Abstract
Progressive respiratory failure due to Pseudomonas aeruginosa is the leading cause of morbidity and mortality in patients with cystic fibrosis. The pulmonary delivery of antimicrobial agents provides high concentrations of drug directly to the site of infection and attains pharmacokinetic-pharmacodynamic indices exceeding those which can be achieved with systemic dosing. MP-376 is a new formulation of levofloxacin that enables the safe aerosol delivery of high concentrations of drug to pulmonary tissues. In vivo studies were conducted to demonstrate the efficacy of MP-376 in models of mouse pulmonary infection. The superiority of aerosol dosing over systemic dosing was demonstrated in models of both acute and chronic lung infection. In a model of acute lung infection, aerosol treatment with MP-376 once or twice daily reduced the lung bacterial load to a greater extent than aerosol tobramycin or aztreonam did when they were administered at similar or higher doses. The bacterial killing by aerosol MP-376 observed in the lung in the model of acute pulmonary infection translated to improved survival (P < 0.05). In a model of chronic pulmonary infection, aerosol MP-376 had antimicrobial effects superior to those of aztreonam (P < 0.05) and effects similar to those of tobramycin (P > 0.05). In summary, these data show that aerosol MP-376 has in vivo activity when it is used to treat acute and chronic lung infections caused by P. aeruginosa.
Pseudomonas aeruginosa, a gram-negative opportunistic pathogen, remains an important cause of pulmonary infection in patients with cystic fibrosis (CF). A majority of patients with CF become infected with P. aeruginosa by the age of 18 years (5, 11). Chronic infection with P. aeruginosa is associated with morbidity and mortality in patients with CF (7, 8, 14, 17). In addition to chronic infection with P. aeruginosa in patients with CF, recent studies have also demonstrated an important role of chronic infection with P. aeruginosa in patients with chronic obstructive pulmonary disease (COPD) (16, 20).
Since achievement of an adequate antimicrobial concentration is a requirement for the successful treatment of chronic pulmonary infections, aerosol antibiotic therapy has become a valuable approach to treatment in patients with CF. Compared to systemic dosing, aerosol treatment provides higher pulmonary concentrations of antibiotics and reduces the systemic level of exposure to the drug (3, 6, 27). Tobramycin inhalation solution is currently the only aerosol antibiotic approved for use for the treatment of bacterial infections in patients with CF. With the aerosol administration of tobramycin, the potential for systemic toxicity is reduced and a clinical benefit has been shown over several cycles of treatment (23); its long-term use, however, has been associated with multiple-antibiotic-resistant P. aeruginosa strains (19, 27). Thus, there is a need for the development of different classes of aerosol antibiotics for the treatment of chronic lung infections in patients with CF.
The fluoroquinolone levofloxacin has potent activity against key pathogens in patients with CF, including P. aeruginosa, with no loss of potency in CF sputum (13). Pharmacokinetic (PK)-pharmacodynamic (PD) studies with systemically administered levofloxacin have shown that bactericidal activity and clinical efficacy are linked to the area under the concentration-time curve (AUC)/MIC and maximum concentration of drug in plasma (Cmax)/MIC ratios (9, 12, 22). In addition, both in vitro and in vivo studies have shown that high levels of exposure (Cmax) relative to the MIC can reduce the rate of selection of drug-resistant bacteria in vivo (12, 13).
Since aerosol administration of antibiotics produces drug levels in pulmonary tissues that are higher than those that can be produced with systemic dosing and that these increased local levels are associated with improved efficacy, a novel aerosol formulation of levofloxacin, MP-376, was developed. MP-376 is a levofloxacin solution formulated with divalent cations and permeant ions for inhalational use. The purpose of the studies described here was to determine the efficacy of aerosol MP-376 in models of acute and chronic lung infection due to P. aeruginosa.
(This work was presented in part at the 21st and 22nd Annual North American Cystic Fibrosis Conferences, October 2007 and 2008, respectively [25, 26].)
MATERIALS AND METHODS
Antimicrobial agents.
Levofloxacin (LKT Laboratories, St. Paul, MN), tobramycin (Sicor Pharmaceuticals, Irvine, CA), and aztreonam (MP Biomedicals, Solon, OH) were purchased from independent vendors. MP-376 was prepared at Mpex Pharmaceuticals, Inc. Prior to the initiation of each experiment, fresh stock solutions of each antibiotic were prepared. MP-376 was diluted in water, levofloxacin and tobramycin were diluted in 0.9% saline, and aztreonam was diluted in 7% sodium bicarbonate in water.
