Abstract
Multidrug-resistant Acinetobacter baumannii infections represent a growing problem, especially in traumatic wounds and burns suffered by military personnel injured in Middle Eastern conflicts. Effective treatment with traditional antibiotics can be extremely difficult, and new antimicrobial approaches are being investigated. One of these alternatives to antimicrobials could be the combination of nontoxic photosensitizers (PSs) and visible light, known as photodynamic therapy (PDT). We report on the establishment of a new mouse model of full-thickness thermal burns infected with a bioluminescent derivative of a clinical Iraqi isolate of A. baumannii and its PDT treatment by topical application of a PS produced by the covalent conjugation of chlorin(e6) to polyethylenimine, followed by illumination of the burn surface with red light. Application of 108 A. baumannii cells to the surface of 10-s burns made on the dorsal surface of shaved female BALB/c mice led to chronic infections that lasted, on average, 22 days and that were characterized by a remarkably stable bacterial bioluminescence. PDT carried out on day 0 soon after application of the bacteria gave over 3 log units of loss of bacterial luminescence in a light exposure-dependent manner, while PDT carried out on day 1 and day 2 gave an approximately 1.7-log reduction. The application of PS dissolved in 10% or 20% dimethyl sulfoxide without light gave only a modest reduction in the bacterial luminescence from mouse burns. Some bacterial regrowth in the treated burn was observed but was generally modest. It was also found that PDT did not lead to the inhibition of wound healing. The data suggest that PDT may be an effective new treatment for multidrug-resistant localized A. baumannii infections.
Acinetobacter baumannii is a gram-negative pathogenic bacterium that has recently attracted much attention due to its remarkable acquisition of multidrug resistance (18, 19). It has been reported to have caused intractable infections in traumatic wounds and burns suffered by military personnel injured in recent Middle Eastern conflicts (24, 25). Photodynamic therapy (PDT) is a rapidly expanding approach to the treatment of diseases because it eliminates unwanted cells, such as malignant cancer cells and infectious microbial cells. PDT involves the combination of nontoxic photosensitizers (PSs) and harmless visible light that, in the presence of oxygen, give reactive oxygen species by energy or electron transfer from the PS excited state that are able to oxidize biomolecules and thereby kill cells (17). The use of PDT to treat localized infections generally involves the topical application of a PS into the infected tissue, followed by illumination with red or near-infrared light that is able to penetrate the tissue (2, 9). Selectivity for bacteria over host tissue can be obtained by the appropriate chemical design of the PS to ensure that the molecule will preferentially bind to bacterial cells rather than mammalian cells. It has been determined by many researchers that the most important features of this molecular design are a combination of an overall cationic charge and water solubility (11, 16). Cationic charge is even more important in the case of gram-negative bacteria that possess a double membrane structure because that structure excludes many anionic and uncharged lipophilic molecules that can effectively penetrate gram-positive bacteria and fungal cells (14).
We have previously reported that a covalent conjugate between the tetrapyrrole molecule known as chlorin(e6) (ce6) and the synthetic cationic polymer polyethylenimine (PEI) is a highly effective PS with activity against gram-negative species (27).
In this report we describe the establishment of a mouse model of A. baumannii infection in full-thickness thermal burns. We used a multidrug-resistant clinical isolate from a U.S. soldier that was transformed with a plasmid that encodes the entire lux operon from Photorhabdus luminescens and that allows bioluminescence imaging of the course of the infection noninvasively in real time. The topical application of PEI-ce6 was carried out, followed by illumination with red light after 15 min.
MATERIALS AND METHODS
Strain and culture conditions.
The initial strain that we obtained was A. baumannii ATCC BAA 747 (ATCC, Manassas, VA). The A. baumannii strain that we employed in the infection was a multidrug-resistant clinical isolate from an injured U.S. soldier deployed to Iraq. A. baumannii was grown in brain heart infusion medium in an orbital shaking incubator (37°C, 100 rpm) to an optical density at 600 nm of 0.6 to 0.8, which corresponds to 108 cells/ml (mid-log phase). This suspension was then centrifuged, washed with phosphate-buffered saline (PBS), and resuspended in PBS at a density of 2 × 109 cells/ml. Bacterial luminescence was routinely measured by the use of 100-μl aliquots of bacterial suspensions in 96-well black-sided plates and a luminescence plate reader (MicroBeta Trilux 1450; PerkinElmer Life and Analytical Sciences Inc., Wellesley, MA).
