Abstract
CEM-101 is a novel fluoroketolide with lower MICs than those of telithromycin and macrolides. Our aim was to assess the cellular accumulation and intracellular activity of CEM-101 using models developed for analyzing the pharmacokinetics and pharmacological properties of antibiotics against phagocytized bacteria. We used THP-1 macrophages and Staphylococcus aureus (ATCC 25923 [methicillin (meticillin) sensitive]), Listeria monocytogenes (strain EGD), and Legionella pneumophila (ATCC 33153). CEM-101 reached cellular-to-extracellular-concentration ratios of about 350 within 24 h (versus approximately 20, 30, and 160 for telithromycin, clarithromycin, and azithromycin, respectively). This intracellular accumulation was suppressed by incubation at a pH of ≤6 and by monensin (proton ionophore) and was unaffected by verapamil (P-glycoprotein inhibitor; twofold accumulation increase for azithromycin) or gemfibrozil. While keeping with the general properties of the macrolide antibiotics in terms of maximal efficacy (Emax; approximately 1-log10-CFU decrease compared to the postphagocytosis inoculum after a 24-h incubation), CEM-101 showed significantly greater potency against phagocytized S. aureus than telithromycin, clarithromycin, and azithromycin (for which the 50% effective concentration [EC50] and static concentrations were about 3-, 6-, and 15-fold lower, respectively). CEM-101 was also about 50-fold and 100-fold more potent than azithromycin against phagocytized L. monocytogenes and L. pneumophila, respectively. These differences in EC50s and static concentrations between drugs were minimized when data were expressed as multiples of the MIC, demonstrating the critical role of intrinsic drug activity (MIC) in eliciting the antibacterial intracellular effects, whereas accumulation per se was unimportant. CEM-101 should show enhanced in vivo potency if used at doses similar to those of the comparators tested here.
Macrolides have long been known for their large volume of distribution (3, 28), which is related to their ability to accumulate inside eukaryotic cells by diffusion/segregation in acidic compartments, namely, lysosomes and related vacuoles (8, 9). As a consequence, macrolides have been considered advantageous for the treatment of infections localized in these compartments (32, 48). In a more general context, they are widely recommended for infections caused by typical intracellular pathogens such Legionella and Chlamydia, based on a large array of both in vitro (7, 17) and clinical (16, 19, 33, 38, 42) data. However, direct quantitative comparisons between intracellular and extracellular activities using facultative intracellular pathogens, such as Staphylococcus aureus or Listeria monocytogenes, suggest that macrolides express only a minimal fraction of their antibacterial potential intracellularly (5, 34, 36), especially considering their great intracellular accumulation. This minimized antibacterial potential against organisms replicating in phagolysosomes and related vacuoles is most likely related to acidic pH, which is known to reduce the activity of macrolides. Another factor is that some organisms, such as L. monocytogenes, may actually replicate in other subcellular compartments. In addition, certain macrolides, such as azithromycin, are subject to active efflux from macrophages (35), which further contributes to suboptimal intracellular activity (34). Key progress in the discovery of new macrolides over the last 20 years has been heralded mainly by the observation that 11,12-carbamate analogs of clarithromycin carrying a lipophilic side chain show improved activity compared to the parent compound (15). Together with the removal of the cladinose (1), this led to the discovery and development of the ketolides, with telithromycin being the first candidate to receive clinical approval. The primary improvement generated by ketolides is activity against erythromycin-resistant organisms (45, 49), but no true improvement in their activity in the intracellular environment has been achieved.
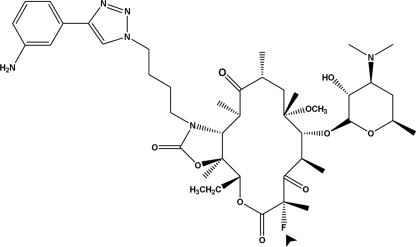
CEM-101 is a novel fluoroketolide containing an 11,12-carbamate-butyl-[1,2,3]-triazolyl-aminophenyl side chain (Fig. 1). CEM-101 demonstrates enhanced potency compared to telithromycin, with activity against telithromycin-intermediate and telithromycin-resistant organisms. This enhanced activity of CEM-101 is probably due to a higher binding affinity to the ribosome (22).
FIG. 1.
Structural formula of CEM-101. The molecule possesses an 11,12-carbamate-butyl-[1,2,3]-triazolyl-aminophenyl side chain and a fluorine atom (arrowhead) substituting position 2 of the macrocycle. The molecule is probably monocationic at neutral or moderately acidic pH, as the calculated pKa of the aminophenyl group is less than 4 (versus ∼8.5 for the desosamine).
This enhanced activity prompted us to assess the cellular accumulation and intracellular activity of CEM-101 using models that have been developed for the study of the intracellular pharmacodynamics of antibiotics (4, 20). Our data show that CEM-101 maintains the maximal efficacy of known macrolides and shows greater potency against intracellular forms of Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila than telithromycin, azithromycin, and clarithromycin. This improved potency of CEM-101 results from the combination of its higher intrinsic activity against S. aureus and possibly also L. pneumophila and the decreased detrimental effect of acidic pH on its activity.
MATERIALS AND METHODS
Cell lines.
Experiments were performed with THP-1 cells (ATCC TIB-202; American Tissue Culture Collection, Manassas, VA), a human myelomonocytic cell line displaying macrophage-like activity, as described earlier (5).
Assay of the cell-associated macrolides and calculation of the apparent cellular-to-extracellular-concentration ratios.
Macrolides were assayed by a microbiological method, using S. aureus ATCC 25923 as a test organism and according to the general procedure described previously (35). Cell proteins were measured in parallel using the Folin-Ciocalteu/biuret method (23). The cell-associated contents in macrolides were expressed by reference to the total cell protein content and converted into apparent concentrations using a conversion factor of 5 μl per mg of cell protein, an average value found for many cultured cells.
