Abstract
Two types of phosphorodiamidate morpholino oligomers (PMOs) were tested for inhibition of growth of Salmonella enterica serovar Typhimurium. Both PMOs have the same 11-base sequence that is antisense to the region near the start codon of acpP, which is essential for lipid biosynthesis and viability. To the 3′ end of each is attached the membrane-penetrating peptide (RXR)4XB (R, X, and B indicate arginine, 6-aminohexanoic acid, and β-alanine, respectively). One peptide-PMO (AcpP PPMO) has no charge on the PMO moiety. The second PPMO has three cations (piperazine) attached to the phosphorodiamidate linkages (3+Pip-AcpP PPMO). A scrambled-sequence PPMO (Scr PPMO) was synthesized for each type of PMO. The MICs of AcpP PPMO, 3+Pip-AcpP PPMO, and either one of the Scr PPMOs were 1.25 μM (7 μg/ml), 0.156 μM (0.94 μg/ml), and >160 μM (>900 μg/ml), respectively. 3+Pip-AcpP PPMO at 1.25 or 2.5 μM significantly reduced the growth rates of pure cultures, whereas AcpP PPMO or either Scr PPMO had no effect. However, the viable cell count was significantly reduced at either concentration of 3+Pip-AcpP PPMO or AcpP PPMO, but not with either Scr PPMO. In other experiments, macrophages were infected intracellularly with S. enterica and treated with 3 μM 3+Pip-AcpP PPMO. Intracellular bacteria were reduced >99% with 3+Pip-AcpP PPMO, whereas intracellular bacteria increased 3 orders of magnitude in untreated or Scr PPMO-treated cultures. We conclude that either AcpP PPMO or 3+Pip-AcpP PPMO inhibited growth of S. enterica in pure culture and that 3+Pip-AcpP PPMO reduced intracellular viability of S. enterica in macrophages.
Salmonella is a food- and waterborne bacterial pathogen that causes a variety of diseases, including gastroenteritis and typhoid fever. It is an intracellular bacterium that invades, replicates, and grows inside epithelial cells and macrophages. Emerging antibiotic-resistant strains of Salmonella enterica serovar Typhi (27, 32) and Typhimurium (11, 19) have increased the need to discover new and effective antibiotics against resistant strains.
Synthetic oligonucleotide mimetics are an experimental class of compounds that modulate expression of specific genes (12, 18, 21, 29, 30, 33). Some examples are peptide nucleic acids, phosphorothioate oligonucleotides, locked nucleic acids, 2′-O-methyloligoribonucleotides, and phosphorodiamidate morpholino oligonucleotides (PMOs). The specificity of action in bacteria is determined by complementary base pairing between the synthetic oligonucleotide and its target, which is usually, but not always, an RNA (12, 33).
Antisense PMOs and peptide nucleic acids have been shown to inhibit growth in pure cultures of a few types of bacteria (16, 17, 22, 23, 26), including S. enterica (31). There are few, if any, reports of inhibition of intracellular bacterial growth using antisense oligomers.
Antisense oligomers require assistance to gain entry into bacterial cells because of their relatively high molecular weights and polar characteristics. Recently, antisense oligomers have been conjugated to membrane-penetrating peptides, which are composed of repeating patterns of cationic and nonpolar residues. Peptide-oligomer conjugates are significantly more effective in inhibiting expression of their specific targets than their nonconjugated counterparts (13, 16). Apparently the membrane-penetrating peptide carries its cargo (the antisense oligomer) across the gram-negative outer membrane after which it traverses the plasma membrane by an unknown mechanism.
Cationic charges were introduced into the linkages between the bases of PMO (B. Geller, B. Mellbye, D. Weller, J. Hassinger, and M. Reeves, submitted for publication). One type of cationic linkage included a side moiety of piperazine (Pip). It was hypothesized that cationic PMOs with Pip linkages would obviate the need for membrane-penetrating peptides. Although this was found not to be true, peptide conjugates of PMOs with Pip (Pip-PMOs) were significantly more potent than the peptide conjugate of the equivalent noncharged PMO. In the present report, a Pip-PMO-peptide conjugate is tested against S. enterica and compared to the equivalent noncharged PMO-peptide conjugate.
