Abstract
MIV-210 is a prodrug of 3′-fluoro-2′,3′-dideoxyguanosine with high oral bioavailability in humans and potent activity against hepatitis B virus (HBV). Woodchucks infected with woodchuck hepatitis virus (WHV) represent an accurate model of HBV infection that is utilized for evaluation of the efficacy and safety of novel anti-HBV agents. Oral administration of MIV-210 at 20 or 60 mg/kg of body weight/day induced a rapid virological response in chronically infected woodchucks, reducing serum WHV DNA levels by 4.75 log10 and 5.72 log10, respectively, in 2 weeks. A progressive decline in WHV viremia occurred throughout the 10-week therapy, giving final reductions of 7.23 log10 and 7.68 log10 in the 20- and 60-mg/kg/day groups, respectively. Further, a daily dose of 10 mg/kg decreased the serum WHV load 400-fold after 4 weeks of treatment, and a dose of 5 mg/kg/day was sufficient to maintain this antiviral effect during the following 6-week period. MIV-210 at 20 or 60 mg/kg/day reduced the liver WHV DNA load 200- to 2,500-fold from pretreatment levels and, importantly, led to a 2.0 log10 drop in the hepatic content of WHV covalently closed circular DNA. The treatment with 60 mg/kg/day was well tolerated. Liver biopsy specimens obtained after the 10-week treatment with 20 or 60 mg/kg/day and after the 10-week follow-up showed hepatocyte and mitochondrial ultrastructures comparable to those in the placebo-treated group. It was concluded that MIV-210 is highly effective against chronic WHV infection. These findings, together with the previously demonstrated inhibitory activity of MIV-210 against lamivudine-, adefovir-, and entecavir-resistant HBV variants, make MIV-210 a highly valuable candidate for further testing as an agent against chronic hepatitis B.
Infection with hepatitis B virus (HBV) is a major public health problem, responsible for an estimated 1 million deaths per year worldwide. Approximately 2 billion people have been or currently are infected with the virus. Among them, about 370 million have serum HBV surface antigen (HBsAg)-positive chronic hepatitis B (CHB), which progresses to cirrhosis or hepatocellular carcinoma (HCC) in 15 to 40% of the cases (23). In certain areas of the world, such as the Asia-Pacific rim, where vertical transmission is the dominant route of infection, chronic HBV infection is highly prevalent (>10%) (16).
Although the HBV vaccine is effective at reducing the number of newly infected people, those already infected are in great need of effective antiviral therapy. The nucleoside and nucleotide analogues, such as lamivudine, adefovir dipivoxil, entecavir, telbivudine, and tenofovir disoproxil fumarate (TDF), have improved the management of chronic HBV infection. However, not all patients with CHB respond well, and the continuous expansion of HBV quasispecies leads to the emergence of drug-resistant variants that are refractory to current therapies. These variants are selected by the action of specific HBV inhibitors during prolonged clinical administration. For instance, the M204V/I mutation is often associated with a compensatory mutation at L180M and with lamivudine breakthrough in as many as 66% of patients after 4 years of lamivudine therapy (15). This mutation also confers cross-drug resistance in various degrees to the entire class of pyrimidine analogues, including emtricitabine and telbivudine (45). Furthermore, prolonged entecavir exposure can lead to the selection of drug-resistant mutants in patients who already harbor lamivudine-resistant HBV (41), resulting in dual resistance. The nucleotide analogues, such as adefovir, select the N236T mutation located in the D domain of the HBV polymerase, although at a rate lower than that of the emergence of lamivudine-resistant mutants (43). However, the risk for the development of resistance can increase dramatically in patients who already have a lamivudine-resistant HBV variant (17). Therefore, there is a need for new antiviral drugs with a high barrier to suppress the development of viral resistance and with activity against the mutants resistant to present therapies.
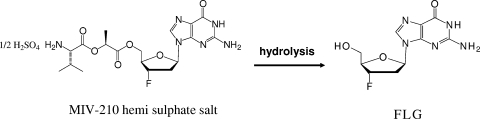
MIV-210 [(C18H25FN6O6)2 · H2SO4] (Fig. 1) is a prodrug of the nucleoside analogue 2′,3′-dideoxy-3′-fluoroguanosine (FLG) that displays high oral bioavailability in humans (8). FLG is a potent inhibitor both of human immunodeficiency virus and of the hepadnaviruses, to which HBV belongs (7, 22, 36, 44). FLG exhibits a broad spectrum of activity not only against resistant HBV variants selected by lamivudine and adefovir but also against more-complex drug resistance patterns potentially selected by the sequential or combination use of adefovir and lamivudine (10, 20) or by that of lamivudine and entecavir (S. A. Locarnini, personal communication).
FIG. 1.
Chemical structures of MIV-210 and FLG.
Woodchuck hepatitis virus (WHV) is more closely related to HBV molecularly and pathogenically than is duck hepatitis B virus. Woodchucks infected with WHV develop acute hepatitis that may progress to chronic hepatitis and HCC, which are overall remarkably similar to the conditions induced by HBV infection in humans (28, 35). WHV and its natural host, the eastern North American woodchuck (Marmota monax), have become an important experimental system providing highly predictive information not only on the efficacy of novel anti-HBV agents (1, 4, 6, 11-14, 25, 32) but also for preclinical drug safety assessment, including the evaluation of potential organ injury and hepatic toxicity (5, 40). Information on antiviral effect and safety obtained from the woodchuck model has predicted the outcomes of CHB treatment well (13).
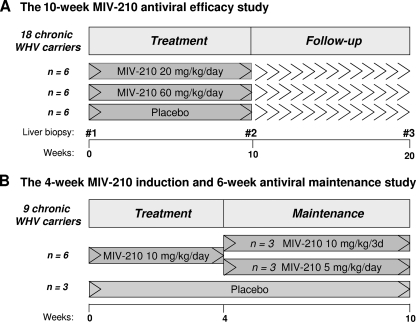
The objectives of the current study were to determine the dose-related antiviral efficacy and safety of MIV-210 in woodchucks that were chronically infected with WHV and had progressing chronic hepatitis. Two placebo-controlled antiviral efficacy studies were arranged. In the first study, daily oral administration of 20 or 60 mg of MIV-210 per kg of body weight for 10 weeks was followed by a 10-week observation period. In the second 10-week study, a 4-week induction treatment with a daily dose of 10 mg/kg was followed by administration of 10 mg/kg of MIV-210 every third day or 5 mg/kg daily for 6 weeks in order to maintain and/or advance the antiviral effect induced in the initial phase of the treatment.
MATERIALS AND METHODS
Woodchucks.
All woodchucks investigated in this study were housed in the Woodchuck Viral Hepatitis Research Facility at Memorial University, St. John's, Newfoundland, Canada, under environmental and biosafety conditions specifically established for this species. Experimental procedures and protocols were approved by the institutional President's Committee on Animals Bioethics and Care.
Woodchucks with serum WHV surface antigen (WHsAg)-positive chronic WHV infection were quarantined for 8 weeks prior to the experiments. During this time, the animals underwent extensive physical, biochemical, and serological evaluations, including quantification of serum and hepatic WHV DNA loads (see below). They were weighed weekly during quarantine and throughout the treatment and follow-up periods. In addition, for the assessment of MIV-210 pharmacokinetics, all woodchucks with chronic WHV infection, which were subsequently included in the MIV-210 antiviral efficacy study, and four healthy, WHV-naïve woodchucks were examined. The absence of WHV in the healthy animals was confirmed by the negativity of serological markers of WHV infection (WHsAg and antibodies to WHV core antigen) and the absence of WHV DNA in randomly selected serum and liver biopsy samples tested by a highly sensitive PCR-nucleic acid hybridization (PCR-NAH) assay (sensitivity, <10 virus genome equivalents [vge]/ml) as described elsewhere (3, 30) and briefly outlined below.
One woodchuck (WM 3231) with chronic WHV infection showed spontaneous clearance of serum WHsAg and a transient decrease in the serum WHV DNA level to 3.58 log10 vge/ml during the MIV-210 oral uptake experiment. However, serum WHV DNA rebounded to a high level (8.79 log10 vge/ml) in this animal at the time of acquisition of the first liver biopsy specimen, i.e., prior to the initiation of the 10-week antiviral efficacy study (see Fig. 2A). Consequently, the animal was enrolled in the 20-mg/kg MIV-210 study group. Of note, woodchucks with progressively increasing levels of gamma glutamyltransferase (GGT) in serum, a biochemical indication of HCC development (28), were excluded from the study, except for WM 3176, in which elevated GGT levels were found after the initiation of the experiment and which was included in the placebo-treated group. All woodchucks had serum WHV DNA loads in the range between 8.79 log10 and 11.45 log10 vge/ml before the initiation of MIV-210 treatment. It should be noted that serum WHV DNA loads very closely reflect the levels of WHV viremia, as was established in a series of previous experiments in which WHV DNA was quantified in parallel in both untreated and DNase-treated (30) serum samples from several woodchucks chronically infected with WHV (data not shown).
FIG. 2.
