Abstract
The study of polyomavirus has benefited immensely from two scientific methodologies, cell culture and in vitro studies on one side and the use of transgenic mice as experimental models on the other. Both approaches allowed us to identify cellular products targeted by the viruses, the consequences of these interactions at the phenotypic and molecular level, and thus the potential roles of the targets within their normal cellular context. In particular, cell culture and in vitro reports suggest a model explaining partially how SV40 large T antigen contributes to oncogenic transformation. In most cases, T antigen induces cell cycle entry by inactivation of the Rb proteins (pRb, p130, and p107), thus activating E2F-dependent transcription and subsequent S-phase entry. Simultaneously, T antigen blocks p53 activity and therefore prevents the ensuing cell-cycle arrest and apoptosis. For the most part, studies of T antigen expression in transgenic mice support this model, but the use of T antigen mutants and their expression in different tissue and cell type settings have expanded our knowledge of the model system and raised important questions regarding tumorigenic mechanisms functioning in vivo.
Keywords: SV40, large T antigen, transgenic, model system, mutant analysis
Introduction
Two major very exciting areas of scientific research came together in the early 1980s and resulted in the production of models to investigate cancer. On one hand, the technology to produce transgenic mice was being developed rapidly [1] and, at the same time, many viral and cellular oncogenes were being discovered and subsequently cloned. It soon became evident that the expression of SV40 sequences including the large T antigen (T antigen) in mice resulted in tumor development in particular tissues [2, 3]. Up until that point cancer models had been based on cell lines obtained from human or animal tumors and maintained in tissue culture, or from the subcutaneous inoculation of those cells into immunodeficient mice. Although obviously useful, these models did not offer a good approach to understanding the genetics and the molecular pathways involved in different types of tumorigenesis, nor to the relevance to particular tumors and their site of origin “in vivo”. The arrival of transgenic mice expressing T antigen made clear, not only that T antigen expression was capable of driving resting cells into active proliferation status, but also that this trait was hereditary and the mouse progeny presented cancer predisposition. As a result, SV40 T antigen has been expressed in multiple tissues of transgenic mice to mimic tumorigenic processes.
T antigen has been expressed under the control of numerous promoters directing its expression to different cells and/or tissue types, including mammary glands, pancreas, liver, prostate, salivary glands, intestine, brain, lung, kidney, eye, smooth muscle, cartilage and bone [4, 5]. Some particular systems are of special interest due to their clinical relevance. For instance, the phenotype induced by expression of T antigen in the prostate closely resembles specific tumorigenic conditions in humans. In other instances, the exhaustive characterization of transgenic mice expressing full length T antigen and mutant derivatives of the oncogene have helped to dissect the molecular mechanisms that must be disrupted in a normal cell in order for it to progress through different stages of tumorigenesis (e.g. pancreas, intestine, choroid plexus epithelium, eye lenses). Space constrains force us to concentrate on these last systems.
The model system
SV40 depends heavily on the cell machinery to pursue its own genome replication and transcription, as the viral genome is relatively small. In particular, the virus requires that the host cell re-enters S-phase in order to use the cellular machinery for its own replication. Although not a normal part of the viral life cycle, the viral DNA can sometimes integrate into the chromosome of a nonpermissive species and maintain the expression of the viral T antigens, and transformation of the host cell follows. Upon transformation with SV40, cells become capable of proliferation and survival in a number of environments that are otherwise growth-restrictive for non-transformed cells. For instance, transformed cells can grow in the absence of serum, form dense foci of multilayered cells in culture, grow independently of anchorage, or induce tumors when injected into immuno-compromised mice.
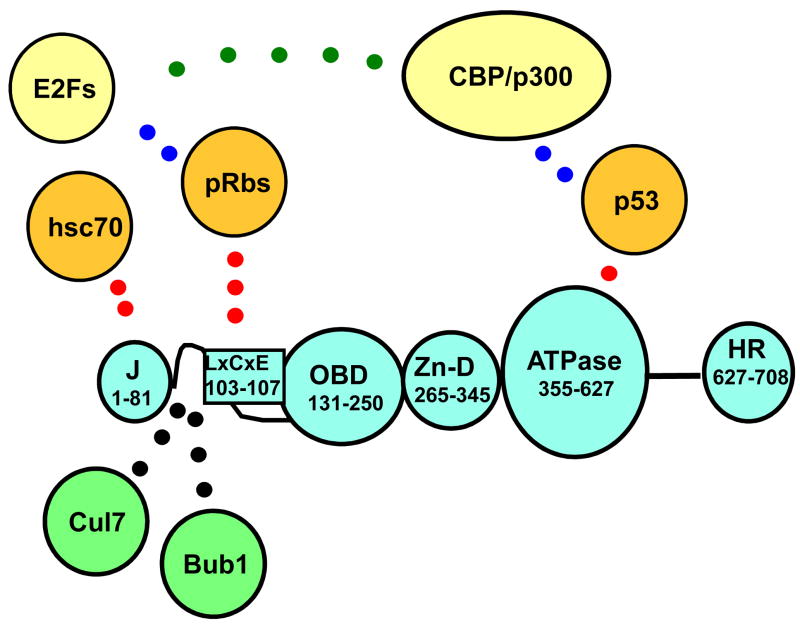
Although the full transformation potential of SV40 is mediated by two virus-encoded proteins -the 708 amino acid large T antigen and the 174 amino acid small t antigen-, large T antigen “per se” is necessary and often sufficient to induce and maintain transformation. The virus needs this extremely versatile protein for DNA replication, transcriptional control and virion assembly. These functions are carried out through the independent and cooperative action of five structural domains in T antigen, which include a J domain at the amino-terminus, a sequence-specific DNA binding domain (OBD, for origin binding domain), a Zn-binding domain, an ATPase domain (AAA+), and the host-range domain (Figure 1).