Introduction of bioluminescence genes into the clinical isolate of A. baumannii.
The bioluminescence genes (luxCDABE operon) were originally cloned from P. luminescens (29). The luxCDE genes encode an enzyme complex that synthesizes the luciferase substrate decanal using precursors from the fatty acid cycle. The luxAB genes encode the luciferase enzyme, which catalyzes the light-emitting reaction. The luxCDABE operon, which was contained in plasmid pMF 385, was initially cloned into A. baumannii ATCC BAA 747. Plasmid pMF 385 was proved to be a stable genetic reporter in the gram-negative organisms Escherichia coli and Pseudomonas aeruginosa (10). Plasmid pMF 385 was introduced into the clinical A. baumannii strain by following mostly standard molecular cloning protocols (23). Small-scale preparations of plasmids was done with a Fast Plasmid Eppendorf kit (5Prime, Gaithersburg, MD). The electroporation of A. baumannii was performed with a Gene Pulser apparatus (Bio-Rad Laboratories, Munich, Germany). Selection for the electrotransformants was performed on Luria-Bertrani (LB) plates containing carbenicillin (250 μg/ml). The bacteria were plated directly onto LB agar plates, and luciferase-expressing colonies were selected by bioluminescence imaging.
Bioluminescence imaging.
The bioluminescence imaging system (Hamamatsu Photonics KK, Bridgewater, NJ) has been described in detail elsewhere (10). In short, it consists of an intensified charge-coupled-device camera mounted in a light-tight specimen chamber fitted with a light-emitting diode, a setup that allowed a background grayscale image of the entire mouse to be captured. In the photon-counting mode, an image of the light emitted from the bacteria was captured by using an integration time of 2 min at a maximum setting on the image-intensifier control module. By use of ARGUS software (Hamamatsu), the luminescence image was presented as a false-color image superimposed on top of the grayscale reference image. The image-processing component of the software calculated the total pixel values (in relative luminescence units [RLU]) from the luminescence images of the infected wound area.
Mouse model of full-thickness thermal burns.
Adult female BALB/c mice (age, 6 to 8 weeks; weight, 17 to 21 g; Charles River Laboratories, Wilmington, MA) were used in the study. The animals were housed one per cage, were maintained on a 12-h light and 12-h dark cycle, and had access to food and water ad libitum. All animal procedures were approved by the Subcommittee on Research Animal Care (IACUC) of the Massachusetts General Hospital and met the guidelines of the National Institutes of Health. The mice received buprenorphine (0.03 mg/kg of body weight subcutaneously twice a day) for 3 days after the burn for pain relief. The mice were euthanized according to the protocol when their condition was assessed to be moribund.
Before the creation of burns, the mice were anesthetized by the intraperitoneal (i.p.) injection of a ketamine-xylazine cocktail, and then the dorsal surface was shaved. Burns were created by applying two preheated (≈95°C) brass blocks (Small Parts, Inc., Miami, FL) to the opposing sides of an elevated skin fold on the dorsal surface of the mice (26) for 10 s (nonlethal, full-thickness, third-degree burns). The combined brass block area was 15 mm by 10 mm, giving an area of 150 mm2 and corresponding to 4% of the total body surface area (6). Immediately after the creation of the burns, the mice were resuscitated with i.p. injections of 0.5 ml sterile saline (Phoenix Scientific Inc., St. Joseph, MO) to prevent dehydration.
Establishment of infection.
Bacterial infection took place as described by Ha and Jin (7). Five minutes after the creation of the burns (to allow the burns to cool), a suspension (50 μl) of A. baumannii in sterile PBS containing 108 cells was inoculated onto the surface of each burn with a pipette tip and was then smeared onto the burn surface with an inoculating loop. The mice were imaged with the luminescence camera immediately after application of the bacteria to ensure that the bacterial inoculum applied to each burn was consistent.
Preparation of PEI-ce6 conjugate.
