Abstract
Candida albicans readily forms biofilms on the surface on indwelling medical devices, and these biofilms serve as a source of local and systemic infections. It is estimated that 27% of nosocomial C. albicans bloodstream infections are polymicrobial, with Staphylococcus aureus as the third most common organism isolated in conjunction with C. albicans. We tested whether S. aureus and C. albicans are able to form a polymicrobial biofilm. Although S. aureus formed poor monoculture biofilms in serum, it formed a substantial polymicrobial biofilm in the presence of C. albicans. In terms of architecture, S. aureus formed microcolonies on the surface of the biofilm, with C. albicans serving as the underlying scaffolding. In addition, S. aureus matrix staining revealed a different phenotype in polymicrobial versus monomicrobial biofilms, suggesting that S. aureus may become coated in the matrix secreted by C. albicans. S. aureus resistance to vancomycin was enhanced within the polymicrobial biofilm, required viable C. albicans, and was in part mediated by C. albicans matrix. However, the growth or sensitivity to amphotericin B of C. albicans is not altered in the polymicrobial biofilm.
There is increasing evidence in the literature for the importance of polymicrobial infections in which microorganisms interact in a synergistic or inhibitory fashion, impacting pathogenesis and the health of the patient. It was originally estimated that over half of infections originated from biofilms (12). However, the NIH currently estimates that biofilms account for over 80% of all infections in the body (NIH SBIR/STTR Study and Control of Microbial Biofilms program announcement, release date 21 April 1999, http://grants.nih.gov/grants/guide/pa-files/PA-99-084.html). Biofilms are communities of microbes embedded in a polysaccharide matrix adhered to a biotic or abiotic surface. The biofilm-associated microorganisms are refractory to both antimicrobial agents and the host immune response. Polymicrobial biofilms represent an understudied and clinically relevant health problem, with the potential to serve as an infectious reservoir for a variety of microorganisms, including bacteria and fungi.
Biofilms can form on indwelling medical devices and serve as a source of nosocomial bloodstream infections, which prolong hospitalization and are the 10th leading cause of death in the United States (30). Candida albicans is the fourth leading cause of bloodstream infections and the third most commonly isolated organism from intravascular catheters and is associated with the highest incidence of mortality (13, 30). C. albicans readily forms biofilms on a wide variety of polymers used to make indwelling medical devices, such as dental materials, stents, shunts, prostheses (voice, heart valve, knee, etc.), implants (lens, breast, denture, penile, etc.), endotracheal tubes, pacemakers, and catheters (reviewed in reference 23). There is some evidence to suggest that a large proportion of device-related Candida albicans infections involve biofilms (14, 15, 23). In a prospective study of catheter colonization, C. albicans ranked second in the ratio of colonization to invasive disease (13). C. albicans biofilms have a unique gene expression pattern (18, 24, 31) and are more resistant to antifungal treatment than planktonic cells (23, 26). A unique feature of C. albicans biofilms is the morphological heterogeneity of the biofilm cells, which results in a complex three-dimensional biofilm architecture (11). C. albicans biofilm formation is initiated upon contact with an appropriate polymeric surface under morphogenesis-inducing growth conditions. Serum is the classical clinically relevant inducer of morphogenesis, although other media can be used in vitro. During early biofilm formation, yeast cells adhere to an appropriate surface and initiate germ tube formation. The intermediate phase is characterized by continued hyphal elongation and extracellular matrix production, which is composed primarily of glucose along with proteins and other sugars (2). Mature biofilms consist of a yeast base, with hyphal elements encased in matrix extending away from the surface forming a sticky net-like structure (11). Newly formed daughter yeast cells grow out of hyphal elements and are released, seeding new niches for biofilm formation or infection.
It is estimated that 27% of nosocomial C. albicans bloodstream infections are polymicrobial, with Staphylococcus aureus as the third most common organism isolated in conjunction with C. albicans (22). Interestingly, the combined effect of C. albicans and S. aureus results in synergism and increased mortality in mice (5-9). Although the infectious parameters of polymicrobial infections in humans are not well characterized, eliminating the infection may involve a more complex antimicrobial regimen. It has previously been demonstrated that a mixed species biofilm of C. albicans and Staphylococcus epidermidis enhances the growth of S. epidermidis and increases the resistance of S. epidermidis to vancomycin (1, 16). It is worth mentioning that biofilm formation in S. epidermidis and biofilm formation in S. aureus are not equivalent. Compared with S. epidermidis, S. aureus does not form biofilms as readily on abiotic surfaces, requiring precoating and nutrient supplementation (10). However, S. aureus is a more clinically important pathogen, with higher rates of device-related systemic infection and mortality (reviewed in references 20 and 25). Therefore, we sought to determine whether C. albicans and S. aureus could form polymicrobial biofilms and whether these biofilms exhibited altered antimicrobial sensitivity.
MATERIALS AND METHODS
C. albicans strains and handling.
C. albicans strain SC5314 was subcultured from freezer stocks onto Sabouraud dextrose agar (SDA) plates and incubated at 30°C overnight to generate C. albicans yeast used for the experiments. All subsequent liquid subcultures, grown in Sabouraud dextrose broth (SDB), were derived from colonies isolated from these plates.
S. aureus strain and handling.
S. aureus ATCC 25923 is a clinical isolate and is capable of biofilm formation in vitro (29). For each experiment, strains were subcultured from freezer stocks onto brain heart infusion (BHI) agar plates and incubated at 37°C overnight. All subsequent liquid subcultures were derived from colonies isolated from these plates.
Biofilm formation.