Figure 1. Domain structure of SV40 T antigen.
Different protein domains of T antigen (blue) and the corresponding amino acids are indicated: J domain (J); Rb-protein binding region (LXCXE); origin binding site (OBD); Zinc finger domain (Zn-D); ATPase region contaning the p53 binding region (ATPase); host range domain (HR). Established cellular targets are depicted in orange and their regions of interaction with T antigen indicated (red dots). Known interacting proteins whose role has not been established by transgenic analysis are depicted in green. Cellular components (yellow) that might associate with T antigen through other proteins as well as their possible interactions are also indicated.
In addition, T antigen is able to induce transformation in cell culture and in vivo transgenic systems, a phenomenon effected through its interaction with at least five cellular targets: hsc70, the three Rb tumor suppressor proteins (pRb, p107, and p130), and the tumor suppressor p53 [4, 6]. T antigen binds p53 through interactions with exposed amino acids on the surface of its ATPase domain [7]. Similarly, the three Rb-proteins bind to an LXCXE motif located in the flexible linker between the J domain and the OBD. Finally, the J domain governs recruitment and activation of hsc70, a cellular chaperone [8–10]. T antigen has been shown to interact with another three targets, and these interactions could contribute to transformation as well, but they have been less studied. Two of these factors, the checkpoint kinase Bub1 and the cullin Cul7 interact with T antigen via the flexible linker near the LXCXE motif [11–14] (Figure 1). Finally, the transcriptional adapter proteins CBP/p300 [15–17], bind T antigen through interactions with p53 and could also contribute to transformation. In addition, small t antigen contributes to transformation through its interaction with the cellular phosphatase PP2A [18, 19].
The study of T antigen in cell culture systems has clearly established that the ability of this oncogene to re-activate the cell cycle and to transform multiple cell types is linked to its capacity of tampering with the Rb family, as well as its ability to prevent the ensuing apoptosis by binding and inactivating the tumor suppressor p53. Both functions then allow uncontrolled cell proliferation. Nevertheless, some exceptions are found in tumorigenesis induced by T antigen in transgenic systems, which will be discussed below.
Tissue System
Brain
SV40 T antigen has been used to induce tumorigenesis in several cell types of the brain although, by far, the most studied is the choroid plexus epithelium (CPE). Located within the brain ventricles, the choroid plexus is a continuous epithelial layer that produces and filters the cerebrospinal fluid, in fact cushioning and protecting the brain from foreign substances, immune cells and/or metabolic waste. The continuous epithelial layer lining the ventricles contain multiple tight junctions on the apical surface of the cells, thus creating an effective blood-cerebrospinal fluid barrier.
Regarding T antigen function, the CPE is a particularly interesting system, as the expression of different T antigen mutants in this tissue have yielded numerous insights into the molecular mechanisms necessary to disrupt quiescence and re-enter the cell cycle. Expression of T antigen in the CPE induces the formation of rapidly developing tumors, a phenotype that does not require the expression of small t and results in the death of the mice prior to or around 1 month of age [20, 21]. If an amino-terminal truncated version of T antigen (dl137, T121) is expressed in the CPE, similar tumors arise but at a much lower rate [20, 22]. A mutant T121 protein carrying a defective LXCXE motif, and thus unable to interfere with the Rb pathway, is unable to induce tumors, indicating that blocking the Rb pathway is essential to induce CPE proliferation [22]. The fact that tumors arise at a much slower pace in T121-expressing animals indicates that the progression of CPE tumorigenesis is linked to a cellular function targeted by the carboxy terminus end of T antigen [22]. Indeed, blocking the p53-mediated apoptosis induced by T121 allows for fast tumor proliferation [23], and upregulation of the transcriptional activator E2F1 in response to pRb inactivation seems to be required to induce p53 and the subsequent apoptosis [24].
Therefore, at least in the CPE, disruption of the Rb pathway by T antigen releases the brakes that halt cell proliferation in quiescent cells. Abnormal entry into S-phase and upregulation of E2F1 then trigger a p53-mediated response aimed to eliminate the abnormally proliferating cells, but T antigen is also capable of preventing this apoptosis by binding and inactivating p53 itself, thus inducing fast and aggressive tumors.
Atm (ataxia telangiectasia mutated) is a serine/threonine protein kinase that phosphorylates several proteins in response to DNA damage. This phosphorylation initiates a series of events leading to cell cycle arrest, DNA repair or apoptosis. In particular, the Atm function upstream of p53 has been shown to be essential to control the apoptosis induced by DNA damage. Nevertheless, the removal of Atm does not seem to be affect the apoptosis induced by T antigen in the CPE [25]. Thus, the apoptotic responses triggered by different stimuli (e.g. DNA damage versus oncogenic stimuli), despite being mediated by p53, do not necessarily utilize the same molecular mechanisms.
Other cell types in the brain have been used to investigate oncogenesis mediated by T antigen. For instance, conditional expression of the truncated T mutant T121 in astrocytes leads to aberrant proliferation and extensive apoptosis [26]. Surprisingly and in contrast with the phenotype induced in CPE cells, development of astrocytomas is not dependent on p53, but instead is affected by the amount of the tumor suppressor PTEN present [26]. Thus, in this case, the apoptosis mediated by T antigen does not rely on p53, and T antigen triggers other signals in the cell destined to counteract its abnormal effects on cell proliferation.