The PEI-ce6 conjugate was prepared by a modification of the method described previously (27). In brief, cross-linked PEI (high molecular weight [10,000 to 25,000]; catalog number 40,872-7; Aldrich) was reacted with ce6 (Frontier Scientific, Logan, UT) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (Sigma) in borate buffer (0.1 M, pH 8.5) for 24 h in the dark. The crude conjugate was purified by precipitation from acetone at −20°C, followed by exhaustive (2 days, three changes) dialysis (molecular mass cutoff, 2 kDa) against distilled water containing 0.1% Triton X-100. The substitution ratio was calculated from the absorption spectrum of the conjugate (0.1 M NaOH, 1% sodium dodecyl sulfate) to be an average of 1 ce6/PEI chain by assuming that the absorption coefficient of conjugated ce6 is the same as that of free ce6 (ɛ400 = 150,000 M−1 cm−1) and according to the results of the trinitrobenzene sulfonic acid assay for free amino groups (8).
PDT.
PEI-ce6 was added at 30 min, 24 h, or 48 h after infection. The conjugate was added as 50 μl of a solution in PBS-dimethyl sulfoxide (DMSO) cocktail (10% or 20% DMSO, 800 to 900 μM ce6 equivalent), which was added to the burns that would be treated by the use of PDT or with PS only (dark controls). After a further 15 min to allow the conjugate to bind to and penetrate the bacteria, the mice were again imaged by using a luminescence camera to quantify any dark toxicity of the conjugate to the bacteria. The mice were then illuminated with 660 ± 15 nm light delivered by a noncoherent light source (LumaCare, Newport Beach, CA). The light power was routinely measured with a power meter (Coherent, Portland, OR), and the power density used was 100 mW/cm2. The mice were given a total light exposure of up to 240 J/cm2 in aliquots, with luminescence imaging taking place after exposure to each aliquot of light. Immediately after PDT, the mice were resuscitated with a second i.p. injection of 0.5 ml sterile saline to prevent dehydration.
Mouse follow-up.
The bacterial luminescence from the mouse burns was recorded daily until the bioluminescence disappeared or the animals were determined to be moribund and euthanized. The burn area was measured each day with a vernier caliper until the wound healed or the animal was euthanized. When the animals were euthanized, blood samples were also taken from the heart of the dead mice and streaked onto brain heart infusion agar plates.
Statistical methods.
In a two-dimensional coordinate system, the area-under-the-curve data, which represent the time courses of bacterial luminescence of the burn or the time courses of the burn area, were calculated by the use of numerical integration (1). Differences in the areas under the curves between the control and the treatment groups and between different treatments groups were compared for statistical significance by a t test. Survival analysis was performed by the Kaplan-Meier method (12). Survival curves were compared, and the differences in the survival rates were tested for significance by the use of a log-rank test (20). Linear correlation was tested for significance by the use of the Vassar-Stats program (http://faculty.vassar.edu/lowry/corr_stats.html). P values of <0.05 were considered significant.
RESULTS
Correlation of bacterial luminescence to CFU of A. baumannii.
By using a luminescence plate reader in combination with a colony formation assay, a good linear correlation (R = 0.9972, P < 0.0001) between the bacterial luminescence and the numbers of CFU (6 orders of magnitude) of the A. baumannii strain used in the present study was found (Fig. 1).
FIG. 1.
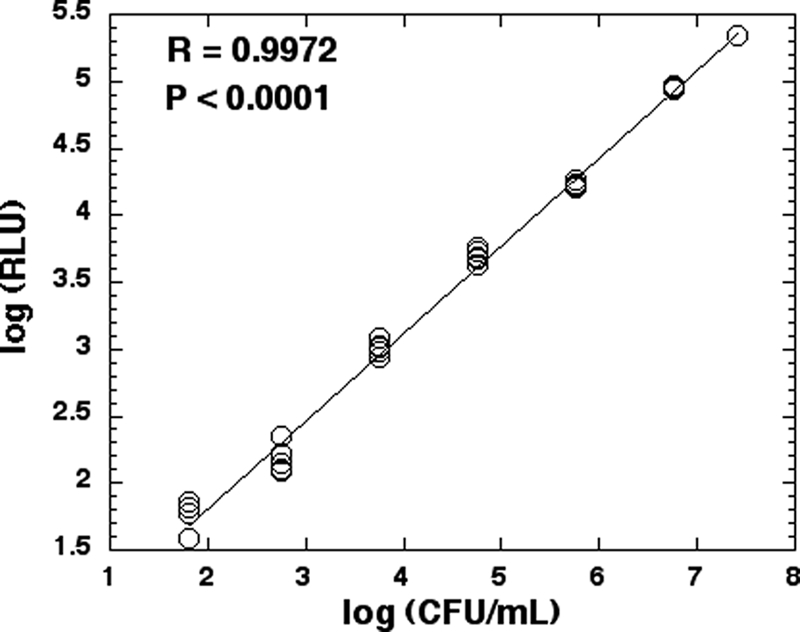
Linear correlation between bioluminescence (RLU) and CFU (CFU/ml) of A. baumannii.
