Abstract
A retrospective survey was conducted to characterize β-lactamases in a collection of 43 ceftazidime-resistant Pseudomonas aeruginosa isolates recovered from patients with bloodstream infections hospitalized at a Brazilian teaching hospital between January and December 2005. Resistance rates for carbapenems, aminoglycosides, and quinolones were over 80%, with only colistin remaining active against all isolates. Pulsed-field gel electrophoresis analysis identified seven different genotypes. AmpC overproduction was found to be the sole β-lactamase-mediated mechanism responsible for ceftazidime resistance in four isolates (9.3%). Nine isolates (20.9%) produced an extended-spectrum β-lactamase (ESBL), either GES-1 (n = 7, 16.3%) or CTX-M-2 (n = 2, 4.6%). Carbapenemase activity was detected in 30 (70%) additional isolates. Among those isolates, two isolates (4.6%) produced the ESBL GES-5, possessing the ability to hydrolyze imipenem; a single isolate (2.3%) produced the metallo-β-lactamase (MBL) IMP-1; and 27 isolates produced the MBL SPM-1 (62.8%). None of the isolates coproduced both ESBL and MBL. Insertion sequence elements ISCR4 and ISCR1 were associated with blaSPM-1 and blaCTX-M-2 genes, respectively, whereas the blaGES-1 and blaGES-5 genes were part of class 1 integron structures. This study underlines the spread of MBL- and ESBL-producing P. aeruginosa isolates as an important source of ceftazidime resistance in Brazil.
Pseudomonas aeruginosa is a leading cause of hospital- acquired infections. Acquisition of β-lactamases, such as class A extended-spectrum β-lactamases (ESBLs) and class B metallo-β-lactamases (MBLs), by P. aeruginosa nosocomial isolates is detrimental to antimicrobial therapy in hospitalized patients (19).
The ESBLs reported for P. aeruginosa are SHV, TEM, PER, VEB, BEL, GES, and, more recently, CTX-M types (1, 7, 8, 16, 20, 23, 29). The GES-type enzymes are unusual since point amino acid changes in their active sites may extend their hydrolytic activity to carbapenems (31, 39, 40). ESBL production in P. aeruginosa has been documented in Brazil (2, 5, 21), but its prevalence remains unknown.
Five types of acquired MBLs have been identified in P. aeruginosa: IMP, VIM, SPM, GIM, and AIM (41, 42). In Brazil, IMP-, VIM-, and SPM-producing P. aeruginosa clinical isolates have been identified (35). In addition, SPM producers have been reported as endemic in Brazilian territory due to dissemination of a single clone (10).
The aim of this study was to investigate the diversity and frequency of both ESBL and MBL production and to characterize the genetic support of those acquired β-lactamase genes in a collection of ceftazidime-resistant P. aeruginosa clinical isolates from Brazil, taken as a model of a developing country.
MATERIALS AND METHODS
Bacterial strains.
A total of 154 consecutive P. aeruginosa isolates were recovered from patients with bloodstream infections hospitalized at Hospital São Paulo between January and December 2005. A single isolate per patient was retained for this study. Among those isolates, 43 (28%) were ceftazidime resistant by the CLSI disk diffusion method (inhibition zone of ≤14 mm and MIC of ≥32 μg/ml) and thus were further characterized. Escherichia coli TOP10 was used as a recipient strain in cloning experiments (23). Transformation experiments were performed using both E. coli TOP10 and P. aeruginosa PAO1 as the recipients.
Clinical data.
Clinical data including age, comorbidities, unit of the hospital, site of infection, therapeutic regimen, and final disposition (death or discharge) have been collected for each patient.
Susceptibility testing and screening for AmpC overproducers and/or ESBL production.
Antibiotic susceptibility profiles of the 43 P. aeruginosa isolates were determined by the agar dilution method according to the CLSI guidelines (3). AmpC overproducers were identified by testing susceptibility to ceftazidime on Mueller-Hinton plates supplemented with 250 μg/ml cloxacillin (18, 33). Detection of ESBL production was carried out by a double disk synergy method testing ceftazidime, aztreonam, and cefepime at a distance of 15 mm from ticarcillin-clavulanic acid disks, on Mueller-Hinton plates supplemented or not with cloxacillin-containing plates (28).
Screening for carbapenemase activity.
Hydrolysis of imipenem was assessed by UV spectrophotometry assays, as described previously (10, 11, 25). Briefly, 10 ml of an overnight broth culture was harvested and then disrupted by sonication. Whole-protein extracts were obtained after centrifugation. Hydrolytic activity of 20 μl of the crude extract was determined against 100 μM imipenem in 100 mM phosphate buffer (pH 7.0), and measurements were carried out at a wavelength of 297 nm.
PCR amplification for detection of ESBL and MBL genes; analysis of the genetic environment and sequencing.