Bacteria that grow within eukaryotic host cells pose a challenging situation for antisense antibiotics. Not only must the oligomer penetrate the bacterial cell, but it must first enter the eukaryotic cell and retain its structural integrity within the endosome. Cationic membrane-penetrating peptides are generally thought to enter eukaryotic cells by first binding to proteoglycans and then being internalized by endocytosis (1, 20, 28). Recently we reported that a conjugate of a PMO and the membrane-penetrating peptide (RXR)4XB (X is 6-aminohexanoic acid, and B is β-alanine) was effective in delivering antisense PMOs into Escherichia coli (25). PMO conjugates of (RXR)4XB have been shown previously to enter mammalian cells, where they can modulate gene expression (4, 7, 10, 24, 36). These results suggest that PMO-(RXR)4XB conjugates have potential for use against intracellular bacteria.
In this report, we test the hypothesis that peptide-PMOs (PPMOs) targeted to a specific, essential gene (acpP, which is required for synthesis of lipids), can inhibit intracellular growth of S. enterica.
MATERIALS AND METHODS
Bacterial strains.
Salmonella enterica serovar Typhimurium 14028s was used in all experiments. Liquid cultures were grown aerobically at 37°C in either Mueller-Hinton II broth (for MIC assay) or LB broth (for growth curves and to infect tissue cultures).
PMO.
Peptide-PMOs were synthesized at AVI BioPharma (Corvallis, OR) as described previously (31; Geller et al., submitted). The base sequence of all AcpP PMOs is 5′-CTTCGATAGTG-3′, which is complementary to bases 6 to 16 of the coding region of acpP. The base sequence of all scrambled-sequence (Scr) control PPMOs is 5′-TCTCAGATGGT-3′. The positions of Pip are indicated by the plus symbol: AcpP, 5′-C+TTCGA+TAG+TG-3′; scrambled, 5′-TC+TCAGA+TGG+T-3′. (RXR)4XB is attached by its carboxy terminus to the 3′ end of each PMO. The amino terminus of the peptide moiety is acetylated.
MIC.
MIC was determined by the microdilution method (8). To control nonspecific toxicity, Scr PPMOs with a scrambled base sequence that is not complementary to any gene in S. enterica were also tested. In all cases, the MIC of the control PPMO was above the limit of measurement, which was 160 μM.
Growth curves.
Single-colony, overnight (18-h) cultures in LB broth were diluted 2 × 10−2 into LB broth. The starting concentration of bacterial cells was 9.0 × 107 CFU/ml (standard deviation, 0.8 × 107 CFU/ml). PPMO was immediately added to a final concentration of 1.25 μM or 2.5 μM. The diluted cultures were grown aerobically at 37°C. Growth was monitored at various times after addition of the PMO by measuring the optical density (1-cm light path) of the cultures in a spectrophotometer (600 nm). Samples were removed after 24 h, diluted, and spread on LB broth plates to determine the number of viable cells.
Tissue culture.