Testing of MICs for bacterial strains.
P. aeruginosa ATCC 27853 and NH57388A (a gift from Niels Hoiby) were used in the MIC testing studies. MICs were determined by a broth microdilution assay according to CLSI reference methods (2). Assays were performed in a final volume of 100 μl. The bacterial suspensions were adjusted to yield a cell density of 5 × 105 CFU/ml. The antibiotics were prepared at a concentration equivalent to twofold the highest desired final concentration in culture medium and were then diluted directly into 96-well microtiter plates. Microtiter plates were incubated for 24 h at 35°C and were read by using a microtiter plate reader (Molecular Devices) at 600 nm, as well as by visual observation by using a microtiter plate reading mirror. The MIC was defined as the lowest concentration of antibiotic at which the visible growth of the organism was completely inhibited.
Mice.
Female Swiss mice (age, 5 to 6 weeks) were obtained from Harlan West Coast (Germantown, CA). All studies were performed under protocols approved by an institutional animal care and use committee.
Preparation of pseudomonal alginate.
P. aeruginosa NH57388A was cultured in 50 ml Mueller-hinton broth (MHB) for 24 to 28 h at 37°C with shaking (170 rpm). The bacterial cells were harvested by centrifugation (23,000 × g, 30 min, 4°C) and resuspended in 3 to 6 ml of MHB. The supernatant was collected and placed in an 80°C water bath for 30 min. Alginate was precipitated by adding the supernatant to 150 ml of ice-cold 99% ethanol. The precipitated alginate was collected with a sterile bacterial loop and washed several times in sterile saline. The purified alginate was then resuspended in 10 ml of sterile saline and stirred vigorously to form a homogeneous suspension. The alginate concentration was measured and adjusted to a concentration of 2 to 3 mg/ml (24).
Aerosol administration of antibiotics.
The antibiotics were aerosolized with a microspray aerosol device (MicroSprayer, model IA-C; PennCentury, Philadelphia, PA) attached to an FMJ-250 high-pressure syringe (PennCentury). This device produces a 16- to 22-μm mass medium-diameter spray. For administration, each mouse was anesthetized (5% isoflurane in oxygen running at 4 liters/min) and positioned securely at a 45 to 50° angle by the upper teeth, the microspray aerosol tip was inserted to the bifurcation, and a 50-μl volume was administered.
Pharmacokinetics.
Mice (n = 3/time point) were administered a single aerosol dose of MP-376 at 60 mg/kg of body weight or a intraperitoneal (i.p.) dose of levofloxacin at 20 mg/kg. The mice were killed at 0.08, 0.16, 0.25, 0.5, 0.75, 1.0, 2.0, 3.0, and 4.0 h after dosing; and their lungs were collected. Levofloxacin lung homogenate concentrations administered as levofloxacin or MP-376 were measured by a high-pressure liquid chromatography method. Analytical standards (0.05 to 100 mg/liter) were prepared in fresh mouse lung homogenate collected from untreated animals. Lung homogenate or standards for both compounds were mixed with double the volume of 4% trichloroacetic acid, vortexed, and then centrifuged at 12,000 rpm for 10 min with a refrigerated Eppendorf 5415c centrifuge set at 4 to 10°C. Aliquots of the supernatant (25 μl) were injected directly onto the high-pressure liquid chromatograph by using a temperature-controlled autoinjector set at 10°C. Standard curves of the peak area versus the standard concentration were constructed, and the data were fit by weighted linear regression (Excel software; Microsoft, Seattle, WA). The concentrations of levofloxacin in the lung homogenate were calculated from these standard curves. The lung PK parameters were determined by using the WinNonlin program (Pharsight, Mountain View, CA).
Model of acute mouse lung infection.
