Abstract
The potential for reutericyclin derivatives to be used as topical antibiotics to treat staphylococcal skin infections was investigated. All reutericyclins inhibited the growth of clinical isolates of drug-resistant Staphylococcus aureus. Unlike the standard topical agent mupirocin, most reutericyclin derivatives eradicated staphylococcal biofilms. Moreover, two compounds formulated in hydrophilic petrolatum (10%, wt/wt) were efficacious in treating S. aureus superficial skin infections in mice. These data exemplify the prospect of developing reutericyclins as new topical antibiotics.
The topical antibiotic mupirocin has been widely used to treat both primary and secondary skin infections and to decontaminate the nares of methicillin-resistant Staphylococcus aureus (MRSA) (5). However, the future clinical utility of this drug is severely challenged by the high prevalence of mupirocin resistance among staphylococci (5). There is therefore an ongoing need for the discovery of alternative topical agents to treat MRSA carriage and dermatological infections caused by S. aureus.
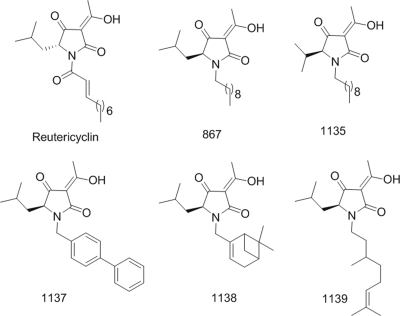
Naturally occurring tetramic acids (2,4-pyrrolidinedione) represent one potential source for obtaining new antibacterials (13). Although the antibacterial activities of tetramic acids were recognized more than 50 years ago, there are no antibiotics of this type currently available (13). Within this class of underexplored antibiotics is the amphipathic molecule reutericyclin, which is produced by Lactobacillus reuteri and exhibits activity against only gram-positive pathogens (6). This results from the dissipation of the bacterial transmembrane proton potential (6). However, like many pharmacologically active natural products, reutericyclin is difficult to synthesize because of its chemically unstable α,β-unsaturated lipophilic moiety (Fig. 1), making it unattractive for development as a chemotherapeutic agent (13). To overcome this problem, we recently reported a set of easily synthesizable, chemically stable analogs of reutericyclin that retained activity against S. aureus and other gram-positive pathogens (13). Since the chemotherapeutic potential of reutericyclins has not been evaluated (6, 13) and mupirocin resistance is rising (5), we investigated whether these chemotypes could be considered for future development as topical agents to primarily control skin infections caused by S. aureus.
FIG. 1.
Structures of reutericyclin and chemically stable derivatives. The numbers of additional carbon bonds making up the aliphatic chains are shown beside the parentheses.
The activities of five reutericyclin analogs (867, 1135, 1136, 1137, and 1138) with structurally different motifs were examined alongside reutericyclin (Fig. 1). Vancomycin and mupirocin were used as controls. MICs were determined by microdilution in Mueller-Hinton broth against a panel of contemporary strains of S. aureus (n = 51) (Table 1). Since Streptococcus pyogenes is also a common skin pathogen, MICs against a set of recent skin isolates (n = 14) were determined in brain heart infusion broth. The effect of serum on antibiotic activities was evaluated by microdilution MICs in Mueller-Hinton broth containing 50% human serum. Minimum biofilm eradication concentrations and minimum biofilm inhibitory concentrations were obtained against biofilm cells as described previously (3). The effects of the analogs on exponentially growing S. aureus 8325 and membrane integrity were evaluated as described previously (11). Cytotoxicity was evaluated by exposing normal CCD-32Sk human skin fibroblast cells (ATCC, Manassas, VA) to antibiotics for 48 h, followed by an MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay as described previously (8).
TABLE 1.