(A) Scheme for the 10-week antiviral efficacy study with daily p.o. MIV-210 doses of 20 mg/kg and 60 mg/kg. (B) Scheme for the 4-week antiviral induction and 6-week maintenance study. MIV-210 was given at 10 mg/kg/day p.o. for the first 4 weeks and then for 6 weeks either at 10 mg/kg p.o. every third day (10 mg/kg/3d) or at 5.0 mg/kg daily.
Compound.
MIV-210 (lagociclovir valactate) is Medivir's proprietary candidate drug. The substance was produced by DuPont Chemoswed (Malmö, Sweden). MIV-210 was dissolved at the desired concentration (based on individual animal weight) in cherry-flavored water and was administered in a 2-ml volume. For this purpose, the dissolved drug was taken up in a disposable syringe and given orally. Woodchucks are fond of a cherry-like taste and would take the whole volume without spilling. Placebo-treated animals received cherry-flavored water alone.
MIV-210 pharmacokinetic study.
MIV-210 was given orally to four healthy, WHV-naïve woodchucks first at 10 mg/kg and then, after a 14-day washout period, at 40 mg/kg. In addition, all chronic carriers of WHV enrolled in this study received a single oral dose of 60 mg/kg. Blood samples were collected at time zero, 30 min, 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, and 12 h. During the first 6 h of blood sampling, each woodchuck was kept in a restraining box without access to food or water. During the break between the 6- and 12-h collections, the animal was returned to the cage and had unrestricted access to food and water. Serum MIV-210 and FLG contents were quantified in Medivir's laboratories.
MIV-210 antiviral efficacy studies.
In the first study, designated “the 10-week antiviral efficacy study,” 18 chronic carriers of WHV were randomized into three study groups of six animals each: (i) placebo-treated animals, (ii) animals treated with MIV-210 at 20 mg/kg/day, and (iii) animals treated with MIV-210 at 60 mg/kg/day. MIV-210 was dosed for 10 weeks, followed by a 10-week observation period after the cessation of the drug or placebo treatment, as outlined in Fig. 2A.
In the second study, termed “the 4-week antiviral induction study,” nine chronic carriers of WHV were randomized into three groups. Three animals were treated with placebo, while the others received MIV-210 daily at 10 mg/kg for 4 weeks. Subsequently, the MIV-210-treated group was randomly divided into two subgroups of three animals each; one subgroup received 10 mg/kg of MIV-210 every third day for 6 weeks, and the other received 5 mg/kg of MIV-210 daily for 6 weeks, as shown in Fig. 2B.
Sample collection.
Animals chronically infected with WHV were bled at least on two occasions during the quarantine period and weekly throughout the treatment and follow-up periods. The blood was obtained from the digitalis vein under isoflurane inhalant anesthesia. Samples of fresh blood were used immediately for hematological examinations, while serum samples were used for the determination of WHV DNA and WHsAg contents and for clinical biochemistry evaluations.
Liver biopsy specimens were obtained by surgical laparotomy under general inhalant anesthesia from all woodchucks included in the 10-week antiviral efficacy study. The first liver sample was obtained prior to the initiation of therapy, the second at the end of the 10-week treatment period, and the third at the end of the 10-week follow-up period (Fig. 2A). Liver tissue fragments were also cryopreserved for WHV nucleic acid analyses, and others were fixed in formalin for histological examination or in Karnovsky's buffer for ultrastructural examination.
Determination of serum WHsAg and WHV DNA reactivities and of intrahepatic WHV DNA and covalently closed circular DNA (cccDNA) loads.
Serum was tested for the presence of WHsAg using an in-house specific enzyme-linked immunoassay, as reported previously (30). For WHV DNA identification, total DNA was extracted from a 100-μl serum sample or from approximately 50 mg of liver tissue by using proteinase K and the phenol-chloroform-isoamyl alcohol method (3, 30). In the first instance, WHV DNA in the serum or liver was quantified by a dot blot hybridization assay (sensitivity, ∼106 WHV vge/ml) using 10-fold serial dilutions of the samples tested and radiolabeled recombinant complete WHV DNA as a probe (30). As quantification standards, 10-fold serial dilutions of radiolabeled recombinant WHV DNA were applied (30). The WHV DNA load was estimated by performing densitometric analyses of phosphorimage signals and comparing their intensities to those of the quantification standards run with each assay. When WHV DNA loads were below the detection limit of the dot blot hybridization assay, the virus DNA levels were determined by direct and, if required, nested PCR-NAH, as reported previously (3, 30). The sensitivity of the direct PCR-NAH assay was ∼103 vge/ml, and that of nested PCR-NAH was <10 vge/ml (30).
WHV cccDNA, representing a virus genome replicative intermediate, was detected in liver tissue samples by the specific PCR amplification assay (sensitivity, ∼102 vge/μg) as described previously (3, 18). Briefly, DNA was digested with mung bean nuclease prior to amplification with oligonucleotide primers spanning the nicked region of the WHV genome. The specificity of the PCR amplicons was routinely confirmed by NAH and semiquantified by densitometric analysis. The levels of WHV cccDNA detected in liver biopsy specimens obtained after 10 weeks of treatment and after the 10-week follow-up were compared to those found in the liver sample collected from the same animal prior to treatment.
Hematological and clinical biochemistry evaluations.
Samples of fresh blood were analyzed for hematology parameters, such as hematocrit (HCT), hemoglobulin (HGB), white blood cell count (WBC), granulocyte count (Grans), lymphocyte and monocyte counts (L/M), and platelet count (PLT), using the Idexx QBC VetAutoread hematology system (Vettest S.A., Neuchâtel, Switzerland). The values obtained for the hematological parameters were compared to those determined for the same animal before MIV-210 treatment and to those from a large cohort (n = 36) of healthy woodchucks examined prior to this study. Clinical biochemistry parameters, including levels of alanine aminotransferase, GGT, alkaline phosphatase, amylase, lipase, and lactate in serum, were determined by the Vettest assay system (Vettest S.A.). The results were compared to the range of normal values from healthy woodchucks (28).
Histological and ultrastructural examinations.
For histology, a fragment of each liver biopsy specimen was processed with paraffin. Paraffin sections were stained with hematoxylin-eosin, Masson's trichrome stain, and periodic acid-Schiff stain and were impregnated with silver. Morphological alterations were determined according to criteria described previously (29, 30). The histological degree of hepatitis, reflecting the severity of inflammatory liver injury, was enumerated on the basis of grades assigned separately for hepatocellular, extrahepatocellular, intralobular, and portal alterations, taking into consideration an impression of the global picture of liver pathology.
For ultrastructural examination, small tissue fragments of all three liver biopsy specimens obtained from each of four randomly selected chronic WHV carriers from three groups investigated in the 10-week antiviral efficacy study were processed with Epon. Ultrathin sections were examined for ultrastructural alterations, taking into consideration the findings for the placebo-treated animals.
Statistical analysis.
A two-tailed, unpaired Student t test with 95% confidence intervals was used to compare the means of the results for the sample groups investigated. P values of ≤0.05 were considered statistically significant.
RESULTS
Pharmacokinetics of orally administered MIV-210.
Four healthy woodchucks received, per os (p.o.), first 10 mg/kg of MIV-210 and then, after a 2-week washout period, 40 mg/kg of MIV-210. The maximum concentrations of the resulting FLG in serum (Cmax) were 0.54 ± 0.14 μM and 2.10 ± 1.50 μM, and the times to Cmax (Tmax) were 4.10 ± 1.0 h and 3.3 ± 1.5 h, while the half-lives (t1/2) were 5.44 ± 1.2 h and 4.2 ± 1.6 h for animals receiving 10 mg/kg and 40 mg/kg, respectively. The corresponding mean areas under the concentration-time curve from 0 h to infinity (AUC0-∞) for FLG were 5.15 ± 1.0 μM · h/liter for the 10-mg/kg dose and 10.5 ± 4.2 μM · h/liter for the 40-mg/kg dose (Table 1). In addition, 14 chronic carriers of WHV with histologically confirmed chronic hepatitis received 60 mg/kg of MIV-210 once p.o. The Cmax of FLG was 3.50 ± 1.9 μM, and the Tmax was 2.90 ± 1.1 h. The corresponding mean AUC0-∞ was 20.1 ± 11.3 μM · h/liter. On the basis of these results, exposure, as determined by the maximum concentration in plasma, increased from 1.0 μM to 3.50 μM at the orally given single MIV-210 dose of 20 mg/kg and 60 mg/kg.
TABLE 1.
Average pharmacokinetic parameters for FLG after oral administration of MIV-210 to healthy and chronically WHV infected woodchucks
| Pharmacokinetic parameter for FLG | Value (mean ± SD) for:
|
||
|---|---|---|---|
| Healthy woodchucks (n = 4) receiving MIV-210 at:
|
WHV-infected woodchucks (n = 14) receiving MIV-210 at 60 mg/kg | ||
| 10 mg/kg | 40 mg/kg | ||
| Cmax (μM) | 0.54 ± 0.14 | 2.10 ± 1.5 | 3.50 ± 1.9 |
| Tmax (h) | 4.10 ± 1.0 | 3.30 ± 1.5 | 2.90 ± 1.1 |
| AUC0-∞ (μM · h/liter) | 5.15 ± 1.0 | 10.50 ± 4.2 | 20.1 ± 11.3 |
| t1/2 (h) | 5.44 ± 1.2 | 4.20 ± 1.6 | 4.7 ± 0.8 |
The 10-week antiviral efficacy study.
