Abstract
The present multicenter, randomized crossover study compared the safety and efficacy of continuous infusion with those of short infusions of ceftazidime in patients with cystic fibrosis. Patients with chronic Pseudomonas aeruginosa colonization received two successive courses of intravenous tobramycin and ceftazidime (200 mg/kg of body weight/day) for pulmonary exacerbation administered as thrice-daily short infusions or as a continuous infusion. The primary endpoint was the variation in the forced expiratory volume in 1 s (FEV1) during the course of antibiotic treatment. Sixty-nine of the 70 patients enrolled in the study received at least one course of antibiotic treatment. The improvement in FEV1 at the end of therapy was not statistically different between the two treatment procedures (+7.6% after continuous infusion and +5.5% after short infusions) but was better after continuous ceftazidime treatment in patients harboring resistant isolates (P < 0.05). The interval between the course of antibiotic treatments was longer after the continuous infusion than after the short infusion of ceftazidime (P = 0.04). The mean serum ceftazidime concentration during the continuous infusion was 56.2 ± 23.2 μg/ml; the mean peak and trough concentrations during the short infusions were 216.3 ± 71.5 and 12.1 ± 8.7 μg/ml, respectively. The susceptibility profiles of the P. aeruginosa isolates remained unchanged and were similar for both regimens. Quality-of-life scores were similar whatever the treatment procedure, but 82% of the patients preferred the continuous-infusion regimen. Adverse events were not significantly different between the two regimens. In conclusion, the continuous infusion of ceftazidime did not increase its toxicity and appeared to be as efficient as short infusions in patients with cystic fibrosis as a whole, but it gave better results in patients harboring resistant isolates of P. aeruginosa.
Although there is currently no curative treatment for cystic fibrosis (CF), the life expectancy of CF patients has progressively increased, with the median survival time reaching 38 years (6). Progress in antibiotic treatment may partly account for this improvement, because morbidity and mortality in patients with CF mostly result from chronic respiratory tract infections, particularly infections with Pseudomonas aeruginosa. Acute exacerbations due to P. aeruginosa infection are treated with intravenous (i.v.) antibiotics, mostly combinations of a β-lactam, such as ceftazidime, and an aminoglycoside.
In conventional treatment regimens, ceftazidime is administered in the form of thrice-daily short infusions (SIs), each of which lasts 30 min. However, the use of a 24-h continuous infusion (CI) of β-lactams has been proposed, because β-lactams are time-dependent antibiotics and they have little, if any, postantibiotic effect against gram-negative bacteria. Several reports have even concluded that the duration of the period during which the serum concentration of β-lactam antibiotics exceeds the MIC determines the clinical outcome (3, 7, 14, 18, 19). Given the short half-life of ceftazidime, both pharmacodynamic and pharmacokinetic considerations suggest that CI might be more effective than SIs (1, 16, 17, 29).
A few clinical studies have considered a 24-h CI of ceftazidime in CF patients (4, 22, 33). Those studies reported good clinical results and few complications but included only a small number of patients.
We carried out a randomized controlled trial to compare the safety and efficacy of courses of tobramycin and ceftazidime administered i.v. as either thrice-daily SIs or a 24-h CI in CF patients with acute exacerbation of chronic pulmonary P. aeruginosa infection.
MATERIALS AND METHODS
Patients.
CF patients over the age of 8 years with chronic P. aeruginosa infection of the respiratory tract and at least two courses of i.v. antibiotics in the year before enrollment were enrolled at the time of a pulmonary exacerbation, as defined by Fuchs et al. (10). Noninclusion criteria were allergy to ceftazidime or tobramycin, bronchial colonization with Burkholderia cepacia, renal impairment, and a history of lung transplantation. The study was approved by the local ethics committee, and written informed consent was obtained from adult patients and from the parents of pediatric patients.
Study design.
The study was a multicenter, randomized, crossover trial. Each patient received two successive i.v. antibiotic courses during two different pulmonary exacerbations. The allocation sequence was generated by centralized randomization: ceftazidime SIs followed by ceftazidime CI or ceftazidime CI followed by ceftazidime SIs. No minimum time was imposed between the two courses. Each patient was monitored until a third intravenous antibiotic course was required. The ceftazidime SIs were delivered as thrice-daily 30-min infusions in 100 ml of 0.9% sodium chloride, and the ceftazidime CI was delivered in 230 ml of 0.9% sodium chloride over 23 h. The daily dose of ceftazidime was 200 mg/kg of body weight, with a maximum dose of 12 g being administered. For the ceftazidime CI, a loading dose of 60 mg/kg (maximum, 2 g) was used. All patients also received tobramycin (10 mg/kg) in the form of one 30-min infusion per day. Portable devices were used: the Intermate SV 200 portable device (Baxter) for the 30-min SIs of ceftazidime and tobramycin and the Infusor LV10 portable device (Baxter) for the CI of ceftazidime. Oral ciprofloxacin treatment was authorized, provided that it was administered in an identical manner during both courses of antibiotic treatment. The patients were treated at home or in the hospital for 14 to 21 days, which was determined by the investigators, but were treated in the same way for both courses. The primary outcome measure was the change in the forced expiratory volume in 1 s (FEV1) between the beginning and the end of the i.v. antibiotic course and is expressed as a percentage of the predicted normal value. FEV1 has been accepted as a primary endpoint in clinical trials with patients with CF (9) and has been shown to be reproducible in the same patient at different times of follow-up (24). In our adult CF center, the mean coefficient of variation for FEV1 between three successive measurements in patients in a stable state was found to be 3.1% (standard deviation [SD], 1.6%). The secondary outcome measures were the interval between two successive i.v. antibiotic treatment courses (calculated from the last day of the first i.v. course to the first day of the second i.v. course); quality-of-life scores validated in French (Cystic Fibrosis Questionnaire score of 14+ for teenagers and adults and Cystic Fibrosis Questionnaire Child score of P for children aged 8 to 13 years) (11); and C-reactive protein, white blood cell, hepatic enzyme, and creatinine levels. At the end of the study, the patients were asked which regimen they would prefer for future treatment.