RAW264.7 murine macrophages (American Type Culture Collection, Manassas, VA; catalogue number TIB-71) were grown in Dulbecco modified Eagle medium (DMEM) (Gibco-BRL, Rockville, MD) supplemented with 10% fetal bovine serum (HyClone, Ogden, UT), glutamine (Gibco-BRL), sodium pyruvate (Gibco-BRL), and nonessential amino acids (Gibco-BRL) at 37°C in an atmosphere containing 5% CO2. Salmonellae were prepared by diluting a 15-h culture 5 ×10−2 into LB broth and growing aerobically at 37°C for 3 h. Exponential-phase bacteria were harvested by centrifugation at 5,000 × g for 5 min and resuspended in DMEM. Approximately 3 × 105 bacterial cells were added to each culture of 3 ×106 macrophages in a total volume of 1 ml. The multiplicity of infection varied from 0.08 to 0.1. The infected cultures were centrifuged at 250 × g for 5 min and then incubated for 30 min at 37°C and 5% CO2. The cells were washed four times with phosphate-buffered saline (PBS) and then incubated for 1 h at 37°C and 5% CO2 in DMEM plus 10 μg/ml gentamicin to kill extracellular bacteria. The cells were again washed two times in PBS and then treated with 3 μM peptide-PMO in DMEM plus 10 μg/ml gentamicin. After 2, 18, and 42 h of treatment, one culture from each treatment group and one untreated culture were washed two times with PBS, and the cells were removed by trypsinization for 10 min. Viable macrophages were counted in a hemocytometer using trypan blue, and then the culture was lysed in 0.1% Triton X-100. The lysate was diluted and spread on LB plates to innumerate viable bacteria. The growth medium plus gentamicin and peptide-PMO were replaced at 2 and 18 h posttreatment to the cultures that were not harvested and lysed. For a control to show the bactericidal efficacy of gentamicin on extracellular bacteria, a control culture without macrophages was infected with 3 × 105 bacteria and incubated with DMEM plus 10 μg/ml gentamicin. At 2, 18, and 42 h posttreatment, samples were diluted and spread on LB plates, but there were no colonies detected at any time point. The experiment was repeated three times.
Statistical analysis.
Treatment group means were analyzed by one-way analysis of variance using GraphPad InStat 3.0 (San Diego, CA).
RESULTS
Growth curves.
Two different PPMOs targeting acpP were added separately at the same concentration (2.5 μM) to growing cultures of S. enterica serovar Typhimurium 14028s. Optical densities were monitored at various times for 24 h. Both PPMOs are 11 bases in length, have the same base sequence that is antisense to the region immediately 3′ of the start codon of acpP mRNA, and have the membrane-penetrating peptide (RXR)4XB attached to the 3′ end of the PMO. The PPMOs differ in that the AcpP PPMO has no charge on the PMO moiety, whereas the PMO with three cations (piperazine) attached to the phosphorodiamidate linkages (3+Pip-AcpP PPMO) has three positive charges on the PMO moiety (Fig. 1). The positions of Pip in the interbase linkages is indicated in Materials and Methods. For a control for nonspecific effects, identical cultures were grown with 2.5 μM scrambled base sequence (Scr) PPMOs.
FIG. 1.
Structures of PMOs used in this study. The drawing on the left illustrates the noncharged PMO, which is representative of the type used for AcpP PPMO. The drawing on the right illustrates the Pip derivative (Pip-PMO). See Materials and Methods for the positions of modifications. All PMOs are covalently attached at the 3′ end to the carboxy terminus of the peptide (RXR)4XB (not shown).
The growth rate and final optical density of the culture with 3+Pip-AcpP PPMO were lower than those of the untreated culture (Fig. 2A). There was no apparent decrease in the growth rate or final optical density of cultures with AcpP PPMO or with either one of the Scr PPMOs.
FIG. 2.
Growth of Salmonella in pure culture. Growing cultures of S. enterica serovar Typhimurium were not treated (open squares) or treated with 2.5 μM AcpP PPMO (open triangles), 3+Pip-AcpP PPMO (solid triangles), Scr PPMO (open circles), or 3+Pip-Scr PPMO (X). Growth was monitored by optical density (A) and viable cell count at 24 h (B). The open bar in panel B represents the viable cell count immediately before treatment with PPMO. Error bars indicate standard deviation (n = 3). OD600, optical density at 600 nm.
The viable cell count was measured after 24 h of growth. The viable cell count of the culture with 3+Pip-AcpP PPMO was significantly (P < 0.001) lower by over 4 orders of magnitude than the counts for untreated or 3+Pip-Scr PPMO-treated cultures (Fig. 2B). The culture with AcpP PPMO, despite no apparent effect on the growth rate or final optical density (Fig. 2A), had a significantly (P < 0.001) lower viable cell count by more than 10-fold compared to untreated or Scr PPMO-treated cultures (Fig. 2B). A similar effect has been observed in cultures of E. coli treated with AcpP PMOs (9, 14). There was no significant difference (P > 0.05) in viable cells of cultures treated with either one of the Scr PPMOs compared to the untreated culture.