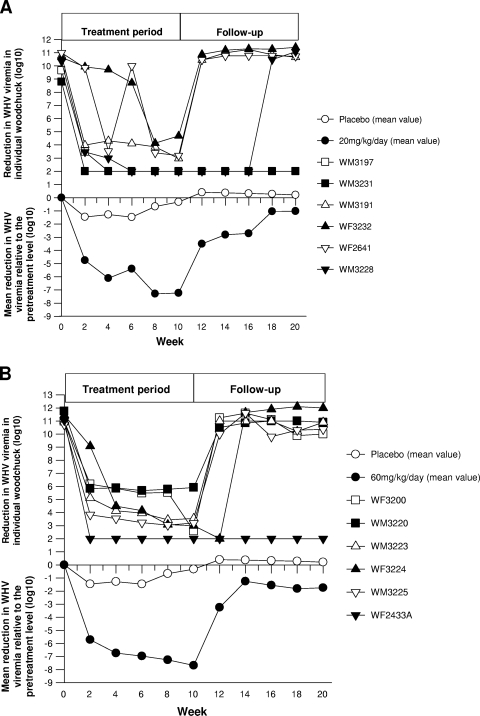
During the 10-week treatment period, two groups of woodchucks with chronic WHV infection received MIV-210 at a daily dose of 20 mg/kg (n = 6) or 60 mg/kg (n = 6), while a control group (n = 6) was treated with the placebo (Fig. 2A). A profound virological response was evident during the first 2 weeks and noticeably increased further until 4 weeks of treatment with both MIV-210 doses. During this time, serum viremia, as measured by the serum WHV DNA load, declined rapidly in 8 out of 12 animals from levels ranging from 8.79 log10 to 11.45 log10 vge/ml before treatment to below the detection limit of the dot blot hybridization assay (<106 vge/ml). Taking into account all six animals in each treatment group, the average reductions in WHV viremia were 6.11 log10 and 6.74 log10 (i.e., more than 1 million-fold) after 4 weeks of treatment with 20 mg/kg/day and 60 mg/kg/day, respectively (Table 2). This rapid antiviral effect was followed by a progressive decline in the WHV DNA level throughout the remaining 10-week treatment period, resulting in total mean reductions of 7.23 log10 and 7.68 log10 at the end of the 10-week treatment in the 20-mg/kg/day and 60-mg/kg/day groups, respectively (Table 2; Fig. 3A and B). The placebo-treated animals showed no significant changes in their WHV viremia levels (Fig. 3A and B).
TABLE 2.
Mean serum WHV DNA levels prior to, during, and following the 10-week MIV-210 antiviral efficacy study
| Time | Placebo
|
MIV-210 treatment at:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 mg/kg/day
|
60 mg/kg/day
|
||||||||
| No. of samples | Mean log10 WHV DNA load (vge/ml) | Range of WHV DNA load (vge/ml) | No. of samples | Mean log10 WHV DNA load (vge/ml) | Range of WHV DNA load (vge/ml) | No. of samples | Mean log10 WHV DNA load (vge/ml) | Range of WHV DNA load (vge/ml) | |
| Pretreatment | 6 | 10.61 | 1.6 × 1010-6.2 × 1010 | 6 | 10.20 | 6.2 × 108b-9.9 × 1010 | 6 | 11.06 | 3.0 × 1010-2.8 × 1011 |
| During treatment | |||||||||
| Wk 2 | 6 | 9.15 | 5.5 × 103a-4.5 × 1010 | 6 | 5.45 | <102-8.5 × 109 | 6 | 5.34 | <102-1.2 × 109 |
| Wk 4 | 6 | 9.33 | 7.6 × 103a-12.0 × 1010 | 6 | 4.09 | <102-5.2 × 109 | 6 | 4.32 | <102-7.6 × 105 |
| Wk 6 | 6 | 9.14 | 5.6 × 103a-9.1 × 1010 | 6 | 4.80 | <102-1.1 × 1010 | 6 | 4.09 | <102-4.8 × 105 |
| Wk 8 | 5 | 9.94 | 1.0 × 108-5.6 × 1010 | 6 | 2.91 | <102-1.4 × 104 | 6 | 3.80 | <102-6.1 × 105 |
| Wk 10 | 5 | 10.29 | 8.0 × 109-4.7 × 1010 | 5 | 2.97 | <102-5.0 × 104 | 6 | 3.38 | <102-6.2 × 105 |
| After cessation of treatment | |||||||||
| Wk 2 | 4 | 11.0 | 5.0 × 1010-2.3 × 1011 | 5 | 7.15 | <102-7.6 × 1010 | 6 | 7.81 | <102-2.8 × 1011 |
| Wk 4 | 4 | 10.98 | 5.0 × 1010-2.3 × 1011 | 5 | 7.39 | <102-1.6 × 1011 | 6 | 9.81 | <102-4.5 × 1011 |
| Wk 6 | 4 | 10.93 | 3.5 × 1010-1.2 × 1011 | 5 | 7.39 | <102-1.6 × 1011 | 6 | 9.50 | <102-9.5 × 1011 |
| Wk 8 | 4 | 10.89 | 4.4 × 1010-1.7 × 1011 | 5 | 9.16 | <102-2.0 × 1011 | 6 | 9.25 | <102-1.3 × 1012 |
| Wk 10 | 4 | 10.82 | 3.5 × 1010-1.2 × 1011 | 5 | 9.18 | <102-2.8 × 1011 | 6 | 9.31 | <102-1.1 × 1012 |
Value from WM 2637, in which the serum WHV DNA level declined between weeks 2 and 6 and rebounded to a high level at week 8.
Value indicates the level of WHV viremia at the time of acquisition of the first liver biopsy specimen for WM 3231, which cleared WHs antigenemia.
FIG. 3.
Effects of MIV-210 on the serum WHV loads of woodchucks with chronic WHV hepatitis treated with an oral dose of 20 mg/kg/day (A) or 60 mg/kg/day (B) or with placebo during the 10-week treatment period and the 10-week follow-up period. Changes in WHV DNA loads in individual animals (upper panels) and mean changes in WHV viremia (lower panels) in sequential serum samples obtained from each of the three study groups were determined by quantifying WHV DNA using a dot blot hybridization test and, when that test was nonreactive, a PCR-NAH assay. The results are expressed in log10 virus genome equivalents per milliliter.
Analysis of the 20-mg/kg/day MIV-210 treatment group showed that one WHV carrier (WM 3231), which cleared serum WHsAg but carried WHV DNA in serum at a high level (i.e., 8.79 log10 vge/ml) before the initiation of treatment (Fig. 3A), became faintly WHV DNA reactive (<10 vge/ml) after 2 weeks of treatment. At the end of the second week of treatment, four out of six animals exhibited a >6.0 log10 reduction in WHV viremia, while 0.71 log10 to 1.15 log10 reductions were seen for two animals. However, during the subsequent 8-week treatment period, significant improvement was observed for those two woodchucks, and 5.95 log10 to 7.80 log10 declines in WHV DNA loads occurred at the end of the 10-week treatment. Within 2 weeks after the cessation of 20-mg/kg/day p.o. administration, WHV viremia had bounced back to pretreatment levels in four out of five animals and remained at this level throughout the 10-week follow-up. However, in one animal (WM 3228), the rebound of WHV viremia was delayed for an additional 6 weeks, during which only trace amounts of WHV DNA (<10 vge/ml) were detectable in serum (Fig. 3A). The >8.0 log10 suppression of WHV DNA in serum in the absence of drug pressure for an additional 6 weeks after the completion of the treatment suggested that MIV-210 therapy could exert a prolonged antiviral effect in some situations. Thus, treatment with MIV-210 appeared not only to reduce WHV viremia effectively in one chronically infected woodchuck (WM 3228) but also to maintain WHV DNA at a very low level (between <10 and 230 vge/ml) throughout the 10-week treatment and follow-up period in another animal (WM 3231), which cleared WHs antigenemia.
In the group of animals receiving MIV-210 at 60 mg/kg/day, a rapid and profound virological suppression, similar to that seen in the 20-mg/kg/day treatment group, was observed. Five out of six animals in the 60-mg/kg/day group showed a 4.3 to 9.0 log10 decline in WHV viremia after 2 weeks of therapy from the pretreatment levels of 10.48 to 11.45 log10 vge/ml (Table 2; Fig. 3B). Animal WF 3224 showed a relatively weak initial response, achieving a 2.37 log10 reduction at week 2 of treatment. However, serum WHV DNA levels in this animal declined progressively, with an overall reduction of 8.45 log10 at the end of the 10-week treatment period. In one animal (WF 2433A) with a high level of WHV viremia (11.04 log10 vge/ml) and a high intrahepatic WHV DNA load (7.43 log10 vge/μg of total DNA) before treatment, a sharp drop of more than 9.0 log10 in the serum WHV DNA level was achieved in just 2 weeks of treatment. This suppression was maintained throughout the 10-week treatment period and the subsequent 10-week follow-up (Fig. 3B). In fact, the serum WHV DNA level remained undetectable in this animal even during an extended 16-week observation period when measured by a very sensitive nested PCR-NAH assay, suggesting a potentially complete suppression of chronic WHV infection. However, liver biopsy samples collected at the ends of the 10-week treatment period and the 10-week follow-up period remained WHV DNA reactive at a low level of 3.41 to 3.45 log10 vge/μg of total DNA (Table 3).