Overnight cultures generated as described above were washed twice in sterile 1× phosphate-buffered saline (PBS) by centrifugation at 4,000 rpm for 10 min. Suspensions of C. albicans were prepared in 0.01% trypan blue and counted with a hemocytometer. S. aureus cells were enumerated using the live-dead stain BacLight (Molecular Probes Invitrogen, Carlsbad, CA). Microbes were diluted in different media for biofilm formation: 50% bovine serum (50% BS), 50% heat-inactivated bovine serum (50% BS-HI), or BHI. C. albicans was diluted to a final concentration of 106 CFU/ml, and S. aureus was diluted to a final concentration of 106 to 108 CFU/ml. C. albicans (100 μl) and S. aureus (10 μl) were added to the wells of a 96-well tissue culture-treated polystyrene plate. Bacterial and fungal suspensions were mixed in the wells by pipetting up and down, and the plates were incubated at 37°C for 24 h. This resulted in a final organism concentration in the wells of 106 CFU/ml for C. albicans and 105 to 107 CFU/ml for S. aureus. Control wells contained C. albicans alone, S. aureus alone, or medium alone.
Biofilm adhesion assay.
S. aureus ATCC 25923 was diluted to 106 CFU/ml in 50% BS, 50% BS-HI, or BHI, and 100-μl aliquots were added to a 96-well tissue culture-treated plate. The plates were incubated at 37°C for 1, 2, 4, 6, and 24 h. For each time point, two identical but separate plates were set up. At each time point, one plate was washed with 1× PBS (three times) to remove nonadherent cells, while the control plate was not washed. Bacterial levels in washed and unwashed plates were determined by the CFU assay.
CFU assay.
Biofilms were washed in 1× PBS to remove nonadherent cells. Cells were resuspended in 100 μl of 1× PBS by sonicating for 10 min and followed by pipetting up and down. Microscopic analysis prior to plating revealed that the resulting cell suspension consisted of a mixture of single yeast cells and hyphae. We cannot rule out the possibility that some hyphae were sheared and could lead to experimental variability. However, the fact that we obtained excellent reproducibility from experiment to experiment using CFU analysis validates the usefulness of this assay. Tenfold dilutions of each well were made and plated on SDA supplemented with ampicillin (0.008 mg/ml) and erythromycin (0.075 mg/ml) and on BHI agar supplemented with amphotericin B (Amp B) (0.025 mg/ml). These concentrations of antimicrobials were tested and were effective at preventing growth of the nontargeted microbe. The plates were incubated at 37°C overnight and left on the benchtop for 1 week to monitor outgrowth of potential slowly growing colonies resulting from cells damaged by antimicrobial drugs.
Planktonic antimicrobial assay.
C. albicans SC5314 (103 CFU/ml) alone, S. aureus ATCC 25923 (103 CFU/ml) alone, or C. albicans (103 CFU/ml) and S. aureus (103 CFU/ml) together were added to 96-well, deep-well polypropylene plates (Fisher Scientific, Hanover, IL) in 50% bovine serum. Bacterial and fungal suspensions were mixed in the wells by pipetting up and down. Amphotericin B alone, vancomycin alone, or Amp B and vancomycin combined were added to the wells. The plates were incubated at 35°C in an orbital shaker at 175 rpm for 18 h, and fungal and bacterial viability was monitored by the CFU assay.
Biofilm antimicrobial assay.
Biofilms were formed as described above. After a 24-h incubation at 37°C, plates were washed three times with 1× PBS to remove nonadherent cells. Stock solutions of Amp B and vancomycin (Fisher, Hanover Park, IL) were prepared in dimethyl sulfoxide and diluted to working concentrations in 50% BS. Antimicrobials (100 μl) were added to the washed biofilm. The plates were incubated for an additional 24 h, and fungal and bacterial viability was monitored by the CFU assay.
Transwell assays.
C. albicans was separated from S. aureus using a 0.4-μm polyester (PET) Transwell membrane (Corning Life Sciences, Acton, MA). S. aureus (1 × 107 CFU/ml) was added to the bottom of the well, and C. albicans (1 × 106 CFU/ml) was added to the top of the membrane. After a 24-h incubation at 37°C, the bottom and top (membrane) of the Transwell membrane were washed with 1× PBS. Stocks of antimicrobials were prepared as described above and diluted in 50% BS. Vancomycin was added to the bottom of the Transwell membrane, and Amp B was added to the top (membrane) of the Transwell membrane. The plates were incubated for 24 h at 37°C, and growth in both the bottom well and membrane of the Transwell membrane was determined by the CFU assay. Controls without Transwell inserts were also included.
Fluorescence microscopy.
Biofilms were formed in permanox chamber slides (Nalge Nunc, Rochester, NY) pretreated with BS. After the final PBS wash, fluorescent stains were added directly to chamber slides. The following stains were used: SYTO 9 (Molecular Probes, Eugene, OR) stains live yeast and bacteria green; concanavalin A (ConA)-Texas Red conjugate (Molecular Probes, Eugene, OR) stains biofilm matrix red; and calcofluor white (Fluka, Seelze, Germany) stains fungal cell walls blue. The final concentrations of stains were as follows: 6.6 μM for SYTO 9, 50 μg/ml for ConA-Texas Red, and 1 mg/ml for calcofluor white. The slides were examined with the Zeiss Apotome imaging system for confocal fluorescence microscopy and the Nikon Eclipse E800 with Metamorph software for conventional fluorescence microscopy.
SEM.
Biofilms were formed in eight-well chamber slides as previously described. After 24 h, slides were washed with 1× PBS (three times) to remove planktonic cells. The slides were prepared for scanning electron microscopy (SEM) using a previously published protocol (17). The slides were placed in 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) for 2 h. The slides were then dehydrated by successive 10-min incubations in 35% ethanol, 50% ethanol, 70% ethanol, and 95% ethanol followed by six successive 10-min incubations in 100% ethanol. The slides were dried in 100% hexamethyldisilazane (Electron Microscopy Sciences, Hatfield, PA) for 20 min. The slides were cut into 0.5-cm by 0.5-cm squares, affixed to SEM stubs with double-sided tape, and coated with gold/palladium. Specimens were evaluated with the Hitachi S-2400 SEM at the Laboratory of Analytical Electron Microscopy at Wayne State University. The voltage was set at 15 kV and viewed at a magnification from ×1,000 to ×10,000.