The viable cell count of the culture with 3+Pip-AcpP PPMO was also significantly (P < 0.001) reduced nearly 3 orders of magnitude compared to the starting CFU/ml immediately prior to adding the PPMO, which was 9.0 × 107 CFU/ml.
MIC.
The MICs were determined for both AcpP PPMOs using S. enterica 14028s as the indicator strain (Table 1). Both PPMOs inhibited growth at concentrations that are similar to or less than the MIC of ampicillin. The 3+Pip-AcpP PPMO was eight times more potent than the AcpP PPMO on a molar basis. Scr PPMOs with either a neutral PMO or 3+Pip-PMO moiety had no effect on growth at 160 μM or less.
TABLE 1.
MICs of PPMOs using Salmonella enterica 14028s
| PPMO or antibiotic | MIC
|
|
|---|---|---|
| μM | μg/ml | |
| AcpP PPMO | 1.25 | 7 |
| 3+Pip-AcpP PPMO | 0.156 | 0.94 |
| Scr PPMO | >160 | >900 |
| 3+Pip-Scr PPMO | >160 | >1,000 |
| Antibiotic ampicillin | 20 | 7 |
Intracellular growth in macrophages.
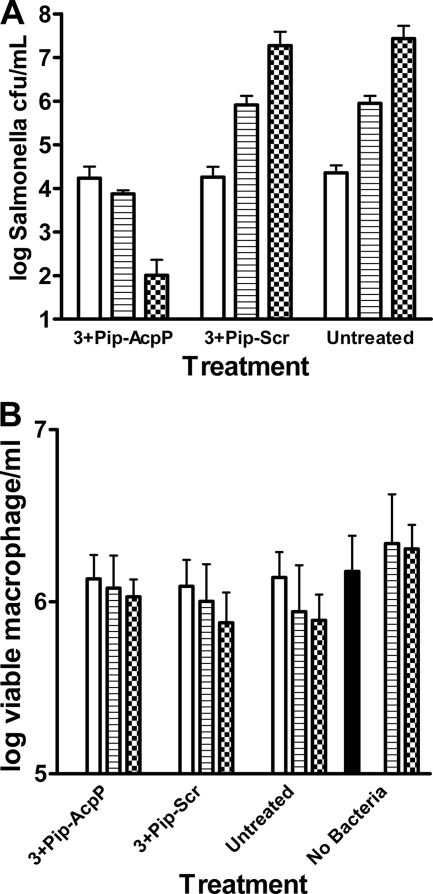
Mouse macrophages were infected intracellularly with S. enterica 14028s and treated 2 h postinfection with 3 μM 3+Pip-AcpP PPMO. Prior to treatment, extracellular bacteria were removed by washing the macrophages and then adding gentamicin to the culture medium. The viabilities of bacteria and macrophages were measured at 2, 18, and 42 h postinfection. Controls included cultures infected but not treated, infected and treated with 3+Pip-Scr PPMO, and uninfected macrophages.
The results show that intracellular bacteria decreased significantly in the culture treated with 3+Pip-AcpP PPMO (Fig. 3A). Between 2 and 18 h of treatment, bacteria were reduced 56%. At 18 h posttreatment, there was a highly significant (P < 0.001) difference in intracellular bacteria between the culture treated with 3+Pip-AcpP PPMO and either one of the other two cultures. After 42 h of treatment with 3+Pip-AcpP PPMO, the number of CFU decreased over 2 orders of magnitude, and the differences between the culture treated with 3+Pip-AcpP PPMO and either the untreated or Scr-treated culture were highly significant (P < 0.01).
FIG. 3.