Specific primers were used under standard PCR conditions to detect ESBL- and MBL-encoding genes, namely, blaTEM, blaSHV, blaCTX-M, blaGES, blaPER, blaVEB, blaBEL, blaKPC, blaIMP, blaVIM, blaSPM, blaGIM, and blaAIM (7, 12, 15, 16, 17, 20, 23, 26, 29, 30, 38, 42). The genetic environment of blaIMP was determined by PCR using the previously published specific primers to anneal at the 5′ and 3′ conserved sequences (CSs) of class 1 integrons, followed by sequencing (24). The genetic environment of blaCTX-M-2 was determined by PCR and further sequencing using specific primers for the insertion sequence ISCR1 located upstream and for the qacEΔ1 and sul1 tandem gene (17). The genetic context of blaSPM-1 was determined by using primers hybridizing with ISCR4, as described previously (27). For direct DNA sequencing, PCR products were purified using PCR purification columns (Qiagen, Courtaboeuf, France). Sequencing reactions were performed using specific primers and an automated ABI 337 sequencer (Applied Biosystems, Foster City, CA). The nucleotide and deduced protein sequences were analyzed with software available over the Internet at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov).
PFGE analysis.
Genetic relatedness among the ceftazidime-resistant P. aeruginosa isolates was evaluated by pulsed-field gel electrophoresis (PFGE) using restriction enzyme SpeI (GE Healthcare, Orsay, France) as described previously (27). Analysis of PFGE patterns was performed by visual inspection of photographs of ethidium bromide-stained gels. The isolates were classified according to the criteria described by Tenover et al. (36). The P. aeruginosa isolate 48-1997 (38), corresponding to the SPM-producing national clone, was included in PFGE experiments for direct comparison of genotypes.
Genetic support of β-lactamase-encoding genes.
Plasmid extraction was performed by the Kieser technique (13). E. coli NCTC50192, harboring four plasmids of 154, 66, 38, and 7 kb, was used as a size marker for plasmids. Transformation assays were performed by electroporation with plasmid extracts from the identified positive isolates, and both E. coli TOP10 and P. aeruginosa PAO1 were used as recipient strains. Selection was performed on agar plates supplemented with 50 μg/ml amoxicillin (amoxicilline) and 50 μg/ml ticarcillin for E. coli and P. aeruginosa, respectively. DNA-DNA hybridization of plasmid extracts was performed with a Southern transfer onto a Hybond N+ nylon membrane (GE Healthcare) as previously described. Labeling of the probe and signal detection were carried out using an enhanced chemiluminescence nonradioactive labeling and detection kit according to the manufacturer's instructions (GE Healthcare). The genetic localization of the β-lactamase-encoding genes was also attempted by using the endonuclease I-CeuI technique, as described previously (14).
Cloning experiments.
Total DNA from P. aeruginosa 35 was HindIII restricted, ligated into the corresponding site of plasmid pBK-CMV, and then transformed in the E. coli TOP10 reference strain by electroporation as described previously (25). Recombinant plasmids were selected on Trypticase soy agar plates containing 1 μg/ml imipenem and 30 μg/ml kanamycin. The cloned DNA fragment of recombinant plasmid p35 was sequenced on both strands.
RESULTS
Clinical data, susceptibility testing, and screening for AmpC overproducers and ESBL producers.
Forty-three out of the 154 P. aeruginosa isolates (28%) displayed ceftazidime resistance. Table 1 summarizes the clinical data, antimicrobial susceptibility profiles, and molecular typing of those 43 isolates. They were collected mainly from patients hospitalized in intensive care units (25 isolates), the emergency room (10 isolates), and the pediatric oncology unit (four isolates) (Table 1). In summary, the retrospective analysis of medical records showed that 24 patients (55.8%) had received adequate antimicrobial therapy, while empirical treatment was not optimal for 14 patients (32.5%). Additionally, the medical records of five patients (11.6%) were not available. Among the 24 patients who had received adequate empirical treatment or for whom antimicrobial therapy was correctly modified according to the results of susceptibility testing, 10 were discharged after 7 or more days of antimicrobial therapy, nine died after 7 or more days of treatment, and five died before 7 days of treatment. Among the 14 patients who had received inadequate empirical therapy, 10 died before results of antimicrobial susceptibility testing, whereas four patients were discharged. Notably, in quite a high number of cases, antibiotics such as carbapenems and the last-resort antibiotic polymyxin B had been used, although unsuccessfully for 20 out of 38 patients. Corresponding clinical data are presented in Table 1.
TABLE 1.