Intestine
The mammalian intestine is an elongated, tubular structure, whose internal lining is responsible for absorbing the nutrients in food and for disposing of the remaining debris. To accomplish this, the small intestine depends on different specialized cell types: The absorptive enterocytes constitute the majority of the intestinal epithelium, while the secretory cells (goblet, Paneth, endocrine) supply the necessary factors to control and help digestion, and the smooth muscle cells provide continued peristalsis to move the food along and out of the gut. While most of the specialized, differentiated cells of the small intestine (enterocytes, endocrine, goblet) reside in finger-shaped structures termed “villi”, the cells responsible for regenerating this constantly renewing epithelium, together with the defensive Paneth cells, are located in pouch structures called crypts of Lieberküm. Following food digestion, the absorption of the remaining water, sodium and traces of nutrients is accomplished in the large intestine.
Numerous groups have reported the generation of transgenic mice expressing T antigen in all intestinal cell types of the small intestine, including endocrine cells [27–29], Paneth cells [30], goblet cells [31], smooth muscle cells [32] and enterocytes [33–35]. Although entry of quiescent cells into S-phase is the usual consequence of T antigen expression in different cell types, including enterocytes, some cells do not follow ectopic proliferation with mitosis and either involute and apoptose, or directly undergo apoptosis [30, 31]. In part because enterocytes constitute the majority (95%) of the intestinal epithelium, the expression of T antigen in this cell type has proven particularly useful to analyze the molecular players involved in intestinal tumorigenesis. We will therefore discuss in detail this transgenic system.
Transgenic mice expressing the early region of SV40 in intestinal enterocytes under the control of the intestinal fatty acid binding promoter were first generated in 1992 [35], and characterized in detail subsequently, both morphologically and at the molecular level [33, 35, 36]. Ectopic expression of T antigen in enterocytes drives these resting cells to exit quiescence and re-enter the S-phase of the cell cycle, resulting in a hyperplastic intestine, but the enterocytes are still fully functional and retain the molecular markers associated to their differentiation status [33, 35].
It is important to mention that, while the initial observations indicated a lack of neoplastic transformation of the intestine in the presence of T antigen [35], subsequent studies have shown that indeed the initial hyperplastic phenotype apparently progresses to dysplasia in older animals [33, 36] Another way to accelerate the progression to dysplasia can be achieved by co-expressing an activated ras oncogene with T antigen [37]. The fact that two lines of transgenic mice show very different phenotypic outcomes varying from simple intestinal hyperplasia to overt dysplasia [33, 36, 37] could be explained by the relative amounts and/or site of ectopic expression of T antigen in the different transgenic lines (see table II in [33]; Rathi el al, unpublished results). This point will be further developed in the discussion.
Numerous mutants of T antigen have been expressed in intestinal enterocytes under the same conditions, allowing us to dissect the molecular pathways required for the oncogenic transformation of this cell type. In the first place, a truncated amino terminus version of T antigen (N136), capable of binding and disrupting the Rb functions but lacking the capacity of interaction with p53, is sufficient to force enterocytes to exit quiescence and to develop hyperplastic intestines [38]; Rathi et al, unpublished results). Furthermore, T antigen mutants containing either a defective LXCXE motif, thus lacking the capacity to bind Rb proteins, or a non-functional J domain, thus unable to disrupt Rb-E2F complexes, are also unable to induce a discernible phenotype in enterocytes [38–40]. These results assert the very prominent role played by the Rb family in the control of abnormal proliferation in enterocytes.
In fact, other methods of genetic inactivation have confirmed that at least two members of the Rb family must be removed in order to induce ectopic proliferation in enterocytes [41]. Intriguingly, there are no clinical reports linking alterations of the Rb proteins to intestinal tumorigenesis. Perhaps tumorigenesis of the small intestine is associated to alterations occurring in precursor cells and not in differentiated ones -such as enterocytes-, and perhaps the control of cell proliferation is very different in both types of systems. One clear possibility is that the “normal” cell proliferation, such as that happening in stem and/or progenitor cells, differs substantially from tumorigenic proliferation. Indeed, the proliferation triggered by T antigen in the differentiated enterocytes is linked to an increase in steady state levels of the transcription factors E2F2 and E2F3 [38]. The resulting hyperplastic phenotype is ameliorated but not fully rescued by depletion of the E2F2 gene, and is independent of E2F3a expression [38]. In contrast, incorporation of BrdU in the intestinal crypts is not affected by the depletion of E2F2 [38].
Surprisingly, several lines of evidence indicate that p53 does not play a role in the tumorigenesis induced by T antigen in enterocytes. On one hand, amino-terminus truncation mutants of T antigen unable to bind and disrupt the p53 protein are still capable of producing hyperplasia and dysplasia in murine enterocytes [34, 36]; Rathi et al, unpublished results), and the genetic removal of p53 does not increase or affect the frequency of this hyperplastic phenotype [36]. In addition, the presence of T antigen in enterocytes fails to stabilize p53, which remains undetectable in villi of either normal or T antigen-expressing mice [36]. Apparently p53 is not a mediator of homeostasis or cell proliferation in the differentiated enterocytes, although it could play a role in the apoptosis of progenitor cells induced by DNA damaging agents. In fact, gamma-irradiation induces p53 expression in the base of intestinal crypts, where progenitor cells are located [42, 43]. The absence of a role played by p53 in enterocytes is in clear contrast with other systems, such as the choroid plexus, where both p53 and E2F1 are mediators of the tumorigenic response elicited by T antigen. These results emphasize the importance of understanding the different tumorigenic responses produced in different cell types.
Albeit fully capable of inducing hyperplasia of the intestine, amino terminal truncations of T antigen induce intestinal dysplasia with lower penetrance than that induced by full length T antigen (Rathi et al, unpublished results). This fact could be linked to the intestinal epithelial location where T antigen is expressed. Despite the fact that both transgenic systems use the same promoter sequences to direct expression to intestinal enterocytes and, indeed, that the corresponding protein is found in the transgenic villi, we also have found significant expression of T antigen in the uppermost region of the crypts. This could mean that, in addition to quiescent and differentiated enterocytes, T antigen is targeting cells at different stages of the cell cycle (e.g. still undergoing proliferation) or with different capabilities to differentiate, and therefore the outcome of the oncogene effects might depend on its interactions with a different subset of cell components.