Intracellular growth of Salmonella in macrophages. Identical cultures of macrophages were infected with Salmonella for 30 min. Extracellular bacteria were removed by washing and treatment with gentamicin. After 1 h of incubation in gentamicin, the macrophages were washed again and then treated with 3 μM 3+Pip-AcpP-(RXR)4XB or 3 μM 3+Pip-Scr-(RXR)4XB. Untreated and noninfected (no bacteria) control cultures were also included. At 2 h (open bars), 18 h (striped bars), and 42 h (checkered bars) posttreatment, the numbers of viable Salmonella (A) and macrophages (B) were counted from one culture of each treatment group and control. The solid black bar in panel B indicates the number of macrophages in one identical culture immediately prior to infection. The error bars represent standard deviations (n = 3).
In comparison, the number of viable intracellular bacteria increased significantly in the culture treated with 3+Pip-Scr PPMO or in the untreated culture (Fig. 3A). Between 2 and 18 h, intracellular bacteria increased in both of these controls by nearly 2 orders of magnitude. After 42 h, the number of viable intracellular bacteria had increased by more than 3 orders of magnitude. There was no significant difference in the number of CFU between untreated and 3+Pip-Scr PPMO cultures at any time point.
Analysis of the viable macrophages from these cultures indicates no significant difference (P > 0.05) over the 42 h of treatment (Fig. 3B). All infected cultures showed a slight decrease in viable macrophages, whereas the uninfected culture showed a statistically insignificant increase in viable cells over the 42-h monitoring period.
DISCUSSION
The most important result of these experiments is that a PMO-peptide conjugate inhibited intracellular growth and viability of S. enterica in macrophages. This shows that a cationic PMO-peptide conjugate can enter macrophages, apparently enter the Salmonella-containing vacuoles, cross two bacterial membranes, and effectively deliver the PMO cargo to its target in the bacterial cytoplasm.
PMO-(RXR)4 conjugates have been shown previously to efficiently enter eukaryotic cells (1, 4, 34, 35). In fact, this was the rationale for choosing the (RXR)4 peptide for these experiments with intracellular bacteria. Although other arginine-rich, amphipathic peptides are also effective in carrying PMOs into eukaryotic cells, (RXR)4 or closely related derivatives are the most favorable tested thus far (2, 3, 34). (RXR)4-PMOs have been shown to accumulate in endosomes (3). The unusual 6-aminohexanoic acid and β-alanine impart stability against degradation (35), which would likely be important for structural integrity and antibiotic action in the harsh, proteolytic environment of the Salmonella-containing vacuole.
Our results show that 3+Pip-AcpP PPMO reduced the number of intracellular bacteria. However, it is unclear whether the killing of bacteria was caused directly by the PPMO or whether the effects of the PPMO on bacterial growth rate enabled the macrophages to kill the bacteria. The 2-log-unit reduction in viable cells in pure culture compared to the starting concentration of viable cells at 0 h (Fig. 2B) shows that the 3+Pip-AcpP PPMO can directly kill bacteria, at least in pure culture conditions.
In pure culture, 2.5 μM AcpP PPMO had no apparent effect on the growth rate or final culture density (Fig. 2A), although it significantly reduced the number of viable cells from the 24-h culture. Previous results with AcpP PMOs and cultures of E. coli have also shown a similar effect (9, 14). The cause of this effect is unknown. We speculate that at relatively low concentrations of PPMO (near the MIC), the rate of uptake of oligomer into the bacterial cell is sufficiently low that the concentration of acyl carrier protein cannot be reduced to the point where growth is affected within the short number of generations required to reach stationary phase. Under our growth conditions, this was only 5.7 generations. When the cells from the 24-h culture are plated, the PPMO already accumulated within the cells would continue to inhibit translation of acyl carrier protein. As the acyl carrier protein is diluted with each cell division, it eventually becomes limiting for growth. This limit would presumably occur before the colony becomes visible. Some of our results are consistent with this speculation. Under the growth conditions used to measure the MIC, the cultures were diluted 300 times more than those used to measure the growth curves. By definition, there was no visible growth in cultures with 1.25 μM or higher AcpP PPMO using the dilution specified in the standard protocol for measuring MIC (8). In addition, increasing the concentration of AcpP PPMO to 50 μM from 2.5 μM resulted in a significant reduction in growth rate under conditions used to measure the growth curve (data not shown).