Matrix isolation.
C. albicans biofilm matrix was isolated using a previously published protocol with slight modifications (27). Briefly, biofilms were grown in two separate polysterene, tissue culture-treated T-175 flasks in 250 ml of 50% BS. The 24-h biofilms were gently washed with 1× PBS to remove nonadherent cells, and 12.5 ml of 50% BS was added to each flask. The biofilm was scraped from the flasks with a cell scraper. The flasks were sonicated for 10 min, and an additional 12.5 ml of 50% BS was added to the flasks to generate a matrix-containing medium equivalent to that used in the polymicrobial biofilms. The contents of the flask were transferred to a sterile Sorvall centrifuge tube and vortexed for 3 min and then centrifuged for 20 min at 12,000 × g. The supernatant was carefully removed and transferred to a fresh sterile Sorvall tube. Centrifugation and transfer steps were repeated two more times. After the final spin, 800 μl of the supernatant was plated onto SDA and incubated overnight to ensure minimal yeast growth. To ensure elimination of yeast cells, the matrix was treated with Amp B (20 μg/ml) prior to use in experiments. Isolation of matrix was monitored by staining supernatant with ConA-Texas Red followed by fluorescence microscopy.
Statistical analysis.
The Student t test (two-tailed, unequal variance) was used to analyze the significance of differences between two experimental groups. Data with a P value of 0.05 or less were considered to be significant.
RESULTS
S. aureus growth and biofilm formation in serum.
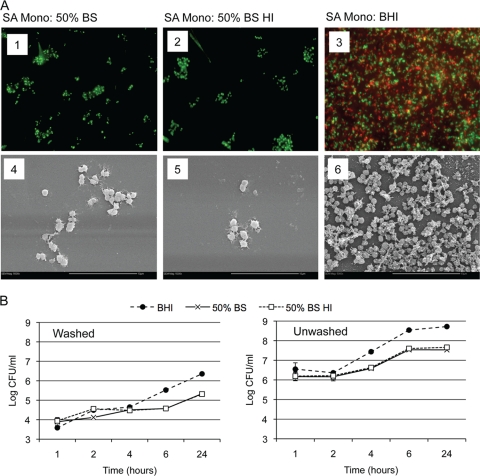
C. albicans readily forms monomicrobial biofilms in serum, a clinically relevant medium. We therefore chose to use this medium to investigate polymicrobial biofilm formation. Traditionally, S. aureus biofilms are formed in rich media, such as BHI broth or Trypticase soy broth (21). Therefore, we monitored growth and biofilm formation in 50% BS, 50% BS-HI, and BHI. We first performed growth curves in the different media (data not shown). Although bacterial growth rates and maximum growth levels were increased in BHI, both sera (BS and BS-HI) were able to support the growth of S. aureus. We then tested whether S. aureus could form a monomicrobial biofilm in serum on an abiotic surface (96-well tissue culture-treated polystyrene plates). We monitored biofilm formation in 50% BS, 50% BS-HI, and BHI by fluorescence microscopy and scanning electron microscopy. Monomicrobial S. aureus biofilms were stained with SYTO 9 (viable bacteria stain green) and ConA-Texas Red (extracellular matrix stains red). After 24 h at 37°C, S. aureus formed a substantial biofilm and extracellular matrix production in BHI by microscopic examination (Fig. 1A, panels 3 and 6). However, in the presence of serum, the abiotic surface was sparsely populated, and no extracellular matrix was observed, indicating a lack of biofilm formation (Fig. 1A, panels 1, 2, 4, and 5). Macroscopic analysis of the 96-well plates also confirmed these results. After the plates were washed, a visible film was observed in BHI but not in serum (data not shown). This indicates that S. aureus is unable to form a biofilm in serum.
FIG. 1.
S. aureus biofilm growth in serum and BHI. S. aureus strain ATCC 29523 was grown overnight at 37°C in BHI broth. Cells were washed, counted and diluted into different media: bovine serum (BS), heat-inactivated bovine serum (BS HI), or BHI. (A) For biofilm formation, cultures were diluted to 107 CFU/ml, and 300-μl aliquots were added to tissue culture-treated chamber slides and incubated at 37°C for 24 h without shaking. Biofilm formation was monitored by fluorescence microscopy and SEM. (Panels 1 to 3) For fluorescence microscopy, biofilms were stained with SYTO 9 (viable microbial cells stain green) and ConA-Texas Red (extracellular matrix stains red). SA, S. aureus; Mono, monomicrobial biofilm. (Panels 4 to 6) For SEM, biofilms formed on chamber slides were processed for SEM, cut, and mounted into stubs. Magnifications, ×600 (panels 1 to 3) and ×5,000 (panels 4 to 6). Bars, 10 μm. (B) For biofilm formation, cultures were diluted to 106 CFU/ml, and 100-μl aliquots were added to tissue culture-treated 96-well plates and incubated at 37°C for 24 h without shaking. Biofilm formation was monitored by the CFU assay. Washed plates were washed after 1 h to remove nonadherent cells, while unwashed plates were left alone. Biofilms were disrupted by sonication, and the number of CFU was determined by plating. Experiments were performed in duplicate, and the results are averages of two separate trials.
