Abstract
Malachite green, a synthetic antimicrobial dye, has been used for over 50 years in mycobacterial culture medium to inhibit the growth of contaminants. The molecular basis of mycobacterial resistance to malachite green is unknown, although the presence of malachite green-reducing enzymes in the cell envelope has been suggested. The objective of this study was to investigate the role of lipoproteins in resistance of Mycobacterium tuberculosis to malachite green. The replication of an M. tuberculosis lipoprotein signal peptidase II (lspA) mutant (ΔlspA::lspAmut) on Middlebrook agar with and without 1 mg/liter malachite green was investigated. The lspA mutant was also compared with wild-type M. tuberculosis in the decolorization rate of malachite green and sensitivity to sodium dodecyl sulfate (SDS) detergent and first-line antituberculosis drugs. The lspA mutant has a 104-fold reduction in CFU-forming efficiency on Middlebrook agar with malachite green. Malachite green is decolorized faster in the presence of the lspA mutant than wild-type bacteria. The lspA mutant is hypersensitive to SDS detergent and shows increased sensitivity to first-line antituberculosis drugs. In summary, lipoprotein processing by LspA is essential for resistance of M. tuberculosis to malachite green. A cell wall permeability defect is likely responsible for the hypersensitivity of lspA mutant to malachite green.
Pathogenic mycobacteria are responsible for millions of human infections each year. Mycobacterium tuberculosis, the causative agent of tuberculosis, accounts for the majority of these cases (12). Recovery of viable organisms from clinical specimens is essential for accurate identification and antibiotic susceptibility testing of mycobacteria (11). However, contamination of primary cultures with rapidly growing bacteria and fungi is a major concern in the mycobacteriology laboratory, particularly for slowly growing mycobacteria (11). Because mycobacteria are relatively resistant to malachite green, it was introduced into mycobacterial solid media, including Middlebrook agar and Lowenstein-Jensen medium, to inhibit the growth of contaminants (21).
Malachite green, a member of the triphenylmethane dye family (Fig. 1a), is a potent antibacterial, antifungal, and antiparasitic agent and has been used extensively in the aquaculture industry for its antimicrobial activity (1). A related dye, crystal violet, is used in surgical solutions and as a topical antimicrobial for treatment of human skin infections (13, 14). Malachite green is active in its oxidized form and inactivated upon reduction or decolorization to leukomalachite green (9). The mechanism of its action is through poisoning of respiratory chain proteins resulting in irreversible uncoupling of oxidative phosphorylation (1). An earlier study had proposed that malachite green-reducing enzymes in the mycobacterial cell wall conferred resistance to malachite green (20). This hypothesis was based on the observation that reduced malachite green accumulated in the lipid fraction of mycobacterial cell wall, although attempts to isolate reducing enzymes were unsuccessful. A more recent study showed that a protein in the membrane fraction of mycobacterial cell wall is responsible for decolorization of malachite green (9). These studies suggest that mycobacterial cell envelope plays an essential role in resistance of mycobacteria to malachite green, but the molecular basis of resistance to triphenylmethane dyes remains undefined. The cell wall of mycobacteria, which consists of an intricate composition and assembly of complex lipids, carbohydrates, proteins, and lipoproteins (4), is known to provide an impermeable physical barrier to chemicals and antibiotics (8). Therefore, it is possible that the mycobacterial cell wall also serves as an impermeable barrier to malachite green.
FIG. 1.