Clinical features of the patients from whom ceftazidime-resistant Pseudomonas aeruginosa isolates were collecteda
| Isolate | Clone | Date of isolation (day/mo/yr) | Hospital unit | Antimicrobial susceptibility | β-Lactamase identified | Age (yr) | Underlying disease | Primary infection | Empirical treatment | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A1 | 1/7/2005 | ICU | CS | SPM-1 | 21 | Acute lymphoblastic leukemia | Bloodstream | FEP, IMP, PMB | Death |
| 2 | A1 | 1/19/2005 | ER | CS, PIP-TZP, ATM | SPM-1 | 61 | None | Bloodstream | RIF, IMP, GM | Death |
| 3 | A1 | 1/19/2005 | ICU | CS | SPM-1 | 17 | Acute myeloid leukemia | Bloodstream | FEP | Death |
| 4 | A1 | 1/27/2005 | ER | CS, ATM | SPM-1 | 62 | Epilepsy | Pneumonia | FEP, IMP | Discharge |
| 5 | A1 | 1/27/2005 | ER | CS, PIP-TZP | SPM-1 | 91 | None | Urinary tract | FEP, IMP, TZP | Death |
| 6 | A1 | 2/10/2005 | Pediatric oncology | CS, ATM | SPM-1 | 3 | NA | NA | NA | NA |
| 7 | A1 | 3/1/2005 | Bone marrow transplant | CS | SPM-1 | 53 | Multiple myeloma | Bloodstream | IMP, PMB | Discharge |
| 8 | A1 | 4/12/2005 | ICU | CS, PIP-TZP | SPM-1 | 48 | Chronic pancreatitis | Bloodstream | IMP, PMB | Death |
| 9 | A1 | 5/3/2005 | Pediatric oncology | CS, ATM | SPM-1 | 14 | NA | NA | NA | NA |
| 10 | A1 | 5/22/2005 | ICU | CS | SPM-1 | 60 | Chronic pancreatitis | Bloodstream | CIP, IMP, PMB | Death |
| 11 | A1 | 5/31/2005 | Hemodialysis | CS, ATM | SPM-1 | 1 | NA | NA | NA | NA |
| 12 | A1 | 5/31/2005 | Hemodialysis | CS, PIP-TZP, ATM | SPM-1 | 1 | Chronic renal failure | Bloodstream | CIP, MEM, PMB | Discharge |
| 13 | A1 | 6/28/2005 | ICU | CS | SPM-1 | 31 | Acute myeloid leukemia | Bloodstream | FEP, IMP | Death |
| 14 | A1 | 7/7/2005 | ICU | CS, ATM | SPM-1 | 37 | HIV, HCV | Bloodstream | PMB | Discharge |
| 15 | A1 | 8/23/2005 | Pediatric oncology | CS, PIP-TZP | SPM-1 | 10 | NA | NA | NA | NA |
| 16 | A1 | 9/6/2005 | ICU | CS, PIP-TZP | SPM-1 | 74 | None | Intra-abdominal | IMP | Death |
| 17 | A1 | 9/20/2005 | ICU | CS, ATM | SPM-1 | 70 | Multiple myeloma | Pneumonia and urinary tract | FEP, IMP, PMB | Death |
| 18 | A1 | 10/27/2005 | ICU | CS, PIP-TZP, ATM | SPM-1 | 70 | Lymphoma | Pneumonia | PMB | Death |
| 19 | A1 | 11/10/2005 | ICU | CS, PIP-TZP, ATM | SPM-1 | 38 | Aortic paraganglioma | Pneumonia | IMP, TZP, PMB | Discharge |
| 20 | A1 | 12/15/2005 | ICU | CS | SPM-1 | 76 | None | Pneumonia | FEP, IMP, PMB | Death |
| 21 | A1 | 12/15/2005 | ICU | CS, PIP-TZP, ATM | SPM-1 | 70 | Chronic renal failure | Pneumonia | FEP, IMP, PMB | Discharge |
| 22 | A1 | 12/17/2005 | ICU | CS, PIP-TZP, ATM | SPM-1 | 70 | None | Pneumonia | PMB | Death |
| 23 | A1 | 12/20/2005 | Pediatric oncology | CS | SPM-1 | 18 | NA | NA | NA | NA |
| 24 | A2 | 1/27/2005 | ICU | CS, PIP-TZP | SPM-1 | 29 | Lymphoma | Intra-abdominal | IMP, PMB | Death |
| 25 | A2 | 7/15/2005 | ICU | CS | SPM-1 | 78 | Hepatitis C | Pneumonia | IMP | Death |
| 26 | A2 | 11/22/2005 | ICU | CS, ATM, AMK | SPM-1 | 64 | COPD | Intra-abdominal | FEP, IMP, PMB | Death |
| 27 | A2 | 11/29/2005 | ER | CS, PIP-TZP, ATM, AMK | SPM-1 | 74 | None | Bloodstream | CRO, NIT | Discharge |
| 28 | B | 1/13/2005 | ER | CS, PIP-TZP, IMP | GES-1 | 79 | Wallenberg syndrome | Pneumonia | FEP | Death |
| 29 | B | 1/19/2005 | ER | CS, ATM, IMP, MEM, CIP, TM, AMK, GM, NET | GES-1 | 62 | Chronic renal failure | Pneumonia | FEP, IMP, PMB, GM | Death |