Microbiologic evaluation.
Samples of sputum collected at the beginning and the end of each antibiotic treatment course were used to establish bacteriological cultures. P. aeruginosa isolates were classified according to the Recommendations of the French Society for Microbiology in three categories of susceptibility to ceftazidime (27): the susceptible isolates were those with MICs of ≤4 mg/liter, the resistant isolates were those with MICs of >32 mg/liter, and the intermediate isolates were those with MICs of >4 and ≤32 mg/liter. When a patient had several P. aeruginosa isolates with different susceptibilities, the patient was considered to be in the category with the most resistant isolate (i.e., patients were classified in the resistant category if they had at least one resistant isolate, in the intermediate category if they had at least one intermediate isolate without a resistant isolate, and in the susceptible category if they had only susceptible isolates). Other pathogens were also identified.
Measurement of ceftazidime concentrations.
Plasma ceftazidime concentrations were determined in volunteer patients once a steady-state concentration (Css) had been reached for the ceftazidime CI and at the following times after the beginning of the infusion for the ceftazidime SIs: before (trough concentration [Ctrough]), 30 min (maximum concentration of drug in plasma [Cmax]), and 4 h (concentration at 4 h [C4]). This made possible calculation of the mean concentrations at the different sampling times for each regimen. The blood samples were centrifuged, and plasma for the determination of the ceftazidime concentration was stored at −80°C until analysis. We used high-performance liquid chromatography with a reverse-phase column (particle size, 5 μm; 4.6 by 250 mm; RP-18 Lichrospher; Merck, Darmstadt, Germany) and UV detection at 254 nm. The mobile phase was a mixture of acetonitrile and phosphate buffer (4/96, vol/vol) (12). In our laboratory, the coefficients of correlation for three different concentrations of ceftazidime (12, 45, and 120 μg/ml) were 5.7%, 4.2%, and 3.1%, respectively, for interassay variability and 4.8%, 3.2%, and 2.8%, respectively, for intra-assay variability.
Statistical analysis.
The trial was designed as an equivalence study. We estimated an SD for the change in FEV1 between the start and the end of each treatment of 12% (31). The regimens were regarded as equivalent if the difference in the change in FEV1 (by use of the 90% confidence interval) between treatments was less than 5% of the predicted normal FEV1. We estimated that with 120 patients randomized, the study would have an 82% power to deem the regimens equivalent if they were truly identical. The data were analyzed according to a preestablished analysis plan with SAS (version 8.2) software (SAS Institute, Cary, NC). Changes in FEV1 between the beginning and the end of the treatment course were assessed by analysis of variance. The treatment effect and the sequence effect were tested as fixed effects, and the effect of the treatment-sequence interaction was also evaluated. The results are expressed as the means ± SDs for quantitative data, and the results for groups were compared by means of two-tailed t tests or two-tailed matched t tests, as appropriate. Qualitative data were compared by the chi-square test or Fisher's exact test, as appropriate. P values of <0.05 were considered statistically significant. Data analyses were planned on the basis of the intention-to-treat (ITT) population (all treated patients), defined as the main population for analysis, but the per protocol (PP) population (all patients who had finished the study without a major deviation) was also studied.
RESULTS
Patients.
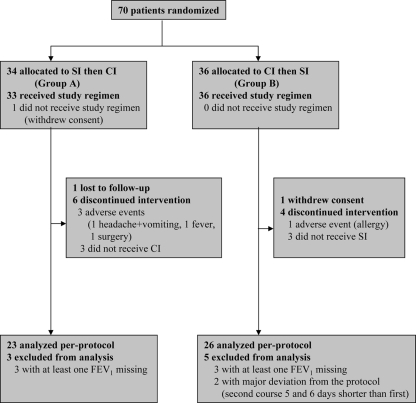
Seventy patients from 15 CF centers in France (8 adult centers and 7 pediatric centers) were enrolled and randomized in the study between September 2001 and May 2003 (Fig. 1): 34 were allocated to thrice-daily ceftazidime SIs for the first course and the ceftazidime CI for the second course (group A), and 36 were allocated to the CI for the first course and the SIs for the second course (group B). No statistically significant difference was found between the two groups for any of the variables studied, including FEV1 (Table 1).