TABLE 3.
Mean WHV DNA loads in liver samples collected prior to and after the 10-week treatment with MIV-210 and after the 10-week observation period following the cessation of treatment
| Time of liver biopsy | Placebo
|
Treatment with MIV-210 at:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 mg/kg/day
|
60 mg/kg/day
|
||||||||
| No. of biopsy specimens | Mean log10 WHV DNA load (vge/μg) | Range of WHV DNA load (vge/μg) | No. of biopsy specimens | Mean log10 WHV DNA load (vge/μg) | Range of WHV DNA load (vge/μg) | No. of biopsy specimens | Mean log10 WHV DNA load (vge/μg) | Range of WHV DNA load (vge/μg) | |
| Pretreatment | 6 | 7.67 | 2.9 × 107-7.5 × 107 | 6 | 6.27 | 1.2 × 103a-1.3 × 108 | 6 | 7.69 | 2.6 × 107b-6.9 × 107 |
| After 10-wk treatment | 6 | 7.78 | 3.0 × 107-1.2 × 108 | 6 | 3.89 | 4.6 × 102a-1.8 × 107 | 6 | 4.30 | 2.6 × 103b-1.5 × 107 |
| After 10-wk follow-up | 4 | 7.71 | 3.2 × 107-1.0 × 108 | 5 | 6.66 | 2.3 × 102a-9.6 × 107 | 6 | 7.04 | 2.8 × 103b-1.2 × 108 |
Value from WM 3231, which cleared WHs antigenemia.
Value from WF 2433A, in which WHV viremia diminished rapidly to <102 vge/ml after 2 weeks of treatment with MIV-210 at 60 mg/kg/day p.o. and the suppression was maintained at a comparably low level of viremia beyond the 10-week treatment period.
The 4-week antiviral induction study.
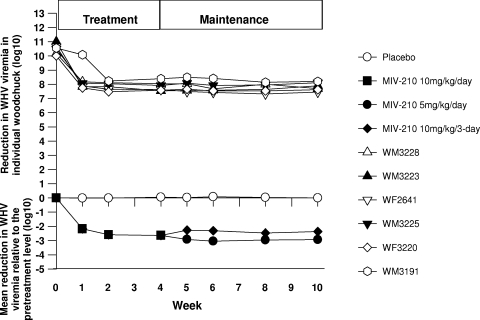
Administration of MIV-210 at doses of 20 mg/kg/day and 60 mg/kg/day showed minimal, if any, dose-related virus response, suggesting that a saturation level of MIV-210 dosing was achieved even at 20 mg/kg/day. This motivated a second study in which nine chronic carriers of WHV were enrolled and divided randomly into two study groups, a placebo-treated group (n = 3) and a group treated with MIV-210 at 10 mg/kg/day (n = 6), for 4 weeks. After this induction period, the group dosed with 10 mg/kg/day was divided into two subgroups (Fig. 2B). Serum samples were tested for WHV DNA by the dot blot hybridization assay. During the 4-week induction period, the dose of 10 mg/kg/day exerted a less pronounced antiviral effect than the previously tested treatment with 20 mg/kg/day. Thus, there were 2.59 log10 and 2.64 log10 reductions in WHV viremia at weeks 2 and 4, respectively, compared to pretreatment serum WHV DNA levels ranging from 10.0 to 11.0 log10 vge/ml (Fig. 4). No change in serum WHV DNA loads was observed in the placebo-treated group. Interestingly, when the treatment was continued with either 10 mg/kg of MIV-210 every third day or 5 mg/kg daily for a further 6 weeks, the suppression of the serum WHV DNA load was maintained, indicating that the therapeutic benefit achieved during the 4-week induction period can be extended by a lower dose of MIV-210 (Fig. 4).
FIG. 4.
Anti-WHV efficacy of MIV-210 given orally to woodchucks with chronic WHV infection at 10 mg/kg/day for 4 weeks and then at doses of 10 mg/kg p.o. every third day (n = 3) or 5.0 mg/kg p.o. per day (n = 3) for a further 6 weeks. Animals treated daily with placebo p.o. (n = 3) for 10 weeks were included as controls. Changes in WHV viremia in individual animals (upper panels) and mean changes in WHV viremia (lower panels) during the 4-week induction period for animals treated with MIV-210 (n = 6) and during the subsequent 6-week antiviral maintenance treatments, and for placebo-treated animals, were determined by dot blot hybridization and, when that test was nonreactive, by PCR-NAH. The results are expressed in log10 virus genome equivalents per milliliter.
At the end of the 10-week treatment, a beneficial antiviral effect was observed in the subgroup dosed daily with 5 mg/kg (n = 3): a mean 2.93 log10 reduction in the WHV DNA load. This finding suggested that MIV-210 at 5 mg/kg/day was also capable of exerting a therapeutic benefit by persistently suppressing serum WHV DNA levels (Fig. 4). The subgroup receiving doses of 10 mg/kg every third day (n = 3) maintained WHV DNA suppression at a mean 2.37 log10 reduction, which was less than the reduction found after daily administration of MIV-210 at the same dose. Nonetheless, this treatment was sufficient to maintain an antiviral response.
Effect of MIV-210 on intrahepatic WHV DNA and WHV cccDNA levels.
In order to assess the effect of MIV-210 on the hepatic WHV content, WHV DNA loads were quantified in liver biopsy specimens obtained before treatment, upon completion of the 10-week treatment, and at the end of the 10-week follow-up period from all woodchucks belonging to the 20-mg/kg/day and 60-mg/kg/day treatment groups and to the placebo-treated group (Fig. 2A). The progressive suppression of WHV viremia observed during the 10-week dosing period was reflected as an appreciable decline in the intrahepatic WHV DNA load. Thus, after 10 weeks of therapy, the hepatic virus genome content decreased, resulting in mean declines of 2.38 log10 (n = 6) and 3.39 log10 (n = 6) from the pretreatment levels in the 20-mg/kg/day and 60-mg/kg/day groups, respectively (Table 3). Thus, MIV-210 not only was highly effective at reducing serum WHV levels but also was able to suppress WHV in hepatocytes, giving a 200- to 2,500-fold reduction in the intrahepatic WHV DNA content. No change in intrahepatic WHV DNA loads was noticed in the placebo-treated group, where mean liver WHV DNA levels were 7.67 log10 vge/μg of total DNA before treatment (n = 6), 7.78 log10 vge/μg of total DNA after 10 weeks of treatment (n = 6), and 7.71 log10 vge/μg of total hepatic DNA at the end of the 10-week follow-up (n = 4) (Table 3). After the cessation of MIV-210 treatment, the liver WHV DNA levels returned gradually to the pretreatment levels in most of the animals. However, two interesting exceptions were found. WM 3231, which cleared WHs antigenemia, and WF 2433A, in which suppression of serum WHV DNA was protracted beyond the 10-week treatment period, displayed low intrahepatic WHV DNA levels, 230 and 2,800 vge/μg of total DNA, respectively, at the end of the 10-week follow-up. Although the number of animals was too small to permit any far-reaching conclusions, it is noteworthy that the progression of chronic infection in the liver was halted in some cases.
Since MIV-210 treatment resulted in a sharp reduction in serum WHV levels and a decline in intrahepatic WHV DNA loads, the level of WHV cccDNA was measured in order to determine the potential effect of MIV-210 treatment on WHV replication. For this purpose, WHV cccDNA was quantified in liver biopsy specimens collected before treatment, after 10 weeks of treatment with 20 mg/kg/day or 60 mg/kg/day, and upon completion of the 10-week follow-up period. A similar evaluation was performed for the animals treated with the placebo (Fig. 2A). In the placebo-treated group, mean hepatic WHV cccDNA levels remained nearly unchanged from the pretreatment levels (5.34 log10 vge/μg of total DNA [n = 6] before treatment, 5.38 log10 vge/μg of total DNA [n = 6] after 10 weeks of treatment, and 5.49 log10 vge/μg of total DNA [n = 4]) after the 10-week follow-up) (Table 4). In contrast, a reduction of approximately 100-fold in the mean hepatic WHV cccDNA content was detected in the MIV-210-treated animals at the end of the 10-week treatment period (Table 4). Notably, WF 2433A, in which suppression of WHV viremia was protracted even in the absence of MIV-210 administration, displayed a >4.0 log10 reduction in the hepatic WHV cccDNA level upon completion of the 10-week treatment, and the level remained >3.0 log10 reduced beyond that period.
TABLE 4.