| 30 | B | 1/28/2005 | ER | CS, PIP-TZP | GES-1 | 79 | Chronic myeloid leukemia | Pneumonia | FEP, IMP, TZP | Discharge |
| 31 | B | 8/2/2005 | ER | CS, PIP-TZP, IMP | GES-1 | 49 | Esophageal cancer | Pneumonia | PMB | Discharge |
| 32 | B | 8/17/2005 | ER | CS | GES-1 | 27 | Mediastinal teratoma | Bloodstream | CIP, FEP | Death |
| 33 | B | 9/20/2005 | ICU | CS, IMP | GES-1 | 54 | Bladder cancer | Pneumonia | FEP, IMP, PMB, TZP | Discharge |
| 34 | C1 | 2/1/2005 | Surgery | CS | GES-5 | 60 | Chronic obstructive pyelonephritis | Intra-abdominal | IMP | Death |
| 35 | C1 | 7/5/2005 | ICU | CS, ATM | GES-5 | 44 | None | Pneumonia | IMP, PMB | Discharge |
| 36 | C2 | 5/22/2005 | ER | CS, PIP-TZP, IMP, MEM | GES-1 | 47 | Bladder cancer | Urinary tract | FEP | Discharge |
| 37 | D1 | 1/19/2005 | ICU | CS, CIP, TM, AMK, GM, NET | AmpC overproduction | 66 | None | Bloodstream | IMP, TZP | Death |
| 38 | D2 | 2/15/2005 | ICU | CS, CIP, TM, AMK, GM, NET | AmpC overproduction | 9 mo | Autoimmune endocrine disease | Pneumonia | CIP, MEM | Death |
| 39 | D2 | 4/12/2005 | ICU | CS, IMP, CIP, TM, AMK, GM, NET | AmpC overproduction | 79 | None | Pneumonia | FEP, IMP, PMB | Death |
| 40 | E | 3/22/2005 | ICU | CS, ATM | CTX-M-2 | 46 | HIV, HBV, HCV, pulmonary tuberculosis | Bloodstream | FEP, CIP | Discharge |
| 41 | E | 3/30/2005 | ICU | CS, ATM, IMP | CTX-M-2 | 36 | HIV | Urinary tract | PMB | Discharge |
| 42 | F | 5/31/2005 | ICU | CS, PIP-TZP, ATM | IMP-1 | 63 | Pancreatic cancer | Pneumonia | IMP, PMB | Death |
| 43 | G | 8/12/2005 | ICU | CS, TM, AMK, GM, NET | AmpC overproduction | 39 | Severe asthma | Bloodstream | CAZ, GM | Death |
Abbreviations: AMK, amikacin; ATM, aztreonam; CAZ, ceftazidime; CIP, ciprofloxacin; CRO, ceftriaxone; CS, colistin; FEP, cefepime; GM, gentamicin; IMP, imipenem; MEM, meropenem; NET, netilmicin; NIT, nitrofurantoin; PMB, polymyxin B; TM, tobramycin; PIP, piperacillin; TZP, tazobactam; RIF, rifampin (rifampicin); COPD, chronic obstructive pulmonary disease; ER, emergency room; HBV, hepatitis B virus infection; HCV, hepatitis C virus infection; HIV, human immunodeficiency virus infection; ICU, intensive care unit; NA, not available.
The 43 ceftazidime-resistant isolates were also resistant to ticarcillin, ticarcillin-clavulanic acid, cefpirome, and cefepime. The highest susceptibility rates were obtained for colistin (100%), followed by aztreonam (44.1%), piperacillin-tazobactam (39.5%), piperacillin (34.8%), imipenem (18.6%), and amikacin (18.6%). Only for piperacillin and piperacillin- tazobactam were susceptibility rates obtained following the breakpoints recommended by the European Committee on Antimicrobial Susceptibility Testing (an isolate is deemed susceptible when the MIC is ≤16 mg/liter). Overproduction of AmpC was identified to be likely the sole enzymatic mechanism responsible for ceftazidime resistance in four isolates (9.3%). Nine isolates were classified as ESBL producers.
Imipenem hydrolysis was observed for 30 isolates, all of them resistant to imipenem. A high imipenem hydrolysis rate was obtained for 28 isolates (average specific activity of 0.2 U·mg of protein−1), and a lower but significant rate was obtained for two isolates (average specific activity of 0.007 U·mg of protein−1).