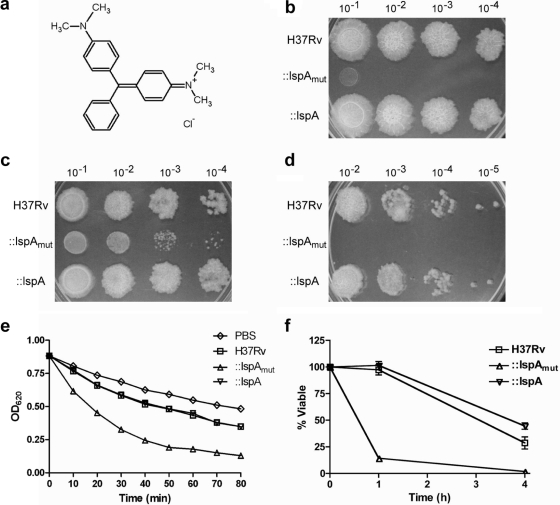
Sensitivity of the M. tuberculosis lspA mutant to malachite green. (a) Chemical structure of malachite green; (b to d) growth of the wild type (H37Rv), the ΔlspA::ΔlspAmut mutant (::lspAmut), and the ΔlspA::ΔlspA strain (::lspA) on Middlebrook 7H9 agar with (b) or without (c) malachite green (0.001 g/liter) and on Middlebrook 7H11 agar (d). Bacterial dilutions are shown on top. Images were taken after 3 weeks of incubation. (e) Decolorization of malachite green in the presence of live bacteria. OD620, optical density at 620 nm. (f) Bacterial viability after exposure to 0.05% SDS. Data points show means ± standard deviations of triplicates. All data are representative of three independent experiments.
Bacterial lipoproteins make up an abundant class of membrane-anchored cell wall proteins with a broad range of functions, including antibiotic resistance, substrate binding and transport, adherence, protein export and folding, cell signaling, and sporulation (19). The M. tuberculosis H37Rv genome encodes 99 lipoproteins, of which only a few have been functionally characterized (18). Lipoprotein precursors are synthesized in the cytoplasm and targeted to the cell wall, where they undergo lipid modification by lipoprotein diacylglycerol transferase (Lgt) followed by removal of the signal sequence by lipoprotein signal peptidase II (LspA) (19). In gram-negative bacteria, lipoproteins undergo a second acyl modification by lipoprotein N-acyltransferase (Lnt) to produce triacylated lipoproteins (22). The M. tuberculosis genome encodes a functional LspA that is dispensable for replication in vitro but is essential for optimal growth in the mouse lung (2, 15).
Given that lipoproteins serve diverse cell wall functions, we investigated the role of lipoprotein processing in resistance of M. tuberculosis to malachite green. We show that LspA is essential for the replication of M. tuberculosis on malachite green-containing medium. Further, the sensitivity of the lspA mutant to malachite green appears to be due to a cell wall permeability defect.
MATERIALS AND METHODS
Media.
H37Rv cultures were grown in Middlebrook 7H9 broth (DifCo) supplemented with 0.2% glycerol, 10% oleic acid-albumin-dextrose-catalase (OADC; DifCo), and 0.05% Tween 80. Middlebrook 7H9 agar had the same composition as 7H9 broth plus 1.5% Bacto agar.
Bacteria.
The construction of the M. tuberculosis H37Rv lspA mutant (ΔlspA::lspAmut) and a complemented strain (ΔlspA::lspA) was previously described (2, 3). Briefly, the lspA null mutant was complemented with an integrating plasmid constructs encoding wild-type or truncated LspA and the two flanking genes (ansA and Rv1540). Functional inactivity of LspA in the ΔlspA::lspAmut strain was confirmed by Western immunoblotting, using an antibody to MPT83, a well-characterized lipoprotein of M. tuberculosis (2, 15). The lspA mutant was propagated in Middlebrook 7H9 broth or agar, without malachite green.
Malachite green decolorization assay.
Bacterial cultures grown to mid-log phase were resuspended in phosphate-buffered saline (PBS) to an optical density of 0.5 at a wavelength of 580 nm. Freshly prepared malachite green was added to 4 ml of bacteria to obtain a final concentration of 10 mg/liter. To prevent photoreduction of malachite green, experiments were performed in dim light and tubes were covered with aluminum foil. The absorbance at wavelength of 620 nm was measured in duplicates at the indicated time points. Background absorbance due to cell scatter was subtracted from absorbance due to cells plus malachite green.
SDS sensitivity assay.
Bacterial cultures grown to mid-log phase were resuspended in fresh growth media and subjected to low-speed centrifugation at 800 rpm for 8 min. The supernatants were diluted with growth media to an optical density of 0.05 at a wavelength of 580 nm and treated with 0.05% sodium dodecyl sulfate (SDS) in triplicate. At the indicated time points, bacterial CFU counts were enumerated on Middlebrook agar.
Drug susceptibility testing.
The susceptibility testing was performed with the MGIT 960 system (Becton, Dickinson and Company, Sparks, MD). A cell suspension of 0.5 McFarland standard was used for each strain to inoculate drug-containing MGIT tubes. A 1:100 dilution was also used to inoculate the growth control for each strain. Drug susceptibility results were interpreted by the MGIT 960 system automatically when the growth units of a growth control reached 400 within 4 to 13 days.
Statistical analysis.
Student's t test was used to determine significant differences between groups.
RESULTS AND DISCUSSION
Lipoprotein processing is essential for resistance of M. tuberculosis to malachite green.
To determine if lipoprotein processing is required for resistance of M. tuberculosis to malachite green, equal densities of the lspA mutant (ΔlspA::lspAmut), the wild-type (H3Rv), and the complemented strain (ΔlspA::lspA) were plated on Middlebrook 7H9 agar with and without 1 mg/liter malachite green. Interestingly, after 3 weeks of incubation on Middlebrook 7H9 agar with malachite, the lspA mutant showed a 104-fold reduction in CFU compared to the wild type and the complemented strain (Fig. 1b). The lspA mutant formed microcolonies on Middlebrook 7H9 agar without malachite green, but there was no reduction in colony counts compared to wild-type bacteria (Fig. 1c). The lspA mutant was also severely attenuated on Middlebrook 7H11 agar, a commercially available solid agar formulation (Fig. 1d). Comparison of the ingredients in the Middlebrook 7H9 and 7H11 agars revealed the additional presence of Cu2+ (1 mg/liter), CaCl2 (0.5 mg/liter), and Zn2+ (1 mg/liter) in 7H9 but not in 7H11 agar and malachite green (1 mg/liter) and pancreatic digest of casein (1 g/liter) in 7H11 but not 7H9 agar. Mixing studies showed that the addition of CuSO4 (1 mg/liter), CaCl2 (0.5 mg/liter), and ZnSO4 (1 mg/liter) to Middlebrook 7H11 could not restore the growth of the lspA mutant (data not shown). Therefore, the growth inhibition seen on Middlebrook 7H11 agar was entirely attributed to malachite green.
The lspA mutant has a cell wall permeability defect.
To determine if the hypersensitivity of the lspA mutant to malachite green was due to loss of malachite green-reducing activity, the rate of malachite green reduction, which results in loss of its characteristic green color and decreased absorbance at 620 nm (20), was measured in the presence of live bacteria. As shown in Fig. 1e, in the presence of wild-type bacteria, the rate of malachite green decolorization during the first 40 min was 1.4-fold greater than the spontaneous decolorization rate in PBS. In contrast, in the presence of the lspA mutant, the rate of malachite green decolorization was 2.5-fold faster than in PBS. The decolorization of malachite green seen with wild-type M. tuberculosis, although slow, is consistent with previous studies showing that mycobacteria decolorize malachite green (9, 20). However, since malachite green decolorization occurs at a significantly faster rate in the presence of the lspA mutant than wild-type bacteria (P = 0.02), the loss of malachite green-reducing enzymes cannot be the principal reason for its sensitivity to malachite green. Instead, we hypothesized that increased cell envelope permeability is the basis of increased malachite green decolorization with the lspA mutant.
To assess the integrity of the cell wall in the lspA mutant, we quantified its sensitivity to the membrane-disrupting detergent SDS. Treatment of the lspA mutant with 0.05% SDS resulted in 86% and 99% loss of viability at 1 and 4 h posttreatment, respectively (Fig. 1f). In contrast, the wild type and the complemented strain were unaffected at 1 h and only moderately affected at 4 h posttreatment. The lspA mutant was significantly more sensitive to SDS than the wild type at 1 h (P = 0.005) and 4 h (P = 0.04) posttreatment.
The lspA mutant is hypersensitive to antituberculosis drugs.
To further assess the effect of the lspA mutation on the activities of first-line antituberculosis drugs, the MICs of rifampin, isoniazid, streptomycin, and ethambutol were determined by a standardized method using the MGIT 960 system. The results show that mutation of lspA in M. tuberculosis results in increased sensitivity to rifampin, isoniazid, and streptomycin (Table 1). Since these drugs have distinct intracellular targets, the increased sensitivity of the lspA mutant to these drugs is consistent with increased access to their targets secondary to increased permeability of the cell wall.
TABLE 1.
MICs of first-line antituberculosis drugs for the lspA mutant
| Strain | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Rifampin | Isoniazid | Streptomycin | Ethambutol | |
| H37Rv | 0.25 | 0.05 | 0.5 | 1.25 |
| ΔlspA::lspAmut | 0.125 | 0.025 | 0.25 | 1.25 |
| ΔlspA::lspA complemented strain | 0.25 | 0.05 | 0.5 | 1.25 |
Altogether, our results suggest that the impermeable cell wall structure is largely responsible for the resistance of M. tuberculosis to malachite green. Increased permeability in the lspA mutant resulting in increased susceptibility to malachite green is consistent with an intracellular target, such as the respiratory chain enzymes, for the drug mechanism (1). Ongoing research will determine whether the cell wall defect in the lspA mutant is due to accumulation of immature lipoproteins or due to functional inactivation or mislocalization of individual lipoproteins. Potential mechanisms of lipoprotein contribution to cell wall integrity include trafficking of cell wall lipids, such as that suggested for phthiocerol dimycocerosates by LppX (5, 17), noncovalent interactions with proximal cell wall domains analogous to those of gram-negative peptidoglycan-associated lipoproteins (19), and organization and maintenance of the cell wall.
Our findings also have implications for pathogenesis research on M. tuberculosis. Given that the majority of mutagenesis libraries have been created on Middlebrook agar with malachite green (7H10 and 7H11) (6, 7, 10, 16), these libraries are likely depleted of malachite green-susceptible mutants, including those with deficiencies in lipoproteins or cell wall permeability. In order for these mutants to be represented in such libraries, compensatory second-site mutations would have to be present. Screening of mutagenesis libraries made on malachite green-free medium may reveal mutants that were previously selected against by malachite green.
Acknowledgments
We thank Ellen Yeh for commenting on the manuscript.
This work was supported by the following grants from the National Institutes of Health: K08 AI06110 to N.B. and R01 AI046097 to J.D.E.
Footnotes
Published ahead of print on 13 July 2009.
REFERENCES
- 1.Alderman, D. J. 1985. Malachite green: a review. J. Fish Dis. 8:289-298. [Google Scholar]
- 2.Banaiee, N., E. Z. Kincaid, U. Buchwald, W. R. Jacobs, Jr., and J. D. Ernst. 2006. Potent inhibition of macrophage responses to IFN-gamma by live virulent Mycobacterium tuberculosis is independent of mature mycobacterial lipoproteins but dependent on TLR2. J. Immunol. 176:3019-3027. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal, A., T. Kobayashi, L. M. Pierini, N. Banaei, J. D. Ernst, K. Miyake, and S. Ehrt. 2009. RP105 facilitates macrophage activation by Mycobacterium tuberculosis lipoproteins. Cell Host Microbe 5:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. [DOI] [PubMed] [Google Scholar]
- 5.Camacho, L. R., P. Constant, C. Raynaud, M. A. Laneelle, J. A. Triccas, B. Gicquel, M. Daffe, and C. Guilhot. 2001. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 276:19845-19854. [DOI] [PubMed] [Google Scholar]
- 6.Cox, J. S., B. Chen, M. McNeil, and W. R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79-83. [DOI] [PubMed] [Google Scholar]
- 7.Darwin, K. H., S. Ehrt, J. C. Gutierrez-Ramos, N. Weich, and C. F. Nathan. 2003. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302:1963-1966. [DOI] [PubMed] [Google Scholar]
- 8.Jarlier, V., and H. Nikaido. 1994. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol. Lett. 123:11-18. [DOI] [PubMed] [Google Scholar]
- 9.Jones, J. J., and J. O. Falkinham III. 2003. Decolorization of malachite green and crystal violet by waterborne pathogenic mycobacteria. Antimicrob. Agents Chemother. 47:2323-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamichhane, G., M. Zignol, N. J. Blades, D. E. Geiman, A. Dougherty, J. Grosset, K. W. Broman, and W. R. Bishai. 2003. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 100:7213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray, P. R., and E. J. Baron (ed.). 2007. Manual of clinical microbiology. ASM Press, Washington, DC.
- 12.Raviglione, M. C. 2003. The TB epidemic from 1992 to 2002. Tuberculosis (Edinburgh) 83:4-14. [DOI] [PubMed] [Google Scholar]
- 13.Safranek, T. J., W. R. Jarvis, L. A. Carson, L. B. Cusick, L. A. Bland, J. M. Swenson, and V. A. Silcox. 1987. Mycobacterium chelonae wound infections after plastic surgery employing contaminated gentian violet skin-marking solution. N. Engl. J. Med. 317:197-201. [DOI] [PubMed] [Google Scholar]
- 14.Saji, M., S. Taguchi, K. Uchiyama, E. Osono, N. Hayama, and H. Ohkuni. 1995. Efficacy of gentian violet in the eradication of methicillin-resistant Staphylococcus aureus from skin lesions. J. Hosp. Infect. 31:225-228. [DOI] [PubMed] [Google Scholar]
- 15.Sander, P., M. Rezwan, B. Walker, S. K. Rampini, R. M. Kroppenstedt, S. Ehlers, C. Keller, J. R. Keeble, M. Hagemeier, M. J. Colston, B. Springer, and E. C. Bottger. 2004. Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 52:1543-1552. [DOI] [PubMed] [Google Scholar]
- 16.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 17.Sulzenbacher, G., S. Canaan, Y. Bordat, O. Neyrolles, G. Stadthagen, V. Roig-Zamboni, J. Rauzier, D. Maurin, F. Laval, M. Daffe, C. Cambillau, B. Gicquel, Y. Bourne, and M. Jackson. 2006. LppX is a lipoprotein required for the translocation of phthiocerol dimycocerosates to the surface of Mycobacterium tuberculosis. EMBO J. 25:1436-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutcliffe, I. C., and D. J. Harrington. 2004. Lipoproteins of Mycobacterium tuberculosis: an abundant and functionally diverse class of cell envelope components. FEMS Microbiol. Rev. 28:645-659. [DOI] [PubMed] [Google Scholar]
- 19.Sutcliffe, I. C., and R. R. B. Russell. 1995. Lipoproteins of gram-positive bacteria. J. Bacteriol. 177:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarnok, I., and P. Czanik. 1959. Malachite green reducing enzyme in mycobacteria. Nature 183:549-550. [DOI] [PubMed] [Google Scholar]
- 21.Tarshis, M. S., M. V. Parker, and W. B. Dunham. 1955. Blood media for cultivation of Mycobacterium tuberculosis. X. Results with malachite green, penicillin and sodium tellurite and comparison of the blood agar-MGP, blood agar-PST and Lowenstein-Jensen media under routine diagnostic conditions. Acta Tuberc. Scand. 31:92-105. [PubMed] [Google Scholar]
- 22.Tokunaga, M., H. Tokunaga, and H. C. Wu. 1982. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc. Natl. Acad. Sci. USA 79:2255-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]