Mean hepatic WHV cccDNA contents prior to, during, and following the 10-week MIV-210 antiviral efficacy study
| Time of liver biopsy | Placebo
|
MIV-210 treatment at:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 mg/kg/day
|
60 mg/kg/day
|
||||||||
| No. of biopsy specimens | Mean log10 WHV cccDNA load (vge/μg) | Range of WHV cccDNA load (vge/μg) | No. of biopsy specimens | Mean log10 WHV cccDNA load (vge/μg) | Range of WHV cccDNA load (vge/μg) | No. of biopsy specimens | Mean log10 WHV cccDNA load (vge/μg) | Range of WHV cccDNA load (vge/μg) | |
| Pretreatment | 6 | 5.34 | 3.4 × 104-9.1 × 105 | 6 | 4.65 | 3.0 × 102a-5.8 × 105 | 6 | 5.25 | 7.0 × 104-3.1 × 105 |
| After 10-wk treatment | 6 | 5.38 | 8.4 × 104-7.4 × 105 | 6 | 2.82 | 0.6 × 101a-5.9 × 105 | 6 | 3.23 | 0.3 × 102b-7.4 × 104 |
| After 10-wk follow-up | 4 | 5.49 | 1.1 × 105-6.1 × 105 | 5 | 4.89 | <1 × 102a-6.5 × 105 | 6 | 4.74 | 2.4 × 102b-0.2 × 105 |
Value from WM 3231, which cleared WHs antigenemia.
Value from WF 2433A, in which WHV viremia rapidly declined to <102 vge/ml after 2 weeks of treatment with MIV-210 at 60 mg/kg/day p.o. and the suppression was maintained at a comparably low level of viremia beyond the 10-week treatment period.
Liver histology and ultrastructure.
The histological severity of hepatitis was graded in liver biopsy specimens obtained before treatment, after 10 weeks of treatment, and after the 10-week follow-up period. Advanced chronic hepatitis accompanied by hepatocellular injury of variable severity was found in the placebo-treated group and in the majority of chronic WHV carriers treated with MIV-210. This overall lack of improvement in liver morphology could be due to the relatively short course of MIV-210 therapy. However, significant improvements in liver histology were seen for WM 3231 and WF 2433A. As indicated above, these two animals showed either clearance of WHsAg from serum or a prolonged suppression of serum WHV DNA levels, as well as less-severe liver inflammatory injury at the end of the study period.
Ultrastructural examination of liver biopsy specimens was focused on evaluations of a potential induction of mitochondrial alterations in relation to MIV-210 administration. Liver tissue samples collected before treatment, after 10 weeks of treatment, and after 10 weeks of follow-up from four animals from each group treated with placebo or with 20 or 60 mg/kg/day of MIV-210 were subjected to this examination. In the majority of the biopsy materials analyzed, the range of ultrastructural alterations encountered was typical of viral hepatitis. A blind analysis of sequential liver biopsy specimens from individual animals revealed no noticeable difference in the range or severity of ultrastructural changes that could have been connected to MIV-210 treatment. In both MIV-210- and placebo-treated animals, there was evidence of occasional limited mitochondrial changes at all time points of sample collection. This implied that there was no relation between treatment with MIV-210 and the hepatic mitochondrial alterations occasionally seen in woodchucks chronically infected with WHV.
Drug safety.
During 10 weeks of MIV-210 treatment and 10 weeks of follow-up, food and water consumption was comparable to that of the placebo-treated animals. No appreciable changes were seen in the body weights of the MIV-210-treated woodchucks during the treatment and follow-up periods. The average weights before and after the 10-week treatment period were 4,441 and 3,686 g for the placebo group, 4,733 and 3,653 g for the 20-mg/kg/day treatment group, and 4,075 and 3,278 g for the 60-mg/kg/day group. The observed weight loss in both the MIV-210- and placebo-treated groups were due to seasonal physiological fluctuations in woodchuck body weight, since treatments were conducted during the winter and early spring. No significant differences in hematological parameters were found between animals treated with placebo and those treated with 20 or 60 mg/kg of MIV-210 (Table 5). Differences, if any, were found only between mean values obtained for MIV-210-treated animals before treatment and those obtained after the 10-week follow-up; mean values at the end of the treatment were comparable to pretreatment values. Thus, some changes in HCT were observed after the 10-week observation period for the group treated with MIV-210 at 20 mg/kg/day (mean ± standard error of the mean [SEM], 37.3% ± 4.8% [n = 5]) or 60 mg/kg/day (mean ± SEM, 35.3% ± 4.9% [n = 6]) compared to the pretreatment values (mean ± SEM, 44.1% ± 3.0% [n = 6] and 47.1% ± 7.1% [n = 6], respectively) (P, <0.008 and <0.05, respectively). Mean values for WBC, Grans, and L/M after the 10-week follow-up also were lower than pretreatment values (P ≤ 0.02) for woodchucks treated with MIV-210 at 20 mg/kg/day but not for those receiving 60 mg/kg/day (Table 5). It should be emphasized that all these values, either for MIV-210-treated or for placebo-treated animals during treatment or after follow-up, were within the normal ranges for healthy woodchucks (n = 36) examined in our previous studies (data not shown).
TABLE 5.
Mean hematological values before treatment, following the 10-week antiviral efficacy study with MIV-210 given daily at 20 mg/kg or 60 mg/kg, and at the end of the 10-week follow-up period after the cessation of treatment
| Treatment groupa and parameter (unit of measurement) | Value (mean ± SEM)
|
||
|---|---|---|---|
| Wk 0 (before treatment) | Treatment wk 10 | After the 10-wk follow-upb | |
| Placebo | |||
| HCT (%) | 40.0 ± 2.6 | 36.4 ± 4.7 | 36.1 ± 3.1 |
| HGB (g/dl) | 13.6 ± 1.3 | 12.5 ± 3.4 | 12.3 ± 1.1 |
| WBC (109/liter) | 8.7 ± 1.8 | 6.2 ± 1.2 | 5.5 ± 1.2* |
| Grans (109/liter) | 8.8 ± 3.3 | 6.7 ± 2.7 | 6.7 ± 2.0 |
| L/M (109/liter) | 4.7 ± 2.4 | 2.6 ± 0.9 | 2.2 ± 0.4 |
| PLT (109/liter) | 408.8 ± 188 | 431.4 ± 127.6 | 441.7 ± 118.8 |
| MIV-210 at 20 mg/kg/day | |||
| HCT (%) | 44.1 ± 3.0 | 37.3 ± 6.1 | 37.3 ± 4.8*** |
| HGB (g/dl) | 14.5 ± 1.2 | 12.7 ± 5.0 | 12.7 ± 1.7 |
| WBC (109/liter) | 11.8 ± 2.9 | 11.5 ± 5.3 | 7.2 ± 1.6** |
| Grans (109/liter) | 11.6 ± 4.1 | 12.1 ± 6.4 | 7.4 ± 2.2** |
| L/M (109/liter) | 9.9 ± 4.1 | 5.1 ± 3.5 | 4.4 ± 1.9** |
| PLT (109/liter) | 338.8 ± 119.4 | 509 ± 152 | 400 ± 68.7c |
| MIV-210 at 60 mg/kg/day | |||
| HCT (%) | 47.1 ± 7.1 | 36.7 ± 5.2 | 35.3 ± 4.9* |
| HGB (g/dl) | 15.2 ± 1.8 | 12.4 ± 1.6 | 12.2 ± 1.7 |
| WBC (109/liter) | 13.5 ± 5.5 | 6.3 ± 1.7 | 7.0 ± 1.8 |
| Grans (109/liter) | 15.7 ± 8.0 | 6.3 ± 2.7 | 6.9 ± 2.7 |
| L/M (109/liter) | 10.0 ± 7.0 | 3.1 ± 1.3 | 3.5 ± 2.2 |
| PLT (109/liter) | 465.8 ± 102.7 | 393.4 ± 155.6 | 404.3 ± 78.6 |
In all groups, there were six animals before treatment and five animals at treatment week 10. At the end of the 10-week follow-up, there were four animals in the placebo group, five animals in the group receiving MIV-210 at 20 mg/kg/day, and six animals in the group receiving MIV-210 at 60 mg/kg/day.
Asterisks indicate that the differences between mean values calculated for individual hematological parameters after the 10-week follow-up and those obtained prior to treatment (week zero) were significant at P values of ≤0.008 (***), ≤0.02 (**), or ≤0.05 (*). No significant differences were found between the placebo-treated and MIV-210-treated animals at the end of the 10-week treatment period.
For this measurement, n = 4.
Furthermore, no treatment-related differences between the levels of alanine aminotransferase, alkaline phosphatase, amylase, and lipase in the MIV-210-treated groups and the placebo-treated group were seen. The serum GGT level, an indicator of advanced liver disease connected with the development of HCC in woodchucks, was 3.4 IU/liter for the placebo-treated animals compared to a mean 0.9 IU/liter for the woodchucks treated with MIV-210 at 20 mg/kg/day and 60 mg/kg/day (P = 0.067). In serum samples obtained immediately prior to the first liver biopsy (see Fig. 2A), there appeared to be some transient increase in the serum lactate level over the normal range of levels of this enzyme in healthy woodchucks. During 10 weeks of treatment with MIV-210, mean serum lactate levels of 13.5 mg/dl for placebo-treated animals and 11.0 mg/dl for woodchucks treated with 20 mg/kg/day and 60 mg/kg/day were obtained, indicating that no change (P = 0.96) could be correlated with the administration of MIV-210.