Identification of acquired β-lactamase genes.
The blaGES-1 (n = 7) and blaCTX-M-2 (n = 2) genes were identified in nine isolates exhibiting an ESBL phenotype. In addition, the blaGES-5 gene, which encodes an unusual ESBL with carbapenemase activity, was identified in two isolates which did not display any ESBL phenotype but corresponded to the two isolates for which weak carbapenem hydrolysis was detected. The MBL-encoding genes blaSPM-1 and blaIMP-1 were identified in 27 isolates (62.8%) and one isolate (2.3%), respectively, corresponding to those isolates for which a high imipenem hydrolysis rate had been noted.
Clonal relationship.
PFGE analysis performed with the 43 P. aeruginosa clinical isolates showed seven main genotypes. The 27 SPM-1-producing isolates belonged to a single genotype, A (corresponding to the genotype of the Brazilian epidemic clone), which can be divided into two subtypes, A1 and A2. GES-1-producing isolates belonged to either genotype B (six isolates) or genotype C1 (one isolate). The two GES-5-producing isolates belonged to genotype C2, which was closely related to genotype C1 (two-band difference). The four AmpC overproducers belonged to genotypes G (one isolate) and D (three isolates), the latter being subclassified into two subtypes, D1 (one isolate) and D2 (two isolates). Genotypes E and F corresponded to the two CTX-M-2-producing isolates and the single IMP-1-producing isolate, respectively (Table 1).
Genetics of β-lactamase-encoding genes.
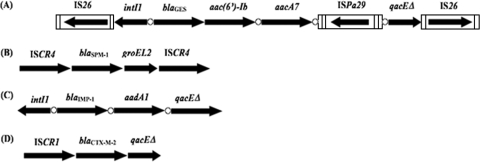
PCR mapping and sequencing revealed that the blaCTX-M-2 gene was preceded by ISCR1 and followed by the qacEΔ1 and sul1 tandem genes (Fig. 1), as previously reported for Enterobacteriaceae (34). ISCR elements are insertion sequences transposing in a particular way since they can mobilize adjacent sequences by rolling-circle transposition (37). Analysis of the SPM-1-producing strains showed that the blaSPM-1 gene was preceded by ISCR4 and followed by groEL and ISCR4 (Fig. 1) with a perfect identity compared to the sequences previously reported from blaSPM-1-producing P. aeruginosa isolates (27). Sequencing of the 5′ CS-3′ CS amplicon obtained from isolate 42 showed that the blaIMP-1 gene was located at the first position of a class 1 integron possessing the strong promoter configuration with Pant and P2. Class 1 integrons are DNA structures that may integrate or excise antibiotic resistance genes as a form of gene cassettes (4). The blaIMP-1 gene was associated with the aadA1 gene cassette encoding resistance to aminoglycosides in that class 1 integron (Fig. 1).
FIG. 1.
Schematic representation of the genetic environment of β-lactamase-encoding genes identified in P. aeruginosa isolates. The coding genes are represented by arrows indicating their translation orientations, and 59-bes are indicated as white circles. (A) Genetic environment of blaGES-1 and blaGES-5. (B) Genetic environment of blaSPM-1. (C) Genetic environment of blaIMP-1. (D) Genetic environment of blaCTX-M-2.
Using the I-CeuI technique, no conclusive results regarding the genetic location of blaCTX-M-2, blaSPM-1, and blaIMP-1 genes were obtained. No plasmid was identified in the two blaCTX-M-2-positive isolates as well as in all blaSPM-1-positive isolates, whereas a single plasmid of 100 kb was identified in the blaIMP-1-positive isolate. Southern blot hybridization of plasmid DNA extracted from blaCTX-M-2, blaSPM-1, and blaIMP-1-positive isolates using the corresponding probes did not give a positive signal. In addition, the transfer of these resistance determinants to both E. coli and P. aeruginosa PAO1 recipient strains remained unsuccessful.
Detailed analysis of blaGES-positive isolates.
In order to detail the environment of the blaGES genes in P. aeruginosa isolates, a cloning step was necessary. Cloning of HindIII-digested total DNA from isolate 35 resulted in a blaGES-5-positive recombinant plasmid containing an insert of approximately 10 kb. Sequencing of the insert revealed that the blaGES-5 gene was located at the first position of a class 1 integron, followed by the aacA7 and aacA4 gene cassettes. The 59-base element (59-be) of the aacA4 gene cassette was interrupted by a new insertion sequence element named ISPa29 belonging to the IS1111 family, members of which have been shown to insert themselves into the recombination sites of gene cassettes by site-specific recombination (32). ISPa29 was 1,534 bp long, possessed 12-bp-long imperfect subterminal inverted repeats, and was not bracketed by any target site duplication as commonly observed for IS1111 family members (Fig. 1) (32). Its transposase shared 63% identity with that of IS1492 identified from Pseudomonas putida. The ISPa29 sequence has been deposited on the IS Biotoul website (http://www-is.biotoul.fr). This class 1 integron was bracketed by two copies of IS26, located in opposite orientations as drawn in Fig. 1, thus constituting a composite transposon structure, as already found for another β-lactamase gene (blaVEB-1)-positive integron, In53 (22).