Mouse model of A. baumannii infection in burns.
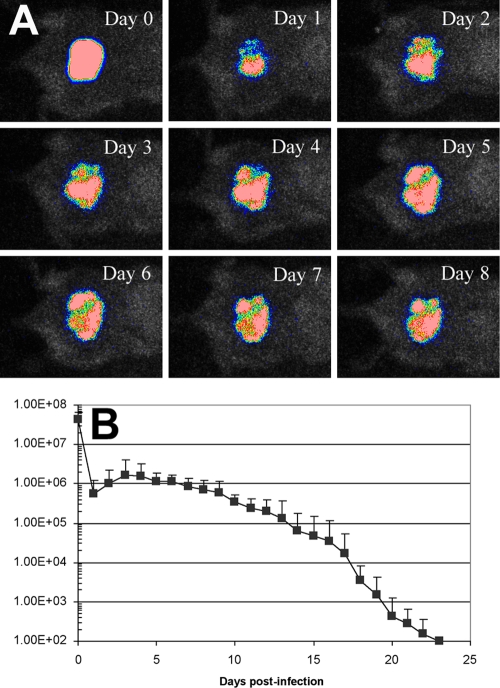
By applying 108 CFU of A. baumannii onto 10-s burns made on the dorsal surface of adult female BALB/c mice, a stable infection was developed in the mouse burns, as characterized by bacterial luminescence imaging. Figure 2A shows the successive bacterial luminescence images of a representative mouse burn infected with A. baumannii. After an initial reduction in the signal on day 1, the bacterial luminescence rebounded on day 2 and remained strong and stable until day 8 (Fig. 2A) and detectable until day 22. Figure 2B depicts the time course of the mean bacterial luminescence values of the infected mouse burns (n = 19). It can be seen that the infection was sustained for over 3 weeks in this model.
FIG. 2.
(A) Successive luminescence images of a representative mouse burn infected with luminescent A. baumannii; (B) time course of mean bacterial luminescence value of the infected mouse burn (n = 19).
Therapeutic responses of A. baumannii infection in mouse burns to PDT.
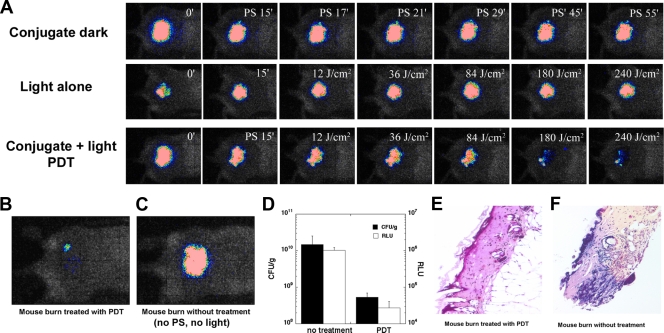
Figure 3A shows the PDT dose-response of the bacterial luminescence of a representative mouse burn infected with A. baumannii and treated with PDT on day 1 (24 h) after infection, a mouse burn treated with PS only (dark control), and a mouse burn treated with light only (light control). For the light control, PBS (with the same amount of DMSO) instead of PS solution was applied to the burn. PDT induced an approximately 1.8-log-unit reduction in bacterial luminescence from the mouse burn, while during the same period of time, less than a 0.9-log-unit reduction of bacterial luminescence was observed for the dark control. The bacterial luminescence of the control treated with light alone increased by a factor of 2 during the same period.