It has been previously reported that serum can interfere with the initial attachment of bacteria to an abiotic surface (19). We therefore examined biofilm formation in plates that were washed or not washed after an initial 1-h attachment period (Fig. 1B). Interestingly, similar numbers of bacteria were washed away in BHI and serum after 1 h (Fig. 1B, left panel). Similar growth rates were observed in the washed plates through 4 h. However, by 24 h, S. aureus grown in BHI had initiated early biofilm formation; we observed a light film macroscopically, dense population and extracellular matrix production microscopically, and increased CFU compared with S. aureus grown in serum (Fig. 1B, left panel, and data not shown). Similar increases in CFU were observed in washed and unwashed plates by 24 h for each medium. However, BHI supported more robust increases compared with serum (2 log units versus 1 log unit). This indicates that serum is not interfering with the initial attachment of S. aureus but that serum does not support subsequent biofilm formation and biofilm growth.
C. albicans-S. aureus polymicrobial biofilm formation in serum.
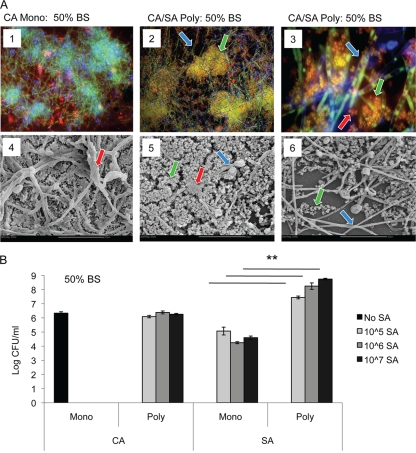
We next tested whether C. albicans and S. aureus could form a polymicrobial biofilm in serum. Polymicrobial biofilms were formed by adding C. albicans and S. aureus concurrently to 96-well tissue culture-treated plates in 50% BS, which were incubated 24 h at 37°C. Biofilm formation was monitored by fluorescence microscopy by staining with SYTO 9 (stains live microbial cells green), calcofluor white (stains fungal cell wall blue), and ConA-Texas Red (stains matrix red). As expected, the monomicrobial Candida biofilm is composed of a dense mat of hyphal and yeast forms surrounded by extracellular matrix (Fig. 2A, panels 1 and 4). Despite the fact that S. aureus cannot form a biofilm in serum, coculture of C. albicans and S. aureus produced a polymicrobial biofilm. At lower magnification, fungi and bacteria are found interspersed within the biofilm, and extracellular matrix is evident. In addition, S. aureus forms microcolonies on the surface of the C. albicans biofilm (Fig. 2A, panels 2 and 5, green arrows). At higher magnification, it is apparent that S. aureus is preferentially associated with the hyphal forms of C. albicans and individual bacterial cells are surrounded with a red halo of matrix material (Fig. 2A, panels 3 and 6). We observed no heterogeneity in the matrix halo phenotype among S. aureus in the polymicrobial biofilms, ruling out the possibility that clumps of multiple S. aureus cells lead to the observed phenotype.
FIG. 2.
Polymicrobial C. albicans-S. aureus biofilm formation in serum. C. albicans SC5314 was grown overnight in SDB at 30°C. S. aureus ATCC 29523 was grown overnight at 37°C in BHI. Both species were washed, counted, and diluted in different media. C. albicans (106 CFU/ml) and S. aureus (107 CFU/ml) were concurrently added to 96-well tissue culture-treated chamber slides and incubated for 24 h at 37°C. Biofilm formation was monitored by fluorescence microscopy and SEM (A) and CFU analysis (B). (A, panels 1 to 3) For fluorescence microscopy, biofilms were stained with calcofluor white (blue stain and blue arrows show fungal cell wall), SYTO 9 (green stain and green arrows show viable microbial cells), and ConA-Texas Red (red stain and red arrows show extracellular matrix). CA, C. albicans; Mono, monomicrobial biofilm; CA/SA Poly, C. albicans and S. aureus polymicrobial biofilm. (Panels 4 to 6) For SEM, biofilms formed on chamber slides were processed for SEM, cut, and mounted into stubs. Magnifications, ×200 (panels 1 and 2), ×1,000 (panel 3), and ×2,000 (panels 4 to 6). Bars, 20 μm. (B) For the CFU assay, plates were washed to remove nonadherent cells. Biofilms were disrupted by sonication, and the number of CFU was determined by plating onto selective media. Experiments were performed in sextuplicate. The data represent the averages of four experiments. Values that were significantly different (P < 0.01) for the S. aureus monomicrobial biofilm versus the S. aureus polymicrobial biofilm are indicated by the bars and asterisks.
We also monitored fungal and bacterial growth within the biofilms by the CFU assay using selective plates to determine fungal versus bacterial levels. C. albicans levels remained the same in monomicrobial and polymicrobial biofilms (Fig. 2B). However, S. aureus levels were significantly increased in the polymicrobial biofilm compared to S. aureus monomicrobial growth in serum (Fig. 2B). In fact, similar levels of S. aureus are observed in a monomicrobial biofilm in BHI and a polymicrobial biofilm with C. albicans in serum (Fig. 2B and 1B, right panel). These data, along with the fluorescence microscopy, indicate that C. albicans and S. aureus form a polymicrobial biofilm in serum, a medium that normally cannot support biofilm formation by S. aureus.
Antimicrobial sensitivity in monomicrobial and polymicrobial biofilms.