Activities of tetramic acids against strains of S. aureus and S. pyogenes
| Organism (no. of strains) | Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| MSSA (10)a | 867 | 0.4-0.8 | 0.4 | 0.8 |
| 1135 | 0.4-1.6 | 0.8 | 0.8 | |
| 1137 | 0.8-1.6 | 0.8 | 1.6 | |
| 1138 | 1.6-3.12 | 1.6 | 3.12 | |
| 1139 | 0.2-1.6 | 0.8 | 1.6 | |
| Reutericyclin | 0.1-1.6 | 0.8 | 1.6 | |
| Mupirocin | 0.05-0.2 | 0.12 | 0.2 | |
| Vancomycin | 0.4-0.8 | 0.8 | 0.8 | |
| MRSA (31)b | 867 | 0.2-1.6 | 0.8 | 1.6 |
| 1135 | 0.4-1.6 | 0.8 | 1.6 | |
| 1137 | 0.4-1.6 | 1.6 | 1.6 | |
| 1138 | 0.4-3.12 | 3.12 | 3.12 | |
| 1139 | 0.1-1.6 | 0.8 | 1.6 | |
| Reutericyclin | 1.6-6.25 | 3.12 | 6.25 | |
| Mupirocin | 0.1-0.8 | 0.2 | 0.4 | |
| Vancomycin | 0.2-6.25 | 0.8 | 3.12 | |
| Mupirocin- | 867 | 0.2-1.6 | 0.8 | 1.6 |
| resistant | 1135 | 0.8-3.12 | 1.6 | 3.12 |
| MRSA (10)c | 1137 | 0.8-1.6 | 1.6 | 1.6 |
| 1138 | 0.4-6.25 | 3.12 | 3.12 | |
| 1139 | 0.8-1.6 | 0.8 | 1.6 | |
| Reutericyclin | 0.8-3.12 | 1.6 | 3.12 | |
| Mupirocin | 12.5->200 | >200 | >200 | |
| Vancomycin | 0.2-0.8 | 0.8 | 0.8 | |
| S. pyogenes (14)d | 867 | 0.024-0.8 | 0.2 | 0.8 |
| 1135 | 0.012-1.6 | 0.2 | 0.8 | |
| 1137 | 0.012-1.6 | 0.2 | 0.8 | |
| 1138 | 0.05-3.12 | 0.4 | 1.6 | |
| 1139 | 0.05-1.6 | 0.4 | 0.8 | |
| Reutericyclin | 0.012-0.4 | 0.4 | 0.4 | |
| Mupirocin | 0.012-0.024 | 0.024 | 0.024 | |
| Vancomycin | 0.05-0.4 | 0.4 | 0.4 | |
Except for oxacillin susceptibility, the resistance phenotypes of all recent methicillin-susceptible S. aureus (MSSA) isolates are undetermined.
The MRSA test panel included 10 recent isolates with undetermined resistances except to oxacillin, 3 strains with intermediate vancomycin resistance, 15 strains resistant to clindamycin, 10 strains resistant to ciprofloxacin, 7 strains resistant to gentamicin, 6 strains resistant to tetracycline, 2 strains resistant to trimethoprim-sulfamethoxazole, and 2 strains resistant to linezolid.
This group consists of six high- and four low-level mupirocin-resistant strains.
This group includes two macrolide-resistant strains.
The therapeutic efficacies of tetramic acids were evaluated using a previously reported model for staphylococcal skin infection (10), which we modified by using BALB/c male mice and the wound isolate S. aureus ATCC 29213. These experiments were approved by the University of Tennessee Animal Care and Use Committee. Briefly, a 2-cm2 dorsal area was tape stripped with Tensoplast adhesive bandage (Smith and Nephew) to remove the epidermis, and infection was started by inoculating the damaged skin with ca. 107 cells. At 4 h postinfection, therapy was administered by topically applying ca. 50 mg of (i) a mineral oil-hydrophilic petrolatum ointment vehicle (i.e., a placebo with no additional excipients), (ii) 2% (wt/wt) mupirocin in vehicle, or (iii) 2 or 10% (wt/wt) of tetramic acid compound in vehicle. Treatment was continued through day 4, with drug application twice daily at 8-h intervals (morning and evening). On the morning of day 5, infected skin lesions were homogenized and plated to determine the viable counts as described previously (10). The results were analyzed by one-way analysis of variance, followed by Tukey's test at a P value of <0.01.