The MIC indicates that both AcpP PPMO and 3+Pip-AcpP PPMO have a potency greater than that for ampicillin. These MICs provide target concentrations that may needed to achieve clinical efficacy. Previous pharmacokinetic analysis in rats indicate that single bolus treatment of a similar PPMO achieved serum concentrations of 1 to 100 μg/ml in rat serum, depending on the dose (6). This suggests that serum concentrations of PPMOs may exceed the MICs shown in Table 1 and that the potency of the PPMOs may be adequate to have an effect in vivo. Further testing of PPMOs in animal models of intracellular infections will be required to address the issue of in vivo efficacy.
The reason for the lower MIC of 3+Pip-AcpP PPMO compared to the noncharged AcpP PPMO is unknown. Perhaps the more uniform distribution of cationic charges along the length of the conjugate enables it to interact more favorably (for transfer across the membrane) with the anionic charges of the membrane lipids. Alternatively, the positive charges on the Pip-PMO may cause it to bind more tightly to its anionic mRNA target.
The novelty of the structure and antisense strategy of PPMOs suggests that they may play a role in addressing the urgent need for new antibiotics to combat antibiotic-resistant bacteria, particularly for gram-negative bacteria (15). PPMOs have been tested in numerous antibiotic-resistant genera and species, including Salmonella, E. coli, Klebsiella pneumoniae, and Burkholderia, and in every case the antibiotic-resistant strains were susceptible to PPMOs (B. Geller, unpublished data). We have found that a PPMO targeted to an essential gene in Mycobacterium avium inhibited intracellular growth (B. Geller and L. Bermudez, unpublished data). We are confident that PPMOs will be applicable to many kinds of bacterial infections, including those caused by intracellular and antibiotic-resistant bacteria.
Acknowledgments
Georgi Mitev thanks the Howard Hughes Medical Institute for an undergraduate research fellowship that supported his work on this project. Bruce Geller was employed at both AVI BioPharma and Oregon State University during the course of these experiments. This work was funded by AVI BioPharma.
We thank Shannon Oda for excellent technical assistance, Hong Moulton for critically reading the manuscript, the entire chemistry department at AVI BioPharma for making and purifying the PPMOs, and the biology support staff at AVI BioPharma.
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Abes, R., A. A. Aruzumanov, H. M. Moulton, S. Abes, G. D. Ivanova, P. L. Iversen, M. J. Gait, and B. Lebleu. 2007. Cell-penetrating-peptide-based delivery of oligonucleotides: an overview. Biochem. Soc. Trans. 35:775-779. [DOI] [PubMed] [Google Scholar]
- 2.Abes, R., A. Aruzumanov, H. Moulton, S. Abes, G. Ivanova, M. J. Gait, P. L. Iversen, and B. Lebleu. 2008. Arginine-rich cell penetrating peptides: design, structure-activity, and applications to alter pre-mRNA splicing by steric-block oligonucleotides. J. Pept. Sci. 14:455-460. [DOI] [PubMed] [Google Scholar]
- 3.Abes, R., H. M. Moulton, P. Clair, S.-T. Yang, S. Abes, K. Melikov, P. Prevot, D. S. Youngblood, P. L. Iversen, L. V. Chernomordik, and B. Lebleu. 2008. Delivery of steric block morpholino oligomers by (R-X-R)4 peptides: structure-activity studies. Nucleic Acids Res. 36:6343-6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abes, S., H. M. Moulton, P. Clair, P. Prevot, D. S. Youngblood, R. P. Wu, P. L. Iversen, and B. Lebleu. 2006. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J. Control Release 116:304-313. [DOI] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Amantana, A., H. M. Moulton, M. L. Cate, M. T. Reddy, T. Whitehead, J. N. Hassinger, D. S. Youngblood, and P. L. Iversen. 2007. Pharmacokinetics, biodistribution, stability and toxicity of a cell-penetrating peptide-morpholino oligomer conjugate. Bioconj. Chem. 18:1325-1331. [DOI] [PubMed] [Google Scholar]