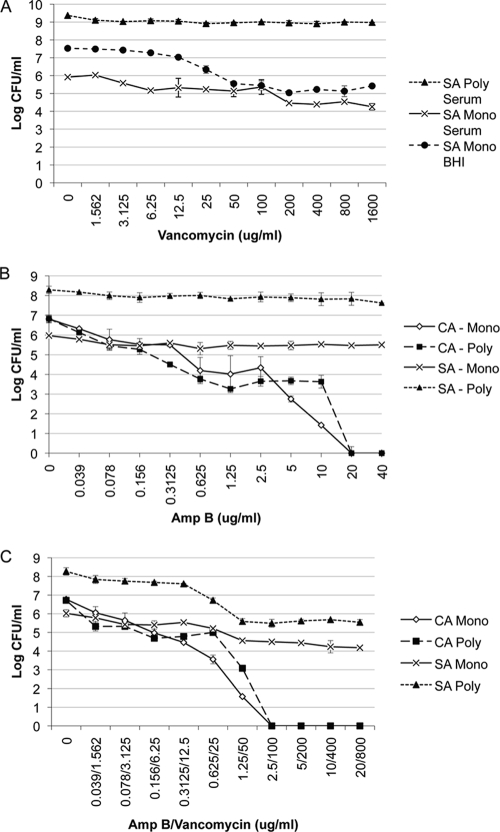
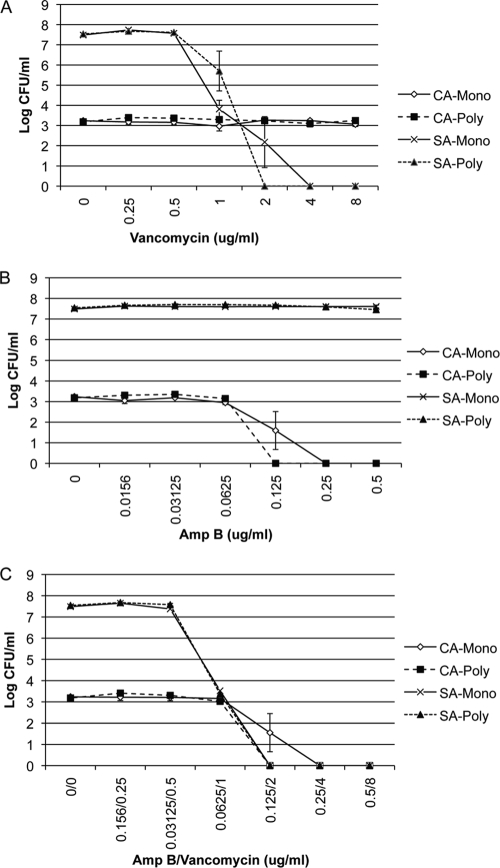
Biofilms are notoriously resistant to antimicrobial drugs compared with planktonic cells. Therefore, we sought to determine whether the sensitivity to antimicrobial drugs of either C. albicans or S. aureus or both C. albicans and S. aureus was influenced within the polymicrobial biofilm compared with a monoculture biofilm. To monitor S. aureus sensitivity to vancomycin, biofilms were formed in 50% BS or BHI, and vancomycin was added at 24 h. After incubation for 24 h in the presence of drug, fungal and bacterial viability was monitored by the CFU assay by selective plating. S. aureus grown in serum responded to vancomycin in a dose-dependent manner (Fig. 3A). S. aureus biofilms formed in BHI were more resistant to killing by vancomycin. However, S. aureus in the polymicrobial biofilm does not respond to vancomycin, even at the highest concentration tested (Fig. 3A), indicating that C. albicans facilitates S. aureus biofilm formation, leading to subsequent increased resistance to antibiotics. C. albicans is not affected by vancomycin, and the number of CFU remained the same in the monomicrobial and polymicrobial biofilms (results not shown). We next measured the effect of S. aureus on the susceptibility of C. albicans to Amp B within the polymicrobial biofilm. Compared with the monomicrobial C. albicans biofilm, the polymicrobial biofilm displays no differences in susceptibility to Amp B (Fig. 3B).
FIG. 3.
Effect of polymicrobial biofilm formation on antimicrobial drug resistance. Polymicrobial biofilms were formed as described in the legend to Fig. 4 using C. albicans SC5314 (106 CFU/ml) and S. aureus ATCC 29523 (107 CFU/ml) added concurrently to 96-well tissue culture-treated plates in 50% BS. Various concentrations (in micrograms per milliliter) of vancomycin alone (A), amphotericin B alone (B), or a combination of both drugs (C) were tested. After incubation for 24 h at 37°C, the number of CFU was determined by plating onto selective media. Experiments were performed in duplicate, and the data represent the averages of two independent experiments. CA, C. albicans; SA, S. aureus; Mono, monomicrobial biofilm; Poly, polymicrobial biofilm.
To determine whether killing of C. albicans in the polymicrobial biofilm allows S. aureus to respond to vancomycin, Amp B and vancomycin were simultaneously added to a 24-h polymicrobial biofilm formed in serum. With increasing concentrations of Amp B, C. albicans viability is significantly reduced. At these same high concentrations of antifungal drug, S. aureus in the polymicrobial biofilm is susceptible to killing by vancomycin to a degree similar to that observed in S. aureus monomicrobial biofilms (Fig. 3C). S. aureus bacteria in both monomicrobial and polymicrobial biofilms treated with Amp B were susceptible to killing with 800 μg/ml vancomycin, resulting in a 2-log-unit reduction in CFU. This result is in stark contrast to the vancomycin resistance observed in viable polymicrobial biofilms at this same concentration of antibiotic (Fig. 3A).
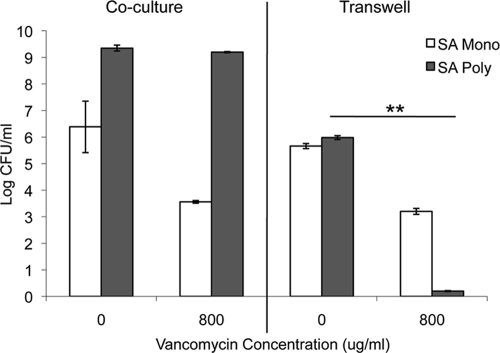
There is the possibility that the effect of C. albicans on S. aureus resistance to vancomycin is due to a C. albicans secreted factor or cross-metabolism within the medium. Therefore, we performed Transwell assays in which S. aureus was separated from C. albicans using a 0.4-μm Transwell filter. S. aureus (107) was added to the bottom of a 24-well tissue culture-treated plate, and the Transwell membrane inserts were placed in the wells. C. albicans (106) was added to the top of the membrane, and the plates were incubated at 37°C. After a 24-h incubation, the plates were washed with 1× PBS (three times), vancomycin was added to the bottom of the wells, and Amp B was added to the top of the membrane. Similar to what is observed for monomicrobial cultures in serum, S. aureus was unable to form a biofilm formation in a Transwell system, with similar growth levels observed in both groups (Fig. 4). In addition, S. aureus did not become coated with matrix, supporting the fact that a biofilm was not formed (data not shown). Moreover, we show that the enhanced resistance to vancomycin is abrogated when S. aureus is physically separated from C. albicans using a Transwell assay. This suggests that the increased resistance is not mediated by factors secreted by C. albicans that diffuse throughout the medium or metabolism of serum components or drug. However, within a polymicrobial biofilm with C. albicans, S. aureus maintained resistance to vancomycin.