P. aeruginosa ATCC 27853 was grown overnight in MHB at 35°C. The bacterial suspensions were adjusted to ca. 1 × 105 to 6 × 105 CFU/ml by correlation of the absorbance at 600 nm with predetermined plate counts. Female Swiss mice were made neutropenic by the i.p. injection of 150 mg/kg cyclophosphamide (Baxter, Deerfield, IL) on days 1 and 3. On day 4, the mice were infected by the intratracheal instillation of 0.05 ml of inoculum with a curved oral gavage tip attached to a 1-ml syringe. Antibiotic treatments were started at 24 h postinfection and were administered once or twice daily (BID) for 24 or 48 h. Antibiotics were aerosolized with a microspray aerosol device. All infections and aerosol treatments were performed while the mice were under isoflurane anesthesia (5% isoflurane in oxygen running at 4 liters/min). An untreated group of mice (n = 8) was killed prior to the initiation of treatment to determine baseline bacterial counts. At 12 to 16 h following administration of the last antibiotic dose, the treated animals (n = 8) were killed by carbon dioxide asphyxiation. The lungs were removed aseptically and homogenized (Pro200 homogenizer; Pro Scientific, Monroe, CT) in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenized lung were plated on Mueller-Hinton agar, and the colonies were counted. For the survival studies, mice (n = 10) were observed for 7 days after the end of treatment or for a total of 9 days postinfection.
Model of chronic mouse lung infection.
P. aeruginosa NH57388A was cultured in 50 ml MHB for 24 to 28 h at 37°C with shaking (170 rpm). The bacterial cells were harvested by centrifugation (23,000 × g, 30 min, 4°C) and resuspended in 3 to 6 ml of MHB (10). The bacterial suspension was diluted (1:10) in the alginate suspension to yield about 108 CFU/ml. The initial establishment of infection was achieved by the establishment of a transient neutropenia by administration of a single 150-mg/kg i.p. dose of cyclophosphamide 4 days prior to infection. On day 4, the mice were infected by use of a curved bead-tipped oral gavage attached to a 1-ml syringe while the mice were under isoflurane anesthesia. Antibiotic treatments were started at 24 h postinfection and were administered BID for three consecutive days. Various concentrations of antibiotics were used, and they were administered either by the i.p. route or by the aerosol route with a microspray device. At 12 to 16 h following the last treatment, the mice were killed, and the colony counts in the lung were determined as described above.
Statistical analysis.
Survival and lung bacterial counts were analyzed by the log-rank test and the Mann-Whitney U test (GraphPad Prism, version 4.03), respectively. A P value of <0.05 was considered statistically significant.
RESULTS
Susceptibility studies.
The MICs of the P. aeruginosa strains used in the animal studies are shown in Table 1. Tobramycin was the most potent antibiotic in vitro, with MICs of <1 μg/ml; MP-376 and levofloxacin had MICs of 1 and 2 μg/ml, respectively, and aztreonam had MICs of 4 μg/ml for both strains.
TABLE 1.
MICs for the strains used in animal models
| Strain | MIC (mg/liter)
|
|||
|---|---|---|---|---|
| MP-376 | Levofloxacin | Tobramycin | Aztreonam | |
| ATCC 27853 | 1 | 1 | 0.25 | 4 |
| NH57388Aa | 2 | 2 | 0.5 | 4 |
Clinical isolate from a patient with CF.
PKs in mice.
The lung PK parameters for MP-376 and levofloxacin are shown in Table 2. Aerosol administration of 60 mg/kg MP-376 produced levofloxacin AUCs and Cmaxs that were 9- and 30-fold higher than those achieved by the dose-normalized i.p. administration of levofloxacin.
TABLE 2.
Dose-normalized lung homogenate PK parameters of levofloxacin administered i.p. or by the aerosol route as MP-376 in mice
| Antimicrobial | Route of administration | Dose (mg/kg) | Cmaxa (mg/kg) | AUCa (mg · h/kg) |
|---|---|---|---|---|
| MP-376 | Aerosol | 60 | 550 | 106 |
| Levofloxacin | i.p. | 20 | 6.2 (18.6) | 4.1 (12.3) |
The values in parentheses were normalized to 60 mg/kg.
Aerosol MP-376 versus systemic levofloxacin in models of acute and lung chronic infection.
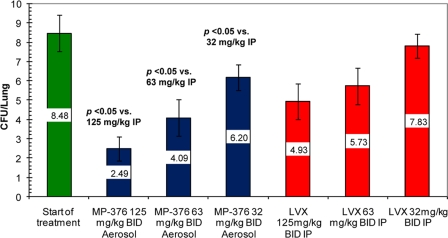
In the model of acute lung infection, aerosol treatment with 125, 62.5, and 32 mg/kg of MP-376 produced 5.9, 4.3, and 2.3 log CFU reductions in the lung bacterial counts, respectively. Systemic treatment with 125, 62.5, and 32 mg/kg of levofloxacin produced 3.5, 2.7, and 0.65 log CFU reductions, respectively (Fig. 1). The reduction in bacterial counts with aerosol MP-376 was greater than that observed with i.p. levofloxacin on a per dose basis (P < 0.05).