- 7.Burrer, R., B. W. Neuman, J. P. Ting, D. A. Stein, H. M. Moulton, P. L. Iversen, P. Kuhn, and M. J. Buchmeier. 2007. Antiviral effects of antisense morpholino oligomers in murine coronavirus infection models. J. Virol. 81:5637-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Clinical and Laboratory Standards Institute document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Deere, J., P. Iversen, and B. L. Geller. 2005. Antisense phosphorodiamidate morpholino oligomer length and target position effects on gene-specific inhibition in Escherichia coli. Antimicrob. Agents Chemother. 49:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge, Q., M. Pastey, D. Kobasa, P. Puthavathana, C. Lupfer, R. K. Bestwick, P. L. Iversen, J. Chen, and D. A. Stein. 2006. Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrob. Agents Chemother. 50:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebreyes, W. A., and C. Altier. 2002. Molecular characterization of multidrug-resistant Salmonella enterica subsp. enterica serovar Typhimurium isolates from swine. J. Clin. Microbiol. 40:2813-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geller, B. L. 2005. Antibacterial antisense. Curr. Opin. Mol. Ther. 7:109-113. [PubMed] [Google Scholar]
- 13.Geller, B. L., J. D. Deere, D. A. Stein, D. Kroeker, H. M. Moulton, and P. L. Iversen. 2003. Inhibition of gene expression in Escherichia coli by antisense phosphorodiamidate morpholino oligomers. Antimicrob. Agents Chemother. 47:3233-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geller, B. L., J. D. Deere, L. Tilley, and P. L. Iversen. 2005. Antisense phosphorodiamidate morpholino oligomer inhibits viability of Escherichia coli in pure culture and in mouse peritonitis. J. Antimicrob. Chemother. 55:983-988. [DOI] [PubMed] [Google Scholar]
- 15.Giske, C. G., D. L. Monnet, O. Cars, and Y. Carmeli on behalf of ReAct-Action on Antibiotic Resistance. 2008. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob. Agents Chemother. 52:813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Good, L., S. K. Awasthi, R. Dryselius, O. Larsson, and P. E. Nielsen. 2001. Bacterial antisense effects of peptide-PNA conjugates. Nat. Biotechnol. 19:360-364. [DOI] [PubMed] [Google Scholar]
- 17.Gruegelsiepe, H., O. Brandt, and R. K. Hartmann. 2006. Antisense inhibition of RNase P: mechanistic aspects and application to live bacteria. J. Biol. Chem. 281:30613-30620. [DOI] [PubMed] [Google Scholar]
- 18.Hebert, C. G., J. J. Valdes, and W. E. Bentley. 2008. Beyond silencing-engineering applications of RNA interference and antisense technology for altering cellular phenotype. Curr. Opin. Biotechnol. 19:500-505. [DOI] [PubMed] [Google Scholar]
- 19.Hermans, A. P., A. M. Beuling, A. H. van Hoek, H. J. Aarts, T. Abee, and M. H. Zwietering. 2006. Distribution of prophages and SGI-1 antibiotic-resistance genes among different Salmonella enterica serovar Typhimurium isolates. Microbiology 152:2137-2147. [DOI] [PubMed] [Google Scholar]
- 20.Jones, S. W., R. Christian, K. Bundell, C. J. Voyce, S. M. Brockbank, P. Newham, and M. A. Lindsay. 2005. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br. J. Pharmacol. 145:1093-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juliano, R., M. R. Alam, V. Dixit, and H. Kang. 2008. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 36:4158-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulyte, A., N. Nekhotiaeva, S. K. Awasthi, and L. Good. 2005. Inhibition of Mycobacterium smegmatis gene expression and growth using antisense peptide nucleic acids. J. Mol. Microbiol. Biotechnol. 9:101-109. [DOI] [PubMed] [Google Scholar]