Further PCR mapping used to target IS26 elements and the gene cassettes for the blaGES-5- and blaGES-1-positive isolates showed that they possessed the same class 1 integron structure. That integron possessed the promoter Pant, whereas promoter P2 was under an inactive form. As observed for the blaGES-1-positive Klebsiella pneumoniae strain ORI-1 from French Guiana (26), the blaGES-1 and blaGES-5 genes identified in our isolates were purified as a form of gene cassette with a truncated 59-be of 19 bp. Again, the I-CeuI technique did not allow us to clarify whether those blaGES-like genes were chromosomally or plasmid located. In addition, plasmid analysis followed by Southern blotting and hybridization with a blaGES-specific probe performed with all blaGES-positive isolates failed to identify any plasmid. In accordance with those latter negative results, repeated attempts to transfer the blaGES-1 and blaGES-5 genes by transformation into E. coli or P. aeruginosa recipient strains failed. Those results strongly suggested the chromosomal location of the blaGES-like genes.
DISCUSSION
The rate of ceftazidime-resistant P. aeruginosa strains (28%) isolated from blood cultures was quite high in this study. A similarly high rate of resistance has been reported for many developing countries worldwide (42). Here we showed that this high rate is mainly due to ESBL- and MBL-encoding genes, together with the clonal spread of several specific clones. Interestingly, AmpC overproduction as a single enzymatic mechanism for ceftazidime resistance was rarely observed; it seemed to be replaced by acquisition of β-lactamases with an expanded spectrum of activity. Worryingly, a very high proportion of those isolates were also resistant to imipenem (82%) and meropenem (95%), suggesting the presence of nonenzymatic mechanisms of carbapenem resistance, such as porin loss and/or overexpression of efflux pumps.
Our study showed that the blaGES-type ESBL genes were identified in 13.9% of the ceftazidime-resistant P. aeruginosa isolates and were divided into two distinct clones. Since blaGES-1 and blaGES-5 genes were identified in the same P. aeruginosa clone, it may correspond to a local evolution of this clone. Considering that GES-5 possesses a wider spectrum of hydrolysis than that of GES-1, it might be speculated that this evolution could be due to a carbapenem-related selective pressure. Notably, those blaGES-1 and blaGES-5 gene cassettes had a truncated 59-be that was identical to that described in the blaGES-1-positive K. pneumoniae ORI-I strain from French Guiana (34). Since French Guiana and Brazil are neighboring countries, those findings may suggest a spread of blaGES-related structures in that part of the world.
The present study underlines the idea that the current spread of CTX-M enzymes may occur not only in the Enterobacteriaceae but also in P. aeruginosa. Interestingly, one recent study carried out in the same Brazilian hospital found that 44.8% of K. pneumoniae clinical isolates were CTX-M-2 producers (6). In addition, a CTX-M-2-producing P. aeruginosa strain that was susceptible to ceftazidime was recently identified in this hospital (22). Such a spread of CTX-Ms may be difficult to detect since many CTX-M variants confer a higher degree of resistance to cefotaxime than to ceftazidime (34). Therefore, since our selection criterion was based on ceftazidime resistance, the rate of CTX-M production among P. aeruginosa isolates identified here might be underestimated.
The genetic environments surrounding the blaCTX-M- and blaGES-type β-lactamase genes in P. aeruginosa were identical to those identified in the Enterobacteriaceae, underlining their common origin. It is likely that the location of the β-lactamase genes in P. aeruginosa results from their transfer from the Enterobacteriaceae.
Overall, the production of SPM-1 carbapenemase was the main source of resistance to ceftazidime, accounting for 62.8% of the isolates. SPM-1-producing P. aeruginosa isolates are widely disseminated in Brazil, with a single clone identified in different cities (10). This clone has been also recently identified in P. aeruginosa isolates recovered from Switzerland (9). We showed here that this SPM-1-producing clone had been responsible for a large outbreak causing septicemia in 27 hospitalized patients, leading to the death of at least 15 of them.
This study underlines the possibility that a variety of β- lactamases with wide spectra of activity may circulate in P. aeruginosa in Brazil, contributing to a multidrug resistance phenotype. Lack of their identification and consequently lack of isolation of carriers may lead to a further dissemination.