FIG. 1.
Comparative antibacterial activities of aerosol MP-376 and i.p. levofloxacin against P. aeruginosa ATCC 27853 in a model of acute lung infection in mice. The treatment groups (n = 8) received two doses of antibiotics over 24 h by the i.p. or the aerosol route. The values shown are the mean log CFU per lung ± standard deviation. Aerosol MP-376 was associated with a greater reduction in bacterial counts than the same dose of levofloxacin given i.p. (P < 0.05 for comparisons at each dose).
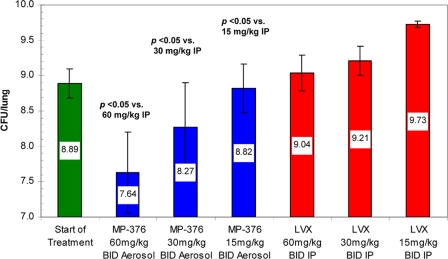
In the chronic lung infection model (Fig. 2), i.p. treatment with 60, 30, and 15 mg/kg of levofloxacin in saline produced 0.15, 0.32, and 0.83 log increases in bacterial counts, respectively. In contrast, aerosol dosing with 60, 30, and 15 mg/kg of MP-376 produced 1.26, 0.62, and 0.07 log decreases in bacterial counts, respectively. Overall, the bacterial load in the lung was significantly lower in mice treated with aerosol MP-376 than in mice treated with systemic levofloxacin on a dose-per-dose basis in both infection models (P < 0.05 for MP-376 versus the results for i.p. levofloxacin).
FIG. 2.
Comparative antibacterial effects of aerosol MP-376 and i.p. levofloxacin against P. aeruginosa NH57388A in a model of chronic infection in mice. The treatment groups (n = 8) received drug BID for 72 h by the aerosol or i.p. route. The values shown are the mean log CFU per lung ± standard deviation. Aerosol MP-376 had a greater antimicrobial effect than the same levofloxacin dose administered i.p. (P < 0.05 for comparison of the aerosolized dose versus the same dose administered i.p.).
Aerosol MP-376, tobramycin, and aztreonam in a model of acute lethal lung infection.
To compare the effects of MP-376, tobramycin, and aztreonam in the model of acute lung infection, mice were infected with P. aeruginosa ATCC 27853 and were treated once or BID by the aerosol route for two consecutive days. Due to toxicity, the maximum dose of tobramycin was limited to 60 mg/kg and the maximum dose of aztreonam was limited to 400 mg/kg. In addition, due to the need for anesthesia for treatment, the maximum number of daily doses was limited to two.
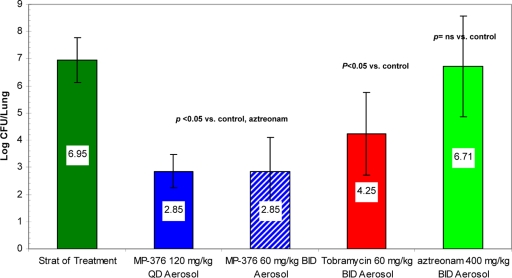
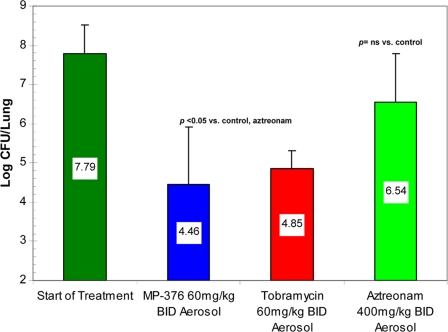
Aerosol dosing with MP-376, tobramycin, and aztreonam produced mean reductions of 4.10, 2.70, and 0.24 log CFU per lung, respectively (P < 0.05 for comparison of MP-376 with aztreonam) (Fig. 3). Of note, administration of the same total daily dose of MP-376 as a single daily dose or a BID dose resulted in similar reductions in the P. aeruginosa counts in the lung.
FIG. 3.
Bactericidal activities of aerosol MP-376, tobramycin, and aztreonam in a model of acute lethal lung infection due to P. aeruginosa ATCC 27853 in mice. The treatment groups (n = 8) received drug twice a day for 48 h by the aerosol route. The values shown are the mean log CFU per lung ± standard deviation. The bacterial counts following the once-daily or BID administration of MP-376 were lower than those following aztreonam treatment (P < 0.05).