In summary, treatment with MIV-210 was well tolerated and caused rapid and pronounced virological responses in woodchucks that were chronic WHV carriers and received a dose of 20 mg/kg/day or 60 mg/kg/day. This antiviral effect was associated with reductions in hepatic WHV DNA loads, as well as with appreciable decreases in hepatic WHV cccDNA levels. Furthermore, a potent antiviral effect was apparent when oral administration of MIV-210 at 5 mg/kg/day was sufficient to maintain suppression of WHV viremia. Significant reductions in WHV loads, lasting for 10 weeks beyond the cessation of therapy, were observed for two woodchucks. These reductions correlated with improvements in liver histology, implying an inhibition of WHV-induced progressive inflammation.
DISCUSSION
Chronic HBV infection remains a major health problem and the most common cause of chronic liver disease globally, responsible for as many as 50% of all cases of cirrhosis and 70 to 90% of HCC cases in China and Southeast Asia. The ultimate goal of antiviral therapy for CHB is to achieve sustained suppression of HBV replication over a long period. The main site of HBV replication is liver parenchyma, although the virus also propagates in the immune system (31). Nonetheless, hepatic tissue and plasma are two compartments of the most abundant virus occurrence in CHB. In the current study, MIV-210 was found to be highly effective at reducing WHV loads in these two compartments, as evidenced by the decreases in WHV DNA loads in serum and WHV DNA and cccDNA contents in liver tissue.
The triphosphate of FLG is a potent inhibitor of hepadnavirus polymerase activity (7, 36). In vitro hydrolysis of MIV-210 leading to the formation of FLG appears to be extremely rapid (t1/2, <5 min) in the blood or plasma of dogs, humans, and rats. The in vivo rate of hydrolysis at physiological temperatures is likely even higher. Therefore, measurement of MIV-210 in the circulation would not be feasible. Oral administration of MIV-210 to both healthy woodchucks and chronic WHV carriers revealed a low oral uptake compared to the high oral bioavailability of >80% observed in humans, and a 25-fold-higher Cmax was found in humans at a similar dose (8). In healthy woodchucks, 10 mg/kg of MIV-210 given p.o. resulted in a mean Cmax of FLG less than 1/3 and 1/10 of the corresponding Cmax reported for a 15-mg/kg p.o. dose of adefovir dipivoxil (4) and a 20-mg/kg p.o. dose of lamivudine (34), respectively. The AUC0-∞ measured for FLG after p.o. administration of the 10-mg/kg dose of MIV-210 ranged from 4.14 to 6.33 μM · h/liter for individual animals (n = 4). These AUC0-∞ were lower than those of 96.6 to 150.6 μM · h/liter reported for a 20-mg/kg p.o. dose of lamivudine given to woodchucks (34). Furthermore, a 15-mg/kg oral dose of adefovir yielded an AUC0-∞ of 61.2 μM · h/liter in woodchucks (4). In the current study, considerable individual variation in AUC0-∞ was observed after the administration of 60 mg/kg MIV-210 to woodchucks with chronic WHV infection; therefore, the plasma FLG concentration has been baseline adjusted (<10% of Cmax), and 4 out of 18 woodchucks were excluded due to high baselines (Table 1). No intravenous dosing was tested. However, on the basis of the low Cmax and in vitro potency in primary woodchuck hepatocytes, where FLG inhibited 99% of WHV multiplication at a concentration of 1.0 μM, doses of 20 mg/kg and 60 mg/kg p.o. were selected to achieve FLG Cmax of 1.0 and 3.0 μM, respectively, in the antiviral efficacy study.
In the present work, the antiviral efficacy of MIV-210 was evaluated in the placebo-controlled studies using different test doses. The treatment-induced response was quantified by measuring WHV genome levels in serum at weekly or biweekly intervals and in the liver before treatment, after 10 weeks of treatment, and upon the completion of the 10-week follow-up. No virus genome sequence was analyzed. All doses of MIV-210 tested, i.e., 20 mg/kg/day and 60 mg/kg/day in the 10-week efficacy study and 10 mg/kg/day and 5 mg/kg/day in the antiviral induction study, induced and maintained the anti-WHV effect. The most evident dose-dependent reduction in serum WHV loads was evident in animals treated p.o. with 20 and 10 mg/kg/day of MIV-210, which led to 6.11 log10 and 2.64 log10 drops after 4 weeks of treatment, respectively. However, there was only a minimal dose-related response during the same period for animal groups treated with 20 and 60 mg/kg/day. It appears that at 20 mg/kg/day, MIV-210 has already reached maximum antiviral potency, most likely as a function of a rate-limited step during anabolic phosphorylation of the triphosphate of FLG. Antiviral efficacy increased with prolongation of the MIV-210 treatment period, as was most clearly seen at the end of the 10-week period of dosing with 20 mg/kg/day, which finally caused >7.0 log10 reductions in WHV viremia.
Compared to other nucleoside analogues tested on woodchucks chronically infected with WHV and based on log10 reductions in plasma WHV DNA loads evaluated by dot blot hybridization assays of comparable sensitivity, MIV-210 appears to be as potent as entecavir and telbivudine, and clearly more potent than lamivudine, adefovir, and tenofovir, for woodchucks chronically infected with WHV. To date, five nucleoside analogues have been approved for HBV treatment: lamivudine, adefovir, entecavir, telbivudine, and TDF. Treatment of chronically infected woodchucks with lamivudine induced a noticeable anti-WHV response, but to variable degrees (11, 13, 25). The most pronounced antiviral response was reported for p.o. treatment with lamivudine at a dose of 15 mg/kg per day, which induced a 5.4 log10 reduction in serum WHV DNA levels after 4 weeks of administration (13). Thus, there appeared to be at least fivefold lower suppression of WHV viremia than that induced by MIV-210 given at 20 mg/kg/day for 4 weeks, which caused a mean 6.11 log10 reduction. In this regard, it is noteworthy that the oral bioavailability of MIV-210 in woodchucks is only 1/3 to 1/10 that of lamivudine (18% to 54%) (34), whereas the two compounds have similar oral uptake levels in humans (>80%) (8, 42).
Treatment of chronically WHV infected woodchucks with 15 mg/kg/day adefovir dipivoxil given p.o. induced decreases in WHV serum loads of 1.6 log10 at 2 weeks and 2.5 log10 at 12 weeks of treatment (4). For comparison, the mean reductions in WHV viremia at 2 weeks and 10 weeks of p.o. treatment with 20 mg/kg/day of MIV-210 were 4.75 log10 and 7.23 log10, respectively (Table 2; Fig. 3A). Oral administration of TDF at 5-mg/kg/day and 15-mg/kg/day doses did not show a dose-related response in chronic WHV carriers, due to a highly variable virological response in the animal group treated with 15 mg/kg/day (27). Overall, WHV serum levels were 1.5 log10 reduced from the pretreatment level at 4 weeks of treatment with TDF. The administration of MIV-210 at a dose of 10 mg/kg/day caused a mean reduction in serum WHV loads of 2.64 log10 (see Fig. 4) after 4 weeks of treatment, despite the lower oral bioavailability of MIV-210 than of TDF (the bioavailability of TDF was between 20 and 30% at 5-mg/kg and 15-mg/kg p.o. doses) (27). Reductions in WHV viremia in infected woodchucks were also reported for the l-nucleoside compound telbivudine (l-dT) and the carbocyclic nucleoside analogue entecavir (1, 6). Serum WHV levels were 7.0 log10 and 8.0 log10 suppressed at week 4 of treatment with 10 mg/kg/day of l-dT and 0.1 mg/kg/day of entecavir, respectively. In the present study, a 7.6 log10 reduction in viremia was seen at week 10 of treatment with MIV-210 at 60 mg/kg/day, but the oral bioavailability of MIV-210 in woodchucks is approximately six- to sevenfold lower than that of l-dT, which has been reported to be about 38% (38). Overall, the antiviral efficacy of the compounds predicted from studies of woodchucks has been successfully translated into anti-HBV efficacy in humans. Thus, treatment with lamivudine, adefovir, or TDF induced appreciable reduction of HBV viremia, causing about 4.0 log10 suppression of HBV DNA in the sera of HBeAg-positive patients with CHB after 1 year of therapy (24, 33, 39), while 1 year of treatment with entecavir or telbivudine gave >6.0 log10 reductions in the sera of HBeAg-positive patients with CHB (2, 9).