FIG. 4.
Effect of separation of S. aureus and C. albicans on biofilm formation and vancomycin resistance using a Transwell system. C. albicans (106 CFU/ml) and S. aureus (107 CFU/ml) were added to either 24-well tissue culture-treated plates or 0.4-μm Transwell plates and incubated for 24 h at 37°C. For Transwell plates, S. aureus was added to the bottom of the well, and C. albicans was added to the top of the membrane to allow passage of secreted factors. Vancomycin (800 μg/ml) was added to the bottom well, and plates were incubated an additional 24 h at 37°C. The plates were washed to remove nonadherent cells, and growth was monitored by the CFU assay. Experiments were performed in duplicate, and the data represent the averages of two independent experiments. Values that were significantly different (P < 0.01) for S. aureus in a polymicrobial biofilm with no drug versus S. aureus in a polymicrobial biofilm with 800 μg/ml vancomycin are indicated by the bar and asterisks. SA, S. aureus; Mono, monomicrobial biofilm; Poly, polymicrobial biofilm.
To determine whether the increased vancomycin resistance of S. aureus observed in polymicrobial biofilms was dependent on biofilm formation, we tested vancomycin resistance in planktonic cultures. Compared with the polymicrobial biofilms, S. aureus in planktonic culture was sensitive to low doses of vancomycin (Fig. 5A). When S. aureus was cocultured with C. albicans during planktonic growth, we observed no differences in sensitivity to vancomycin (Fig. 5A and C). Similarly, no differences were observed in C. albicans sensitivity to Amp B during planktonic growth in the presence and absence of S. aureus (Fig. 5B and C).
FIG. 5.
Effect of planktonic polymicrobial growth on antimicrobial drug resistance. C. albicans SC5314 (103 CFU/ml) alone, S. aureus ATCC 25923 (103 CFU/ml) alone, or C. albicans (103 CFU/ml) and S. aureus (103 CFU/ml) together were added to 96-well, deep-well polypropylene plates in 50% BS. Amp B alone, vancomycin alone, or Amp B and vancomycin combined were added to the wells. The plates were incubated at 35°C in an orbital shaker at 175 rpm for 18 h, and fungal and bacterial viability was monitored by the CFU assay. Experiments were performed in duplicate, and data represent averages of two independent experiments. CA, C. albicans; SA, S. aureus; Mono, monomicrobial biofilm; Poly, polymicrobial biofilm.
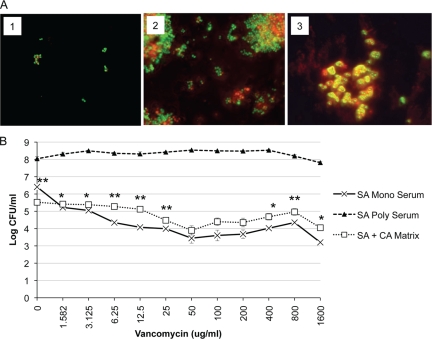
In polymicrobial biofilms, S. aureus is coated with significantly more matrix material than in BHI monoculture S. aureus biofilms, which could be derived from C. albicans (Fig. 1A, panel 3, and Fig. 2A, panel 3). Whether the biofilm was monomicrobial or polymicrobial, the matrix phenotype was distinct and uniform. To determine whether the C. albicans biofilm matrix contributes to vancomycin resistance of S. aureus in a polymicrobial biofilm, C. albicans monoculture matrix was separated from the cellular component of the biofilm and added to S. aureus. We first examined whether C. albicans biofilm matrix was able to coat S. aureus in the absence of viable C. albicans. After incubation for 24 h, we observed matrix-coated S. aureus in serum, similar to what was observed in the polymicrobial biofilms (Fig. 2A, panel 3, and Fig. 6A, panel 3). The Candida matrix coating was also phenotypically different from the S. aureus matrix produced in BHI. Compared with S. aureus biofilm matrix formed in BHI, the Candida matrix coating was thicker and more refractory, producing a halo-like ring surrounding each bacterial cell (Fig. 6A, panels 2 and 3). This unique matrix phenotype matches what is observed with S. aureus in polymicrobial biofilms (Fig. 2A, panel 3), supporting the idea that the matrix coating observed in polymicrobial biofilms could be partially derived from C. albicans. Antimicrobial testing of S. aureus with vancomycin was performed on monoculture S. aureus in C. albicans matrix or in serum alone. A coculture polymicrobial biofilm in serum served as an additional control. Compared with growth in serum alone, S. aureus in serum plus C. albicans matrix was more resistant to vancomycin (Fig. 6B). As seen in previous experiments, S. aureus in the polymicrobial biofilm was highly resistant to vancomycin at all concentrations tested. This suggests that C. albicans extracellular matrix plays a role in the increased resistance of S. aureus in a polymicrobial biofilm but that it is not the only factor involved.
FIG. 6.