FIG. 3.
(A) Dose-response of bacterial luminescence from a representative mouse burn infected with A. baumannii and treated with PS only at 24 h after infection (conjugate dark [control]), a representative mouse burn infected with A. baumannii and treated with light only at 24 h after infection (light alone [control]), and a representative mouse burn infected with A. baumannii and treated with PS and light (conjugate + light PDT) at 24 h after infection; (B) luminescence images, captured on day 5 postinfection, of a mouse burn treated by use of PDT 4 days previously, on day 1; (C) mouse burn without treatment (neither PS nor light was applied); (D) numbers of CFU of A. baumannii and luminescence values (in RLU) from the mouse burns shown in panel B. (E and F) Gram-stained histology sections of the mouse burns shown in panel B.
Figure 3B and C shows the luminescence images captured on day 5 of a mouse burn treated 4 days previously (on day 1) and a no-treatment control (neither PS nor light was applied), respectively. The bacterial luminescence of the nontreated mouse burn (≈1.02 × 106 RLU) was 2 log units higher than that of the treated mouse burn (≈2.73 × 104 RLU). The burned areas of the two mice were then excised. Half of each excised burn was homogenized for determination of the number of bacterial CFU, and the other was sectioned for Gram staining. Quantification of the bacterial CFU (mean of three replicates) (Fig. 3D) indicated that the bacterial bioburden in the nontreated burn (≈1.46 × 1010 CFU/g) was about 2 log units higher than that in the burn treated by use of PDT (5.31 × 108 CFU/g). Therefore, the bacterial luminescence intensity is linearly correlated with the number of bacterial CFU. The Gram-stained histological sections of the two burns showed that there was a much greater bacterial bioburden (dark blue staining) in the nontreated burn (Fig. 3F) than in the burn treated by use of PDT (Fig. 3E).
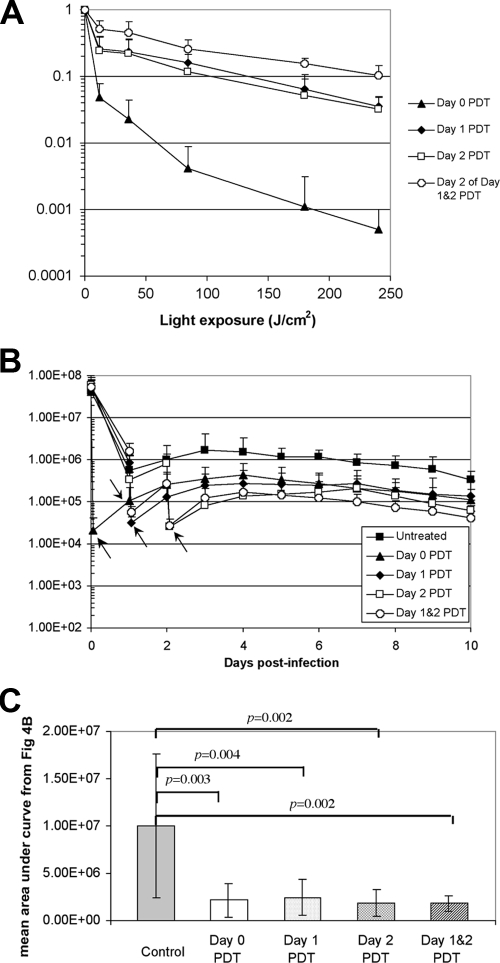
Figure 4A shows the dose-response of the mean bacterial luminescence from the infected mouse burns treated by use of PDT on day 0 (30 min postinfection; n = 7), day 1 (24 h postinfection; n = 11), and day 2 (48 h postinfection; n = 6) and the mean dose-response of the mean bacterial luminescence on day 2 from the mouse burns treated by use of repeated PDT on day 1 and day 2 (n = 9). PDT was carried out on day 0 and gave an average loss of bacterial luminescence of 3.6 log units in a light exposure-dependent manner, while PDT carried out on day 1 or day 2 gave an approximately 1.7-log-unit reduction. When PDT was carried out consecutively on day 1 and day 2, an extra approximately 1-log-unit reduction of bacterial luminescence was achieved on day 2, in addition to the initial 1.7-log-unit reduction on day 1.