It became evident in the course of our study that the reduction in serum WHV loads by treatment with MIV-210 was associated with a significant decrease in hepatic WHV DNA loads and, surprisingly, also with some decline in the WHV cccDNA content. The cccDNA is the first replicative intermediate in the hepadnaviral replication cycle and persists in hepatocytes for a very long time, if not for the lifetime of the infected cell. The eventual loss of HBV cccDNA would be the ultimate goal of an effective anti-HBV therapy. In studies of the woodchuck model of chronic hepatitis, serum WHV titers rebounded to pretreatment levels after cessation of the drug tested, as observed for adefovir, tenofovir, telbivudine, and lamivudine treatments (1, 4, 13, 27). For entecavir, a considerably delayed rebound of WHV viremia was noticed after drug withdrawal, suggesting a reduction in the level of viral transcriptional templates during a 3-month efficacy study (6). After the cessation of the 10-week treatment with MIV-210 at 20 mg/kg/day or 60 mg/kg/day, WHV viremia gradually returned to pretreatment levels in most of the animals within 2 weeks; however, there were some exceptions. Thus, an animal (WM 3228) bearing a high serum WHV load (10.36 log10 vge/ml) before treatment showed that MIV-210 at 20 mg/kg/day induced a robust virological response, causing 7.37 log10 and 8.36 log10 reductions at weeks 2 and 10 of treatment, respectively. The rebound of WHV viremia was delayed in this animal after the cessation of treatment, and a >8.0 log10 suppression in the virus load persisted for an additional 6 weeks (Fig. 3A). A seemingly complete and persistent elimination of WHV viremia (>9.0 log10 reduction) was found in WF 2433A, treated for 10 weeks with 60 mg/kg/day of MIV-210 (Fig. 3B). This was accompanied by an improvement in liver histology over the pretreatment condition. It was a remarkable finding, demonstrating that the relatively short period of treatment with MIV-210 reduced WHV DNA loads from 11.04 log10 vge/ml in serum and 7.41 log10 vge/μg of total DNA in hepatic tissue to <2.0 log10 vge/ml and 3.45 log10 vge/μg of DNA, respectively, levels that were maintained for 10 weeks after drug withdrawal. However, due to the limited number of animals enrolled in the current study and the limited number of those that seemingly eliminated WHV DNA from serum, it is not possible to draw any formal conclusion that the treatment of MIV-210 can clear circulating virus.
At present, the woodchuck model of chronic hepadnavirus infection serves as the most relevant in vivo system not only for identifying the anti-HBV efficacy of test agents but also for assessing their safety in the context of chronic liver inflammation (5, 19, 40). In this regard, while fialuridine (FIAU) administration is associated with a delayed profile of toxicity, including hepatic failure and lactic acidosis, in humans, this toxicity was manifested more accurately in woodchucks than in other animal species (26). In our study, 10 weeks of oral administration of MIV-210 at a dose as high as 60 mg/kg/day was found to be well tolerated by woodchucks with progressing chronic WHV hepatitis. No statistically significant differences in physical and biochemical or hematological parameters were found between the MIV-210- and placebo-treated animals. However, some changes in the levels of HCT, WBC, Grans, and L/M from pretreatment measurements were seen for the same animals at the end of the 10-week follow-up period (see Table 5). Nonetheless, the values remained within the normal ranges encountered in healthy animals. Ultrastructural examination of mitochondrial alterations in hepatic tissue samples collected before, during, and after treatment with MIV-210 at 20 mg/kg/day or 60 mg/kg/day revealed no changes related to the administration of MIV-210. In humans, treatment with MIV-210 also was well tolerated at a single dose of 1,500 mg and at dosing of 150 mg twice a day for 8 days (8). This repeating dosing is likely to be 5 to 10 times higher than the expected clinical dose for HBV-infected patients.
The experience gained from therapy of human immunodeficiency virus infection shows that rational combination chemotherapy has been highly successful. FLG was found to inhibit the elongation of synthesis of hepadnaviral minus-strand DNA during the HBV reverse transcription process (10). Other deoxyguanosine analogues, such as entecavir, have been found to be potent inhibitors of the viral priming process at the initial stage of hepadnaviral transcription (37). Lamivudine-resistant HBV showed cross-resistance to telbivudine and emtricitabine and facilitated the development of more-complex multiple drug-resistant strains, as reported for the emergence of entecavir-related resistant HBV and the outgrowth of adefovir-related mutations at an accelerated pace (17, 20, 41, 45). FLG retained activity against both lamivudine- and adefovir-selected resistant HBV mutants in vitro (21), and the dual complex mutants resistant to both lamivudine and adefovir showed a lack of cross-resistance to FLG (10). FLG also retained its effect against multiple-drug-resistant HBV selected by entecavir in the genetic background of lamivudine resistance (S. A. Locarnini, personal communication). The high potency of MIV-210 and its sustained anti-WHV effect observed in woodchucks with chronic hepatitis, as well as the broad spectrum of activity of MIV-210 against HBV mutants resistant to other antiviral agents, make MIV-210 a highly promising candidate for the treatment of chronic HBV infection in humans.
Acknowledgments
We gratefully acknowledge the technical assistance of Kristina Wikström at Medivir AB. We also thank Colleen L. Trelegan and Matthew Cameron of the Molecular Virology and Hepatology Research Group, Faculty of Medicine, Memorial University, for expert technical assistance and Luke Grenning and Judy Foote, both of the Multidisciplinary Laboratories, Faculty of Medicine, Memorial University, for assistance during laparotomies and for the processing of liver biopsy specimens for histological and ultrastructural examinations.
Footnotes
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Bryant, M. L., E. G. Bridges, L. Placidi, A. Faraj, A. G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J. L. Imbach, B. Hernandez, A. Juodawlkis, B. C. Tennant, B. E. Korba, P. Cote, P. Marion, E. Cretton-Scott, R. F. Schinazi, and J. P. Sommadossi. 2001. Antiviral l-nucleosides specific for hepatitis B virus infection. Antimicrob. Agents Chemother. 45:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, T. T., R. G. Gish, R. De Man, A. Gadano, J. Sollano, Y. C. Chao, A. S. Lok, K. H. Han, Z. Goodman, J. Zhu, A. Cross, D. DeHertogh, R. Wilber, R. Colonno, D. Apelian, and BEHoLD AI463022 Study Group. 2006. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 354:1001-1010. [DOI] [PubMed] [Google Scholar]
- 3.Coffin, C. S., and T. I. Michalak. 1999. Persistence of infectious hepadnavirus in the offspring of woodchuck mothers recovered from viral hepatitis. J. Clin. Investig. 104:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullen, J. M., D. H. Li, C. Brown, E. J. Eisenberg, K. C. Cundy, J. Wolfe, J. Toole, and C. Gibbs. 2001. Antiviral efficacy and pharmacokinetics of oral adefovir dipivoxil in chronically woodchuck hepatitis virus-infected woodchucks. Antimicrob. Agents Chemother. 45:2740-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fourel, I., O. Hantz, K. A. Watanabe, C. Jacquet, B. Chomel, J. J. Fox, and C. Trepo. 1990. Inhibitory effects of 2′-fluorinated arabinosyl-pyrimidine nucleosides on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob. Agents Chemother. 34:473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genovesi, E. V., L. Lamb, I. Medina, D. Taylor, M. Seifer, S. Innaimo, R. J. Colonno, D. N. Standring, and J. M. Clark. 1998. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob. Agents Chemother. 42:3209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hafkemeyer, P., A. Keppler-Hafkemeyer, M. A. al Haya, M. Von Janta-Lipinski, E. Matthes, C. Lehmann, W. B. Offensperger, S. Offensperger, W. Gerok, and H. E. Blum. 1996. Inhibition of duck hepatitis B virus replication by 2′,3′-dideoxy-3′-fluoroguanosine in vitro and in vivo. Antimicrob. Agents Chemother. 40:792-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmenberg, J., T. Larsson, D. Böttiger, E. Augustsson, G. Mardh, and B. Öberg. 2002. Pharmacokinetic evaluation of the hepatitis B nucleoside analogue MIV-210 in human volunteers, abstr. 150. Antivir. Res. 53:3. [Google Scholar]
- 9.Hou, J., Y. K. Yin, D. Xu, D. Yan, J. Nju, X. Zhou, Y. Wang, L. Zhu, Y. He, H. Ren, M. Wan, C. Chen, S. Wu, Y. Chen, J. Xu, Q. Wang, L. Wei, G. Chao, B. F. Constance, G. Harb, N. A. Brown, and J. Jia. 2008. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: results at 1 year of a randomized, double-blind trial. Hepatology 47:447-454. [DOI] [PubMed] [Google Scholar]