All tetramic acids tested were found to be active against the entire panel of clinical S. aureus, which included strains that were resistant to mupirocin, vancomycin, linezolid, clindamycin, and other currently used antibiotics (Table 1). These results indicate that the reported mode of action of reutericyclin (6) is not affected by resistance to routinely used drug classes. However, in the presence of 50% human serum, all reutericyclins were found to be inactive against S. aureus (≥200 μg/ml), indicating that like mupirocin (12), the compounds are highly protein bound. All compounds were active against recent clinical isolates of S. pyogenes (Table 1), which support their potential use to control dermatological infections also caused by this organism. Although reutericyclins target the cytoplasmic membrane (6, 13), our results show that they do not act as nonspecific membrane disrupters in S. aureus. Indeed, at 4× and 32× their MICs, the compounds did not cause bacteriolysis (data not shown) or induce membrane damage in S. aureus, as shown by the lack of leakage of cellular material at an optical density at 260 nm (Table 2). Rather, the compounds were primarily bacteriostatic (data not shown), causing a 2.6- to 2.8-log reduction in cells at 32× their MICs after 24 h.
TABLE 2.
Effects of antibiotics on S. aureus membrane integrity and skin fibroblast cells measured by various assays
| Antibiotic (MIC [μg/ml])a | Absorbance of culture filtrate at 260 nma | MIC (μg/ml) against S. aureus 8235 in DMEMb | Cytotoxic IC50 (μg/ml) against CCD-32Sk in DMEMb | Therapeutic indexc |
|---|---|---|---|---|
| 867 (0.8) | 0.012 ± 0.006 | 25 | 308.4 | 12.3 |
| 1135 (0.8) | 0.065 ± 0.021 | 25 | 356 | 14.4 |
| 1137 (0.8) | 0.041 ± 0.033 | 25 | 529.1 | 21.2 |
| 1138 (1.6) | 0.043 ± 0.023 | 25 | 376.8 | 15.1 |
| 1139 (0.8) | 0.023 ± 0.006 | 25 | 256.1 | 10.2 |
| Reutericyclin (1.6) | 0.05 ± 0.023 | 25 | 263.6 | 10.5 |
| Nisin (12.5) | 0.231 ± 0.047 | NAd | NA | NA |
| Amoxicillin (0.1) | 0.046 ± 0.02 | NA | NA | NA |
S. aureus 8325 resuspended in phosphate-buffered saline was exposed to antibiotics at 4× their Mueller-Hinton broth MICs for 1 h before measuring the absorbance of leaked cellular material at an optical density at 260 nm in the culture filtrate.
Both MICs and cytotoxicities were evaluated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum to allow direct determination of the therapeutic index.
The therapeutic index was calculated as the cytotoxic 50% inhibitory concentration (IC50)/MIC. Estimated therapeutic indices for vancomycin and mupirocin were >25,000 and >1,600, respectively, since the respective MICs in DMEM were 0.8 and 12.5 μg/ml and the cytotoxic IC50s were both >20,000 μg/ml.
NA, not assessed.
Reutericyclin is known to prevent the colonization of sourdough by the biofilm-forming competitor Lactobacillus sanfranciscensis while allowing the producing strain L. reuteri to become stably established (7). This might imply that reutericyclin has a natural role in preventing or eliminating biofilms of competing gram-positive organisms. However, this speculation has not been rigorously tested. We now clearly show (Table 3) that with the exception of compound 1138, all tested reutericyclins were able to kill staphylococci residing in biofilms in the Calgary Biofilm model. The minimum biofilm eradication concentrations were comparable to the control antibiofilm agent rifampin (rifampicin) but superior to mupirocin and vancomycin. Thus, reutericyclins with activities against S. aureus biofilms may be useful for controlling recalcitrant biofilm-mediated skin infections (1). The basis for the inactivity of 1138 against biofilms is unknown, though it is plausible that the bicyclic ring system of 1138 (Fig. 1) affects its solubility and penetration into the biofilm matrix.
TABLE 3.