FIG. 4.
(A) Dose-response of mean bacterial luminescence of the mouse burns infected with A. baumannii and treated by use of PDT at day 0 (30 min), day 1 (24 h), and day 2 (48 h) and consecutive PDT at day 1 (24 h) and day 2 (48 h) after infection; (B) time courses of bacterial luminescence values of the infected burns in different groups of mice (arrows, endpoint of PDT); (C) mean areas under the bioluminescence-versus-time plots (in the two-dimensional coordinate system in panel A) representing the overall bioburden of the mouse burns in different groups.
The time courses (from day 0 to day 10) of the mean bacterial luminescence of the infected burns in the different groups of mice are shown in Fig. 4B. Some bacterial regrowth in the burns treated by use of PDT was observed, but it was generally modest. The average bacterial luminescence intensity of the burns treated by use of PDT after regrowth was at least 1 log unit lower than that of the nontreated burns at the same time points. Statistical comparison of the areas under the bioluminescence-versus-time plots in the two-dimensional coordinate system in Fig. 4B showed that PDT significantly decreased the bacterial bioburden of the infected burns (P < 0.004) (Fig. 4C).
Survival analysis.
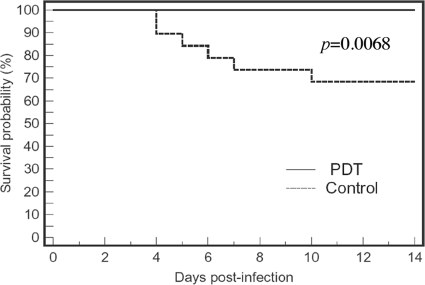
Figure 5 shows the Kaplan-Meier survival curves for the untreated mice (n = 19) and the mice treated by use of PDT (by combining the data for the mice treated by use of PDT on day 0, day 1, day 2, and day 1 and day 2 combined). It can be seen that the mice treated by use of PDT had a survival advantage over the nontreated mice. On day 21 postinfection, the survival rates of the nontreated mice and the mice treated by use of PDT were 68.4% and 100.0%, respectively (P = 0.0068). Nonbioluminescent Escherichia coli was found in the blood samples taken from the hearts of the dead mice, but no luminescent A. baumannii colonies were observed in the cultures of the blood samples.
FIG. 5.
Kaplan-Meier survival curves of A. baumannii-infected mouse burns that were not treatment (neither PS nor light was applied) and that were treated by use of PDT.
Wound healing.
All the mouse burns, including infected burns treated by use of PDT, infected burns not treated by use of PDT, and noninfected burns, demonstrated an initial increase in wound size until day 6; and then the wound size gradually decreased until the complete healing of the burns (data not shown). Statistical comparison of the areas under the burn area-versus-time plots in the two-dimensional coordinate system showed that there was no significant difference among the wound healing rates of noninfected burns, infected burns treated by use of PDT, and infected burns treated by use of PDT on day 1 (P > 0.4).
DISCUSSION
Topical PDT was highly effective in reducing the bacterial luminescence (i.e., inactivating the bacterial cells) in full-thickness mouse burns infected with A. baumannii. Although A. baumannii has developed something of a reputation as a superbug, this reputation does not seem to be derived from any high degree of invasiveness. In our mouse model of a full-thickness burn infection, A. baumannii appeared to form highly stable infections that could last for as long as 3 weeks in the surviving mice. This extended duration represents a very chronic infection in terms of mouse models, and its long-lasting properties are probably due to the high biofilm-forming capacity of the A. baumannii strain (28). It appeared that there was only a limited time during which the organisms causing the infection were capable of growing, as judged by the increasing bacterial luminescence signal. After about 3 days, the infection had reached a stable level and would likely maintain this level for a period of weeks, with only a slow decline being evident.
The results of treatment by use of PDT showed that PDT was the most effective when it was carried out on day 0, soon after the application of the bacteria to the surface of the burn. At that point, the A. baumannii cells had not penetrated deep into the burns and remained superficial, rendering the inactivation of A. baumannii by PDT easier. The treatment of bacterial infections soon after the inoculation of bacteria is analogous to the treatment of bacterial infections at the very early stage of wound contamination. On day 1 or day 2, as suggested by the results of previous studies (13, 22), the bacteria had penetrated deep into the burns and the infection became established by forming a biofilm; as a result, the rate of inactivation of the A. baumannii population by PDT was lower with the same PDT dose in comparison to the rate of inactivation measured on day 0.