Acknowledgments
This work was partially funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, France, and mostly by grants from the European Community (DRESP2 contract, LSHM-CT-2005-018705 and TROCAR HEALTH-F3-2008-223031) and by the INSERM. We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), which gave a PDEE grant to Renata Cristina Picão (protocol #3682/07-2) and to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing a researcher grant to Ana Cristina Gales (process number 307714/2006-3).
Footnotes
Published ahead of print on 13 July 2009.
REFERENCES
- 1.Al Naiemi, N., B. Duim, and A. Bart. 2006. A CTX-M extended-spectrum β-lactamase in Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Med. Microbiol. 55:1607-1608. [DOI] [PubMed] [Google Scholar]
- 2.Castanheira, M., R. E. Mendes, T. R. Walsh, A. C. Gales, and R. N. Jones. 2004. Emergence of the extended-spectrum β-lactamase GES-1 in a Pseudomonas aeruginosa strain from Brazil: report from the SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemother. 48:2344-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing. Nineteenth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Collis, C. M., and R. M. Hall. 1995. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob. Agents Chemother. 39:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Da Fonseca, E. L., V. V. Vieira, R. Cipriano, and A. C. Vicente. 2007. Emergence of blaGES-5 in clinical colistin-only-sensitive (COS) Pseudomonas aeruginosa strain in Brazil. J. Antimicrob. Chemother. 59:576-577. [DOI] [PubMed] [Google Scholar]
- 6.Do Carmo Filho, J. R., R. M. Silva, M. Castanheira, M. C. Tognim, A. C. Gales, and H. S. Sader. 2008. Prevalence and genetic characterization of blaCTX-M among Klebsiella pneumoniae isolates collected in an intensive care unit in Brazil. J. Chemother. 20:600-603. [DOI] [PubMed] [Google Scholar]
- 7.Dubois, V., C. Arpin, P. Noury, C. André, L. Coulange, and C. Quentin. 2005. Prolonged outbreak of infection due to TEM-21-producing strains of Pseudomonas aeruginosa and enterobacteria in a nursing home. J. Clin. Microbiol. 43:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois, V., L. Poirel, C. Marie, C. Arpin, P. Nordmann, and C. Quentin. 2002. Molecular characterization of a novel class 1 integron containing blaGES-1 and a fused product of aac3-Ib/aac6′-Ib′ gene cassettes in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Salabi, A., M. A. Toleman, T. R. Walsh, R. Frei, and T. Bruderer. 2008. The metallo-β-lactamase (MBL) SPM-1 arrives in Europe, abstr. C1-124, p. 92. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 10.Gales, A. C., L. C. Menezes, S. Silbert, and H. S. Sader. 2003. Dissemination in distinct Brazilian regions of an epidemic carbapenem-resistant Pseudomonas aeruginosa producing SPM metallo-β-lactamase. J. Antimicrob. Chemother. 52:699-702. [DOI] [PubMed] [Google Scholar]
- 11.Héritier, C., L. Poirel, D. Aubert, and P. Nordmann. 2003. Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob. Agents Chemother. 47:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirakata, Y., T. Yamaguchi, M. Nakano, K. Izumikawa, M. Mine, S. Aoki, A. Kondoh, J. Matsuda, M. Hirayama, K. Yanagihara, Y. Miyazaki, K. Tomono, Y. Yamada, S. Kamihira, and S. Kohno. 2003. Clinical and bacteriological characteristics of IMP-type metallo-β-lactamase-producing Pseudomonas aeruginosa. Clin. Infect. Dis. 37:26-32. [DOI] [PubMed] [Google Scholar]
- 13.Kieser, T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19-36. [DOI] [PubMed] [Google Scholar]
- 14.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moland, E. S., N. D. Hanson, V. L. Herrera, J. A. Black, T. J. Lockhart, A. Hossain, J. A. Johnson, R. V. Goering, and K. S. Thomson. 2003. Plasmid-mediated, carbapenem-hydrolysing β-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J. Antimicrob. Chemother. 51:711-714. [DOI] [PubMed] [Google Scholar]
- 16.Naas, T., L. Philippon, L. Poirel, E. Ronco, and P. Nordmann. 1999. An SHV-derived extended-spectrum β-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:1281-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagano, N., Y. Nagano, C. Cordevant, N. Shibata, and Y. Arakawa. 2004. Nosocomial transmission of CTX-M-2 β-lactamase-producing Acinetobacter baumannii in a neurosurgery ward. J. Clin. Microbiol. 42:3978-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordmann, P., and H. Mammeri. 2007. Extended-spectrum cephalosporinases: structure, detection and epidemiology. Future Microbiol. 2:297-307. [DOI] [PubMed] [Google Scholar]
- 19.Nordmann, P., T. Naas, N. Fortineau, and L. Poirel. 2007. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr. Opin. Microbiol. 10:436-440. [DOI] [PubMed] [Google Scholar]