Bacterial strains, susceptibility testing, and 24-h dose-response curve studies with broth.
S. aureus ATCC 25923 (methicillin [meticillin] sensitive), L. monocytogenes strain EGD, and L. pneumophila strain ATCC 33153 were used in the present study. MIC determinations were performed in Mueller-Hinton broth (for S. aureus) and tryptic soy broth (for L. monocytogenes) after a 24-h incubation, or in α-ketoglutarate-buffered yeast extract broth (for L. pneumophila) after a 48-h incubation. For S. aureus studies, 24-h concentration-response experiments in acellular medium were performed in Mueller-Hinton broth.
Cell infection and assessment of antibiotic intracellular activities.
Infection of THP-1 cells and assessment of the intracellular activity of antibiotics were performed as described previously (5, 20) for S. aureus and L. monocytogenes, or with minor adaptations for L. pneumophila using (i) a multiplicity of infection of 10 bacteria per macrophage and (ii) gentamicin (50 mg/liter) for 30 to 45 min for the elimination of nonphagocytosed bacteria.
Antibiotics and main reagents.
CEM-101, clarithromycin, and telithromycin were obtained as laboratory standards from Cempra Pharmaceuticals (Chapel Hill, NC) and azithromycin and linezolid from Pfizer Inc., New York, NY. Cell culture media and sera were from Invitrogen Corp. (Carlsbad, CA) and Lonza Inc. (Basel, Switzerland).
Statistical analyses.
Curve-fitting statistical analyses were performed with GraphPad Prism version 4.03 and GraphPad Instat version 3.06 (GraphPad Software, San Diego, CA).
RESULTS
Cellular accumulation and modulation by acidic pH, monensin, and effux transporter inhibitors.
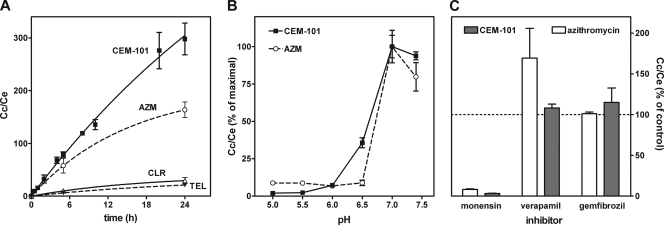
In the first series of experiments, we examined the extent of CEM-101 accumulation in THP-1 macrophages and how this accumulation is influenced by (i) acidic pH, (ii) the addition of monensin, and (iii) efflux transporter inhibitors. As shown in Fig. 2A, the cellular uptake of CEM-101 proceeded almost linearly over time, reaching an accumulation level approximately 350-fold the extracellular concentration within 24 h. Figure 2B shows that the accumulation of both CEM-101 and azithromycin was drastically reduced when the experiments were conducted at acidic pH, with the change occurring almost entirely when the pH was brought from 7 to 6. Figure 2C shows that monensin, which is known to decrease the cellular accumulation of weak organic bases, also almost completely suppressed the accumulation of both CEM-101 and azithromycin. In contrast, verapamil, an inhibitor of the P-glycoprotein efflux transporter (P-gp, also known as MDR1), increased the accumulation of azithromycin without affecting that of CEM-101, whereas gemfibrozil, an inhibitor of multidrug resistance proteins (MRP) and other organic anion transporters did not affect either compound. (Of note, neither verapamil nor gemfibrozil affected the accumulation of telithromycin or clarithromycin [data not shown].) Finally, we examined the efflux of CEM-101 from cells incubated with 10 mg/liter of CEM-101 for 1 h and then transferred into drug-free medium. Efflux proceeded in a bimodal fashion, with half of the cell-associated drug being released within approximately 10 min, and a slower release phase of several hours (data not shown) followed.
FIG. 2.
Accumulation of CEM-101 versus comparators in THP-1 cells at 37°C (all drugs at an extracellular concentration of 10 mg/liter). (A) Kinetics of accumulation (AZM, azithromycin; Cc, intracellular concentration; Ce, extracellular concentration); (B) influence of the pH of the culture medium on the accumulation (30 min) of CEM-101 (solid symbols and solid line) and azithromycin (open symbols and dotted line); (C) influence of monensin (50 μM; 2-h incubation), verapamil (150 μM; 24-h incubation), or gemfibrozil (250 μM; 24-h incubation) on the cellular accumulation of azithromycin and CEM-101. All values are means ± standard deviations (SD) of three independent determinations (when not visible, SD bars are smaller than the symbols).
Susceptibility toward S. aureus ATCC 25923, Listeria monocytogenes EGD, and Legionella pneumophila ATCC 33153.
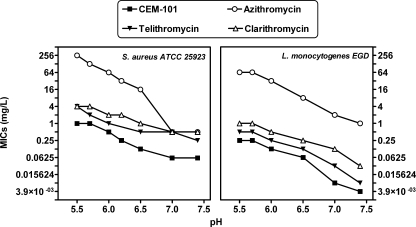
CEM-101 showed lower MICs than azithromycin against the three selected organisms (S. aureus, 0.06 and 0.5 mg/liter; L. monocytogenes, 0.004 and 1 mg/liter; and L. pneumophila, 0.004 and 0.016 mg/liter) in conventional susceptibility testing. The MICs of CEM-101, telithromycin, azithromycin, and clarithromycin against S. aureus and L. monocytogenes were then measured in broths adjusted to pH values ranging from 5.5 to 7.4. The range was selected to cover the values at which the antibiotics could be exposed in the extracellular milieu or intracellularly for the two organisms considered. As illustrated in Fig. 3, all four drugs showed a marked decrease in potency against both organisms when the pH was decreased from 7.4 to 5.5, with azithromycin demonstrating the most significant loss of activity. CEM-101 retained the most activity, consistently showing the lowest MICs throughout the entire pH range investigated, with values (mg/liter) ranging from 0.06 (pH 7.4) to 0.5 (pH 5.5) for S. aureus (ATCC 25923) and 0.0039 (pH 7.4) to 0.25 (pH 5.5) for L. monocytogenes (EDG). For L. pneumophila (data not shown), the MIC of CEM-101 increased from 0.005 to 0.01 and that of azithromycin from approximately 0.01 to 0.25 mg/liter when the pH of the broth was decreased from 7.4 to 6.5 (no determination could be made at lower pH values because of absence of growth).