Antibacterial activities of reutericyclins against staphylococcal biofilms
| Antibiotic | Activity (μg/ml)a for indicated strain
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MRSA ATCC 33591
|
MSSA ATCC 29213
|
S. epidermidis RP62A
|
|||||||
| MIC | MBIC | MBEC | MIC | MBIC | MBEC | MIC | MBIC | MBEC | |
| 867 | 0.8 | 0.8 | 6.25-12.5 | 0.8 | 3.12 | 50 | 0.4 | 0.4 | 50 |
| 1135 | 0.8 | 0.8 | 25 | 0.8 | 1.6 | 25 | 0.8 | 0.8 | 25-200 |
| 1137 | 0.8 | 0.8 | 100 | 0.8 | 3.12 | 100 | 0.8 | 1.6 | 200 |
| 1138 | 1.6 | 1.6 | >400 | 1.6 | 6.2 | ≥400 | 3.12 | 1.6 | >400 |
| 1139 | 0.8 | 0.4-0.8 | 50 | 0.8 | 3.12 | 50 | 0.8 | 0.4 | 200 |
| Reutericyclin | 1.6 | 0.8 | ≥400 | 1.6 | 3.12 | 50 | 1.6 | 0.8-1.6 | 100 |
| Rifampin | 0.012 | <0.2 | 6.25-12.5 | 0.006 | <0.2 | 25 | 0.012 | <0.2 | 50-100 |
| Vancomycin | 1.6 | 1.6 | >400 | 0.8 | 3.12 | >400 | 1.6 | 12.5 | >400 |
| Mupirocin | 0.06 | <0.2 | >400 | 0.1 | <0.2 | >400 | 0.12 | <0.2 | >400 |
Activities are based on two or more test replicates and are given as the range of the minimum and maximum values obtained. MSSA, methicillin-resistant S. aureus; MBIC, minimum biofilm inhibitory concentration; MBEC, minimum biofilm eradication concentration.
Spontaneous mutants could not be selected against reutericyclin, its analogs, or the vancomycin control from a bacterial population (>1010 CFU/ml) of S. aureus 8325 and MRSA N315 at 4× their MICs. This signifies that the reutericyclin class has a lower potential for selecting resistant mutants than the mupirocin control, which selected mutants at frequencies of 7.8 × 10−8 and 2.5 × 10−8. However, like the related tetramic acid vancoresmycin (2), resistant mutants may emerge in subinhibitory concentrations of reutericyclin antibiotics.
The cytotoxicities for tetramic acids against CCD-32Sk skin fibroblasts are shown in Table 2, indicating that reutericyclins selectively inhibit the growth of S. aureus with therapeutic indices comparable to those of topically applied antiseptics (4).
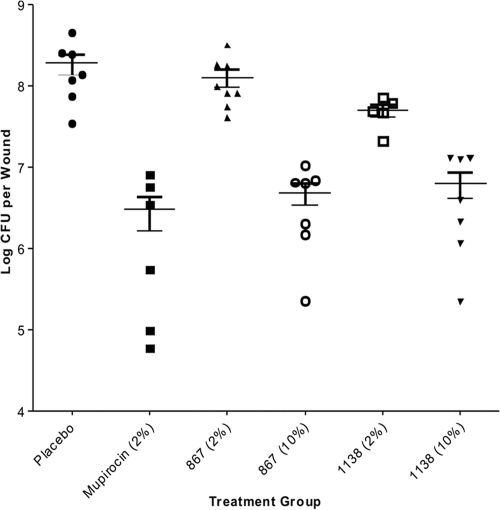
Our animal efficacy studies provide the first description of in vivo efficacy for the reutericyclin class of antibiotics against an S. aureus disease. At 10% (wt/wt), both 867 and 1138 significantly reduced the S. aureus bioburden in infected skin in a manner comparable to that of 2% topical mupirocin (Fig. 2), but limited efficacy was observed when 867 and 1138 were compounded at 2% in the hydrophilic petrolatum ointment. Although these results might imply that reutericyclins are not as efficacious as mupirocin in the murine model used, we believe the observed difference in the efficacies primarily resulted from the slow partitioning of the aqueous dermis by these highly lipophilic molecules. Consequently, alternative drug delivery systems, such as nanoemulsions (9), that are compatible with lipophilic drugs will be required to achieve good dermal penetration and improve the efficacy of reutericyclin antibiotics.
FIG. 2.
Efficacies of antibiotics against S. aureus ATCC 29213 in tape-stripped mice. The number of bacteria recovered from each mouse is shown by the symbol for the corresponding treatment group. The median value for each group is shown by the horizontal bar, and the total numbers of mice per group were as follows: placebo, n = 7; mupirocin (2%), n = 6; 867 (2%), n = 8; 867 (10%), n = 7; 1138 (2%), n = 5; and 1138 (10%), n = 7. The MICs for mupirocin, 867, and 1138 against strain 29213 are 0.1, 0.8, and 1.6 μg/ml, respectively.