Eye
Expression of SV40 T antigen has been directed to numerous cell types within the eye to generate model systems for particular human malignancies. For instance, T antigen was targeted early on to the retina of transgenic mice and causes ocular tumors similar to human retinoblastoma [44], an eye malignancy occurring in young children and caused by inactivating mutations in both alleles of the tumor suppressor RB gene. Due to the relevance of the human pathological condition, enormous efforts have been invested to generate a more specific mouse model and, in particular, to specifically inactivate the RB gene, either in the whole mouse or using conditional strategies in the retina.
The ocular lens has provided a model to further understand the mechanism of tumorigenesis mediated by T antigen. In this system, expression of T antigen results in tumor formation and reduced expression of differentiation markers [45], while expression of the first 191 amino acids of T antigen results in lens cell proliferation and apoptosis, and the animals present microphtalmia, microlentia and lens ablation. As in other systems described above, mutational analysis indicates that T antigen needs to disrupt the Rb pathway to induce proliferation in the fiber cells of the lenses [46, 47], and induction of S-phase does not require the presence of small t [46]. Although the role of p53 and apoptosis in this particular system has not been established, inactivation of specific genes by knock out techniques has confirmed the pivotal roles of pRb controlling cell proliferation and the role of p53 in the ensuing apoptotic response in the lenses [48].
Surprisingly, a mutant T antigen unable to disrupt Rb is still able to block differentiation as measured by crystallin expression [46]. In this case, postmitotic cells fail to differentiate, a phenotype that could be linked to the ability of the T antigen to bind the transcriptional regulators CBP/p300 and, in turn, to inhibit their interaction with c-Maf [46]. In addition to its important role in revealing cellular pathways controlling oncogenic proliferation, these studies indicate how T antigen can be used to identify pathways involved in cell differentiation.
Pancreas
The pancreas is an organ responsible for producing both digestive enzymes (which help the digestion of carbohydrates, proteins and fat when secreted to the small intestine) and several essential hormones (insulin, glucagon, somatostatin and pancreatic polypeptide). To be able to produce all those substances, the pancreas is made of both endocrine and exocrine tissues. All four different, hormone-producing, endocrine cell types are contained in the islets of Langerhans, which account for only 1–2% of the cellular mass in the adult pancreas. The rest of the organ is composed of exocrine acinar tissue, which synthesizes the digestive hydrolases, and of ducts, responsible for secreting buffered fluids to flush the acinar secretions to the intestine.
One of the most studied models of T antigen-induced tumorigenesis, the RIP-Tag2 mice, was generated by directing the oncogene expression to β-cells in the islets of the pancreas [49]. Following T antigen expression, the cells re-enter S-phase, with a subset of islets later developing lesions corresponding to hyperplasia and dysplasia [49]. In this system, it is possible that T antigen levels are not sufficient to completely obliterate all the available pRb. In fact, the T antigen-induced tumorigenic phenotype is accentuated by complete removal of members of the pRb pathway by genetic deletion [50]. Nevertheless, T antigen seems able to effectively suppress all p53-induced apoptosis in this system [51]. The observation that only about half of the islets become hyperplastic in the RIP-Tag2 mice [49] suggests that the oncogene can efficiently initiate tumorigenesis, but that additional mutations or cellular changes are necessary to drive tumor progression. In fact, activation of insulin-like growth factor 2 (IGF2) in this system apparently serves as a survival factor, and T antigen transgenic mice lacking the Igf2 gene show reduced malignancy and higher rates of apoptosis [52]. Furthermore, co-expression in RIP-Tag2 mice of the receptor for both Igf2 and Igf1 (the tyrosine kinase IGF1R) increases the tumorigenic progression [53].
On the other hand, expression of both large T and small t antigens in the acinar cells gives rise to carcinomas and, in some cases, metastasis [54–57]. In this case, truncated amino terminal versions of T antigen are sufficient to induce this phenotype [58], indicating that, similarly to what was observed in enterocytes, disruption of p53 is not required to generate neoplasia in this tissue. This phenotype does not require the presence of small t either.
Finally, the expression of T antigen in endocrine cells of the pancreas results in pancreatic insulinomas and lymphomas [28]. This transformation does not require the presence of small t, but needs a functional J domain in T antigen [28].
Molecular mechanisms of transformation
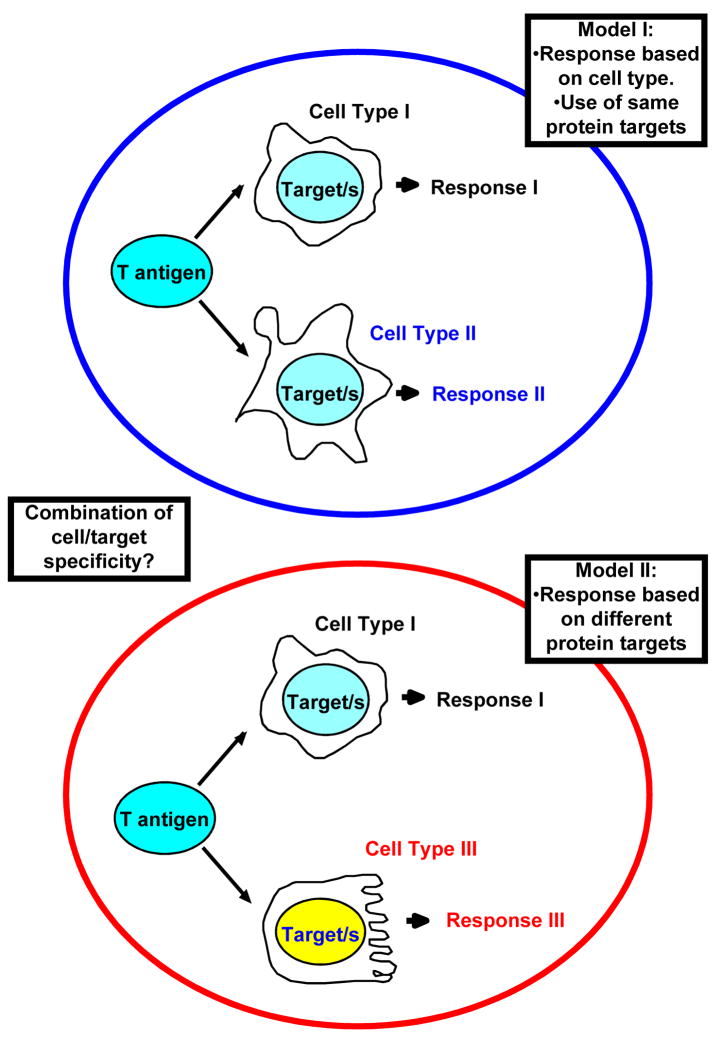
Based on cell culture experiments, it has been suggested that the action of T antigen on Rb proteins is necessary to induce ectopic proliferation and, subsequently, transformation. Disruption of the control imposed by this tumor suppressor triggers an apoptotic response, and full length T antigen is capable of preventing this as well by binding an inactivating p53, the main mediator of the cell damage response. This general modus operandi seems to be in place as well for many of the in vivo transgenic systems studied to date, although notable exceptions have been revealed in the last few years. It is very clear now that the activities of T antigen needed to induce cancer depend largely on the tissue or cell type targeted for expression (Figure 2).
Figure 2. How is SV40 T antigen affecting different types of cells?
Two non mutually-exclusive models are possible. T antigen seems to bind and inactivate or modify the function of a series of cellular partners in multiple instances. Differences observed upon expression of T antigen could be related to the role of such targets in specific cell types, and to secondary consequences in each specific case (Model I). On the other hand, there are several reports indicating that specific targets (e.g. p53) don’t play a role in tumorigenesis mediated by T antigen. A possible alternative scenario would involve differential interactions with alternative cellular components in distinct sets of cells (Model II). A combination of both models is also possible.
Role of Rb proteins in transformation mediated by T antigen
Truncated amino terminal versions of T antigen able to disrupt the Rb pathway are in many cases sufficient to induce ectopic proliferation and tumorigenesis. For instance, the first 121 or 136 amino acids of T antigen induce S-phase re-entry and various degrees of tumorigenesis when expressed in enterocytes, the choroid plexus epithelium, acinar cells of the pancreas or the liver [20, 22, 34, 38, 58, 59]; Rathi et al, unpublished results). Ectopic S-phase and tumorigenic induction seem to require a functional LXCXE motif [22, 38, 39, 59] and, furthermore, an intact J domain region in T antigen [40, 60]. Because the J domain has been shown to function in the “displacement” of Rb-E2F complexes, in particular p130-E2F4 [61], these results strongly support a role for the Rb proteins as mediators of T antigen-induced tumorigenesis.
Although T antigen expression results in the removal of p130 [38], possibly through a proteasome-mediated degradation mechanism [62], its ability to eliminate other Rb proteins is not as clear. For instance, T antigen expression does not affect the steady state levels of pRb in enterocytes, and even results in increased levels of p107 [38]. This last observation is probably the consequence of p107 upregulation at the transcriptional level upon induction of E2F activity, as p107 is a clearly defined E2F-target gene. The current model would predict that pRb bound to T antigen would be inactive, as observed with p53 bound to T antigen, but the role of p107 in T antigen-mediated transformation remains to be elucidated.
The level of inactivation of pRb might play a very relevant role, not only in the induction of tumorigenesis, but in its maintenance and aggressiveness as well. For instance, models of pancreatic islet carcinogenesis show heterogeneous T antigen expression [49] during all stages of tumor progression. If the expression of T antigen is not sufficient to inactivate all existing pRb protein, that threshold could determine the level of cell hyperproliferation. In fact, genetic ablation of the pRb gene (or its regulator p27Kip1) accentuates T antigen-induced tumorigenesis, enhancing the frequency and growth rates of premalignant lesions, solid tumors, and invasive carcinomas [50]. Thus, in this system, inactivation of pRb –perhaps partial- is sufficient to initiate tumorigenesis, but further loss of the protein or its regulators can enhance malignant progression. It is feasible that a stoichiometric imbalance between the levels of T antigen and the available amounts of pRb -or other cellular targets-could result in only partial inhibition of the function and, therefore, in an attenuated phenotype [50]. Consistent with this hypothesis, the level of oncogenic transformation of enterocytes and/or retinal-brain tumors achieved by the expression of full-length T antigen seems to depend on the amounts of oncogene expressed [33, 35, 63]. Alternatively, the state of differentiation of the cells at the time of T antigen ectopic expression might play a significant role in the ensuing tumorigenic process [64].
Role of p53 in T antigen-mediated transformation
Despite the relevance of p53 in cancer, it seems clear that induction of tumorigenesis in numerous cell types does not require the inactivation of p53. Such is the case in specific cells from the intestine, [34, 36]; Rathi et al, unpublished results), pancreas [58], liver [59] and brain [20, 22]. In some cases, removal of p53 might impinge on the rate of tumorigenic progression [23], but in others either it has a modest role [59] or it is not a factor at all [26, 36]; Rathi et al, unpublished results).
In contrast, and unlike full length T antigen, the amino terminal region of the oncogene “per se” is unable to transform B and T lymphoid cells [20, 22]. Instead, the disruption of the p53 pathway is a requisite to induce tumorigenesis in T cells [20, 60]. Cells of lymphoid origin might be particularly sensitive to the status of p53 as programmed cell death plays an essential role in the process of their life span.
Role of E2F factors in transformation mediated by T antigen
Whatever the effects that T antigen produces in Rb proteins, it seems clear that upregulation of E2F factors and/or their transcriptional activity is a consequence of the oncogene’s expression in transgenic tissues. In fact, specific E2F factors have been shown to mediate the phenotype induced by T antigen [24, 38]; Rathi et al, unpublished results; Cantalupo et al, unpublished results). Similarly to observed in cell culture systems, T antigen expression also disrupts the repressive p130-E2F4 complexes present in quiescent cells of whole tissue [38]. Based on these and other observations, a model has emerged suggesting that T antigen disrupts the repressive transcriptional complexes (e.g. p130/E2F4) occupying genes of promoters needed to exit quiescence and thereby releasing “free”, transcriptionally active E2Fs (E2F1–2–3) which, in turn, drive the cells into S phase.
Despite the general similarities, the expression of SV40 T antigen in different transgenic systems seems to utilize some unique cellular partners. For instance, in enterocytes, T antigen expression only minimally upregulates E2F1, and removal of E2F1 from T antigen-expressing intestines does not affect the hyperproliferative status of the tissue [38]. Furthermore, the ectopic expression of E2F1 in normal enterocytes does not induce cell proliferation [39]. This system also lacks a p53-dependent apoptosis response to T antigen expression [36]; Rathi et al, unpublished results). In contrast, the depletion of Rb mediated by T antigen triggers a strong apoptotic response in the choroid plexus, which depends both on E2F1 and p53 [23, 24]. Surprisingly, the presence of p19ARF has no role on this apoptosis [65], despite multiple reports indicating the need of p19ARF to transmit the T antigen oncogenic signal to p53. All these results reinforce the existence of different cell-specific pathways responding to the same oncogenic stimuli.
Discussion and final remarks
Expression of the amino terminus region of T antigen is capable of binding and disrupting the normal functions of all components of the retinoblastoma family, pRb, p107 and p130. Nevertheless, it is imperative to remember that T antigen is capable of interaction with other cellular components through the same region (e.g. Cul7, Bub1). Thus, the use of truncated versions of T antigen cannot be considered fully equivalent to deletion of the Rb family until the contribution of those factors is fully evaluated. Similarly, the conclusions derived from transgenic experiments using amino-terminus truncations of T antigen should not be considered equal to the consequences of removal of all three Rb proteins.
The prevalent view in the field is that, in order to induce tumorigenesis, T antigen must interfere with the two key pathways central to the control of the process, namely pRb and p53. In doing so, T antigen not only induces ectopic proliferation of otherwise resting cells, but it also prevents the apoptosis triggered by aberrant initiation of the S-phase. According to this view, T antigen acts upon the same targets in all cell types, either in vivo or in vitro, and it disrupts pRb and p53. The specific outcomes of these actions could be different depending on the cell type. However, this model does not account for all experimental observations to date. An alternative hypothesis is that T antigen acts on one or more unique targets in each different cell type to mediate transformation (Figure 2). A combination of both models should also be taken into consideration.
Most, if not all, T antigen-expressing transgenic systems described to date target the oncogene expression to quiescent cells, where repressive complexes prevent proliferation and transition through the cell cycle. It is a clear quiescent to proliferative status transition. But the mechanisms required to support continuous proliferation and to prevent cells from exiting the cell cycle (e.g. those operating in progenitor and/or stem cells) might be different or use different molecular players. It remains to be determined if T antigen expression disrupts the same cellular pathways in resting and proliferative cells and what the consequences of these interactions would be. One intriguing possibility is that oncogenic stimuli in progenitor cells could affect systems such as wnt/β-catenin that control the exit from cell proliferation and establishment of differentiation, while the same stimuli in differentiated cells would interact with different factors responsible for the particular cell type.
Acknowledgments
We thank Paul Cantalupo, Nicole Seneca and Jon Schilke for critical reading of the manuscript.
Abbreviations
- T antigen
SV40 large T antigen
- CPE
choroid plexus epithelium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmiter RD, Brinster RL. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–99. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinster RL, et al. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37(2):367–79. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmiter RD, et al. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumours in transgenic mice. Nature. 1985;316(6027):457–60. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24(52):7729–45. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 5.Saenz-Robles MT, Sullivan CS, Pipas JM. Transforming functions of Simian Virus 40. Oncogene. 2001;20(54):7899–907. doi: 10.1038/sj.onc.1204936. [DOI] [PubMed] [Google Scholar]
- 6.Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11(1):15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 7.Lilyestrom W, et al. Crystal structure of SV40 large T-antigen bound to p53: interplay between a viral oncoprotein and a cellular tumor suppressor. Genes Dev. 2006;20(17):2373–82. doi: 10.1101/gad.1456306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodsky JL, Pipas JM. Polyomavirus T antigens: molecular chaperones for multiprotein complexes. J Virol. 1998;72(7):5329–34. doi: 10.1128/jvi.72.7.5329-5334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan CS, Pipas JM. T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis. Microbiol Mol Biol Rev. 2002;66(2):179–202. doi: 10.1128/MMBR.66.2.179-202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan CS, Pipas JM. The virus-chaperone connection. Virology. 2001;287(1):1–8. doi: 10.1006/viro.2001.1038. [DOI] [PubMed] [Google Scholar]
- 11.Ali SH, et al. Cul7/p185/p193 binding to simian virus 40 large T antigen has a role in cellular transformation. J Virol. 2004;78(6):2749–57. doi: 10.1128/JVI.78.6.2749-2757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohrman DC, Imperiale MJ. Simian virus 40 large T antigen stably complexes with a 185-kilodalton host protein. J Virol. 1992;66(3):1752–60. doi: 10.1128/jvi.66.3.1752-1760.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai SC, et al. Simian virus 40 large T antigen binds a novel Bcl-2 homology domain 3-containing proapoptosis protein in the cytoplasm. J Biol Chem. 2000;275(5):3239–46. doi: 10.1074/jbc.275.5.3239. [DOI] [PubMed] [Google Scholar]
- 14.Cotsiki M, et al. Simian virus 40 large T antigen targets the spindle assembly checkpoint protein Bub1. Proc Natl Acad Sci U S A. 2004;101(4):947–52. doi: 10.1073/pnas.0308006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckner R, et al. Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol. 1996;16(7):3454–64. doi: 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lill NL, et al. p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J Virol. 1997;71(1):129–37. doi: 10.1128/jvi.71.1.129-137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poulin DL, Kung AL, DeCaprio JA. p53 targets simian virus 40 large T antigen for acetylation by CBP. J Virol. 2004;78(15):8245–53. doi: 10.1128/JVI.78.15.8245-8253.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn WC, et al. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22(7):2111–23. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mungre S, et al. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J Virol. 1994;68(3):1675–81. doi: 10.1128/jvi.68.3.1675-1681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, et al. T-antigen mutant activities in vivo: roles of p53 and pRB binding in tumorigenesis of the choroid plexus. Oncogene. 1992;7(6):1167–75. [PubMed] [Google Scholar]
- 21.Chen JD, Van Dyke T. Uniform cell-autonomous tumorigenesis of the choroid plexus by papovavirus large T antigens. Mol Cell Biol. 1991;11(12):5968–76. doi: 10.1128/mcb.11.12.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saenz Robles MT, et al. Induction versus progression of brain tumor development: differential functions for the pRB- and p53-targeting domains of simian virus 40 T antigen. Mol Cell Biol. 1994;14(4):2686–98. doi: 10.1128/mcb.14.4.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Symonds H, et al. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994;78(4):703–11. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- 24.Pan H, et al. Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol Cell. 1998;2(3):283–92. doi: 10.1016/s1097-2765(00)80273-7. [DOI] [PubMed] [Google Scholar]
- 25.Liao MJ, et al. Atm is dispensable for p53 apoptosis and tumor suppression triggered by cell cycle dysfunction. Mol Cell Biol. 1999;19(4):3095–102. doi: 10.1128/mcb.19.4.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao A, et al. Astrocyte inactivation of the pRb pathway predisposes mice to malignant astrocytoma development that is accelerated by PTEN mutation. Cancer Cell. 2002;1(2):157–68. doi: 10.1016/s1535-6108(02)00029-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee YC, Asa SL, Drucker DJ. Glucagon gene 5′-flanking sequences direct expression of simian virus 40 large T antigen to the intestine, producing carcinoma of the large bowel in transgenic mice. J Biol Chem. 1992;267(15):10705–8. [PubMed] [Google Scholar]
- 28.Ratineau C, Ronco A, Leiter AB. Role of the amino-terminal domain of simian virus 40 early region in inducing tumors in secretin-expressing cells in transgenic mice. Gastroenterology. 2000;119(5):1305–11. doi: 10.1053/gast.2000.19278. [DOI] [PubMed] [Google Scholar]
- 29.Upchurch BH, et al. Peptide YY expression is an early event in colonic endocrine cell differentiation: evidence from normal and transgenic mice. Development. 1996;122(4):1157–63. doi: 10.1242/dev.122.4.1157. [DOI] [PubMed] [Google Scholar]
- 30.Garabedian EM, et al. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272(38):23729–40. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 31.Gum JR, Jr, et al. Mouse intestinal goblet cells expressing SV40 T antigen directed by the MUC2 mucin gene promoter undergo apoptosis upon migration to the villi. Cancer Res. 2001;61(8):3472–9. [PubMed] [Google Scholar]