FIG. 3.
Comparative susceptibilities of S. aureus ATCC 25923 and L. monocytogenes EGD to CEM-101, telithromycin, azithromycin, and clarithromycin, based on MIC determinations in pH-adjusted broth.
Time and concentration effects against extracellular and intraphagocytic S. aureus.
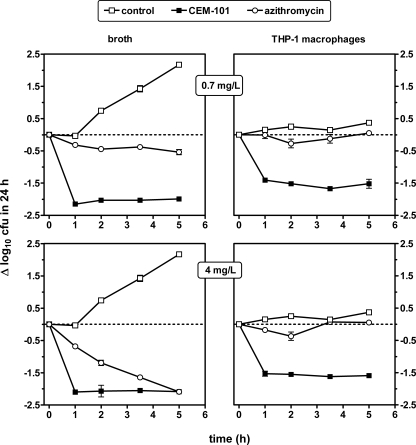
Short-term (6-h) time-kill curves were obtained for CEM-101 in comparison with those for azithromycin against S. aureus (ATCC 25923) in broth and after phagocytosis by THP-1 macrophages using two single fixed concentrations of 0.7 and 4 mg/liter. The lower concentration was chosen to be relevant to the serum concentration of azithromycin (2) and CEM-101 (27, 39), and the higher concentration was selected to be above the MIC of azithromycin for the organisms of interest. Results presented in Fig. 4 show that under these conditions, only CEM-101 was able to significantly decrease CFU in broth as well as in THP-1 macrophages at the 0.7-mg/liter concentration. At the 4-mg/liter concentration in broth, azithromycin eventually achieved the same antibacterial effect as CEM-101, but at a lower rate (5 h compared to 1 h). In THP-1 macrophages, no consistent activity was detected for azithromycin, even at the 4-mg/liter concentration, whereas CEM-101 again achieved a reduction of approximately 1.5 log10 CFU, similar to the magnitude seen at the 0.7-mg/liter concentration. In all situations with CEM-101, the maximal decrease of CFU was obtained within 1 h and was maintained thereafter.
FIG. 4.
Short-term time-kill effect of CEM-101 and azithromycin on S. aureus (ATCC 25923) in broth (left panels; pH 7.4) or after phagocytosis by THP-1 macrophages (right panels). Both drugs were used at an extracellular concentration of either 0.7 (top panels) or 4 (bottom panels) mg/liter. MICs of CEM-101 and azithromycin were 0.06 and 0.5 mg/liter, respectively. All values are means ± standard deviations (SD) of three independent experiments (when not visible, SD bars are smaller than the symbols).
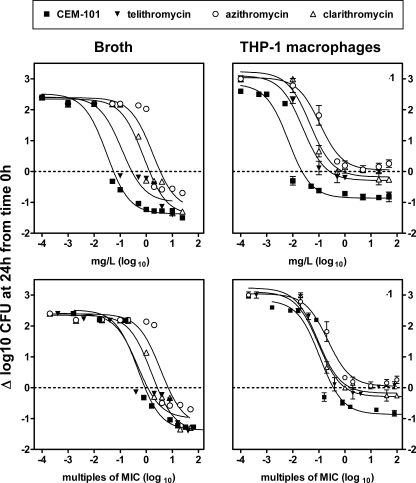
We then performed concentration-response experiments at a fixed time point (24 h) to obtain the pertinent pharmacological descriptors of CEM-101 activity (relative potency [50% effective concentration {EC50}], apparent static concentration [Cs], and relative maximal efficacy [Emax] [see reference 5 for details and illustration]) in comparison with clarithromycin, azithromycin and telithromycin activity. Data are presented in Fig. 5 as a function of (i) weight concentrations (mg/liter) and (ii) multiples of the MICs (as determined in broth at pH 7.4). The numerical values of the corresponding pharmacological descriptors are shown in Table 1. The activities in both broth and THP-1 macrophages developed in a concentration-dependent fashion (following the law of mass action), as denoted by the sigmoidal shape of each best-fit function (Hill equation). In broth, the relative efficacy of CEM-101 (Emax of −1.37 log10) was similar to that of the other drugs (Emax values of −1.00 to −1.41 log10). In THP-1 macrophages, the relative efficacy of CEM-101 was significantly decreased compared to that in broth (Emax of −0.86 log10), but not to the same extent as those of the other drugs, which essentially became bacteriostatic only (Emax values of 0.04 to −0.29 log10). On a weight basis, CEM-101 had higher relative potencies (lower E50 values) and lower static concentrations (lower Cs values) than all three comparator drugs in both broth and in THP-1 macrophages. When the data were analyzed as a function of equipotent concentration (multiples of the MIC), these differences in EC50 values were reduced, indicating that the MIC was the main driving parameter in this context. In broth, even when analyzed as multiples of the MIC, CEM-101 and clarithromycin still showed significantly lower EC50s than telithromycin and azithromycin. Of note, the relative potencies of all four drugs were systematically improved when tested in THP-1 macrophages compared to broth (lower EC50s, even when expressed in multiples of the MIC), indicating a corresponding 4- to 16-fold enhancement of apparent intrinsic activity.