The challenge of discovering antibiotic drug candidates from synthetic libraries has led to a renewed focus of utilizing natural products as scaffolds for obtaining novel antibiotics. In support of this, we have shown herein that reutericyclins represent one potential source for obtaining novel topical antibiotics to combat the growing menace of staphylococcal skin infections.
Acknowledgments
From the University of Tennessee Health Science Center, we thank John S. Jackson and Charles N. May for technical assistance; Hassan Almoazen and Robin E. Lee for helpful discussions; Keith English for providing clinical isolates of MRSA and methicillin-susceptible S. aureus; and James Dale for providing clinical isolates of S. pyogenes. We also thank Elaine S. Walker of East Tennessee State University for providing mupirocin-resistant MRSA. All other clinical strains were from the Network on Antimicrobial Resistance in Staphylococcus aureus.
This work was kindly funded by the University of Tennessee Research Foundation.
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Akiyama, H., T. Hamada, W. K. Huh, O. Yamasaki, T. Oono, W. Fujimoto, and K. Iwatsuki. 2003. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in skin lesions of bullous impetigo, atopic dermatitis and pemphigus foliaceus. Br. J. Dermatol. 148:526-532. [DOI] [PubMed] [Google Scholar]
- 2.Becker, P., R. Hakenbeck, and B. Henrich. 2009. An ABC transporter of Streptococcus pneumoniae involved in susceptibility to vancoresmycin and bacitracin. Antimicrob. Agents Chemother. 53:2034-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damour, O., S. Z. Hua, F. Lasne, M. Villain, P. Rousselle, and C. Collombel. 1992. Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytes. Burns 18:479-485. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande, L. M., A. M. Fix, M. A. Pfaller, and R. N. Jones. 2002. Emerging elevated mupirocin resistance rates among staphylococcal isolates in the SENTRY Antimicrobial Surveillance Program (2000): correlations of results from disk diffusion, Etest and reference dilution methods. Diagn. Microbiol. Infect. Dis. 42:283-290. [DOI] [PubMed] [Google Scholar]
- 6.Ganzle, M. G. 2004. Reutericyclin: biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 64:326-332. [DOI] [PubMed] [Google Scholar]
- 7.Ganzle, M. G., and R. F. Vogel. 2003. Contribution of reutericyclin production to the stable persistence of Lactobacillus reuteri in an industrial sourdough fermentation. Int. J. Food Microbiol. 80:31-45. [DOI] [PubMed] [Google Scholar]
- 8.Hurdle, J. G., R. B. Lee, N. R. Budha, E. I. Carson, J. Qi, M. S. Scherman, S. H. Cho, M. R. McNeil, A. J. Lenaerts, S. G. Franzblau, B. Meibohm, and R. E. Lee. 2008. A microbiological assessment of novel nitrofuranylamides as anti-tuberculosis agents. J. Antimicrob. Chemother. 62:1037-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khandavilli, S., and R. Panchagnula. 2007. Nanoemulsions as versatile formulations for paclitaxel delivery: peroral and dermal delivery studies in rats. J. Investig. Dermatol. 127:154-162. [DOI] [PubMed] [Google Scholar]
- 10.Kugelberg, E., T. Norstrom, T. K. Petersen, T. Duvold, D. I. Andersson, and D. Hughes. 2005. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 49:3435-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliva, B., A. J. O'Neill, K. Miller, W. Stubbings, and I. Chopra. 2004. Anti-staphylococcal activity and mode of action of clofazimine. J. Antimicrob. Chemother. 53:435-440. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland, R., R. J. Boon, K. E. Griffin, P. J. Masters, B. Slocombe, and A. R. White. 1985. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob. Agents Chemother. 27:495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yendapally, R., J. G. Hurdle, E. I. Carson, R. B. Lee, and R. E. Lee. 2008. N-substituted 3-acetyltetramic acid derivatives as antibacterial agents. J. Med. Chem. 51:1487-1491. [DOI] [PubMed] [Google Scholar]