- 23.Kurupati, P., K. S. W. Tan, G. Kumarasinghe, and C. L. Poh. 2007. Inhibition of gene expression and growth by antisense peptide nucleic acids in a multiresistant β-lactamase-producing Klebsiella pneumoniae strain. Antimicrob. Agents Chemother. 51:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClorey, G., H. M. Moulton, P. L. Iversen, S. Fletcher, and S. D. Wilton. 2006. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther. 13:1373-1381. [DOI] [PubMed] [Google Scholar]
- 25.Mellbye, B. L., S. E. Puckett, L. D. Tilley, P. L. Iversen, and B. L. Geller. 2009. Variations in amino acid composition of antisense peptide-phosphorodiamidate morpholino oligomer affect potency against Escherichia coli in vitro and in vivo. Antimicrob. Agents Chemother. 53:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nekhotiaeva, N., S. K. Awasthi, P. E. Nielsen, and L. Good. 2004. Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids. Mol. Ther. 10:252-259. [DOI] [PubMed] [Google Scholar]
- 27.Phan, M. D., C. Kidgell, S. Nair, K. E. Holt, A. K. Turner, J. Hinds, P. Butcher, F. J. Cooke, N. R. Thomson, R. Titball, Z. A. Bhutta, R. Hasan, G. Dougan, and J. Wain. 17 November 2008. Variation in Salmonella enterica serovar Typhi IncH1 plasmids during the global spread of resistant typhoid fever. Antimicrob. Agents Chemother. doi: 10.1128/AAC.00645-08. [DOI] [PMC free article] [PubMed]
- 28.Richard, J. P., K. Melikov, H. Brooks, P. Prevot, B. Lebleu, and L. V. Chernomordik. 2005. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 280:15300-15306. [DOI] [PubMed] [Google Scholar]
- 29.Stein, D. 2008. Inhibition of RNA virus infections with peptide-conjugated morpholino oligomers. Curr. Pharm. Des. 14:2619-2634. [DOI] [PubMed] [Google Scholar]
- 30.Summerton, J. E. 2007. Morpholino, siRNA, and S-DNA compared: impact of structure and mechanism of action on off-target effects and sequence specificity. Curr. Top. Med. Chem. 7:651-660. [DOI] [PubMed] [Google Scholar]
- 31.Tilley, L. D., O. S. Hine, J. A. Kellogg, J. N. Hassinger, D. D. Weller, P. L. Iversen, and B. L. Geller. 2006. Gene-specific effects of antisense phosphorodiamidate morpholino oligomer-peptide conjugates on Escherichia coli and Salmonella enterica serovar Typhimurium in pure culture and in tissue culture. Antimicrob. Agents Chemother. 50:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wain, J., and C. Kidgell. 2004. The emergence of multidrug resistance to antimicrobial agents for the treatment of typhoid fever. Trans. R. Soc. Trop. Med. Hyg. 98:423-430. [DOI] [PubMed] [Google Scholar]
- 33.Woodford, N., and D. W. Wareham on behalf of the UK Antibacterial Antisense Study Group. 11 November 2008. Tackling antibiotic resistance: a dose of common antisense? J. Antimicrob. Chemother. doi: 10.1093/jac/dkn467. [DOI] [PubMed]
- 34.Wu, R. P., D. S. Youngblood, J. N. Hassinger, C. E. Lovejoy, M. H. Nelson, P. L. Iversen, and H. M. Moulton. 2007. Cell-penetrating peptides as transporters for morpholino oligomers: effects of amino acid composition on intracellular delivery and cytotoxicity. Nucleic Acids Res. 35:5182-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youngblood, D. S., S. A. Hatlevig, J. N. Hassinger, P. L. Iversen, and H. M. Moulton. 2007. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells. Bioconj. Chem. 18:50-60. [DOI] [PubMed] [Google Scholar]
- 36.Yuan, J., D. A. Stein, T. Lim, D. Qiu, S. Coughlin, Z. Liu, Y. Wang, R. Blouch, H. M. Moulton, P. L. Iversen, and D. Yang. 2006. Inhibition of coxsackievirus B3 in cell cultures and in mice by peptide-conjugated morpholino oligomers targeting the internal ribosome entry site. J. Virol. 80:11510-11519. [DOI] [PMC free article] [PubMed] [Google Scholar]