FIG. 5.
Concentration-effect relationships for CEM-101, telithromycin, clarithromycin, and azithromycin toward S. aureus (ATCC 25923) in broth (left panels) and after phagocytosis by THP-1 macrophages (right panels). The ordinate shows the change in CFU (Δ log CFU) per ml (broth) or per mg of cell protein (THP-1 macrophages) at 24 h compared to the initial inoculum. The abscissa shows the concentrations of the antibiotics as follows: (i) top panels, weight concentrations (in mg/liter) in broth (left) or in the culture medium (right) and (ii) bottom panels, multiples of the MIC as determined in broth at pH 7.4. All values are means ± standard deviations (SD) of three independent experiments (when not visible, SD bars are smaller than the symbols). Statistical analysis based on global analysis of curve-fitting parameters (one-way analysis of variance); the only significant difference is between CEM-101 and azithromycin in broth (P = 0.04). Numerical values of the pertinent pharmacological descriptors and statistical analysis of their differences are shown in Table 1.
TABLE 1.
Pertinent regression parameters (with confidence intervals) and statistical analysis of the dose-response curves illustrated in Fig. 5a
| Antibiotic | Brothb
|
THP-1 macrophagesc
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emaxd (CI)e | EC50(CI)f
|
Csg
|
R2 | Emax (CI) | EC50(CI)
|
Cs
|
R2 | |||||
| mg/liter | Multiple of MIC | mg/liter | Multiple of MIC | mg/liter | Multiple of MIC | mg/liter | Multiple of MIC | |||||
| CEM-101 | −1.37 (−1.67 to −1.08) a;A | 0.03 (0.02-0.06) a;A | 0.48 (0.26-0.91) a;A | 0.06 | 0.88 | 0.973 | −0.86 (−1.36 to −0.37) a;B | 0.0068 (0.0023-0.020) a;B | 0.11 (0.037-0.32) a;B | 0.022 | 0.35 | 0.927 |
| Telithromycin | −1.00 (−1.78 to −0.22) a;A | 0.12 (0.03-0.52) b;A | 0.46 (0.11-2.06) a;A | 0.29 | 0.96 | 0.892 | −0.29 (−0.70-0.12) b;B | 0.024 (0.007-0.088) b;B | 0.097 (0.027-0.35) a;B | 0.63 | 1.04 | 0.954 |
| Azithromycin | −1.23 (−2.55-0.083) a;A | 1.78 (0.45-7.02) c;A | 3.55 (0.90-14.0) b;A | 3.4 | 6.87 | 0.872 | 0.04 (−0.23-0.32) b;B | 0.11 (0.05-0.22) c;B | 0.22 (0.11-0.45) a;B | >50 | >100 | 0.983 |
| Clarithromycin | −1.41 (−1.95 to −0.87) a;A | 0.80 (0.41-1.56) c;A | 1.59 (0.81-3.1) a,b;A | 1.32 | 2.65 | 0.956 | −0.18 (−0.52-0.16) b;B | 0.046 (0.018-0.12) b,c;B | 0.093 (0.035-0.25) a;B | 0.84 | 1.68 | 0.974 |
The regression parameters described in the table were derived from the analysis of all data points shown in Fig. 5 (data from samples without antibiotics were not used because of evidence of extracellular growth when the extracellular concentration of an antibiotic was lower than 0.01× MIC (5). By analysis of the differences between the parameter values in each column (one-way analysis of variance by the Tuckey test for multiple comparisons of each parameter for all drugs), values with different lowercase letters are significantly different from each other (P < 0.05). By analysis of the differences between broth and THP-1 macrophages per row (unpaired, two-tailed t test), values with different uppercase letters are significantly different from each other (P < 0.05).
Original inoculum (time = 0 h) was (0.97 ± 0.24) × 106 CFU/ml (n = 3).
Original (postphagocytosis) inoculum (time = 0 h) was (2.74 ± 0.55) × 106 CFU/mg protein (n = 3).
Expressed as a decrease in the numbers of CFU (in log10 units) at 24 h from the corresponding original inoculum, as extrapolated for an infinitely high antibiotic concentration; samples yielding less than five counts were considered below detection level.
CI, confidence interval.
Concentration causing a reduction of the inoculum halfway between the initial (E0) and maximal (Emax) values, as obtained from the Hill equation (by using a slope factor of 1).
Concentration resulting in no apparent bacterial growth (number of CFU identical to the original inoculum), as determined by graphical intrapolation.
Modulation of the intracellular activity toward S. aureus by P-gp inhibitors.
As verapamil increased the accumulation of azithromycin but not that of CEM-101, telithromycin, or clarithromycin, we examined whether it would also modulate the activities of these four drugs toward phagocytized S. aureus in a differential fashion. Concentration-effect experiments using the same protocol as that shown in Fig. 5 were therefore performed in the presence of verapamil (100 μM). Verapamil did not modify the response to CEM-101, clarithromycin, or telithromycin; however, it significantly increased both the relative potency (decreased EC50) and the relative efficacy (decreased Emax) of azithromycin. The increased relative potency and efficacy eventually made the azithromycin response indistinguishable from that of CEM-101 (see data in Fig. SP1 in the supplemental material). Gemfibrozil had no effect on any of the four antibiotics.
Activity against intraphagoctic L. monocytogenes and L. pneumophila.
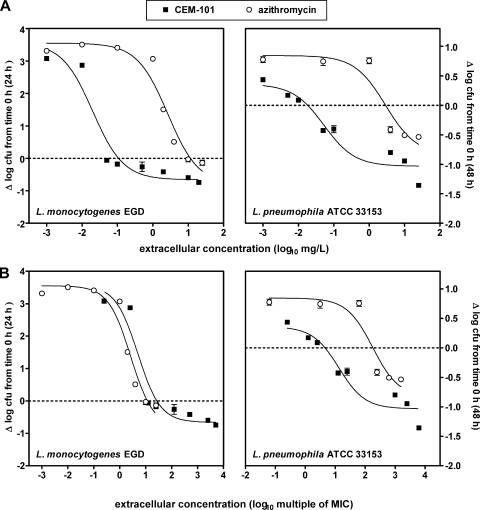
In the last series of experiments, we used the same approach as that used for S. aureus to assess the activities of CEM-101 and azithromycin against phagocytized L. monocytogenes and L. pneumophila to obtain information on concentration-effect relationships and on the corresponding pertinent pharmacological descriptors. As shown in Fig. 6 (see also Table 2 for numerical values), a relationship compatible with the Hill equation was observed in all cases, although the limited growth of L. pneumophila made the fitting of functions somewhat more uncertain. When the data were plotted against weight concentration, it clearly appeared that CEM-101 had a much higher relative potency (lower EC50) than azithromycin for both L. monocytogenes and L. pneumophila. This difference was reduced but nevertheless remained significant when data for L. pneumophila were plotted against multiples of the MIC, indicating that the MIC was an important but not the exclusive driver of intracellular activity against this organism. Conversely, no difference in the responses was seen for L. monocytogenes when data were expressed as multiples of the MIC.
FIG. 6.
Concentration-effect relationships for CEM-101 and azithromycin toward intraphagocytic L. monocytogenes (strain EGD, left panels) and L. pneumophila (strain ATCC 33153, right panels). The ordinate shows the change in CFU (Δ log CFU) per mg of cell protein at 24 h (L. monocytogenes) or 48 h (L. pneumophila) compared to the initial postphagocytosis inoculum. The abscissa shows the concentrations of the antibiotics as follows: (i) top panels, weight concentrations (in mg/liter); (ii) bottom panels, multiples of the MIC as determined in broth at pH 7.4. All values are means ± standard deviations (SD) of three independent experiments (when not visible, SD bars are smaller than the symbols). Numerical values of the pertinent pharmacological descriptors and statistical analysis of their differences are shown in Table 2.
TABLE 2.
Pertinent regression parameters (with confidence intervals) and statistical analysis of the dose-response curves illustrated in Fig. 6a
| Antibiotic |
L. monocytogenes EGDb
|
L. pneumophila ATCC 33153c
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emaxd (CI)e | EC 50 (CI)f
|
Csg
|
R2 | Emax (CI) | EC50 (CI)
|
Cs
|
R2 | |||||
| mg/liter | Multiple of MIC | mg/liter | Multiple of MIC | mg/liter | Multiple of MIC | mg/liter | Multiple of MIC | |||||
| CEM-101 | −0.66 (−1.28 to −0.037) a | 0.020 (0.005-0.073) a | 5.00 (1.36-18.5) a | 0.11 | 0.88 | 0.934 | −1.03 (−1.34 to −0.72) a | 0.052 (0.012-0.23) a | 13.1 (3.02-57.0) a | 0.018 | 4.56 | 0.920 |
| Azithromycin | −0.81 (−2.11-0.48) a | 2.66 (0.91-7.73) b | 2.66 (0.81-3.1) a | 11.6 | 11.6 | 0.953 | −0.83 (−2.00-0.34) a | 2.86 (0.17-48.6) b | 179.0 (10.5-3,038) b | 2.90 | 181 | 0.903 |
The regression parameters described in the table were derived from the analysis of all data points shown in Fig. 6 (data from samples without antibiotics were not used because of evidence of extracellular growth when the extracellular concentration of an antibiotic was lower than 0.01× MIC (5). By analysis of the differences between the parameter values in each column (unpaired, two-tailed t test), values with different lowercase letters are significantly different from each other (P < 0.05).
Original (postphagocytosis) inoculum (time = 0 h; CFU/mg protein) for L. monocytogenes was (1.67 ± 0.22) × 106 (n = 3).
Original (postphagocytosis) inoculum (time = 0 h; CFU/mg protein) for L. pneumophila was (0.94 ± 0.06) × 106.
Expressed as a decrease in the numbers of CFU (in log10 units) at 24 h (L. monocytogenes) or 48 h (L. pneumophila) from the corresponding original inoculum, as extrapolated for an infinitely high antibiotic concentration; samples yielding less than five counts were considered below detection level.
CI, confidence interval.
Concentration causing a reduction of the inoculum halfway between initial (E0) and maximal (Emax) values, as obtained from the Hill equation (by using a slope factor of 1).
Concentration resulting in no apparent bacterial growth (number of CFU identical to the original inoculum), as determined by graphical intrapolation.
DISCUSSION
Lack of effective antimicrobial therapy results in intracellular survival of bacteria, which remains a major cause of bacterial spreading, life-threatening therapeutic failures, and establishment of chronic, relapsing infections. These situations are indeed clearly observed during the course of infections caused by the three organisms studied here, with typical examples being meningitis for L. monocytogenes (14, 18); invasion of lung macrophages for L. pneumophila (12, 41); and endocarditis (37), osteomyelitis (46), and skin structure infections (25) for S. aureus.
While it has long been assumed that intracellular accumulation of an antibiotic is indicative of efficient activity against bacteria, pharmacodynamic evaluation of a large series of commonly used antibiotics has revealed that other parameters, such as intracellular bioavailability and modulation of activity in the infected compartment, are also critical (10, 44). The present study confirms and significantly extends previous observations made with macrolides in this context (5, 11), thanks to the differential behaviors exhibited by CEM-101 compared to those by telithromycin, azithromycin, and clarithromycin.
The main findings in our study are that CEM-101 accumulates to a considerably larger extent than the comparators, including azithromycin, and consistently expresses greater potency (decreased values of EC50 and Cs), while showing maximal efficacy (Emax) similar to those of the comparators tested here. This indicates that the improvements resulting from the structural modifications introduced in CEM-101 relate to modulation of pharmacokinetic properties and intrinsic activity (including its reduced susceptibility to physico-chemical conditions prevailing in the infected compartment) rather than to a change in its mode of action. Thus, by and large, CEM-101 exhibits the essentially bacteriostatic character of macrolides but expresses it better in the intracellular milieu and at considerably lower extracellular concentrations than the comparators.
The cellular accumulation of CEM-101 clearly results from the general mechanism of proton trapping of weak organic bases (13) envisaged for all macrolides (44), as accumulation is almost completely suppressed, in parallel with that of azithromycin, by exposure to acid pH or to the proton ionophore monensin (43, 47). Based on the general model of diffusion/segregation of weak bases in acidic membrane-bound compartments (13), accumulation is determined by the number of ionizable groups and the ratios between the membrane permeability coefficients of the unionized and ionized forms of the drug. While CEM-101 has two ionizable functions, the pKa of the aminophenyltriazole is calculated to be less than 4, making the molecule largely monocationic (as clarithromycin and telithromycin) at neutral and even at lysosomal pH (∼5) (29). In contrast, azithromycin has two ionizable functions, with pKas of >6 (24), and is therefore dicationic intracellularly. CEM-101, however, possesses a fluoro substituent in position 2, which should make it more lipophilic than clarithromycin or telithromycin. Additional biophysical studies aiming at determining the ratio of the permeability constants of the unionized and ionized forms of CEM-101 in comparison with clarithromycin and telithromycin should be very informative in this context, as this parameter is as critical as the number of ionizable functions in determining the level of cellular accumulation of weak organic bases (13). In another context, the greater cellular accumulation of CEM-101 is probably partially due to its insusceptibility to P-gp-mediated efflux (which is expressed by THP-1 macrophages under our culture conditions [21]) in contrast to azithromycin.
Potential improvement in the expression of CEM-101 activity in the intracellular milieu compared with that of azithromycin is strongly suggested by the experiments that examined the effect of acid pH on the MIC of CEM-101 against S. aureus. Intraphagocytic S. aureus survives and multiplies in the acidic environment of phagolysosomes of THP-1 cells (21, 36), which may explain why CEM-101 systematically shows a higher relative potency (lower EC50 and Cs) against this organism than azithromycin. The differential effect of acid pH on CEM-101 and azithromycin is probably also important to explain our observations with intraphagocytic L. pneumophila, as the differences between the two antibiotics cannot be fully explained either by differences in the MICs (as measured in broth by conventional techniques) or by the potentially greater accumulation of CEM-101. Interestingly, recent data suggest that active multiplication of the intracellular forms of L. pneumophila, contrary to what was originally thought, may require their transit and sojourn in an acidic vacuole with an average pH of 5.6 (40). While the MICs of L. pneumophila could not be determined at this low value because of the lack of bacterial growth in vitro, the data obtained at pH 6.5 are suggestive of a reduced inhibitory effect of acidic pH on CEM-101 versus azithromycin. This behavior is not unique to CEM-101, as it is shared by telithromycin and clarithromycin (for which the MIC-pH relationships in broth are essentially parallel to that of CEM-101). This observation is in line with our suggestion that the aminophenyltriazole function does not become significantly ionized within the pH range of 5 to 7.4. What differentiates CEM-101 from these macrolides, however, is its considerably higher intrinsic activity (lower MIC). This allows for sufficient intracellular activity of CEM-101 at low pH, even when in cells at low intracellular concentrations, as evidenced from our data with intraphagocytic S. aureus.
In contrast to what was observed for S. aureus and L. pneumophila, CEM-101 does not express enhanced activity toward intraphagocytic L. monocytogenes beyond what can be attributed to its higher intrinsic activity (lower MIC). This is actually anticipated, since the MIC-pH relationships for the four macrolides against this organism in broth are essentially parallel, and L. monocytogenes is known to sojourn and multiply in the cytosol of many cell types (6, 31), including THP-1 macrophages (30), where pH is close to neutral and where the bulk of CEM-101 is not likely to accumulate.
In conclusion, the present study shows that the intracellular activity of macrolides can be differentiated based on the assessment of their relative potencies and, for organisms residing in compartments with an acid environment, their susceptibility to the negative effect of acid pH on their intrinsic activity. Conversely, and counter to the common belief but also observed in other situations (see reference 26 for a review), we show here that differences in total cell accumulation by themselves do not necessarily translate into commensurate differences in activities against the three organisms examined here. This raises important questions as to which fraction of cell-associated antibiotics is truly active.
The model used here suffers from several limitations related to the use of constant exposure of the infected cells to the drugs (a situation not common in vivo) and our inability to take protein binding into consideration (because of the low protein concentration in the cell culture medium [see the discussions in references 4 and 5]). Nevertheless, it may help in a judicious selection of drug candidates, based on the analysis of the parameters studied here. This may eventually lead to improved therapy for intracellular infections provided that (i) the modifications made to increase the intrinsic activity do not adversely alter the safety profile of the drugs, so that patients can be treated with doses creating extracellular concentrations similar to those of conventional macrolides, and (ii) accumulation and expression of activity in infected cells in vivo are correctly predicted from the present in vitro data.
Supplementary Material
Acknowledgments
We thank Marie-Claire Cambier and Charlotte Misson for dedicated technical assistance.
S.L. is a Postdoctoral Researcher and F.V.B. is a Senior Research Associate of the Belgian Fonds de la Recherche Scientifique (FRS-FNRS). This work was supported by the Fonds de la Recherche Scientifique Médicale (FRSM; grant no. 3.4.597.06) and by a grant-in-aid from Cempra Pharmaceuticals.
Footnotes
Published ahead of print on 29 June 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Agouridas, C., A. Denis, J. M. Auger, Y. Benedetti, A. Bonnefoy, F. Bretin, J. F. Chantot, A. Dussarat, C. Fromentin, S. G. D'Ambrieres, S. Lachaud, P. Laurin, O. Le Martret, V. Loyau, and N. Tessot. 1998. Synthesis and antibacterial activity of ketolides (6-O-methyl-3-oxoerythromycin derivatives): a new class of antibacterials highly potent against macrolide-resistant and -susceptible respiratory pathogens. J. Med. Chem. 41:4080-4100. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. October 2008, posting date. Zmax US product information. Pfizer Labs, New York, NY. http://www.pfizer.com/files/products/uspi_zmax.pdf. Accessed 7 February 2009.
- 3.Bahal, N., and M. C. Nahata. 1992. The new macrolide antibiotics: azithromycin, clarithromycin, dirithromycin, and roxithromycin. Ann. Pharmacother. 26:46-55. [DOI] [PubMed] [Google Scholar]
- 4.Barcia-Macay, M., S. Lemaire, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Evaluation of the extracellular and intracellular activities (human THP-1 macrophages) of telavancin versus vancomycin against methicillin-susceptible, methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:1177-1184. [DOI] [PubMed] [Google Scholar]
- 5.Barcia-Macay, M., C. Seral, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berche, P., J. L. Gaillard, and S. Richard. 1988. Invasiveness and intracellular growth of Listeria monocytogenes. Infection 16(Suppl. 2):S145-S148. [DOI] [PubMed] [Google Scholar]
- 7.Blackman, H. J., C. Yoneda, C. R. Dawson, and J. Schachter. 1977. Antibiotic susceptibility of Chlamydia trachomatis. Antimicrob. Agents Chemother. 12:673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlier, M. B., I. Garcia-Luque, J. P. Montenez, P. M. Tulkens, and J. Piret. 1994. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int. J. Tissue React. 16:211-220. [PubMed] [Google Scholar]
- 9.Carlier, M. B., A. Zenebergh, and P. M. Tulkens. 1987. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J. Antimicrob. Chemother. 20(Suppl. B):47-56. [DOI] [PubMed] [Google Scholar]
- 10.Carryn, S., H. Chanteux, C. Seral, M. P. Mingeot-Leclercq, F. Van Bambeke, and P. M. Tulkens. 2003. Intracellular pharmacodynamics of antibiotics. Infect. Dis. Clin. N. Am. 17:615-634. [DOI] [PubMed] [Google Scholar]
- 11.Carryn, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative intracellular (THP-1 macrophage) and extracellular activities of beta-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob. Agents Chemother. 46:2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cianciotto, N. P. 2001. Pathogenicity of Legionella pneumophila. Int. J. Med. Microbiol. 291:331-343. [DOI] [PubMed] [Google Scholar]
- 13.de Duve, C., T. de Barsy, B. Poole, A. Trouet, P. Tulkens, and F. Van Hoof. 1974. Lysosomotropic agents. Biochem. Pharmacol. 23:2495-2531. [DOI] [PubMed] [Google Scholar]
- 14.Dussurget, O. 2008. New insights into determinants of Listeria monocytogenes virulence. Int. Rev. Cell Mol. Biol. 270:1-38. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes, P. B., W. R. Baker, L. A. Freiberg, D. J. Hardy, and E. J. McDonald. 1989. New macrolides active against Streptococcus pyogenes with inducible or constitutive type of macrolide-lincosamide-streptogramin B resistance. Antimicrob. Agents Chemother. 33:78-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamedani, P., J. Ali, S. Hafeez, R. Bachand, Jr., G. Dawood, S. Quereshi, R. Raza, and Z. Yab. 1991. The safety and efficacy of clarithromycin in patients with Legionella pneumonia. Chest 100:1503-1506. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz, M. A., and S. C. Silverstein. 1983. Intracellular multiplication of Legionnaires' disease bacteria (Legionella pneumophila) in human monocytes is reversibly inhibited by erythromycin and rifampin. J. Clin. Investig. 71:15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Join-Lambert, O. F., S. Ezine, A. Le Monnier, F. Jaubert, M. Okabe, P. Berche, and S. Kayal. 2005. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cell. Microbiol. 7:167-180. [DOI] [PubMed] [Google Scholar]
- 19.Kuzman, I., I. Soldo, S. Schonwald, and J. Culig. 1995. Azithromycin for treatment of community acquired pneumonia caused by Legionella pneumophila: a retrospective study. Scand. J. Infect. Dis. 27:503-505. [DOI] [PubMed] [Google Scholar]
- 20.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2005. Activity of three β-lactams (ertapenem, meropenem and ampicillin) against intraphagocytic Listeria monocytogenes and Staphylococcus aureus. J. Antimicrob. Chemother. 55:897-904. [DOI] [PubMed] [Google Scholar]
- 21.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2007. Modulation of the cellular accumulation and intracellular activity of daptomycin towards phagocytized Staphylococcus aureus by the P-glycoprotein (MDR1) efflux transporter in human THP-1 macrophages and Madin-Darby canine kidney cells. Antimicrob. Agents Chemother. 51:2748-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llano-Sotelo, B., D. Klepacki, and A. S. Mankin. 2009. Binding and action of CEM-10, a new macrolide/ketolide in development for treating infections with macrolide-resistant and macrolide-susceptible bacteria. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother./46th Infect. Dis. Soc. Am. Annu. Meet., abstr. F1-3983.
- 23.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 24.Luan, F., W. Ma, H. Zhang, X. Zhang, M. Liu, Z. Hu, and B. Fan. 2005. Prediction of pKa for neutral and basic drugs based on radial basis function Neural networks and the heuristic method. Pharm. Res. 22:1454-1460. [DOI] [PubMed] [Google Scholar]
- 25.Mempel, M., C. Schnopp, M. Hojka, H. Fesq, S. Weidinger, M. Schaller, H. C. Korting, J. Ring, and D. Abeck. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146:943-951. [DOI] [PubMed] [Google Scholar]
- 26.Mouton, J. W., U. Theuretzbacher, W. A. Craig, P. M. Tulkens, H. Derendorf, and O. Cars. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235-237. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, T. M., M. Gaffney, S. Little, A. M. Slee, and P. Fernandes. 2009. Evaluation of CEM-101, a novel macrolide, in murine infection models. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother./46th Infect. Dis. Soc. Am. Annu. Meet., abstr. F1-3895.
- 28.Nightingale, C. H. 1997. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr. Infect. Dis. J. 16:438-443. [DOI] [PubMed] [Google Scholar]
- 29.Ohkuma, S., and B. Poole. 1978. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 75:3327-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouadrhiri, Y., B. Scorneaux, Y. Sibille, and P. M. Tulkens. 1999. Mechanism of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob. Agents Chemother. 43:1242-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portnoy, D. A., V. Auerbuch, and I. J. Glomski. 2002. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 158:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schentag, J. J., and C. H. Ballow. 1991. Tissue-directed pharmacokinetics. Am. J. Med. 91:5S-11S. [DOI] [PubMed] [Google Scholar]
- 33.Schonwald, S., I. Kuzman, K. Oreskovic, V. Burek, V. Skerk, V. Car, D. Bozinovic, J. Culig, and S. Radosevic. 1999. Azithromycin: single 1.5 g dose in the treatment of patients with atypical pneumonia syndrome-a randomized study. Infection 27:198-202. [DOI] [PubMed] [Google Scholar]
- 34.Seral, C., S. Carryn, P. M. Tulkens, and F. Van Bambeke. 2003. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J. Antimicrob. Chemother. 51:1167-1173. [DOI] [PubMed] [Google Scholar]
- 35.Seral, C., J. M. Michot, H. Chanteux, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2003. Influence of P-glycoprotein inhibitors on accumulation of macrolides in J774 murine macrophages. Antimicrob. Agents Chemother. 47:1047-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seral, C., F. Van Bambeke, and P. M. Tulkens. 2003. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47:2283-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha, B., and M. Herrmann. 2005. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb. Haemost. 94:266-277. [DOI] [PubMed] [Google Scholar]
- 38.Stenberg, K., and P. A. Mardh. 1993. Treatment of concomitant eye and genital chlamydial infection with erythromycin and roxithromycin. Acta Ophthalmol. (Copenhagen) 71:332-335. [DOI] [PubMed] [Google Scholar]
- 39.Still, J. G., K. Clark, T. P. Degenhardt, D. Scott, P. Fernandes, and M. J. Gutierrez. 2009. Single oral dose pharmacokinetics and safety of CEM-101 in healthy subjects. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother./46th Infect. Dis. Soc. Am. Annu. Meet., abstr. F1-3972a.
- 40.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson, M. S., and B. K. Hammer. 2000. Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54:567-613. [DOI] [PubMed] [Google Scholar]
- 42.Tabbara, K. F., A. Abu-el-Asrar, O. al Omar, A. H. Choudhury, and Z. al Faisal. 1996. Single-dose azithromycin in the treatment of trachoma. A randomized, controlled study. Ophthalmology 103:842-846. [DOI] [PubMed] [Google Scholar]
- 43.Tyteca, D., S. P. Van Der, F. Van Bambeke, K. Leys, P. M. Tulkens, P. J. Courtoy, and M. P. Mingeot-Leclercq. 2001. Azithromycin, a lysosomotropic antibiotic, impairs fluid-phase pinocytosis in cultured fibroblasts. Eur. J. Cell Biol. 80:466-478. [DOI] [PubMed] [Google Scholar]
- 44.Van Bambeke, F., M. Barcia-Macay, S. Lemaire, and P. M. Tulkens. 2006. Cellular pharmacodynamics and pharmacokinetics of antibiotics: current views and perspectives. Curr. Opin. Drug Discov. Dev. 9:218-230. [PubMed] [Google Scholar]
- 45.Van Bambeke, F., J. M. Harms, Y. Van Laethem, and P. M. Tulkens. 2008. Ketolides: pharmacological profile and rational positioning in the treatment of respiratory tract infections. Expert. Opin. Pharmacother. 9:267-283. [DOI] [PubMed] [Google Scholar]
- 46.Webb, L. X., W. Wagner, D. Carroll, H. Tyler, F. Coldren, E. Martin, et al. 2007. Osteomyelitis and intraosteoblastic Staphylococcus aureus. J. Surg. Orthop. Adv. 16:73-78. [PubMed] [Google Scholar]
- 47.Yamashiro, D. J., S. R. Fluss, and F. R. Maxfield. 1983. Acidification of endocytic vesicles by an ATP-dependent proton pump. J. Cell Biol. 97:929-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhanel, G. G., M. Dueck, D. J. Hoban, L. M. Vercaigne, J. M. Embil, A. S. Gin, and J. A. Karlowsky. 2001. Review of macrolides and ketolides: focus on respiratory tract infections. Drugs 61:443-498. [DOI] [PubMed] [Google Scholar]
- 49.Zhanel, G. G., and M. N. Neuhauser. 2005. Ketolides (telithromycin, cethromycin), p. 201-221. In V. L. Yu, G. Edwards, P. S. McKinnon, C. Peloquin, and G. D. Morse (ed.), Antimicrobial therapy and vaccines, vol. II. Esun Technologies, LLC, Pittsburgh, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.