Effect of C. albicans biofilm matrix on S. aureus vancomycin resistance. C. albicans biofilm matrix was isolated as described in Materials and Methods. (A) S. aureus (107 CFU/ml) was added to chamber slides in 50% BS (panel 1), BHI (panel 2), or C. albicans biofilm matrix (panel 3). After 24 h, cells were stained with SYTO 9 (viable microbial cells stain green) and ConA-Texas Red (extracellular matrix stain red). (B) S. aureus (107 CFU/ml) was added alone, with C. albicans biofilm matrix, or with C. albicans (106 CFU/ml) to 96-well tissue culture-treated plates in 50% BS. After incubation for 24 h at 37°C, the plates were washed, and vancomycin was added. The plates were incubated for an additional 24 h. The plates were washed to remove nonadherent cells, and growth was monitored by the CFU assay. Experiments were performed in duplicate, and results are representative of two independent experiments. Values that were significantly different (P < 0.01) for S. aureus monomicrobial biofilm in the presence of serum versus S. aureus in the presence of C. albicans matrix are indicated (**). CA, C. albicans; SA, S. aureus; Mono, monomicrobial biofilm; Poly, polymicrobial biofilm.
DISCUSSION
According to the NIH, biofilms are estimated to be responsible for the majority of bacterial and fungal infections in the body (NIH SBIR/STTR Study and Control of Microbial Biofilms program announcement). It is estimated that 27% of nosocomial C. albicans bloodstream infections are polymicrobial, with S. aureus as the third most common organism isolated in conjunction with C. albicans (22). In addition, it has been demonstrated that Candida can form a polymicrobial biofilm with S. epidermidis in vitro, which alters the sensitivity of each species to antimicrobial agents (1). In the present study, we sought to determine whether C. albicans forms polymicrobial biofilms with S. aureus and to begin to characterize these biofilms in terms of structure, conditions affecting biofilm formation, and antimicrobial susceptibility.
We demonstrate that although S. aureus forms poor monoculture biofilms in serum, it forms a substantial polymicrobial biofilm in the presence of C. albicans. Macroscopic examination revealed that while S. aureus can form a visible biofilm when grown in rich media (BHI), serum does not support such growth. This observation was supported by fluorescence microscopy and SEM, which showed sparse growth and lack of extracellular matrix when S. aureus was incubated in serum. In polymicrobial biofilms, C. albicans forms the base of the biofilm with S. aureus adhering preferentially to Candida hyphae within the biofilm. S. aureus also formed microcolonies on the surface of the biofilm. Matrix staining of S. aureus biofilms revealed a different phenotype in polymicrobial versus monomicrobial biofilms. A distinct halo of matrix material was evident surrounding S. aureus within the polymicrobial biofilm, suggesting that S. aureus may become coated in the matrix secreted by C. albicans. Indeed, when S. aureus was incubated in isolated Candida biofilm matrix, the bacteria acquired matrix halos similar to those observed in polymicrobial biofilms. This suggests that S. aureus could become coated with C. albicans biofilm matrix. However, we cannot rule out the possibility that S. aureus may upregulate its own matrix production when grown in coculture. Future studies will address the contribution of fungal versus bacterial matrix in this system.
To rule out potential toxic effects of sera on growth, we monitored planktonic growth of S. aureus in both heat-inactivated and non-heat-inactivated sera. Regardless of the presence or absence of active complement proteins, S. aureus was able to grow in the sera, although not to the same levels observed in BHI. This demonstrates that serum is not toxic but suggests that there is a lack of appropriate nutrients or carbon source in the sera that limits the ability of S. aureus to grow to high levels. Another possibility is that serum interferes with upregulation of genes necessary for biofilm formation. Interestingly, it has been demonstrated that proteolytic by-products of serum albumin produced by C. albicans can be used by S. aureus as a growth medium, but not undigested serum albumin (28). Therefore, C. albicans may be participating in cross-metabolism of serum components, which enhances S. aureus growth and biofilm formation. However, S. aureus cannot form a biofilm in serum in a Transwell system, which would allow passage of cross-metabolized serum components. Therefore, our data suggest that C. albicans metabolism of serum components is not playing a role in S. aureus biofilm formation. An additional explanation is that serum interferes with the attachment of S. aureus to the abiotic plastic surface as has been observed with Pseudomonas aeruginosa (19). However, we examined the initial adhesion period and found that more bacteria remained attached after the bacteria were washed with serum compared with BHI, a medium that promotes biofilm growth in S. aureus. Therefore, this seems an unlikely mechanism to explain the lack of biofilm formation by S. aureus in serum.
We tested whether antimicrobial sensitivity was altered in the polymicrobial biofilms. Although C. albicans displayed no difference in sensitivity to Amp B, S. aureus resistance to vancomycin was greatly enhanced within a polymicrobial biofilm. Moreover, we show that the enhanced resistance to vancomycin is abrogated when S. aureus is physically separated from C. albicans using a Transwell assay. This suggests that the increased resistance is not mediated by C. albicans-secreted factors that diffuse throughout the medium or metabolism of serum components or drug. It should be pointed out that the Transwell membranes permit passage of small diffusible molecules. The intact gel-like C. albicans matrix material is not readily soluble in media and may not pass through the membrane. In addition, the matrix is formed on the top of the biofilm and is separated from the membrane by a dense cellular component. Although individual components of the matrix may pass through the membrane, S. aureus did not become coated with matrix from any source, further supporting the notion that intact matrix did not pass through the membrane. Therefore, we cannot rule out a role for small molecules that become trapped within the matrix. In addition, this suggests that C. albicans facilitation of S. aureus biofilm formation in serum is not a result of cross-metabolism of serum components. However, there is the possibility that C. albicans matrix, which contains glucose, serves as a carbon source for S. aureus, which is not available when separated by the Transwell membrane (2).