- 32.Herring BP, et al. Targeted expression of SV40 large T-antigen to visceral smooth muscle induces proliferation of contractile smooth muscle cells and results in megacolon. J Biol Chem. 1999;274(25):17725–32. doi: 10.1074/jbc.274.25.17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, et al. Transgenic mouse models that explore the multistep hypothesis of intestinal neoplasia. J Cell Biol. 1993;123(4):877–93. doi: 10.1083/jcb.123.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SH, et al. Expression of wild-type and mutant simian virus 40 large tumor antigens in villus-associated enterocytes of transgenic mice. Proc Natl Acad Sci U S A. 1994;91(15):6914–8. doi: 10.1073/pnas.91.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauft SM, et al. Expression of SV-40 T antigen in the small intestinal epithelium of transgenic mice results in proliferative changes in the crypt and reentry of villus-associated enterocytes into the cell cycle but has no apparent effect on cellular differentiation programs and does not cause neoplastic transformation. J Cell Biol. 1992;117(4):825–39. doi: 10.1083/jcb.117.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markovics JA, et al. Intestinal dysplasia induced by simian virus 40 T antigen is independent of p53. J Virol. 2005;79(12):7492–502. doi: 10.1128/JVI.79.12.7492-7502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coopersmith CM, et al. Bi-transgenic mice reveal that K-rasVal12 augments a p53-independent apoptosis when small intestinal villus enterocytes reenter the cell cycle. J Cell Biol. 1997;138(1):167–79. doi: 10.1083/jcb.138.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saenz-Robles MT, et al. Intestinal hyperplasia induced by simian virus 40 large tumor antigen requires E2F2. J Virol. 2007;81(23):13191–9. doi: 10.1128/JVI.01658-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandrasekaran C, Coopersmith CM, Gordon JI. Use of normal and transgenic mice to examine the relationship between terminal differentiation of intestinal epithelial cells and accumulation of their cell cycle regulators. J Biol Chem. 1996;271(45):28414–21. doi: 10.1074/jbc.271.45.28414. [DOI] [PubMed] [Google Scholar]
- 40.Rathi AV, Saenz Robles MT, Pipas JM. Enterocyte proliferation and intestinal hyperplasia induced by simian virus 40 T antigen require a functional J domain. J Virol. 2007;81(17):9481–9. doi: 10.1128/JVI.00922-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haigis K, et al. The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J Biol Chem. 2006;281(1):638–47. doi: 10.1074/jbc.M509053200. [DOI] [PubMed] [Google Scholar]
- 42.Coopersmith CM, Gordon JI. gamma-Ray-induced apoptosis in transgenic mice with proliferative abnormalities in their intestinal epithelium: re-entry of villus enterocytes into the cell cycle does not affect their radioresistance but enhances the radiosensitivity of the crypt by inducing p53. Oncogene. 1997;15(2):131–41. doi: 10.1038/sj.onc.1201176. [DOI] [PubMed] [Google Scholar]
- 43.Komarova EA, et al. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19(33):3791–8. doi: 10.1038/sj.onc.1203717. [DOI] [PubMed] [Google Scholar]
- 44.Windle JJ, et al. Retinoblastoma in transgenic mice. Nature. 1990;343(6259):665–9. doi: 10.1038/343665a0. [DOI] [PubMed] [Google Scholar]
- 45.Mahon KA, et al. Oncogenesis of the lens in transgenic mice. Science. 1987;235(4796):1622–8. doi: 10.1126/science.3029873. [DOI] [PubMed] [Google Scholar]
- 46.Chen Q, et al. Inhibition of lens fiber cell morphogenesis by expression of a mutant SV40 large T antigen that binds CREB-binding protein/p300 but not pRb. J Biol Chem. 2004;279(17):17667–73. doi: 10.1074/jbc.M311678200. [DOI] [PubMed] [Google Scholar]
- 47.Fromm L, et al. The retinoblastoma protein-binding region of simian virus 40 large T antigen alters cell cycle regulation in lenses of transgenic mice. Mol Cell Biol. 1994;14(10):6743–54. doi: 10.1128/mcb.14.10.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgenbesser SD, et al. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994;371(6492):72–4. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315(6015):115–22. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 50.Casanovas O, et al. Incomplete inhibition of the Rb tumor suppressor pathway in the context of inactivated p53 is sufficient for pancreatic islet tumorigenesis. Oncogene. 2005;24(44):6597–604. doi: 10.1038/sj.onc.1208823. [DOI] [PubMed] [Google Scholar]
- 51.Herzig M, Novatchkova M, Christofori G. An unexpected role for p53 in augmenting SV40 large T antigen-mediated tumorigenesis. Biol Chem. 1999;380(2):203–11. doi: 10.1515/BC.1999.028. [DOI] [PubMed] [Google Scholar]
- 52.Christofori G, Naik P, Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994;369(6479):414–8. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- 53.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1(4):339–53. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 54.Ornitz DM, et al. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987;238(4824):188–93. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- 55.Bell RH, Jr, Memoli VA, Longnecker DS. Hyperplasia and tumors of the islets of Langerhans in mice bearing an elastase I-SV40 T-antigen fusion gene. Carcinogenesis. 1990;11(8):1393–8. doi: 10.1093/carcin/11.8.1393. [DOI] [PubMed] [Google Scholar]
- 56.Ceci JD, et al. Transgenic mice carrying a murine amylase 2.2/SV40 T antigen fusion gene develop pancreatic acinar cell and stomach carcinomas. Oncogene. 1991;6(2):323–32. [PubMed] [Google Scholar]
- 57.Ramel S, et al. Inactivation of p53 and the development of tetraploidy in the elastase-SV40 T antigen transgenic mouse pancreas. Pancreas. 1995;11(3):213–22. doi: 10.1097/00006676-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Tevethia MJ, et al. A simian virus 40 large T-antigen segment containing amino acids 1 to 127 and expressed under the control of the rat elastase-1 promoter produces pancreatic acinar carcinomas in transgenic mice. J Virol. 1997;71(11):8157–66. doi: 10.1128/jvi.71.11.8157-8166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennoun M, et al. The amino-terminal region of SV40 large T antigen is sufficient to induce hepatic tumours in mice. Oncogene. 1998;17(10):1253–9. doi: 10.1038/sj.onc.1202047. [DOI] [PubMed] [Google Scholar]
- 60.Symonds HS, et al. Use of transgenic mice reveals cell-specific transformation by a simian virus 40 T-antigen amino-terminal mutant. Mol Cell Biol. 1993;13(6):3255–65. doi: 10.1128/mcb.13.6.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan CS, Cantalupo P, Pipas JM. The molecular chaperone activity of simian virus 40 large T antigen is required to disrupt Rb-E2F family complexes by an ATP-dependent mechanism. Mol Cell Biol. 2000;20(17):6233–43. doi: 10.1128/mcb.20.17.6233-6243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stubdal H, et al. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17(9):4979–90. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.al-Ubaidi MR, et al. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J Cell Biol. 1992;119(6):1681–7. doi: 10.1083/jcb.119.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pichel JG, Lakso M, Westphal H. Timing of SV40 oncogene activation by site-specific recombination determines subsequent tumor progression during murine lens development. Oncogene. 1993;8(12):3333–42. [PubMed] [Google Scholar]
- 65.Tolbert D, et al. p19(ARF) is dispensable for oncogenic stress-induced p53-mediated apoptosis and tumor suppression in vivo. Mol Cell Biol. 2002;22(1):370–7. doi: 10.1128/MCB.22.1.370-377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]