The level of regrowth of A. baumannii in mouse burns treated by use of PDT was modest in comparison to that in the control mouse burns that were not treated. This regrowth may be due to the fact that the bacteria were in the growing phase of the infection, which was from day 1 to day 3. Mice receiving repeated PDT treatments, carried out consecutively on day 1 and day 2, had the lowest bacterial bioburden, although a statistically significant difference was not detected. This was because PDT on day 2 counteracted the effect of bacterial regrowth from day 1 to day 2. If repeated applications of PDT are required due to significant bacterial regrowth, which was rare in the current study with mouse models, daily PDT can be applied until the extent of the infection decreases (the application of PDT several times may be expected).
The significant reduction in the bacterial burden in the infected mouse burns treated by use of PDT subsequently reduced the rate of mortality of the mice. In this study, no mortality occurred in any of the mice treated by use of PDT, while about one-third of the nontreated mice died. We did not observe bioluminescent A. baumannii in the blood samples taken from the dead mice. This is a limitation of this mouse infection model, as bacteremia was observed in the soldiers with burn injuries infected with the same strain of A. baumannii (21). Instead, we found the presence of a nonluminescent E. coli strain in the bloodstreams of the infected mice. We believe that the presence of E. coli in the bloodstream arose by translocation from the large intestine induced by the combination of a third-degree burn and superimposed infection, which caused a deficiency of the gut barrier (3, 5). Previous studies have also shown that infectious insults combined with thermal burns in small-animal models promote bacterial translocation from the gut (15).
It was found in this study that there was no significant difference in the wound healing rates between infected burns not treated by use of PDT and noninfected burns, implying that the A. baumannii strain that we used does not inhibit wound healing. This may also explain why the inactivation of A. baumannii in mouse burns by PDT did not offer any benefit regarding wound healing.
From the data presented above, it can be concluded that PDT has the potential to be an alternative option for the treatment of multidrug-resistant A. baumannii wound infections.
Acknowledgments
This work was supported by the U.S. Air Force MFEL program (contract FA9550-04-1-0079) and the NIH (grant AI050875). T. Dai was supported by a Bullock-Wellman Postdoctoral Fellowship Award.
We are grateful to Victoria Hamrahi for assistance with bacterial identification and to Christopher H. Contag, Tayyaba Hasan, and Albert T. McManus for helpful advice and discussion.
Footnotes
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Davis, P. J., and P. Rabinowitz. 1975. Methods of numerical integration, p. 459. Academic Press, Inc., New York, NY.
- 2.Demidova, T. N., and M. R. Hamblin. 2004. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 17:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaves-Pyles, T., and J. W. Alexander. 2001. Comparison of translocation of different types of microorganisms from the intestinal tract of burned mice. Shock 16:148-152. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Gianotti, L., J. W. Alexander, T. Pyles, L. James, and G. F. Babcock. 1993. Relationship between extent of burn injury and magnitude of microbial translocation from the intestine. J. Burn Care Rehabil. 14:336-342. [DOI] [PubMed] [Google Scholar]
- 6.Gilpin, D. A. 1996. Calculation of a new Meeh constant and experimental determination of burn size. Burns 22:607-611. [DOI] [PubMed] [Google Scholar]
- 7.Ha, U., and S. Jin. 1999. Expression of the soxR gene of Pseudomonas aeruginosa is inducible during infection of burn wounds in mice and is required to cause efficient bacteremia. Infect. Immun. 67:5324-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habeeb, A. F. 1966. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 14:328-336. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamblin, M. R., D. A. O'Donnell, N. Murthy, C. H. Contag, and T. Hasan. 2002. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem. Photobiol. 75:51-57. [DOI] [PubMed] [Google Scholar]
- 11.Jori, G., C. Fabris, M. Soncin, S. Ferro, O. Coppellotti, D. Dei, L. Fantetti, G. Chiti, and G. Roncucci. 2006. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg. Med. 38:468-481. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan, E. L., and P. Meier. 1958. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53:457-481. [Google Scholar]
- 13.Loehfelm, T. W., N. R. Luke, and A. A. Campagnari. 2008. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J. Bacteriol. 190:1036-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik, Z., H. Ladan, and Y. Nitzan. 1992. Photodynamic inactivation of gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B 14:262-266. [DOI] [PubMed] [Google Scholar]
- 15.Manson, W. L., J. M. Coenen, H. J. Klasen, and E. H. Horwitz. 1992. Intestinal bacterial translocation in experimentally burned mice with wounds colonized by Pseudomonas aeruginosa. J. Trauma 33:654-658. [DOI] [PubMed] [Google Scholar]
- 16.Merchat, M., G. Bertolini, P. Giacomini, A. Villanueva, and G. Jori. 1996. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 32:153-157. [DOI] [PubMed] [Google Scholar]
- 17.Mroz, P., and M. R. Hamblin. 2008. Advances in photodynamic therapy: basic, translational and clinical. Artech House, Norwood, MA.