FIG. 1.
Flowchart of study performance.
TABLE 1.
Baseline characteristics of patients (ITT population)
| Characteristic | Group A (n = 33) | Group B (n = 36) |
|---|---|---|
| Mean age (yr) ± SD | 22.6 ± 6.6 | 24.3 ± 7.0 |
| No. (%) male patients | 11 (33) | 10 (28) |
| Mean BMI (kg/m2) ± SD | 18.6 ± 2.6 | 18.7 ± 1.9 |
| Mean FEV1 (% preda) ± SD | 42.6 ± 18.4 | 45.8 ± 19.3 |
| Mean FVC (% pred)b ± SD | 55.6 ± 19.1 | 59.9 ± 19.2 |
| Mean no. of i.v. antibiotic courses/12 mo ± SD | 3.6 ± 1.7 | 3.1 ± 1.4 |
| Mean no. of i.v. antibiotic courses/24 mo ± SD | 6.9 ± 2.7 | 6.3 ± 2.3 |
% pred, percentage of the predicted normal FEV1.
FVC (% pred), percentage of the predicted normal forced vital capacity.
The ITT population comprised 69 patients. Among the patients in group A, 26 patients finished the study, 1 was lost to follow-up before the second i.v. course with the ceftazidime CI, and 6 discontinued the intervention. Among the six patients who discontinued the intervention, one had ceftazidime intolerance with headache and vomiting, one had surgery, one had pulmonary exacerbation requiring a change of i.v. antibiotics, two were colonized with bacteria other than Pseudomonas aeruginosa (methicillin-resistant Staphylococcus aureus and Burkholderia cepacia) and also required a change of i.v. antibiotics, and one had had no indication for a second i.v. antibiotic course at the end of the study. Among the patients in group B, 31 patients finished the study, 1 withdrew consent, and 4 discontinued the intervention. Among the four patients who discontinued the intervention, one had allergy to ceftazidime and three did not receive ceftazidime for the second i.v. antibiotic course. Finally, the PP population comprised 49 patients, as at least one FEV1 reading was missing for 6 patients and 2 patients presented a major deviation to protocol (Fig. 1).
The 20 patients who could not be included in the PP population were not different from the 49 patients in the PP population. Their mean age was 22.5 ± 6.6 years, whereas that for the PP population was 23.3 ± 5.2 years. The mean FEV1 for the patients who could not be included in the PP population was 43.4% ± 16.0% of the predicted normal value, whereas that for the PP population was 43.9% ± 15.5% of the predicted normal value.
A longer duration of therapy and the addition of ciprofloxacin were used for the most severe patients harboring more resistant P. aeruginosa. For the SI treatment, the duration of therapy was 21 days for 10 patients and 14 days for 52 patients, whereas for the CI treatment, it lasted 21 days for 12 patients and 14 days for 52 patients.
Ciprofloxacin was received by 26 patients during the SIs (39%) and 18 patients during CI (29%) in the ITT population (and by 16 patients during the SIs and 14 patients during CI in the PP population). The duration of the antibiotic treatment course in the patients receiving ciprofloxacin was 14 days in all except four patients, whose treatment lasted 21 days for the two modalities of treatment.
Microbiologic results.
The susceptibility profiles of the P. aeruginosa isolates were similar for both regimens and remained unchanged. More than one isolate of P. aeruginosa were identified in 25 of 56 patients (45%) at the beginning of the ceftazidime SIs and in 26 of 57 patients (46%) at the beginning of the ceftazidime CI. As a whole, at the baseline, before the ceftazidime SIs, 29 patients (52%) harbored only susceptible isolates, 12 patients (21%) harbored intermediate isolates, and 15 patients (27%) harbored resistant isolates. Before the ceftazidime CI, 27 patients (47%) harbored only susceptible isolates, 10 patients (17%) harbored intermediate isolates, and 20 patients (36%) harbored resistant isolates. Moreover, among the patients who received the ceftazidime SIs, 23 were infected with Staphylococcus aureus, 4 were infected with Haemophilus influenzae, 2 were infected with Streptococcus spp., 2 were infected with Achromobacter xylosoxidans, 1 was infected with Stenotrophomonas maltophilia, and 1 was infected with Serratia marcescens. Among the patients who received the ceftazidime CI, 19 were infected with Staphylococcus aureus, 5 were infected with Haemophilus influenzae, 1 was infected with a Streptococcus sp., 1 was infected with Stenotrophomonas maltophilia, and 1 was infected with Serratia marcescens.
Efficacy of antibiotic treatment.
Analysis of variance showed an absence of sequence effects and no treatment-sequence interaction effects on FEV1 or on the duration of improvement after treatment. We therefore carried out an analysis of variance without including the effect of the previous treatment. The values of FEV1 at the beginning of the ceftazidime SIs were not statistically different from the values of FEV1 at the beginning of the ceftazidime CI (Table 2). The mean change in the values of FEV1 between the beginning and the end of the i.v. antibiotic treatment course appeared to be similar for both treatment regimens in the ITT population (P = 0.15), whereas it was higher after the CI than after the SIs of ceftazidime (P = 0.02) in the PP population (Table 2). The rates of improvement in FEV1 appeared better after the CI than after the SIs in patients harboring resistant isolates of P. aeruginosa (P < 0.05) (Table 3). The mean difference in the interval between the two successive i.v. antibiotic treatment courses was significantly longer after the ceftazidime CI treatment than after the SI treatment in the ITT population as well as in the PP population (Table 4).
TABLE 2.
Comparison of change in mean FEV1 from start of treatment between treatment groups
| Population | SIs of ceftazidime
|
CI of ceftazidime
|
Mean difference (90% CIb) | P | ||||
|---|---|---|---|---|---|---|---|---|
| Initial FEV1a | Final FEV1 | Mean change | Initial FEV1 | Final FEV1 | Mean change | |||
| ITT (n = 69) | 44.3 (18.6) | 49.8 (21.6) | 5.5 (10.6) | 42.7 (19.1) | 50.3 (21.8) | 7.6 (12.1) | 2.1 (−0.3 to 5.2) | 0.15 |
| PP (n = 49) | 44.4 (18.4) | 50.0 (22.9) | 5.6 (10.1) | 42.8 (19.2) | 52.4 (22.9) | 9.6 (10.6) | 4.0 (1.2 to 6.7) | 0.02 |
FEV1 values are given as the mean percentage of the predicted normal value (SD).
CI, confidence interval.
TABLE 3.
Comparison of change in FEV1 from the start of treatment according to Pseudomonas susceptibility to ceftazidime in both treatment groups
| Ceftazidime regimen | Susceptible strains
|
Intermediate strains
|
Resistant strains
|
|||
|---|---|---|---|---|---|---|
| No. of patients | Change in mean % of predicted FEV1 (SD) | No. of patients | Change in mean % of predicted FEV1 (SD) | No. of patients | Change in mean % of predicted FEV1 (SD) | |
| SIs | 28 | 8.1 (8.4) | 11 | 6.4 (7.6) | 15 | 1.7 (5.6) |
| CI | 26 | 7.9 (9.7) | 10 | 6.0 (8.6) | 18 | 6.2 (6.6)a |
P < 0.05 for comparison of the mean change in FEV1 in patients harboring resistant isolates of P. aeruginosa between the SIs and the CI of ceftazidime.
TABLE 4.
Comparison of time before next i.v. antibiotic course between treatments
| Population | Mean (SD) duration between treatments (mo) for ceftazidime administered as:
|
Mean difference (90% CIa) | P | |
|---|---|---|---|---|
| SIs | CI | |||
| ITT (n = 69) | 2.8 (1.7) | 3.2 (1.9) | 0.4 (0.08 to 0.71) | 0.04 |
| PP (n = 49) | 2.7 (1.6) | 3.1 (1.8) | 0.4 (0.06 to 0.7) | 0.05 |
CI, confidence interval.
Tolerance of antibiotic treatment.
Tolerance of the antibiotic treatment by the ITT population was analyzed. We recorded 124 adverse events (68 during the ceftazidime SIs and 56 during the ceftazidime CI) in 50 patients. Only two of these events were considered to be severe adverse events requiring hospitalization for pulmonary exacerbation (one after the SIs of ceftazidime and one after the CI of ceftazidime). The most frequent adverse events were abdominal pain, nausea and diarrhea (12%), hemoptysis (11.3%), headaches (7.3%), pulmonary exacerbations (6.5%), and tonsillitis (6.5%).
The C-reactive protein concentration and the white blood cell count decreased significantly at the end of each antibiotic course for both regimens. The aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) levels increased significantly at the end of the antibiotic course, with no statistically significant difference between the two regimens being detected. No significant changes in the alkaline phosphatase and the gamma-glutamyl transferase levels were observed (Table 5).
TABLE 5.
Variations in values of biochemical parameters between treatment groups
| Parameter | Mean (SD) change for the following form of ceftazidime administration:
|
Mean difference (90% CIb) | P | |
|---|---|---|---|---|
| SIs | CI | |||
| CRPa concn (mg/liter) | −19.7 (34.9) | −18.7 (33.9) | 1.0 (−11.6 to 13.6) | 0.90 |
| Leukocyte count (no. of cells/mm3) | −1,829 (3,218) | −2,068 (3,289) | −128 (−9,968 to 712) | 0.80 |
| Neutrophil count (no. of cells/mm3) | −2,047 (3,434) | −2,294 (3191) | −84 (−1011 to 842) | 0.88 |
| ALAT concn (IU/liter) | 7.4 (19.8) | 11.9 (24.1) | 4.5 (−2.5 to 11.5) | 0.26 |
| ASAT concn (IU/liter) | 4.7 (14.2) | 8.6 (17.8) | 3.9 (−1.2 to 9.1) | 0.21 |
| Alkaline phosphatase concn (IU/liter) | −35.0 (101.5) | −28.6 (48.0) | 4.5 (−15.8 to 24.8) | 0.71 |
| Gamma GTc concn (IU/liter) | −0.5 (17.3) | 4.6 (24.9) | 5.3 (−1.8 to 12.5) | 0.21 |
| Creatinine concn (μmol/liter) | −0.30 (8.67) | 1.51 (10.77) | 1.81 (−1.65 to 5.27) | 0.39 |
CRP, C-reactive protein.
CI, confidence interval.
Gamma GT, gamma-glutamyl transferase.
Pharmacokinetics.
Pharmacokinetic analyses were carried out for 28 patients. The mean ceftazidime Css during CI was 56.2 ± 23.2 μg/ml (range, 37.0 to 65.9 μg/ml), whereas during the ceftazidime SIs, the mean Ctrough was 12.1 ± 8.7 μg/ml (range, 6.1 to 16.6 μg/ml), the mean Cmax was 216.3 ± 71.5 μg/ml (range, 172.0 to 247.0 μg/ml), and the mean C4 was 40.7 ± 21.5 μg/ml (range, 24.8 to 56.6 μg/ml). The mean Css was therefore significantly higher than the mean Ctrough and the mean C4 (P < 0.05).
Quality-of-life analysis and patient preference.
The quality-of-life scores were similar for both treatments, but 82% of the 57 patients who received the two modalities of treatment said that they preferred the CI to three SIs of ceftazidime.
DISCUSSION
We addressed the question of whether daily ceftazidime CI over 24 h is as good as the conventional thrice-daily treatment when it is combined with i.v. tobramycin administered once daily (26, 31) for the treatment of pulmonary exacerbations in CF patients with chronic bronchial colonization with P. aeruginosa. Our study is the first one to compare the clinical and pharmacological issues related to two different modalities of ceftazidime treatment in such a large number of patients with CF. We found that the ceftazidime CI was at least as effective as SIs in increasing FEV1 by the end of treatment and appeared to be more effective in patients harboring resistant isolates of P. aeruginosa. It was well tolerated.
Unlike tobramycin, which has concentration-dependent antibacterial activity and a postantibiotic effect, β-lactam antibiotics display time-dependent antibacterial activity (15, 28, 29). Intermittent ceftazidime administration might result in undesirably high Cmaxs and low, potentially sub-MIC Ctroughs, whereas the administration of ceftazidime by CI should avoid these fluctuations and keep the percentage of the time that the concentration is greater than the MIC above 100% for the entire duration of treatment (1, 16, 29). Moreover, Alou et al. have stressed the importance of optimizing the time that the concentration of ceftazidime is greater than the MIC for resistant strains (1): they found no difference in the activities against susceptible and intermediate strains achieved by intermittent infusion and CI regimens, whereas only the CI achieved comparable activity over the same time period, minimizing the differences between the activities of the regimens against resistant and susceptible strains. Now the level of resistance to first-line antipseudomonal agents is high in patients with CF and resistance to ceftazidime has been found in 39.6% of CF patient isolates of P. aeruginosa (20), which is not very different from our susceptibility results. Nevertheless, the resistance of P. aeruginosa to ceftazidime was not a noninclusion criterion in our study, as there is evidence that susceptibility testing is not a reliable predictor of clinical success in the treatment of pulmonary exacerbations with i.v. tobramycin and ceftazidime in patients with CF (25). Nevertheless, because of the high rate of resistance among P. aeruginosa isolates, we decided not to reduce the doses of ceftazidime for delivery by CI in comparison to those used for the SI, contrary to the reductions used in previous studies (4, 22, 33), because we thought that higher doses might be more effective.
We observed considerable variability in serum ceftazidime concentrations during intermittent administration (with very high Cmaxs and very low Ctroughs, even below the MIC in some cases), whereas the Css remained permanently above the MIC during CI. We completed pharmacologic studies with more patients with CF than any previously reported study. Our mean steady-state blood ceftazidime concentration of 56.2 ± 23.2 μg/ml was consistent with those found in previous studies: 52.9 ± 18.4 μg/ml in 9 children given a CI of 300 mg/kg/day ceftazidime (8) and 56.1 ± 23.3 μg/ml in 12 patients given a CI of 200 mg/kg/day ceftazidime (5). For lower daily ceftazidime doses of 100 mg/kg/day administered by CI, Csss were reported to be 38.3 μg/ml in 4 patients with CF (13), 28.7 ± 5 μg/ml in 8 adults with CF (32), and 28.5 ± 8.4 μg/ml in 14 children with CF (22). In another study, 5 adults with CF were initially treated with 2 g of ceftazidime thrice daily (dosage range, 102 to 154 mg/kg/day) for 10 days (4). In that study, the dosages for the CI were calculated to achieve serum Csss equal to 6.6 times the MIC for the most resistant P. aeruginosa isolate from the intermittent dosing phase without exceeding 6 g/day. The dosing method resulted in substantially reduced total daily doses (range, 29 to 75%) in four patients during CI.
In our study, we found that the increases in FEV1 were similar after both treatment modalities for the ITT population but higher after CI than after SIs for the PP population. As previously suggested by in vitro pharmacodynamic studies (1), we showed that the improvement in FEV1 was better after ceftazidime CI for patients harboring P. aeruginosa-resistant strains. The interval between antibiotic treatment courses was longer after CI in both study populations. Previous clinical studies concluded that the CI of ceftazidime was effective in patients with CF, but only very small numbers of patients were studied. Moreover, in some of those studies, ceftazidime was administered as monotherapy and CI was not compared with SIs (13, 33). In the study by Vinks et al. (33), 17 adult patients with CF received 33 courses of treatment by the CI of ceftazidime at a dose of 100 mg/kg/day for acute exacerbation of lung infection over a period of 2 years. For the 12 patients for whom data were available, FEV1 had increased by the end of treatment, and this increase persisted for 4 to 6 weeks. Multiple courses of ceftazidime monotherapy by CI did not result in a lasting increase in the frequency of ceftazidime-resistant P. aeruginosa strains (33). Two other studies compared courses of SIs and the CI of ceftazidime combined with tobramycin in 5 adults (4) and with amikacin in 14 children (22) requiring i.v. antibiotic therapy for the pulmonary exacerbation of CF. Patients showed clinical improvement with both regimens, with no significant difference in outcome (including FEV1) occurring between the regimens. As in our study, the pattern of susceptibility of the P. aeruginosa isolates was unaffected and remained similar with the two regimens. In both studies (4, 22), the CI of ceftazidime tended to give a better clinical outcome, but this trend was not significant, probably due to the small sample size. In another study published only in abstract form (23), 42 CF patients were treated with ceftazidime (200 mg/kg/day) thrice daily plus tobramycin once daily or with a CI of ceftazidime (100 mg/kg/day) plus tobramycin once daily in a prospective crossover study. No significant differences in clinical outcomes were observed. None of the previous studies compared the rates of improvement in FEV1 according to the initial susceptibility of the isolates.
In our study, the quality-of-life scores were similar for both ceftazidime regimens and improved at the end of the course of treatment. Most patients preferred CI to thrice-daily SIs. Similarly, all 14 children enrolled in the study of Rappaz et al. said that they were happy with this new treatment approach and would prefer the CI of ceftazidime for subsequent treatment (22). This mode of treatment was also preferred in a German study (23). As most of these treatments took place at home, this made it possible for the nurse to visit the patient only once per day, at a convenient time, rather than three times (one visit very early, at about 6 a.m.; one visit in the middle of the day; and one visit very late, at about 10 p.m.). which is required for SIs. This makes it possible for patients to continue to attend school or work if their general status allows it and limits their social isolation during i.v. antibiotic treatment.
We observed no difference in adverse effects between the two regimens. Transient increases in serum levels of the liver enzymes ASAT and ALAT were observed at the end of each course, but without the significant difference between the CI and SI regimens feared by Plasse et al. (21). No adverse effects of the CI of ceftazidime have ever been reported in patients with CF (4, 22). Ceftazidime (120 g/liter) displays 90% stability for up to 24 h at 25°C but for only 8 h at 37°C (2, 30). Temperature control is therefore critical for the administration of ceftazidime via portable pumps, which should not be carried under clothing for prolonged periods (30). Ceftazidime degradation leads to the release of pyridine, which reaches levels in excess of the threshold of the U.S. Pharmacopeia at 37°C but not at 25°C.
One limitation of our study is that it was not possible to include the intended number of patients due to problems with enrollment because of other competing clinical studies at the CF centers. Nonetheless, we included and analyzed more patients than previous studies in which the CI of ceftazidime was reported to improve the clinical outcome, but without the statistical significance being reported.
Conclusion.
The combination of the CI of ceftazidime and the administration of tobramycin once daily appears to be safe and as effective as thrice-daily ceftazidime SIs. Nevertheless, the ceftazidime CI seems to be of particular interest for patients harboring resistant isolates of P. aeruginosa. It can simplify treatment for patients with CF. Our results have potential implications for the prescription of i.v. antibiotic courses for acute pulmonary exacerbation in patients with CF. Further multicenter studies should evaluate the optimal dose of ceftazidime for use for CI by comparison with the daily dose used for SIs.
Acknowledgments
This study was supported by ANTADIR, Vaincre la Mucoviscidose, GSK, and Roche. Portable pumps were supplied by Baxter.
The pharmaceutical companies played no direct role in data handling or statistical analysis.
We declare that we have no competing interests.
In addition to the authors, the following investigators also participated in the French Ceftazidime Study: Muriel Lebourgeois, Hôpital Necker, Paris, France; Isabelle Pin, CHU Grenoble, Grenoble, France; Vincent Rosner, Hôpital Hautepierre, Strasbourg, France; Marlène Murris-Espins, Hôpital Larrey, Toulouse, France; Martine Reynaud-Gaubert, Hôpital Sainte Marguerite, Marseille, France; Michèle Gérardin, Hôpital Robert Debré, Paris, France; Nadine Desmazes-Dufeu, Hôpital Cochin, Paris, France; Ralph Epaud, Hôpital Trousseau, Paris, France; and Thierry Perez, Hôpital Calmette, Lille, France.
Footnotes
Published ahead of print on 15 June 2009.
REFERENCES
- 1.Alou, L., L. Aguilar, D. Sevillano, M. J. Giménez, O. Echeverría, M. L. Gómez-Lus, and J. Prieto. 2005. Is there a pharmacodynamic need for the use of continuous versus intermittent infusion with ceftazidime against Pseudomonas aeruginosa? An in vitro pharmacodynamic model. J. Antimicrob. Chemother. 55:209-213. [DOI] [PubMed] [Google Scholar]
- 2.Baririan, N., H. Chanteux, E. Viaene, H. Servais, and P. M. Tulkens. 2003. Stability and comparability study of cefepime in comparison with ceftazidime for potential administration by continuous infusion under conditions pertinent to ambulatory treatment of cystic fibrosis patients and to administration in intensive care units. J. Antimicrob. Chemother. 51:651-658. [DOI] [PubMed] [Google Scholar]
- 3.Benko, A. S., D. M. Cappelletty, J. A. Kruse, and M. J. Rybak. 1996. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with suspected gram-negative infections. Antimicrob. Agents Chemother. 40:691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosso, J. A., C. R. Bonapac, P. A. Flume, and R. L. White. 1999. A pilot study of the efficacy of constant-infusion ceftazidime in the treatment of endobronchial infections in adults with cystic fibrosis. Pharmacotherapy 19:620-626. [DOI] [PubMed] [Google Scholar]
- 5.Byl, B., D. Baran, F. Jacobs, A. Herschuelz, and J. P. Thys. 2001. Serum pharmacokinetics and sputum penetration of amikacin 30 mg/kg once daily and of ceftazidime 200 mg/kg/day as a continuous infusion in cystic fibrosis patients. J. Antimicrob. Chemother. 48:325-327. [DOI] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation. 2007. Patient registry 2006. Annual data report to the centers directors. Cystic Fibrosis Foundation, Bethesda, MD.
- 7.Daenen, S., Z. Erjavec, D. R. A. Uges, H. G. De Vries-Hospers, P. De Jonge, and M. R. Halie. 1995. Continuous infusion of ceftazidime in febrile neutropenic patients in acute myeloid leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 14:188-192. [DOI] [PubMed] [Google Scholar]
- 8.David, T. J., and J. Devlin. 1989. Continuous infusion of ceftazidime in cystic fibrosis. Lancet i:1454-1455. [DOI] [PubMed] [Google Scholar]
- 9.Döring, G., J. S. Elborn, M. Johannesson, H. de Jonge, M. Griese, A. Smyth, and H. Heijerman for the Consensus Study Group. 2007. Clinical trials in cystic fibrosis. J. Cyst. Fibros. 6:85-99. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs, H. J., D. S. Borowitz, D. H. Christiansen, E. M. Morris, M. L. Nash, B. W. Ramsey, B. J. Rosenstein, A. L. Smith, and M. E. Wohl. 1994. Effect of aerosolised recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N. Engl. J. Med. 331:637-642. [DOI] [PubMed] [Google Scholar]
- 11.Henry, B., P. Aussage, C. Grosskopf, and J. M. Goehrs. 2003. Development of the Cystic Fibrosis Questionnaire (CFQ) for assessing quality of life in pediatric and adult patients. Qual. Life Res. 12:63-76. [DOI] [PubMed] [Google Scholar]
- 12.Jehl, F., P. Birckel, and H. Monteil. 1987. Hospital routine analysis of penicillins, third-generation cephalosporins and aztreonam by conventional and high-speed high-performance liquid chromatography. J. Chromatogr. 413:109-119. [DOI] [PubMed] [Google Scholar]
- 13.Kuzemko, J., and C. Crawford. 1989. Continuous infusion of ceftazidime in cystic fibrosis. Lancet ii:385. [DOI] [PubMed] [Google Scholar]
- 14.Lipman, J., C. D. Gomersall, T. Gin, G. M. Joynt, and R. J. Young. 1999. Continuous infusion ceftazidime in intensive care: a randomized controlled trial. J. Antimicrob. Chemother. 43:309-311. [DOI] [PubMed] [Google Scholar]
- 15.MacGowan, A. P., and K. E. Bowker. 1998. Continuous infusion of β-lactam antibiotics. Clin. Pharmacokinet. 35:391-402. [DOI] [PubMed] [Google Scholar]
- 16.Manduru, M., L. B. Mihm, R. L. White, L. V. Friedrich, P. A. Flume, and J. A. Bosso. 1997. In vitro pharmacodynamics of ceftazidime against Pseudomonas aeruginosa isolates derived from cystic fibrosis patients. Antimicrob. Agents Chemother. 41:2053-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouton, J. W., and A. A. Vinks. 1996. Is continuous infusion of β-lactam antibiotics worthwhile? Efficacy and pharmacokinetic considerations. J. Antimicrob. Chemother. 38:5-15. [DOI] [PubMed] [Google Scholar]
- 18.Nicolau, D. P., C. H. Nightingale, M. A. Banevicius, Q. Fu, and R. Quintiliani. 1996. Serum bactericidal activity of ceftazidime: continuous infusion versus intermittent injections. Antimicrob. Agents Chemother. 40:61-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pea, F., P. Viale, D. Damiani, F. Pavan, F. Cristini, R. Fanin, and M. Furlanut. 2005. Ceftazidime in acute myeloid leukemia patients with febrile neutropenia: helpfulness of continuous intravenous infusion in maximizing pharmacodynamic exposure. Antimicrob. Agents Chemother. 49:3350-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitt, T. L., M. Sparrow, M. Warner, and M. Stefanidou. 2003. Survey of resistance of Pseudomonas aeruginosa from UK patients with cystic fibrosis to six commonly prescribed antimicrobial agents. Thorax 58:794-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plasse, J. C., C. Chabloz, A. Terrier, and G. Bellon. 2002. Is it safe to administer a continuous infusion of ceftazidime (Fortum®) prepared for 24 hours in cystic fibrosis patients? Pediatr. Pulmonol. 33:232-233. [DOI] [PubMed] [Google Scholar]
- 22.Rappaz, I., L. A. Decosterd, J. Bille, M. Pilet, N. Bélaz, and M. Roulet. 2000. Continuous infusion of ceftazidime with a portable pump is as effective as thrice-a-day bolus in cystic fibrosis children. Eur. J. Pediatr. 159:919-923. [DOI] [PubMed] [Google Scholar]
- 23.Riethmüller, J., A. Busch, P. Franke, R. Ziebach, R. von Butler, and M. Stern. 2000. Pharmacodynamic variation of intravenous antibiotic treatment with ceftazidime and tobramycin in CF patients is equally effective, abstr. 340. Abstr. 13th Eur. Cystic Fibrosis Conf.
- 24.Sanders, D. B., M. Rosenfeld, N. Mayer-Hamblett, D. Stamey, and G. J. Redding. 2007. Reproducibility of spirometry during cystic fibrosis pulmonary exacerbations. Pediatr. Pulmonol 43:1142-1146. [DOI] [PubMed] [Google Scholar]
- 25.Smith, A. L., S. B. Fiel, N. Mayer-Hamblett, B. Ramsey, and J. L. Burns. 2003. Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration. Lack of association in cystic fibrosis. Chest 123:1495-1502. [DOI] [PubMed] [Google Scholar]
- 26.Smyth, A., K. H. V. Tan, P. Hyman-Taylor, M. Mulheran, S. Lewis, D. Stableforth, and A. Knox. 2005. Once versus three-times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis—the TOPIC study: a randomised controlled trial. Lancet 365:573-578. [DOI] [PubMed] [Google Scholar]
- 27.Société Française de Microbiologie. 2003. Report 2003 of the Antibiogramme Committee of the French Society for Microbiology. Société Française de Microbiologie, Paris, France. http://www.sfm.asso.fr.
- 28.Spino, M. 1991. Pharmacokinetics of drugs in cystic fibrosis. Clin. Rev. Allergy 9:169-210. [DOI] [PubMed] [Google Scholar]
- 29.Touw, D. J., A. A. Vinks, J. W. Mouton, and A. M. Horrevorts. 1998. Pharmacokinetic optimisation of antibacterial treatment in patients with cystic fibrosis. Current practice and suggestion for future directions. Clin. Pharmacokinet. 35:437-459. [DOI] [PubMed] [Google Scholar]
- 30.Viaene, E., H. Chanteux, H. Servais, M.P. Mingeot-Leclercq, and P. Tulkens. 2002. Comparative stability studies of antipseudomonal β-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis) and motor-operated syringes (intensive care units). Antimicrob. Agents Chemother. 46:2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vic, P., S. Ategbo, D. Turck, M. O. Husson, V. Launay, G. A. Loeuille, A. Sardet, A. Deschildre, D. Druon, and C. Arrouet-Lagande. 1998. Efficacy, tolerance, and pharmacokinetics of once daily tobramycin for Pseudomonas exacerbations in cystic fibrosis. Arch. Dis. Child. 78:536-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinks, A. A., D. J. Touw, H. G. Heijerman, M. Danhof, G. P. de Leede, and W. Bakker. 1994. Pharmacokinetics of ceftazidime in adult cystic fibrosis patients during continuous infusion and ambulatory treatment at home. Ther. Drug Monit. 16:341-348. [DOI] [PubMed] [Google Scholar]
- 33.Vinks, A. A., R. W. Brimicombe, H. G. Heijerman, and W. Bakker. 1997. Continuous infusion of ceftazidime in cystic fibrosis patients during home treatment: clinical outcome, microbiology and pharmacokinetics. J. Antimicrob. Chemother. 40:125-133. [DOI] [PubMed] [Google Scholar]