As stated above, the results of microscopic analysis suggest that S. aureus becomes coated with Candida matrix within the polymicrobial biofilm. Because it has been suggested that matrix plays a role in drug resistance of C. albicans biofilms, we tested the S. aureus sensitivity to vancomycin in the presence of isolated Candida biofilm matrix. Interestingly, S. aureus resistance to vancomycin was increased after being coated with the isolated Candida matrix. There are several mechanisms whereby Candida matrix could mediate this effect. First, the matrix may limit penetration of the drug, which has been suggested as a mechanism for increased antifungal resistance in C. albicans biofilms (3, 4). Matrix also has been reported to slow the diffusion of drugs in Candida-S. epidermidis polymicrobial biofilms (1). Therefore, one possibility is that the delayed drug exposure may permit enough time for S. aureus to upregulate drug resistance genes. Second, the matrix material itself may alter the growth and gene expression of S. aureus, resulting in upregulation of antimicrobial resistance genes. Future experiments will address whether matrix enhances drug resistance genes in S. aureus.
Acknowledgments
This work was supported by Wayne State University start-up funds, a Francis Foundation grant (M.C.N.), and NIAID (R01 AI72406-01A1).
Footnotes
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Adam, B., G. S. Baillie, and L. J. Douglas. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344-349. [DOI] [PubMed] [Google Scholar]
- 2.Al-Fattani, M. A., and L. J. Douglas. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999-1008. [DOI] [PubMed] [Google Scholar]
- 3.Al-Fattani, M. A., and L. J. Douglas. 2004. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 48:3291-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. [DOI] [PubMed] [Google Scholar]
- 5.Carlson, E. 1983. Effect of strain of Staphylococcus aureus on synergism with Candida albicans resulting in mouse mortality and morbidity. Infect. Immun. 42:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson, E. 1983. Enhancement by Candida albicans of Staphylococcus aureus, Serratia marcescens, and Streptococcus faecalis in the establishment of infection in mice. Infect. Immun. 39:193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson, E. 1982. Synergistic effect of Candida albicans and Staphylococcus aureus on mouse mortality. Infect. Immun. 38:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson, E., and G. Johnson. 1985. Protection by Candida albicans of Staphylococcus aureus in the establishment of dual infection in mice. Infect. Immun. 50:655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson, E. C. 1988. Synergism of Candida albicans and delta toxin producing Staphylococcus aureus on mouse mortality and morbidity: protection by indomethacin. Zentralbl. Bakteriol. Mikrobiol. Hyg. Abt. 1 Orig. A 269:377-386. [DOI] [PubMed] [Google Scholar]
- 10.Cassat, J. E., C. Y. Lee, and M. S. Smeltzer. 2007. Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods Mol. Biol. 391:127-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 13.Crump, J. A., and P. J. Collignon. 2000. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Dominic, R. M., S. Shenoy, and S. Baliga. 2007. Candida biofilms in medical devices: evolving trends. Kathmandu Univ. Med. J. 5:431-436. [PubMed] [Google Scholar]
- 15.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 16.El-Azizi, M. A., S. E. Starks, and N. Khardori. 2004. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J. Appl. Microbiol. 96:1067-1073. [DOI] [PubMed] [Google Scholar]
- 17.Fratesi, S. E., F. L. Lynch, B. L. Kirkland, and L. R. Brown. 2004. Effects of SEM preparation techniques on the appearance of bacteria and biofilms in the Carter Sandstone. J. Sediment. Res. 74:858-867. [Google Scholar]
- 18.García-Sánchez, S., S. Aubert, I. Iraqui, G. Janbon, J. M. Ghigo, and C. d'Enfert. 2004. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot. Cell 3:536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond, A., J. Dertien, J. A. Comer-Hamood, J. A. Griswold, and A. N. Hamood. 7 October 2008. Serum inhibits P. aeruginosa biofilm formation on plastic surfaces and intravenous catheters. J. Surg. Res. doi: 10.1016/j.jss.2008.09.003. [DOI] [PubMed]
- 20.Katneni, R., and S. S. Hedayati. 2007. Central venous catheter-related bacteremia in chronic hemodialysis patients: epidemiology and evidence-based management. Nat. Clin. Pract. Nephrol. 3:256-266. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy, C. A., and J. P. O'Gara. 2004. Contribution of culture media and chemical properties of polystyrene tissue culture plates to biofilm development by Staphylococcus aureus. J. Med. Microbiol. 53:1171-1173. [DOI] [PubMed] [Google Scholar]
- 22.Klotz, S. A., B. S. Chasin, B. Powell, N. K. Gaur, and P. N. Lipke. 2007. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 59:401-406. [DOI] [PubMed] [Google Scholar]
- 23.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murillo, L. A., G. Newport, C. Y. Lan, S. Habelitz, J. Dungan, and N. M. Agabian. 2005. Genome-wide transcription profiling of the early phase of biofilm formation by Candida albicans. Eukaryot. Cell 4:1562-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinilla, J. C., D. F. Ross, T. Martin, and H. Crump. 1983. Study of the incidence of intravascular catheter infection and associated septicemia in critically ill patients. Crit. Care Med. 11:21-25. [DOI] [PubMed] [Google Scholar]
- 26.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 27.Schooling, S. R., and T. J. Beveridge. 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188:5945-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staib, F., and R. Geier. 1971. Proteolysis products of Candida albicans as a substratum for growth of Staphylococcus aureus—a preliminary report. Zentralbl. Bakteriol. Abt. 1 Orig. A 218:374-375. [PubMed] [Google Scholar]
- 29.Stepanovic, S., D. Vukovic, I. Dakic, B. Savic, and M. Svabic-Vlahovic. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175-179. [DOI] [PubMed] [Google Scholar]
- 30.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
- 31.Yeater, K. M., J. Chandra, G. Cheng, P. K. Mukherjee, X. Zhao, S. L. Rodriguez-Zas, K. E. Kwast, M. A. Ghannoum, and L. L. Hoyer. 2007. Temporal analysis of Candida albicans gene expression during biofilm development. Microbiology 153:2373-2385. [DOI] [PubMed] [Google Scholar]