- 18.Peleg, A. Y. 2007. Optimizing therapy for Acinetobacter baumannii. Semin. Respir. Crit. Care Med. 28:662-671. [DOI] [PubMed] [Google Scholar]
- 19.Perez, F., A. Endimiani, and R. A. Bonomo. 2008. Why are we afraid of Acinetobacter baumannii? Expert Rev. Anti-Infect. Ther. 6:269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peto, R., and J. Peto. 1972. Asymptotically efficient rank invariant test procedures. J. R. Stat. Soc. A 135:185-207. [Google Scholar]
- 21.Ressner, R. A., C. K. Murray, M. E. Griffith, M. S. Rasnake, D. R Hospenthal, and S. E Wolf. 2008. Outcomes of bacteremia in burn patients involved in combat operations overseas. J. Am. Coll. Surg. 206:439-444. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Baño, J., S. Martí, S. Soto, F. Fernández-Cuenca, J. M. Cisneros, J. Pachón, A. Pascual, L. Martínez-Martínez, C. McQueary, L. A. Actis, J. Vila, and Spanish Group for the Study of Nosocomial Infections (GEIH). 2008. Biofilm formation in Acinetobacter baumannii: associated features and clinical implications. Clin. Microbiol. Infect. 14:276-278. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 24.Scott, P., G. Deye, A. Srinivasan, C. Murray, K. Moran, E. Hulten, J. Fishbain, D. Craft, S. Riddell, L. Lindler, J. Mancuso, E. Milstrey, C. T. Bautista, J. Patel, A. Ewell, T. Hamilton, C. Gaddy, M. Tenney, G. Christopher, K. Petersen, T. Endy, and B. Petruccelli. 2007. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 44:1577-1584. [DOI] [PubMed] [Google Scholar]
- 25.Scott, P. T. 2004. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002-2004. MMWR Morb. Mortal. Wkly. Rep. 53:1063-1066. [PubMed] [Google Scholar]
- 26.Stevens, E. J., C. M. Ryan, J. S. Friedberg, R. L. Barnhill, M. L. Yarmush, and R. G. Tompkins. 1994. A quantitative model of invasive Pseudomonas infection in burn injury. J. Burn Care Rehabil. 15:232-235. [DOI] [PubMed] [Google Scholar]
- 27.Tegos, G. P., M. Anbe, C. Yang, T. N. Demidova, M. Satti, P. Mroz, S. Janjua, F. Gad, and M. R. Hamblin. 2006. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 50:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallenet, D., P. Nordmann, V. Barbe, L. Poirel, S. Mangenot, E. Bataille, C. Dossat, S. Gas, A. Kreimeyer, P. Lenoble, S. Oztas, J. Poulain, B. Segurens, C. Robert, C. Abergel, J. M. Claverie, D. Raoult, C. Medigue, J. Weissenbach, and S. Cruveiller. 2008. Comparative analysis of acinetobacters: three genomes for three lifestyles. PLoS One 3:e1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi, L., K. W. Cho, and S. C. Tu. 1991. Cloning and nucleotide sequences of lux genes and characterization of luciferase of Xenorhabdus luminescens from a human wound. J. Bacteriol. 173:1399-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]