.brainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14:4825–4830. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol. 1993;264:R544–R554. doi: 10.1152/ajpregu.1993.264.3.R544. [DOI] [PubMed] [Google Scholar]
- Lydic R, McCarley RW, Hobson JA. The time-course of dorsal raphe discharge, PGO waves, and muscle tone averaged across multiple sleep cycles. Brain Res. 1983;274:365–370. doi: 10.1016/0006-8993(83)90720-5. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA, Lorinc Z. Microdialys is of cat pons reveals enhanced acetylcholine release during state-dependent respiratory depression. Am J Physiol. 1991;261:R766–R770. doi: 10.1152/ajpregu.1991.261.3.R766. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in Cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19:3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. c-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;20:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavanji V, Datta S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur J Neurosci. 2003;17:359–370. doi: 10.1046/j.1460-9568.2003.02460.x. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Mechanisms and models of REM sleep control. Arch Ital Biol. 2004;142:429–467. [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Discharge patterns of cat pontine brain stem neurons during desynchronized sleep. J Neurophysiol. 1975;38:751–766. doi: 10.1152/jn.1975.38.4.751. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Merchant-Nancy H, Vazquez J, Garcia F, Druker-Colin R. Brain distribution of c-fos expression as a result of prolonged rapid eye movement (REM) sleep period duration. Brain Res. 1995;681:15–22. doi: 10.1016/0006-8993(95)00275-u. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neurosci. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Dement WC. Cataplectic-like behavior in cats after microinjections of carbachol in pontine reticular formation. Brain Res. 1974;68:335–343. doi: 10.1016/0006-8993(74)90402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem. 1996;271:14214–14220. doi: 10.1074/jbc.271.24.14214. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy: GABA and Neuropeptides in the CNS. Part 1. Vol. 4. Elsevier Science; Amsterdam: 1985. pp. 436–622. [Google Scholar]
- Nestler EJ, Greengard P. Protein phosphorylation and the regulation of neuronal function. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic Neurochemistry: Molecular, cellular, and Medical Aspects. Little Brown; Boston: 1994. pp. 449–474. [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nature Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain atlas in stereotaxic coordinates. Academic Press Inc.; San Diego: 1998. [Google Scholar]
- Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(di-npropylamino)tetralin (8-OH-DPA) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J Neurosci. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrochi JJ, Mamelak AN, Madison RD, Macklis JD, Hobson JA. Mapping neuronal inputs to REM sleep induction sites with carbachol fluorescent microspheres. Science. 1989;245:984–986. doi: 10.1126/science.2475910. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Saha S, Datta S. Two-way active avoidance training -specific increases in phosphorylated cAMP response element-binding protein in the dorsal hippocampus, amygdala, and hypothalamus. Europ J Neurosci. 2005;21:3403–3414. doi: 10.1111/j.1460-9568.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Sisson JC, Verma IM. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schonthal A, Buscher M, Angel P, Rahmsdorf HJ, Ponta H, Hattori K, Chiu R, Karin M, Herrlich P. The Fos and Jun/AP-1 proteins are involved in the downregulation of Fos transcription. Oncogene. 1989;4:629–636. [PubMed] [Google Scholar]
- Semba K. Aminergic and cholinergic afferents to REM sleep induction regions of the pontine reticular formation in the rat. J Comp Neurol. 1993;330:543–556. doi: 10.1002/cne.903300410. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Ann Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Sheng M, Dougan ST, McFadden G, Greenberg ME. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988;8:2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromani PJ, McGinty DJ. Pontine neuronal response to local cholinergic infusion: relation to REM sleep. Brain Res. 1986;386:20–31. doi: 10.1016/0006-8993(86)90137-x. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV, Gillin JC. Distribution of acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation. Sleep. 1988;11:1–16. doi: 10.1093/sleep/11.1.1. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Kilduff TS, Bloom FE, McCarley RW. Cholinergically induced REM sleep triggers Fos-like immunoreactivity in dorsolateral pontine regions associated with REM sleep. Brain Res. 1992;580:351–357. doi: 10.1016/0006-8993(92)90968-f. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Winston S, McCarley RW. Pontine cholinergic neurons show Fos-like immunoreactivity associated with cholinergically induced REM sleep. Mol Brain Res. 1996;38:77–84. doi: 10.1016/0169-328x(95)00325-m. [DOI] [PubMed] [Google Scholar]
- Sohm F, Gaiddon C, Antoine M, Boutilier AL, Loeffler JP. The retinoblastoma susceptibility gene product/Sp1 signalling pathway modulated by Ca2+ /calmodulin kinases II and IV activity. Oncogene. 1998;18:2762–2769. doi: 10.1038/sj.onc.1202634. [DOI] [PubMed] [Google Scholar]
- Shouse MN, Siegel JM. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RH, Kameda SR, Carvalho RC, Takatsu-Coleman AL, Niigaki ST, Abilio VC, Tufik S, Frussa-Filho R. Anxiogenic effect of sleep deprivation in the elevated plus-maze test in mice. Psychopharmacol. 2004;176:115–122. doi: 10.1007/s00213-004-1873-z. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotonergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, CurroDossi R. Neuronal activities in brainstem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Phasic but not tonic REM-selective discharge of periaqueductal gray neurons in freely behaving animals: relevance to postulates of GABAergic inhibition of monoaminergic neurons. Brain Res. 2002;945:276–280. doi: 10.1016/s0006-8993(02)02914-1. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. GABA-ergic neurons of the laterodorsal and pedunculopontine tegmental nuclei of the cat express c-fos during carbachol-induced active sleep. Brain Res. 2001;892:309–319. doi: 10.1016/s0006-8993(00)03264-9. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Verret L, Leger L, Fort P, Luppi P-H. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Europ J Neurosci. 2005;21:2488–2504. doi: 10.1111/j.1460-9568.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brainstem Control of the events of REM sleep. Prog Neurobiol. 1984;22:241–288. doi: 10.1016/0301-0082(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res. 1993;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Reiner PB. The immunohistochemical localization of choline acetyltransferase in the cat brain. Brain Res Bull. 1987;18:371–415. doi: 10.1016/0361-9230(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Vogel GW. A review of REM sleep deprivation. Arch Gen Psychiat. 1975;32:749–761. doi: 10.1001/archpsyc.1975.01760240077006. [DOI] [PubMed] [Google Scholar]
- Wang H-L, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Europ J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA. A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: locus of the sensitive region. Neurosci. 1990a;39:279–293. doi: 10.1016/0306-4522(90)90267-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA. A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: spontaneous and drug-induced neuronal activity. Neurosci. 1990b;39:295–304. doi: 10.1016/0306-4522(90)90268-9. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Mancillas JR, Morales FR, Chase MH. c-fos expression in the pons and medulla of the cat during carbachol-induced active sleep. J Neurosci. 1993;13:2703–2718. doi: 10.1523/JNEUROSCI.13-06-02703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamuy J, Sampogna S, Morales FR, Chase MH. c-fos expression in mesopontine noradrenergic and cholinergic neurons of the cat during carbachol-induced active sleep: a double-labeling study. Sleep Res Online. 1998;1:28–40. [PubMed] [Google Scholar]