- 10.Jacquard, A. C., M. N. Brunelle, C. Pichoud, D. Durantel, S. Carrouée-Durantel, C. Trepo, and F. Zoulim. 2006. In vitro characterization of the anti-hepatitis B virus activity and cross-resistance profile of 2′,3′-dideoxy-3′-fluoroguanosine. Antimicrob. Agents Chemother. 50:955-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korba, B. E., P. Cote, W. Hornbuckle, R. Schinazi, J. D. Gangemi, B. C. Tennant, and J. L. Gerin. 2000. Enhanced antiviral benefit of combination therapy with lamivudine and alpha interferon against WHV replication in chronic carrier woodchucks. Antivir. Ther. 5:95-104. [PubMed] [Google Scholar]
- 12.Korba, B. E., P. Cote, W. Hornbuckle, R. Schinazi, J. L. Gerin, and B. C. Tennant. 2000. Enhanced antiviral benefit of combination therapy with lamivudine and famciclovir against WHV replication in chronic WHV carrier woodchucks. Antivir. Res. 45:19-32. [DOI] [PubMed] [Google Scholar]
- 13.Korba, B. E., P. Cote, W. Hornbuckle, B. C. Tennant, and J. L. Gerin. 2000. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology 31:1165-1175. [DOI] [PubMed] [Google Scholar]
- 14.Korba, B. E., R. F. Schinazi, P. Cote, B. C. Tennant, and J. L. Gerin. 2000. Effect of oral administration of emtricitabine on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob. Agents Chemother. 44:1757-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai, C. L., J. Dienstag, E. Schiff, N. W. Leung, M. Atkins, C. Hunt, N. Brown, M. Woessner, R. Boehme, and L. Condreay. 2003. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. 36:687-696. [DOI] [PubMed] [Google Scholar]
- 16.Lau, G. K. K. 2001. Hepatitis B infection in China. Clin. Liver Dis. 5:361-380. [DOI] [PubMed] [Google Scholar]
- 17.Lee, Y. S., D. J. Suh, Y. S. Lim, S. W. Jung, K. M. Kim, H. C. Lee, Y. H. Chung, Y. S. Lee, W. Yoo, and S. O. Kim. 2006. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 43:1385-1391. [DOI] [PubMed] [Google Scholar]
- 18.Lew, Y.-Y., and T. I. Michalak. 2001. In vitro and in vivo infectivity and pathogenicity of the lymphoid cell-derived woodchuck hepatitis virus. J. Virol. 75:1770-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis, W., B. Griniuviene, K. O. Tankersley, E. S. Levine, R. Montione, L. Engelman, G. de Courten-Myers, M. A. Ascenzi, W. E. Hornbuckle, J. L. Gerin, and B. C. Tennant. 1997. Depletion of mitochondrial DNA, destruction of mitochondria, and accumulation of lipid droplets result from fialuridine treatment in woodchucks (Marmota monax). Lab. Investig. 76:77-87. [PubMed] [Google Scholar]
- 20.Locarnini, S., and N. Warner. 2007. Major causes of antiviral drug resistance and implications for treatment of hepatitis B virus monoinfection and coinfection with HIV. Antivir. Ther. 12(Suppl. 3):H15-H23. [PubMed] [Google Scholar]
- 21.Locarnini, S. A., V. Sozzi, H. Zhang, B. Öberg, and T. Shaw. 2003. 2′,3′-Dideoxy-3′-fluoroguanosine (FLG) has antiviral activity against both wild type and lamivudine resistant hepatitis B virus in vitro: results of assays using the recombinant baculovirus system. Hepatology 38(Suppl.):714A. [Google Scholar]
- 22.Löfgren, B., K. Vickery, Y. Y. Zhang, and E. Nordenfelt. 1996. 2′,3′-Dideoxy-3′-fluoroguanosine inhibits duck hepatitis B virus in vivo. J. Viral Hepat. 3:61-65. [DOI] [PubMed] [Google Scholar]
- 23.Maddrey, W. C. 2000. Hepatitis B: an important public health issue. J. Med. Virol. 61:362-366. [DOI] [PubMed] [Google Scholar]
- 24.Marcellin, P., T. T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, C. L. Brosgart, and Adefovir Dipivoxil 437 Study Group. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 25.Mason, W. S., J. Cullen, G. Moraleda, J. Saputelli, C. E. Aldrich, D. S. Miller, B. Tennant, L. Frick, D. Averett, L. D. Condreay, and A. R. Jilbert. 1998. Lamivudine therapy of WHV-infected woodchucks. Virology 245:18-32. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie, R., M. W. Fried, R. Sallie, H. Conjeevaram, A. M. Di Bisceglie, Y. Park, B. Savarese, D. Kleiner, M. Tsokos, C. Luciano, T. Pruett, J. L. Stotka, S. E. Straus, and J. H. Hoofnagle. 1995. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N. Engl. J. Med. 333:1099-1105. [DOI] [PubMed] [Google Scholar]
- 27.Menne, S., P. J. Cote, B. E. Korba, S. D. Butler, A. L. George, L. A. Tochkov, W. E. Delaney IV, S. Xiong, L. J. Gerin, and C. B. Tennant. 2005. Antiviral effect of oral administration of tenofovir disoproxil fumarate in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 49:2720-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalak, T. I. 1998. The woodchuck animal model of hepatitis B. Viral Hepat. Rev. 4:139-165. [Google Scholar]
- 29.Michalak, T. I., and B. Lin. 1994. Molecular species of hepadnavirus core and envelope polypeptides in hepatocyte plasma membrane of woodchucks with acute and chronic viral hepatitis. Hepatology 20:275-286. [PubMed] [Google Scholar]
- 30.Michalak, T. I., I. U. Pardoe, C. S. Coffin, N. D. Churchill, D. S. Freake, P. Smith, and C. L. Trelegan. 1999. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology 29:928-938. [DOI] [PubMed] [Google Scholar]
- 31.Mulrooney-Cousins, P. M., and T. I. Michalak. 2007. Persistent occult hepatitis B virus infection: experimental findings and clinical implications. World J. Gastroenterol. 13:5682-5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peek, S. F., P. J. Cote, J. R. Jacob, I. A. Toshkov, W. E. Hornbuckle, B. H. Baldwin, F. V. Wells, C. K. Chu, J. L. Gerin, B. C. Tennant, and B. E. Korba. 2001. Antiviral activity of clevudine [l-FMAU, (1-(2-fluoro-5-methyl-β,l-arabinofuranosyl)uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax). Hepatology 33:254-266. [DOI] [PubMed] [Google Scholar]
- 33.Peters, M. G., J. Andersen, P. Lynch, T. Liu, B. Alston-Smith, C. L. Brosgart, J. M. Jacobson, V. A. Johnson, R. B. Pollard, J. E. Rooney, K. E. Sherman, S. Swindells, B. Polsky, and ACTG Protocol A5127 Team. 2006. Randomized controlled study of tenofovir and adefovir in chronic hepatitis B virus and HIV infection: ACTG A5127. Hepatology 44:1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopalan, P., F. D. Boudinot, C. K. Chu, B. C. Tennant, B. H. Baldwin, and R. F. Schinazi. 1996. Pharmacokinetics of (−)-2′-3′-dideoxy-3′-thiacytidine in woodchucks. Antimicrob. Agents Chemother. 40:642-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roggendorf, M., and T. K. Tolle. 1995. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology 38:100-112. [DOI] [PubMed] [Google Scholar]
- 36.Schröder, I., B. Holmgren, B. Öberg, and B. Löfgren. 1998. Inhibition of human and duck hepatitis B virus by 2′,3′-dideoxy-3′-fluoroguanosine in vitro. Antivir. Res. 37:57-66. [DOI] [PubMed] [Google Scholar]
- 37.Seifer, M., R. K. Hamatake, R. J. Colonno, and D. N. Standring. 1998. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob. Agents Chemother. 42:3200-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Standring, D. N., E. G. Bridges, L. Placidi, A. Faraj, A. G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J. L. Imbach, B. Hernandez, A. Juodawlkis, B. C. Tennant, B. E. Korba, P. Cote, E. Cretton-Scott, R. F. Schinazi, M. Myers, M. L. Bryant, and J. P. Sommadossi. 2001. Antiviral beta-l-nucleosides specific for hepatitis B virus infection. Antivir. Chem. Chemother. 12(Suppl. 1):119-129. [PubMed] [Google Scholar]
- 39.Sung, J. J., J. Y. Lai, S. Zeuzem, W. C. Chow, E. J. Heathcote, R. P. Perrillo, C. L. Brosgart, M. A. Woessner, S. A. Scott, D. F. Gray, and S. D. Gardner. 2008. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J. Hepatol. 48:728-735. [DOI] [PubMed] [Google Scholar]
- 40.Tennant, B. C., B. H. Baldwin, L. A. Graham, M. A. Ascenzi, W. E. Hornbuckle, P. H. Rowland, I. A. Tochkov, A. E. Yeager, H. N. Erb, J. M. Colacino, C. Lopez, J. A. Engelhardt, R. R. Bowsher, F. C. Richardson, W. Lewis, P. J. Cote, B. E. Korba, and J. L. Gerin. 1998. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology 28:179-191. [DOI] [PubMed] [Google Scholar]
- 41.Tenney, D. J., S. M. Levine, R. E. Rose, A. W. Walsh, S. P. Weinheimer, L. Discotto, M. Plym, K. Pokornowski, C. F. Yu, P. Angus, A. Ayres, A. Bartholomeusz, W. Sievert, G. Thompson, N. Warner, S. Locarnini, and R. J. Colonno. 2004. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob. Agents Chemother. 48:3498-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Leeuwen, R., J. M. Lange, E. K. Hussey, K. H. Donn, S. T. Hall, A. J. Harker, P. Jonker, and S. A. Danner. 1992. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase I study. AIDS 6:1471-1475. [DOI] [PubMed] [Google Scholar]
- 43.Villeneuve, J. P., D. Durantel, S. Durantel, C. Westland, S. Xiong, C. L. Brosgart, C. S. Gibbs, P. Parvaz, B. Werle, C. Trepo, and F. Zoulim. 2003. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J. Hepatol. 39:1085-1089. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, H., B. Öberg, J. Harmenberg, L. Vrang, C. Rydergård, T. Larsson, and B. Samuelsson. 2002. Inhibition of multiple-drug resistant (MDR) HIV-1 by 3′-fluoro-2′,3′-dideoxyguanosine (FLG), abstr. A-1853. Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother.
- 45.Zoulim, F. 2004. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antivir. Res. 64:1-15. [DOI] [PubMed] [Google Scholar]