- 20.Nordmann, P., E. Ronco, T. Naas, C. Duport, Y. Michel-Briand, and R. Labia. 1993. Characterization of a novel extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrino, F. L., K. R. Netto-dos Santos, L. W. Riley, and B. M. Moreira. 2006. blaGES carrying Pseudomonas aeruginosa isolates from a public hospital in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 10:251-253. [DOI] [PubMed] [Google Scholar]
- 22.Picão, R. C., L. Poirel, A. C. Gales, and P. Nordmann. 2009. Further identification of CTX-M-2 extended-spectrum β-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:2225-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirel, L., L. Brinas, A. Verlinde, L. Ide, and P. Nordmann. 2005. BEL-1, a novel clavulanic acid-inhibited extended-spectrum β-lactamase, and the class 1 integron In120 in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3743-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel, L., L. Cabanne, H. Vahaboglu, and P. Nordmann. 2005. Genetic environment and expression of the extended-spectrum β-lactamase blaPER-1 gene in gram-negative bacteria. Antimicrob. Agents Chemother. 49:1708-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirel, L., M. Guibert, D. Girlich, T. Naas, and P. Nordmann. 1999. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob. Agents Chemother. 43:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirel, L., I. Le Thomas, T. Naas, A. Karim, and P. Nordmann. 2000. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poirel, L., M. Magalhaes, M. Lopes, and P. Nordmann. 2004. Molecular analysis of metallo-β-lactamase gene blaSPM-1-surrounding sequences from disseminated Pseudomonas aeruginosa isolates in Recife, Brazil. Antimicrob. Agents Chemother. 48:1406-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirel, L., O. Menuteau, N. Agoli, C. Cattoen, and P. Nordmann. 2003. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirel, L., T. Naas, M. Guibert, E. B. Chaibi, R. Labia, and P. Nordmann. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poirel, L., T. Naas, D. Nicolas, L. Collet, S. Bellais, J. D. Cavallo, and P. Nordmann. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirel, L., G. F. Weldhagen, T. Naas, C. De Champs, M. G. Dove, and P. Nordmann. 2001. GES-2, a class A β-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob. Agents Chemother. 45:2598-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Post, V., and R. M. Hall. 2009. Insertion sequences in the IS1111 family that target the attC recombination sites of integron-associated gene cassettes. FEMS Microbiol. Lett. 290:182-187. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Martinez, J. M., L. Poirel, and P. Nordmann. 2009. Extended-spectrum cephalosporinases in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:1766-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossolini, G. M., M. M. D'Andrea, and C. Mugnaioli. 2008. The spread of CTX-M-type extended-spectrum β-lactamases. Clin. Microbiol. Infect. 14(Suppl. 1):33-41. [DOI] [PubMed] [Google Scholar]
- 35.Sader, H. S., A. O. Reis, S. Silbert, and A. C. Gales. 2005. IMPs, VIMs and SPMs: the diversity of metallo-β-lactamases produced by carbapenem-resistant Pseudomonas aeruginosa in a Brazilian hospital. Clin. Microbiol. Infect. 11:73-76. [DOI] [PubMed] [Google Scholar]
- 36.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toleman, M. A., P. M. Bennett, and T. R. Walsh. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toleman, M. A., A. M. Simm, T. A. Murphy, A. C. Gales, D. J. Biedenbach, R. N. Jones, and T. R. Walsh. 2002. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: report from the SENTRY antimicrobial surveillance programme. J. Antimicrob. Chemother. 50:673-679. [DOI] [PubMed] [Google Scholar]
- 39.Vourli, S., P. Giakkoupi, V. Miriagou, E. Tzelepi, A. C. Vatopoulos, and L. S. Tzouvelekis. 2004. Novel GES/IBC extended-spectrum β-lactamase variants with carbapenemase activity in clinical enterobacteria. FEMS Microbiol. Lett. 234:209-213. [DOI] [PubMed] [Google Scholar]
- 40.Wachino, J., Y. Doi, K. Yamane, N. Shibata, T. Yagi, T. Kubota, and Y. Arakawa. 2004. Molecular characterization of a cephamycin-hydrolyzing and inhibitor-resistant class A β-lactamase, GES-4, possessing a single G170S substitution in the omega-loop. Antimicrob. Agents Chemother. 48:2905-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh, T. R. 2008. Clinically significant carbapenemases: an update. Curr. Opin. Infect. Dis. 21:367-371. [DOI] [PubMed] [Google Scholar]
- 42.Yong, D., J. M. Bell, B. Ritchie, R. Pratt, M. A. Toleman, and T. R. Walsh. 2007. A novel sub-group metallo-β-lactamase (MBL), AIM-1 emerges in Pseudomonas aeruginosa (PSA) from Australia, abstr. C1-593, p. 75. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed]