Abstract
Racemic 2,4-diaminopyrimidine dihydrophthalazine derivatives BAL0030543, BAL0030544, and BAL0030545 exhibited low in vitro MICs toward small, selected panels of Enterococcus faecalis, Enterococcus faecium, Streptococcus pneumoniae, Moraxella catarrhalis, and Mycobacterium avium, though the compounds were less active against Haemophilus influenzae. The constellation of dihydrofolate reductases (DHFRs) present in 20 enterococci and 40 staphylococci was analyzed and correlated with the antibacterial activities of the dihydrophthalazines and trimethoprim. DHFRs encoded by dfrB, dfrA (S1 isozyme), dfrE, and folA were susceptible to the dihydrophthalazines, whereas DHFRs encoded by dfrG (S3 isozyme) and dfrF were not. Studies with the separated enantiomers of BAL0030543, BAL0030544, and BAL0030545 revealed preferential inhibition of susceptible DHFRs by the (R)-enantiomers. BAL0030543, BAL0030544, and BAL0030545 were well tolerated by mice during 5- and 10-day oral toxicity studies at doses of up to 400 mg/kg of body weight. Using a nonoptimized formulation, the dihydrophthalazines displayed acceptable oral bioavailabilities in mice, and efficacy studies with a septicemia model of mice infected with trimethoprim-resistant, methicillin-resistant Staphylococcus aureus gave 50% effective dose values in the range of 1.6 to 6.25 mg/kg.
Despite sustained efforts to identify new drugs against antibiotic-resistant bacteria, treatment of infections caused by vancomycin-resistant enterococci and methicillin-resistant staphylococci continues to challenge clinicians (5). Enterococci are often associated with urinary tract infections, intra-abdominal abscesses, endocarditis, and bacteremia, whereas staphylococci are common causes of skin and skin structure infections, endocarditis, osteomyelitis, prosthetic joint infections, catheter-related bacteremia, and nosocomial pneumonia. Methicillin-resistant Staphylococcus aureus (MRSA) accounts for approximately 50% of all S. aureus infections in the United States (24). Once confined to healthcare settings, MRSA has emerged as a serious cause of community-acquired skin and skin structure infections (32), and an increased frequency of skin cultures positive for S. aureus is associated with nasal and intestinal colonization (2).
Dihydrofolate reductase (DHFR; EC 1.5.1.3) is an essential enzyme in most pathogenic bacteria, and the clinical success of trimethoprim (28, 33, 41, 43) confirms DHFR as an important chemotherapeutic target. A phenylalanine→tyrosine substitution at position 98 in the wild-type, nontransferable, chromosomally encoded DHFR renders S. aureus resistant to trimethoprim (15); a corresponding tyrosyl residue occurs in transferable plasmid-encoded wild-type staphylococcal DHFR isozymes S1, S2, and S3 (13, 14, 40) and in the enterococcal DHFRs encoded by dfrE and dfrF. The gene encoding DHFR S1 may also be associated with staphylococcal chromosomal cassette mec N1 (SCCmec-N1) (37).
BAL0030543, BAL0030544, and BAL0030545 are novel dihydrophthalazine antifolates with potent activities against staphylococci (6). This report focuses on (i) in vitro activities of the dihydrophthalazines toward other bacterial pathogens, (ii) the relationship between DHFR primary structure and susceptibilities of staphylococci and enterococci toward antifolates, and (iii) the in vivo antimicrobial and pharmacological/toxicological properties of the dihydrophthalazines.
MATERIALS AND METHODS
Antimicrobial compounds.
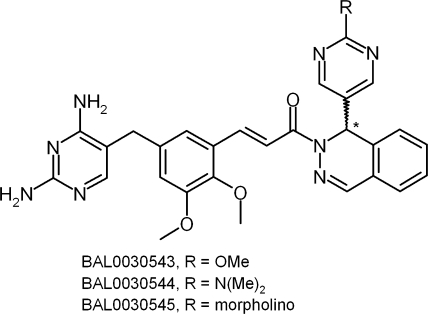
Racemic BAL0030543, BAL0030544, and BAL0030545 (Fig. 1) were prepared at Basilea Pharmaceutica International AG by published procedures (25). Racemates were dissolved in dimethyl sulfoxide (DMSO)-methanol (1:1), and chiral resolution of the racemic mixtures BAL0030543, BAL0030544, and BAL0030545 was achieved by preparative high-performance liquid chromatography using an (R,R) Whelk-O 2 column (25-cm × 21.1-mm internal diameter; Regis Technologies, Inc., Morton Grove, IL) and an isocratic mobile phase (15% H2O plus 70% methanol plus 15% DMSO plus 0.1% triethylamine; flow rate, 1.5 ml/min) with UV detection (254 nm) to obtain the corresponding enantiomers [(RS)-BAL0030543→(R)-BAL0031117 + (S)-BAL0031118; (RS)-BAL0030544→(R)-BAL0031115 + (S)-BAL0031116; and (RS)-BAL0030545→(R)-BAL0031113 + (S)-BAL0031114]. Circular dichroic spectra were obtained for 10 μM ethanolic solutions of dihydrophthalazines over the range of 230 to 500 nm, using a model π*-180 dichrograph (Applied Photophysics, Surrey, United Kingdom).
FIG. 1.
Structures of BAL0030543, BAL0030544, and BAL0030545.
Comparator antibiotics (azithromycin [Azm], clarithromycin [Clr], dapsone, levofloxacin [Lvx], linezolid [Lzd], penicillin G [Pen], rifampin [rifampicin; Rif], streptomycin [Str], and trimethoprim [Tmp]) were obtained from commercial sources. Stock solutions of antibiotics were prepared in DMSO.
Bacterial strains and MICs.
Bacterial strains were either clinical isolates proprietary to the Basilea strain collection or reference strains from the American Type Culture Collection (Manassas, VA). MICs over the range of 0.25 to 128 μg/ml were obtained for enterococci and staphylococci by the microdilution broth method according to Clinical and Laboratory Standards Institute guidelines (7), using Mueller-Hinton medium (Oxoid, Ltd., Basingstoke, United Kingdom); for streptococci and Moraxella catarrhalis, Mueller-Hinton broth was supplemented with 5% (vol/vol) horse serum (Sigma-Aldrich, Buchs SG, Switzerland) (27).
MICs for suspected DHFR inhibitors toward Haemophilus influenzae were obtained using filter-sterilized Haemophilus test medium, prepared by supplementing Mueller-Hinton broth with 5% (wt/vol) yeast extract (Difco) and 15 μg/ml each of NAD+ (Sigma) and hematin (Sigma). MICs for Azm and Lvx were obtained using Mueller-Hinton broth supplemented with 5% (vol/vol) Fildes enrichment (BBL) (35).
Quality control strains (Enterococcus faecalis ATCC 29212 for enterococcal panels, Streptococcus pneumoniae ATCC 49619 for streptococcal panels, H. influenzae ATCC 49247 for H. influenzae and M. catarrhalis panels, and S. aureus ATCC 29213 for staphylococcal panels) were tested in each MIC run to ensure proper performance of the assay (8, 20). Vancomycin MICs were read after a full 24 h of incubation (8).
Mycobacterium avium was subcultured (35°C, 5% CO2) on slants of Middlebrook 7H11 agar (BD Diagnostic Systems, Sparks, MD); cultures were checked using Kinyoun carbolfuchsin-brilliant green (BD), a modified Ziehl-Neelsen method (BD), and neutral red (42). Seven-day MICs (drug range, 0.06 to 32 μg/ml) were determined essentially as described by Thiermann et al. (46). Microtiter plates inoculated with M. avium were loaded into 7-liter GENboxes (bioMérieux SA, Marcy l'Etoile, France) containing a flask of distilled water to maintain humidity during prolonged incubation at 35°C. GENboxes were opened once daily for a few minutes to refresh the atmosphere.
To calculate geometric mean MICs (16), MICs of “≤x” were set equal to “x,” MICs of “≥y” were set equal to “y,” and MICs of “>z” were set equal to “2z.”
DHFR assays.
Bacterial DHFR genes were amplified and cloned into the Qiagen expression vector pQE-30, containing the coding sequence for an N-terminal His6 tag. Overexpression of cloned DHFR was induced by the addition of isopropyl-β-d-thiogalactopyranoside (0.1 mM) to the culture medium, and recombinant proteins were purified over a 5-ml Ni-nitrilotriacetic acid Superflow cartridge (Qiagen AG, Hombrechtikon ZH, Switzerland). Human DHFR was purchased from Sigma-Aldrich. DHFR assays (Sigma-Aldrich) were performed according to the manufacturer's instructions in 96-well flat-bottomed polystyrene microtiter plates. DHFR was diluted to 110 μl with assay buffer (50 mM KH2PO4-K2HPO4 + 100 mM KCl + 1 mM EDTA + 0.05% [vol/vol] Tween 80, pH 5.0) and mixed with 20 μl of blank or inhibitor solution (20 mM in DMSO; final DMSO concentration in assay wells, ≤0.25%). After incubation for 2 min at room temperature, reactions were initiated by the addition of assay buffer (20 μl) containing 60 μM NADPH plus 50 μM dihydrofolic acid, and the optical density at 340 nm was measured at 15-s intervals for 18 min with a SpectraMax 250 plate reader (Molecular Devices Corp., Sunnyvale, CA). Fifty percent inhibitory concentrations (IC50s) were calculated using Prism v4.03 (GraphPad Software, Inc., La Jolla, CA).
DHFR gene analyses.
Oligonucleotide primers were designed for amplification of DHFR genes from (i) S. aureus (dfrA [GenBank accession number Y07536], dfrB [GenBank accession number Z16422], and dfrG [GenBank accession number AB205645]), (ii) Staphylococcus epidermidis (dfrC [GenBank accession number Z48233] and dfrD [GenBank accession number Z50141]), (iii) Enterococcus faecalis (dfrE [GenBank accession number AF028811] and dfrF [GenBank accession number AF028812]), and (iv) Enterococcus faecium (folA [GenBank accession number AAAK03000036; SwissProt accession number P00380]). Additional primers were designed for amplification of a 2.1-kb dfrA-SCCmec-N1 fragment (GenBank accession numbers X13290 and AB063172).
PCR mixtures contained 4 μl of lysed bacterial culture, 25 μl of JumpStart Taq ReadyMix (Sigma-Aldrich), 3 μl each of forward and reverse primers (10 μM in water), and 15 μl of double-distilled water. Taq polymerase was activated by incubation at 95°C for 5 min. A total of 35 PCR cycles were run, with DNA denaturation at 95°C (0.5 min), primer annealing at 45°C (0.5 min), and DNA extension at 72°C (3 min). After the final cycle, samples were incubated for an additional 5 min at 72°C, and then purified PCR fragments (3 to 8 μl; 15 ng/100 bp) were mixed with 2 μl of forward primers (10 μM in water). Water was added to a final volume of 10 μl, and the fragments were sequenced (Microsynth AG, Balgach SG, Switzerland).
Cytotoxicity and genotoxicity.
In vitro cytotoxicity of the dihydrophthalazine antifolates toward HeLa cells (ATCC strain CCL-2) over 10 log2 dilution steps (0.2 to 100 μM) was monitored (440 nm) for 48 h in serum-free medium supplemented with WST-1 (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's protocol. IC50s were obtained by fitting the data to a sigmoidal dose-response model (Prism v4.03).
Ames II assays were performed using Salmonella enterica serovar Typhimurium strains TA98 and TAmix (an equimolar mixture of strains TA7001 and TA7006) (18), with and without metabolic activation by S9 (prepared from livers of phenobarbital- and 5,6-benzoflavone-treated rats). Compounds were dissolved in DMSO and tested over the concentration range of 0.25 to 250 μg/ml. The in vitro micronucleus test (49) was performed with and without S9 activation over the concentration range of 0.5 to 280 μg/ml, using linearly growing V79-4 Chinese hamster lung fibroblasts (ATCC strain CCL-93) (1% DMSO in each assay well).
Pharmacokinetics.
For intravenous (i.v.) administration, BAL0030543, BAL0030544, and BAL0030545 were solubilized in a 5% glucose solution containing Solutol HS 15 (10%) (BASF AG, Ludwigshafen, Germany) and N-methylpyrrolidone (5%) (Fluka Analytical, Buchs SG, Switzerland), whereas for oral administration, the dihydrophthalazines were solubilized in a mixture of Solutol HS 15 (40%), N-methylpyrrolidone (17%), and 5% glucose (43%). Six male NMRI mice (Harlan Netherlands BV, Horst, The Netherlands) received an i.v. bolus of 2.5 mg/kg of body weight (2 ml/kg) of test substance, while four male NMRI mice received 25 mg/kg (10 ml/kg) by gavage. Blood (40 μl) was collected in heparin-coated capillaries from the saphenous veins of two mice at each sampling time over a period of 24 h, admixed with 300 μl of acetonitrile-water (9:1 [vol/vol]), and stored at −20°C. As needed, quenched blood samples were centrifuged, and the supernatants were analyzed for antifolates by liquid chromatography-tandem mass spectrometry (limit of quantification, 5 ng/ml). Areas under the curve (AUCs) were determined by the linear trapezoidal rule, and the bioavailability (%F) of each experimental drug was calculated as the ratio of dose-normalized AUCs after oral and i.v. administration.
Systemic toxicity in mice.
Exploratory toxicity testing of BAL0030543, BAL0030544, and BAL0030545 was performed using male NMRI mice with 5- and 10-day dosing schedules. Daily doses of dihydrophthalazines at 0, 100, or 400 mg/kg were administered by gavage. At the study end, blood samples were collected, animals were euthanized with CO2 and necropsied, and histopathology of selected organs was performed.
Experimental septicemia in mice.
Septicemia was induced in control and treatment groups of male NMRI mice (five animals per group) by intraperitoneal injection of 750 μl per animal of a 1:2 (vol/vol) mixture of bacterial suspension (∼107 bacteria in Mueller-Hinton broth) plus 5% (wt/vol) porcine mucin in demineralized water (34); preliminary experiments showed this to be the minimum inoculum size required to kill 80 to 100% of control mice within 5 days. Emulsions of BAL0030543, BAL0030544, BAL0030545, or Tmp (5 ml/kg) were prepared in 5% glucose containing Solutol HS 15 (10%) and N-methylpyrrolidone (10%), and test compounds or vehicle was administered i.v. at 1, 3, and 5 h postinfection. Antibiotic doses were halved until death of at least three of five animals occurred within 5 days.
RESULTS
MICs.
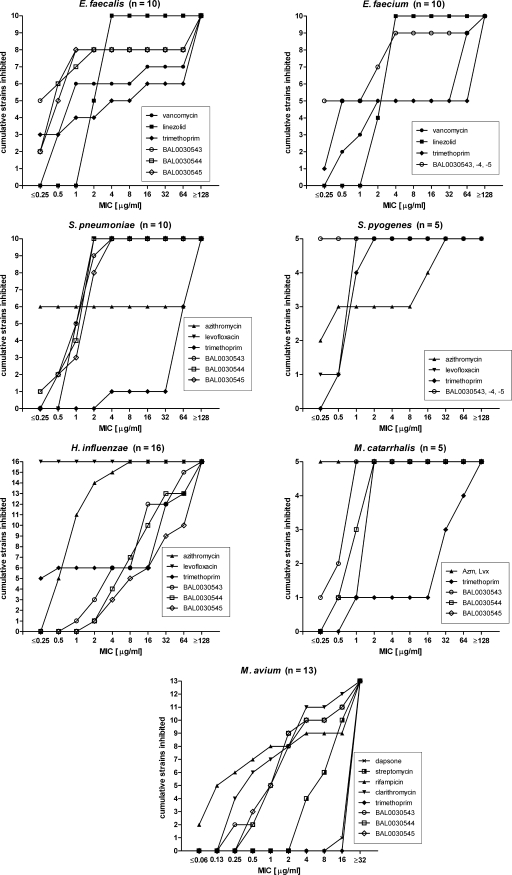
MICs were obtained for BAL0030543, BAL0030544, BAL0030545, and comparators, using small, selected panels of E. faecalis (6 Vans strains, 1 Vani strain, and 3 Vanr strains), E. faecium (5 Vans and 5 Vanr strains), Streptococcus pneumoniae (1 Peni and 9 Penr strains), Streptococcus pyogenes (5 strains), Haemophilus influenzae (6 Tmps and 10 Tmpr strains), Moraxella catarrhalis (5 strains), and Mycobacterium avium (13 strains) isolates. Cumulative numbers of strains of each species inhibited by each drug over the MIC range examined are presented in Fig. 2.
FIG. 2.
Cumulative inhibition of bacterial strains by BAL0030543, BAL0030544, BAL0030545, and comparators.
There was no correspondence between Van susceptibility and susceptibility to dihydrophthalazines among the enterococci; geometric mean MICs for BAL0030543, BAL0030544, and BAL0030545 were lower than those for Tmp, Lzd, and Van.
The pneumococci proved to be much more sensitive to the dihydrophthalazines and to Lvx than to Tmp or, in 4/10 cases, to Azm, whereas the experimental antifolates were more potent than either Azm, Lvx, or Tmp toward the S. pyogenes panel. However, in vitro activities of BAL0030543, BAL0030544, and BAL0030545 toward H. influenzae were inferior to those of Lvx, Azm, and Tmp (for nearly half of the strains). For four of five M. catarrhalis strains, the experimental antifolates were much more potent than Tmp, although Azm and Lvx were even more active against this species.
Rif and Clr were the most active drugs against M. avium, followed by the dihydrophthalazines and Str. No MICs of <16 μg/ml were observed for dapsone, a dihydropteroate synthase inhibitor reportedly active against M. avium (23). No MICs for Tmp of <32 μg/ml were encountered, concordant with the reported intrinsic resistance of M. avium to this antibiotic (44). BAL0030543, BAL0030544, and BAL0030545 each inhibited growth of 10/13 (77%) M. avium strains examined at concentrations of ≤4 μg/ml; there was no obvious correlation between the susceptibility of M. avium strains to Clr, Rif, or Str and susceptibility to the dihydrophthalazines (Basilea Pharmaceutica International AG, unpublished data).
Effect of thymidine on the MIC.
Substantial increases in the MICs of Tmp, BAL0030543, BAL0030544, and BAL0030545 toward E. faecalis, E. faecium, S. pneumoniae, and H. influenzae strains generally followed from supplementing Mueller-Hinton broth with 50 or 250 μg/ml of thymidine (Table 1). Similarly elevated MICs of antifolates in the presence of thymidine were also observed for strains of Staphylococcus epidermidis and S. aureus (Basilea Pharmaceutica International AG, unpublished data).
TABLE 1.
Effects of supplemental thymidine on MICs of Tmp and dihydrophthalazine antifolates
| Drug + thymidine concn (μg/ml) | MIC range (μg/ml) (no. of strains tested)b
|
|||
|---|---|---|---|---|
| E. faecalis | E. faecium | S. pneumoniae | H. influenzae | |
| Tmp + 0 | ≤0.25-16 (4a) | ≤0.25-0.5 (5a) | ≥64 (7) | ≤0.25-0.5 (5) |
| Tmp + 50 | >128 (4) | >128 (5) | ND | 2->128 (5) |
| Tmp + 250 | >128 (4) | >128 (5) | ND | 4->128 (5) |
| BAL0030543 + 0 | ≤0.25-1 (6) | ≤0.25-4 (8) | 1-4 (7) | 1-4 (5) |
| BAL0030543 + 50 | ≥128 (6) | >128 (8) | 64->128 (7) | 32->128 (5) |
| BAL0030543 + 250 | ≥128 (6) | >128 (8) | 64->128 (7) | 32->128 (5) |
| BAL0030544 + 0 | 0.5-2 (6) | ≤0.25-4 (8) | 1-2 (7) | 2-8 (5) |
| BAL0030544 + 50 | ≥128 (6) | ≥128 (8) | ≥128 (7) | 32->128 (5) |
| BAL0030544 + 250 | ≥128 (6) | >128 (8) | ≥128 (7) | 32->128 (5) |
| BAL0030545 + 0 | 0.5-1 (6) | ≤0.25-4 (8) | 2-4 (7) | 2-16 (5) |
| BAL0030545 + 50 | ≥128 (6) | >128 (8) | ≥128 (7) | ≥128 (5) |
| BAL0030545 + 250 | ≥128 (6) | >128 (8) | ≥128 (7) | ≥128 (5) |
Strains for which Tmp MICs were ≥64 μg/ml were not tested.
ND, not done.
DHFR gene analysis.
DHFR genes from 10 E. faecalis, 10 E. faecium, 19 S. aureus, and 21 S. epidermidis strains were cloned, and their amino acid sequences were correlated with MICs of Tmp, BAL0030543, BAL0030544, and BAL0030545 toward the strains (Tables 2 and 3).
TABLE 2.
DHFR gene analysis and MIC ranges of Tmp and dihydrophthalazine antifolates toward selected enterococci
| Species | No. of strains | Van phenotype | MIC range (μg/ml)
|
Presence of DHFR genea
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Tmp | BAL0030543 | BAL0030544 | BAL0030545 | dfrE | folA | dfrF | |||
| E. faecalis | 1 | Vanr | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | + | − | − |
| 4 | Vans/r | ≤0.25-1 | ≤0.25 | ≤0.25-0.5 | ≤0.25-0.5 | + (K88N, A5T, or H153R) | − | − | |
| 1 | Vans | 16 | 0.5 | 0.5 | 1 | + (A5T + K88N + F102Y) | − | − | |
| 2 | Vanr | 128 | 1 | 1-2 | 1 | + | − | − | |
| 2 | Vans | >128 | >128 | >128 | >128 | + | − | + | |
| E. faecium | 2 | Vans | 0.5 | ≤0.25 | ≤0.25 | ≤0.25 | − | + | − |
| 2 | Vans | ≤0.25-0.5 | ≤0.25 | ≤0.25 | ≤0.25 | − | + (M145I or R52H) | − | |
| 1 | Vans | 0.5 | ≤0.25 | ≤0.25 | ≤0.25 | + (A5T + K88N) | + (M145I) | − | |
| 4 | Vanr | >128 | 2-4 | 2-4 | 2-4 | − | + | − | |
| 1 | Vanr | >128 | >128 | >128 | >128 | − | + | + | |
The presence and absence of particular DHFR genes are indicated by “+” and “−”, respectively; amino acids differing from those in the wild-type enzyme are shown in parentheses.
TABLE 3.
DHFR gene analysis and MIC ranges of Tmp and dihydrophthalazine antifolates toward selected staphylococci
| Species | No. of strains | Met phenotypea | MIC range (μg/ml)
|
Presence of DHFR geneb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmp | BAL0030543 | BAL0030544 | BAL0030545 | dfrB | dfrC | dfrA (S1) | dfrD (S2) | dfrG (S3) | dfrA within SCCmec-N1 | |||
| S. aureus | 2 | Mets | ≤0.25 | ≤0.25 | 1 | 0.5 | + | − | − | ND | ||
| 1 | Metr | >128 | 0.5 | 1 | 1 | + (F98Y) | − | − | − | |||
| 3 | Metr | 128 | 1-2 | 2 | 0.5-2 | + | + | − | + | |||
| 11 | Metr | >128 | 1-4 | 1-4 | 1-4 | + | + | − | − | |||
| 2 | Metr | >128 | 32-64 | 64-128 | 64-128 | + | − | + | ND | |||
| S. epidermidis | 2 | Mets | ≤0.25-1 | ≤0.25 | <0.25-0.5 | <0.25-1 | + | − | − | ND | ||
| 3 | Mets | >128 | 0.5-4 | 1-4 | 1-4 | + (F98Y) | − | − | ND | |||
| 1 | Mets | >128 | ≤0.25 | 1 | 1 | + (F98Y) | + | − | ND | |||
| 5 | Mets | 32->128 | 0.5 | 1 | 1 | + | + | − | ND | |||
| 10 | Metr | >128 | 0.5-2 | 0.5-2 | 1-2 | + | + | − | − | |||
Met, methicillin.
The presence and absence of particular DHFR genes are indicated by “+” and “−”, respectively; the presence of an F98Y mutation and the presence of dfrA within SCCmec-N1 are indicated. ND, not done.
For E. faecalis, high MICs (>128 μg/ml) for all antifolates were associated with the presence of a plasmid-carried dfrF gene, whereas elevated MICs for Tmp but not dihydrophthalazines occurred in enterococci lacking dfrF. The amino acid substitutions detected in DHFRs encoded by chromosomal dfrE genes appeared to have no effect on susceptibility toward Tmp, with the possible exception of one E. faecalis strain (Tmp MIC, 16 μg/ml) wherein the dfrE gene encoded a Phe102Tyr mutation analogous to the Phe98Tyr mutation responsible for Tmp resistance in some staphylococci.
Chromosomal DHFR genes dfrB (S. aureus) and dfrC (S. epidermidis) were detected in all 40 staphylococci surveyed. Five of the chromosomally encoded DHFRs contained the Phe98Tyr mutation, and all of these strains were highly resistant to Tmp; however, elevated MICs (≥32 μg/ml) for the dihydrophthalazines toward S. aureus seemed to depend upon the presence of the S3 DHFR isozyme encoded by the plasmid-borne dfrG gene. Strains expressing the S1 DHFR isozyme encoded by the dfrA gene were highly resistant to Tmp (MICs of ≥128 μg/ml) but had low MICs (≤4 μg/ml) for BAL0030543, BAL0030544, and BAL0030545. In three S. aureus strains, the dfrA gene was not borne on a plasmid but rather was associated with the chromosomal SCCmec-N1 element, which also confers methicillin resistance. No dfrD genes were detected in the S. epidermidis strains surveyed, and no S. aureus strains harbored both dfrA and dfrG genes.
Inhibition of DHFRs by racemic and chiral dihydrophthalazine antifolates.
BAL0030543, BAL0030544, and BAL0030545 were highly inhibitory toward both the staphylococcal wild-type DfrB isozyme and its Phe98Tyr mutant, as well as the type S1 isozyme encoded by dfrA, whereas Tmp failed to inhibit either the DfrB Phe98Tyr mutant or the S1 enzyme.
The racemic dihydrophthalazines were resolved to their constituent enantiomers by chiral preparative high-performance liquid chromatography, and circular dichrograms revealed that the enantiomers clustered into two groups: BAL0031113, BAL031115, and BAL0031117 each had a negative peak at 280 nm and a positive peak at 330 nm, whereas BAL0031114, BAL0031116, and BAL0031118 each had a positive peak at 280 nm and a negative peak at 330 nm. By correlating these Cotton effects with those of closely related 2,4-diaminopyrimidine dihydrophthalazines whose absolute configurations had been established (50), BAL0031113, BAL0031115, and BAL0031117 were assigned the (R)-configuration, and BAL0031114, BAL0031116, and BAL0031118 were assigned the (S)-configuration. The (R)-enantiomers were consistently more active than the (S)-enantiomers toward a panel of staphylococcal DHFRs (Table 4). These results match those obtained for whole cells, for which MICs for the (R)-enantiomers were consistently lower than those for the (S)-enantiomers (Table 5). Neither Tmp nor any of the dihydrophthalazines significantly inhibited human DHFR at the highest antifolate concentration tested (52 μM) (Table 4).
TABLE 4.
DHFR enzyme inhibition data
| Compound | IC50 (nM)a
|
% Human DHFR activityb | ||
|---|---|---|---|---|
| Wild-type DfrB (Tmps) | DfrB F98Y (Tmpr) | DfrA (S1) (Tmpr) | ||
| (RS)-BAL0030543 | 5.2 | 22.6 | 121.1 | 46.0 |
| (R)-BAL0031117 | 3.9 | 13.5 | 98.3 | 40.5 |
| (S)-BAL0031118 | 12.5 | 1146 | 1327 | 66.5 |
| (RS)-BAL0030544 | 11.3 | 70.2 | ND | ND |
| (R)-BAL0031115 | 9.2 | 40.9 | 35.1 | 68.7 |
| (S)-BAL0031116 | 37.6 | 2917 | 1009 | 88.5 |
| (RS)-BAL0030545 | 12.2 | 124.4 | 153.6 | 75.9 |
| (R)-BAL0031113 | 8.9 | 64.4 | 98.0 | 75.6 |
| (S)-BAL0031114 | 46.8 | 5705 | 2586 | 93.4 |
| Tmp | 25.7 | 10243 | 32997 | 74.0 |
IC50s were determined for S. aureus enzymes. ND, not done.
The activity of the human DHFR enzyme was determined in the presence of a 52 μM concentration of compound.
TABLE 5.
MICs of antifolates toward staphylococci
| Compound | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
|
S. aureus
|
S. epidermidis
|
||||
| ATCC 29213 (Vans Mets TmpsdfrB) | ATCC 43300 (Vans Metr TmpsdfrB) | VRS1 (Vanr Metr TmprdfrB dfrA)a | ATCC 14990 (Vans Mets TmpsdfrC) | BAS000635 (Vans Mets TmprdfrC dfrA) | |
| (RS)-BAL0030543 | 0.5 | 0.25 | 4 | 0.125 | 4 |
| (R)-BAL0031117 | 0.25 | 0.125 | 2 | 0.06 | 1 |
| (S)-BAL0031118 | 2 | 1 | >8 | 0.5 | >8 |
| (RS)-BAL0030544 | 0.5 | 1 | 2 | 0.25 | 2 |
| (R)-BAL0031115 | 0.5 | 0.5 | 2 | 0.25 | 2 |
| (S)-BAL0031116 | 2 | 2 | >8 | 0.5 | >8 |
| (RS)-BAL0030545 | 1 | 0.5 | 2 | 0.25 | 2 |
| (R)-BAL0031113 | 1 | 1 | 2 | 0.25 | 2 |
| (S)-BAL0031114 | 4 | 2 | >8 | 1 | >8 |
| Tmp | 1 | 1 | >128 | <0.25 | >128 |
VRS1, Vanr strain of S. aureus from Michigan (obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus, Eurofins Medinet, Inc., Chantilly, VA).
Cytotoxicity and genotoxicity.
BAL0030543, BAL0030544, and BAL0030545 had cytotoxicity IC50s toward HeLa cells in the range of 12 to 25 μM. Genotoxicity studies revealed that exposure to the dihydrophthalazines did not increase the number of revertant colonies of S. enterica serovar Typhimurium or promote the induction of micronucleated fibroblasts.
Pharmacokinetics and toxicity.
Oral bioavailabilities of BAL0030543, BAL0030544, and BAL0030545 in uninfected male NMRI mice were 25 to 30% for nonoptimized formulations; preliminary pharmacokinetic parameters are listed in Table 6. Dihydrophthalazine absorption from the gastrointestinal tract was delayed, with peak blood concentrations appearing only by 7 h postgavage. Daily administration of dihydrophthalazines for 5 or 10 days was generally well tolerated at doses of up to 400 mg/kg, with no clinical findings or relevant effect on body weights. Minimal hepatotoxicity (<10% increased liver weight, inflammatory infiltrates, single-cell necrosis, and increased mitosis) was noted for 400 mg/kg of BAL0030543 and BAL0030545 after both 5- and 10-day exposures. Plasma exposure was severalfold lower in animals treated with BAL0030545 than in animals treated with BAL0030543 or BAL0030544 (Table 6).
TABLE 6.
Preliminary pharmacokinetic parameters for dihydrophthalazine antifolates in uninfected male NMRI mice receiving 25 mg/kg of compound orally
| Antifolate | Cmax (μg/ml) | tmax (h) | AUC0-t (μg·h/ml) | F (%) |
|---|---|---|---|---|
| BAL0030543 | 3.24 | 0.5 | 10.63 | 25 |
| BAL0030544 | 5.39 | 2 | 23.4 | 26 |
| BAL0030545 | 0.99 | 6 | 5.59 | 29 |
Efficacy studies with a mouse model of septicemia.
The in vivo efficacies of BAL0030543, BAL0030544, and BAL0030545 were investigated after intraperitoneal infection with Tmps and Tmpr strains of S. aureus (Table 7). For Tmps strain S. aureus ATCC 29213, complete protection of the animals was afforded for all three experimental antifolates dosed i.v. at 3.1 mg/kg, whereas at 1.6 mg/kg all animals succumbed to the infection, giving a 50% effective dose (ED50) range of 1.6 to 3.1 mg/kg for the dihydrophthalazines; the corresponding ED50 for Tmp was >3.1 mg/kg. When mice were infected with Tmpr strain S. aureus BAS003908, an ED50 range of 6.25 to 12.5 mg/kg was obtained for i.v. Tmp, whereas i.v. BAL0030543 and BAL0030544 had an ED50 range of 3.1 to 6.25 mg/kg and i.v. BAL0030545 had an ED50 range of 1.6 to 3.1 mg/kg.
TABLE 7.
MIC and ED50 values for dihydrophthalazine antifolates and Tmp in a mouse model of septicemia
| Compound |
S. aureus ATCC 29213 (Tmps)
|
S. aureus BAS003908 (Tmpr)
|
||
|---|---|---|---|---|
| MIC (μg/ml) | ED50 range (mg/kg) | MIC (μg/ml) | ED50 range (mg/kg) | |
| Tmp | <0.25 | >3.1 | >128 | 6.25-12.5 |
| BAL0030543 | <0.25 | 1.6-3.1 | 0.5 | 3.1-6.25 |
| BAL0030544 | 1 | 1.6-3.1 | 1 | 3.1-6.25 |
| BAL0030545 | 0.5 | 1.6-3.1 | 1 | 1.6-3.1 |
Due to the fast onset of septicemia and the slow absorption of dihydrophthalazines from the gastrointestinal tract, no protective activity was observed when dihydrophthalazines were administered orally to mice after they had been infected with S. aureus. However, when mice were dosed prophylactically with 50 mg/kg of BAL0030545 4 h before infection with Tmps strain S. aureus ATCC 43300, followed by 50 mg/kg of BAL0030545 at 1 h and 3 h postinfection, all five infected animals survived for 5 days, whereas two of three untreated control animals died within 1 day.
DISCUSSION
Dihydrophthalazine antifolates BAL0030543, BAL0030544, and BAL0030545 have high in vitro activities against methicillin-susceptible S. aureus, MRSA, and coagulase-negative staphylococci (6). The activities of these compounds were further explored using small, select “challenge panels” of other clinically relevant pathogens whose in vitro MICs for vancomycin (E. faecalis) or sulfamethoxazole-Tmp (H. influenzae and S. pneumoniae) were disproportionately skewed toward values higher than those commonly encountered in the clinic (3, 4, 12, 39). The results revealed that the in vitro activities of BAL0030543, BAL0030544, and BAL0030545 toward enterococci matched that of Lzd and exceeded that of Van. MICs of the dihydrophthalazines for pneumococci, group A streptococci, and M. catarrhalis were far lower than those of Tmp and comparable to those of Lvx. The dihydrophthalazines also showed very interesting activities against M. avium, an established pulmonary pathogen (30, 38) which has been implicated in inflammatory bowel diseases (22, 29) and which is the subject of considerable effort vis-à-vis antifolate therapy (1, 21). However, BAL0030543, BAL0030544, and BAL0030545 showed relatively poor in vitro activities toward H. influenzae.
Since DHFR inhibitors exert their antibacterial effects largely through interference with dTMP biosynthesis, providing bacterial cells with an alternative source of thymidine (or, in some cases, thymine) can specifically reverse the inhibitory action of antifolates (26, 31). The substantial increases in MICs observed when assay media were supplemented with thymidine are consistent with expectations predicated on structural considerations that the 2,4-diaminopyrimidine dihydrophthalazine derivatives indeed function as DHFR inhibitors. Dramatically elevated MICs of BAL0030543, BAL0030544, and BAL0030545 were also observed for strains of S. pneumoniae, a species reportedly unable to utilize exogenous thymidine (10), though the thymidine concentrations employed in the present study were much higher than those used by Coll et al. (10).
DHFR genes from 40 staphylococci and 20 enterococci were cloned and sequenced. Most of the staphylococci surveyed harbored either the dfrA gene (30/40 strains) or the dfrG gene (2/40 strains), encoding Tmpr DHFR isozymes. Only the two S. aureus strains producing the S3 isozyme had elevated MICs for dihydrophthalazines. The S2 isozyme, encoded by dfrD, was not detected among the strains examined; this particular DHFR appears to be closely related to DHFR isozymes encoded by dfrG and by dfrF (11, 40), which confer resistance to BAL0030543, BAL0030544, and BAL0030545, in which case strains expressing the very rare dfrD gene may be expected to be nonsusceptible to dihydrophthalazine antifolates.
BAL0030543, BAL0030544, and BAL0030545 are racemic mixtures. Whereas slight differences were observed in the inhibitory activities of the two separated enantiomers toward the wild-type chromosomal DHFR encoded by dfrB, very dramatic differences were observed for the (S)-enantiomers relative to the racemates or (R)-enantiomers toward DHFRs encoded by dfrB (F98Y) and dfrA.
IC50s of the dihydrophthalazines for HeLa cells are on the order of 10 μg/ml, approximately 2 log2 dilution steps higher than the geometric mean MICs for most of the species examined in this study, suggesting a narrow therapeutic window for infections caused by these microorganisms. However, different cell types from the same mammalian species and analogous cell types from different mammalian species often display very different cytotoxicities in response to the same compound (36). Of greater relevance is that no untoward toxicity or histopathology was observed in mice dosed daily with up to 400 mg/kg of BAL0030543, BAL0030544, or BAL0030545 for 5 or 10 days, nor was BAL0030543, BAL0030544, or BAL0030545 (with or without metabolic activation) mutagenic or clastogenic in genotoxicity assays performed in vitro. Whereas the dihydrophthalazines and Tmp had similar activities against Tmps S. aureus in a mouse model of septicemia, the dihydrophthalazines were significantly more potent than Tmp against Tmpr S. aureus, despite the relatively high thymidine titer in murine blood, which can lead to poor efficacies for DHFR inhibitors tested in this species (45). Future studies on the efficacy of the dihydrophthalazines in murine models might benefit from the use of thymidine kinase-deficient infectious agents (19, 47) or from pretreatment of animals with thymidine phosphorylase (9, 17, 48).
The attractive in vitro antibacterial activities of BAL0030543, BAL0030544, and BAL0030545, considered in conjunction with their favorable oral bioavailabilities and encouraging pharmacological/toxicological properties, warrant further investigation of these molecules for use against infections caused by multiresistant gram-positive cocci, including indications for which long-term therapy is warranted.
Acknowledgments
We thank Franck Danel for obtaining circular dichrograms, Claude Nuoffer for performing cytotoxicity assays, Christina Würmlin for genotoxicity studies, and Dominique Klauer, Pascal Fullhardt, and Klaus Gebhardt for toxicology, pharmacokinetic, and mouse efficacy studies.
Footnotes
Published ahead of print on 22 June 2009.
REFERENCES
- 1.Barrow, E. W., W. J. Suling, L. E. Seitz, R. C. Reynolds, and W. W. Barrow. 2006. New antifolate inhibitors for Mycobacterium avium. Med. Chem. 2:505-510. [DOI] [PubMed] [Google Scholar]
- 2.Bhalla, A., D. C. Aron, and C. J. Donskey. 2007. Staphylococcus aureus intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect. Dis. 7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biedenbach, D. J., J. M. Bell, H. S. Sader, J. D. Turnidge, and R. N. Jones. 2009. Activities of dalbavancin against a worldwide collection of 81,673 gram-positive bacterial isolates. Antimicrob. Agents Chemother. 53:1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasi, F., D. J. Farrell, and L. Dubreuil. 2009. Antibacterial activity of telithromycin and comparators against pathogens isolated from patients with community-acquired respiratory tract infections: the Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin Study year 5 (2003-2004). Diagn. Microbiol. Infect. Dis. 63:302-308. [DOI] [PubMed] [Google Scholar]
- 5.Bogdanovich, T., L. M. Ednie, S. Shapiro, and P. C. Appelbaum. 2005. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 49:4210-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, C., L. M. Ednie, G. Lin, K. Smith, K. Kosowska-Shick, P. McGhee, B. Dewasse, L. Beachel, P. Caspers, B. Gaucher, G. Mert, S. Shapiro, and P. C. Appelbaum. 2009. Antistaphylococcal activity of dihydrophthalazine antifolates, a family of novel antibacterial drugs. Antimicrob. Agents Chemother. 53:1353-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. M7-A7. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement (M100-S18). Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Cobben, D. C. P., P. H. Elsinga, A. van Waarde, and P. L. Jager. 2003. Letter to the editor. Cancer Res. 63:8558-8559. [PubMed] [Google Scholar]
- 10.Coll, P. F., V. R. Ausina, J. V. Vernis, B. O. Mirelis, and G. P. Prats. 1984. Exogenous thymidine and reversal of the inhibitory effect of sulfamethoxazole-trimethoprim on streptococci. Eur. J. Clin. Microbiol. 3:424-426. [DOI] [PubMed] [Google Scholar]
- 11.Coque, T. M., K. V. Singh, G. M. Weinstock, and B. E. Murray. 1999. Characterization of dihydrofolate reductase genes from trimethoprim-susceptible and trimethoprim-resistant strains of Enterococcus faecalis. Antimicrob. Agents Chemother. 43:141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critchley, I. A., S. D. Brown, M. M. Traczewski, G. S. Tillotson, and N. Janjic. 2007. National and regional assessment of antimicrobial resistance among community-acquired respiratory tract pathogens identified in a 2005-2006 U.S. faropenem surveillance study. Antimicrob. Agents Chemother. 51:4382-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dale, G. E., C. Broger, P. G. Hartman, H. Langen, M. G. P. Page, R. L. Then, and D. Stüber. 1995. Characterization of the gene for the chromosomal dihydrofolate reductase (DHFR) of Staphylococcus epidermidis ATCC 14990: the origin of the trimethoprim-resistant S1 DHFR from Staphylococcus aureus? J. Bacteriol. 177:2965-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale, G. E., H. Langen, M. G. P. Page, R. L. Then, and D. Stüber. 1995. Cloning and characterization of a novel, plasmid-encoded trimethoprim-resistant dihydrofolate reductase from Staphylococcus haemolyticus MUR313. Antimicrob. Agents Chemother. 39:1920-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale, G. E., C. Broger, A. D'Arcy, P. G. Hartman, R. DeHoogt, S. Jolidon, I. Kompis, A. M. Labhardt, H. Langen, H. Locher, M. G. P. Page, D. Stüber, R. L. Then, B. Wipf, and C. Oefner. 1997. A single amino acid substitution in Staphylococcus aureus dihydrofolate reductase determines trimethoprim resistance. J. Mol. Biol. 266:23-30. [DOI] [PubMed] [Google Scholar]
- 16.Davies, B. I. 1990. The importance of the geometric mean MIC. J. Antimicrob. Chemother. 25:471-472. [DOI] [PubMed] [Google Scholar]
- 17.Ferone, R., S. R. Bushby, J. J. Burchall, W. D. Moore, and D. Smith. 1975. Identification of Harper-Cawston factor as thymidine phosphorylase and removal from media of substances interfering with susceptibility testing to sulfonamides and diaminopyrimidines. Antimicrob. Agents Chemother. 7:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flückiger-Isler, S., M. Baumeister, K. Braun, V. Gervais, N. Hasler-Nguyen, R. Reimann, J. Van Gompel, H.-G. Wunderlich, and G. Engelhardt. 2004. Assessment of the performance of the Ames II assay: a collaborative study with 19 coded compounds. Mutat. Res. 558:181-197. [DOI] [PubMed] [Google Scholar]
- 19.Fu, K. P., E. F. Kimble, R. J. Coldreck, and E. A. Konopka. 1984. Antimicrobial susceptibility, growth kinetic and pathogenicity of thymidine-requiring Streptococcus species. Chemotherapy 30:373-378. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs, P. C., A. L. Barry, S. D. Brown, and the Antimicrobial Susceptibility Testing QC Group. 1997. Interpretative criteria and quality control parameters for testing of susceptibilities of Haemophilus influenzae and Streptococcus pneumoniae to trimethoprim and trimethoprim-sulfamethoxazole. J. Clin. Microbiol. 35:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangjee, A., Y. Zeng, T. Talreja, J. J. McGuire, R. L. Kisliuk, and S. F. Queener. 2007. Design and synthesis of classical and nonclassical 6-arylthio-2,4-diamino-5-ethylpyrrolo[2,3-d]pyrimidines as antifolates. J. Med. Chem. 50:3046-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golan, L., A. Livneh-Kol, E. Gonen, S. Yagel, I. Rosenshine, and N. Y. Shpigel. 2009. Mycobacterium avium paratuberculosis invades human small-intestinal goblet cells and elicits inflammation. J. Infect. Dis. 199:350-354. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez, A. H., O. G. Berlin, and D. A. Bruckner. 1989. In-vitro activity of dapsone and two potentiators against Mycobacterium avium complex. J. Antimicrob. Chemother. 24:19-22. [DOI] [PubMed] [Google Scholar]
- 24.Grim, S. A., R. P. Rapp, C. A. Martin, and M. E. Evans. 2005. Trimethoprim-sulfamethoxazole as a viable treatment option for infections caused by methicillin-resistant Staphylococcus aureus. Pharmacotherapy 25:253-264. [DOI] [PubMed] [Google Scholar]
- 25.Guerry, P., C. Hubschwerlen, S. Jolidon, J.-L. Specklin, and P. Wyss. September 2000. Substituted 2,4-diaminopyrimidines. U.S. patent 6,114,330.
- 26.Hamilton-Miller, J. M. T. 1988. Reversal of activity of trimethoprim against gram-positive cocci by thymidine, thymine and ‘folates.’ J. Antimicrob. Chemother. 22:35-39. [DOI] [PubMed] [Google Scholar]
- 27.Heller, S., L. Kellenberger, and S. Shapiro. 2007. Antipropionibacterial activity of BAL19403, a novel macrolide antibiotic. Antimicrob. Agents Chemother. 51:1956-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, M. I., D. Merrilees, W. A. Robson, T. Lennon, J. Masters, K. E. Orr, J. N. S. Matthews, and D. E. Neal. 2007. Oral ciprofloxacin or trimethoprim reduces bacteriuria after flexible cystoscopy. Br. J. Urol. Int. 100:826-829. [DOI] [PubMed] [Google Scholar]
- 29.Juste, R. A., N. Elguezabal, A. Pavón, J. M. Garrido, M. Geijo, I. Sevilla, J. L. Cabriada, A. Tejada, F. García-Campos, R. Casado, I. Ochotorena, and A. Izeta. 2009. Association between Mycobacterium avium subsp. paratuberculosis DNA in blood and cellular and humoral immune response in inflammatory bowel disease patients and controls. Int. J. Infect. Dis. 13:247-254. [DOI] [PubMed] [Google Scholar]
- 30.Kasperbauer, S. H., and C. L. Daley. 2008. Diagnosis and treatment of infections due to Mycobacterium avium complex. Semin. Respir. Crit. Care Med. 29:569-576. [DOI] [PubMed] [Google Scholar]
- 31.Koch, A. E., and J. J. Burchall. 1971. Reversal of the antimicrobial activity of trimethoprim by thymidine in commercially prepared media. Appl. Microbiol. 22:812-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, L. G., F. Perdreau-Remington, G. Rieg, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 33.Minassian, M. A., D. A. Lewis, D. Chattopadhyay, B. Bovill, G. J. Duckworth, and J. D. Williams. 1998. A comparison between single-dose fosfomycin trometamol (Monuril®) and a 5-day course of trimethoprim in the treatment of uncomplicated lower urinary tract infection in women. Int. J. Antimicrob. Agents 10:39-47. [DOI] [PubMed] [Google Scholar]
- 34.Nungester, W. J., A. A. Wolf, and L. F. Jourdonais. 1932. Effect of gastric mucin on virulence of bacteria in intraperitoneal infections in the mouse. Proc. Soc. Exp. Biol. Med. 30:121-122. [Google Scholar]
- 35.Pankuch, G. A., D. B. Hoellman, G. Lin, S. Bajaksouzian, M. R. Jacobs, and P. C. Appelbaum. 1998. Activity of HMR 3647 compared to those of five agents against Haemophilus influenzae and Moraxella catarrhalis by MIC determination and time-kill assay. Antimicrob. Agents Chemother. 42:3032-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peternel, L., M. Kotnik, A. Preželj, and U. Urleb. 2009. Comparison of 3 cytotoxicity screening assays and their application to the selection of novel antibacterial hits. J. Biomol. Screen. 14:142-150. [DOI] [PubMed] [Google Scholar]
- 37.Qi, W., M. Ender, F. O'Brien, A. Imhof, C. Ruef, N. McCallum, and B. Berger-Bächi. 2005. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Zürich, Switzerland (2003): prevalence of type IV SCCmec and a new SCCmec element associated with isolates from intravenous drug users. J. Clin. Microbiol. 43:5164-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez, J., C. Mason, J. Ali, and F. A. Lopez. 2008. Mycobacterium avium complex pulmonary disease: management options in HIV-negative patients. J. La. State Med. Soc. 160:248-254. [PubMed] [Google Scholar]
- 39.Reynolds, R., J. Shackcloth, D. Felmingham, A. MacGowan, et al. 2003. Comparison of BSAC agar dilution and NCCLS broth microdilution MIC methods for in vitro susceptibility testing of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis: the BSAC Respiratory Resistance Surveillance Programme. J. Antimicrob. Chemother. 52:925-930. [DOI] [PubMed] [Google Scholar]
- 40.Sekiguchi, J., P. Tharavichitkul, T. Miyoshi-Akiyama, V. Chupia, T. Fujino, M. Araake, A. Irie, K. Morita, T. Kuratsuji, and T. Kirikae. 2005. Cloning and characterization of a novel trimethoprim-resistant dihydrofolate reductase from a nosocomial isolate of Staphylococcus aureus CM.S2. Antimicrob. Agents Chemother. 49:3948-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivde, S. R., R. P. D. Cooke, W. A. O'Neill, A. G. A. Cowie, W. T. Lawrence, and G. M. Watson. 2002. Trimethoprim versus gentamicin for the prevention of bacteriuria following transrectal biopsy of the prostate—do patients need additional anaerobic cover? Urol. Int. 69:106-110. [DOI] [PubMed] [Google Scholar]
- 42.Soto, C. Y., N. Andreu, I. Gibert, and M. Luquin. 2002. Simple and rapid differentiation of Mycobacterium tuberculosis H37Ra from M. tuberculosis clinical isolates through two cytochemical tests using neutral red and Nile blue stains. J. Clin. Microbiol. 40:3021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stamm, W. E., G. W. Counts, K. F. Wagner, D. Martin, D. Gregory, M. McKevitt, M. Turck, and K. K. Holmes. 1980. Antimicrobial prophylaxis of recurrent urinary tract infections: a double-blind, placebo-controlled trial. Ann. Intern. Med. 92:770-775. [DOI] [PubMed] [Google Scholar]
- 44.Suling, W. J., R. C. Reynolds, E. W. Barrow, L. N. Wilson, J. R. Piper, and W. W. Barrow. 1998. Susceptibilities of Mycobacterium tuberculosis and Mycobacterium avium complex to lipophilic deazapteridine derivatives, inhibitors of dihydrofolate reductase. J. Antimicrob. Chemother. 42:811-815. [DOI] [PubMed] [Google Scholar]
- 45.Then, R. 1993. Discussion: microbiology. J. Chemother. 5:444-446. [DOI] [PubMed] [Google Scholar]
- 46.Thiermann, S., J. Munzinger, and T. Bodmer. 2002. Comparison of phenotypic and genotypic methods for the detection of clarithromycin resistance in Mycobacterium avium. J. Antimicrob. Chemother. 49:679-681. [DOI] [PubMed] [Google Scholar]
- 47.Tokunaga, T., K. Oka, A. Takemoto, Y. Ohtsubo, N. Gotoh, and T. Nishino. 1997. Efficacy of trimethoprim in murine experimental infection with a thymidine kinase-deficient mutant of Escherichia coli. Antimicrob. Agents Chemother. 41:1042-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Waarde, A., D. C. P. Cobben, A. J. H. Suurmeijer, B. Maas, W. Vaalburg, E. F. J. de Vries, P. L. Jager, H. J. Hoekstra, and P. H. Elsinga. 2004. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J. Nucl. Med. 45:695-700. [PubMed] [Google Scholar]
- 49.von der Hude, W., S. Kalweit, G. Engelhardt, S. McKiernan, P. Kasper, R. Slacik-Erben, H. G. Miltenburger, N. Honarvar, R. Fahrig, B. Görlitz, S. Albertini, S. Kirchner, D. Utesch, F. Pötter-Locher, H. Stopper, and S. Madle. 2000. In vitro micronucleus assay with Chinese hamster V79 cells—results of a collaborative study with in situ exposure to 26 chemical substances. Mutat. Res. 468:137-163. [DOI] [PubMed] [Google Scholar]
- 50.Wyss, P. C., P. Guerry, P. G. Hartman, C. Hubschwerlen, S. Jolidon, H. Locher, J.-L. Specklin, and H. Stalder. 1999. Anti-MRSA dihydrofolate reductase inhibitors: synthesis and SAR, abstr. 1800. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.