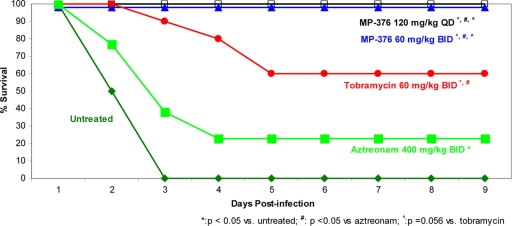
Survival was monitored over 9 days. As shown in Fig. 4, all untreated mice succumbed to the infection after 3 days. Treatment with 800 mg/kg/day (400 mg/kg BID) aerosol aztreonam resulted in the lowest survival rate among the survival rates achieved with the antibiotics used in this study (20%), and the result of treatment with 800 mg/kg/day (400 mg/kg BID) aerosol aztreonam was not significantly different from that of no treatment (P > 0.05). Treatment with 120 mg/kg/day (60 mg/kg BID) tobramycin produced a 60% survival rate, which was statistically different from that for the controls (P < 0.05). Treatment with 120 mg/kg/day MP-376 as either 120 mg/kg once a day or 60 mg/kg BID produced a 100% survival rate, which was significantly different from that for no treatment (controls) or aztreonam treatment (P < 0.05) and which showed a trend better than that achieved with tobramycin (P = 0.056).
FIG. 4.
Effects of aerosol MP-376, tobramycin, and ATM on survival in a model of lethal lung infection due to P. aeruginosa ATCC 27853. Five groups of infected mice (n = 10) were treated with MP-376, tobramycin, and aztreonam by the use of an aerosol microspray device for 48 h. Survival was monitored through day 9 following infection. Both regimens of MP-376 resulted in 100% survival and were superior to aztreonam and tobramycin.
Aerosol MP-376, tobramycin, and aztreonam in a model of chronic lung infection.
Aerosol MP-376, tobramycin, and aztreonam produced mean log CFU reductions of 3.3, 2.9, and 1.25, respectively (Fig. 5). Aerosolized doses of either tobramycin or MP-376 produced bacterial counts significantly lower than those achieved with aztreonam or no treatment (control) (P < 0.05).
FIG. 5.
Bactericidal activities of aerosol MP-376, tobramycin, and aztreonam in a model of chronic lung infection due to P. aeruginosa NH57388A. The treatment groups were treated BID for three consecutive days by the aerosol route. The values shown are the mean log CFU per lung ± standard deviation. Aerosol MP-376 resulted in lower bacterial counts than aztreonam or no treatment (control) (P < 0.05).
DISCUSSION
Patients with CF suffer from chronic infection of the lower respiratory tract that can be caused by one or multiple bacteria, including P. aeruginosa. The systemic administration of antibiotics is a proven approach for the treatment of pulmonary infections, but it has limitations, including toxicity and low levels of penetration into the airway lumen and intraluminal secretions (15, 18, 21, 29, 30). Aerosol antibiotic therapy has overcome many of these limitations by directly targeting the site of infection and has usefulness for the management of chronic pulmonary infections due to P. aeruginosa (3).
Levofloxacin is a fluoroquinolone with potent activity against key pathogens in patients with CF and COPD and demonstrated efficacy against pulmonary infections due to gram-positive and gram-negative pathogens. Studies with levofloxacin demonstrate that it has concentration-dependent bacterial killing, with the AUC/MIC and the Cmax/MIC ratios being the key PK-PD indices for the establishment of efficacy and reducing the emergence of resistance (9, 12, 22).
MP-376 is a novel formulation of levofloxacin for aerosol administration in clinical development for the management of chronic pulmonary infections in patients with CF and COPD. The aerosol delivery of MP-376 directly to the lung has the potential to increase the local concentration of levofloxacin at the site of infection, thereby enhancing the level of bacterial killing compared to that achieved by systemic administration. Our in vivo studies show that aerosol dosing of MP-376 produces greater bacterial killing than systemic dosing in models of both acute and chronic P. aeruginosa lung infection.
Tobramycin inhalation solution is currently the only FDA-approved antibiotic for the inhalation treatment of chronic lung infections caused by P. aeruginosa in patients with CF. Studies of long-term treatment with aerosol tobramycin have shown that it selects for resistant bacteria and has a limited effect on reducing the bacterial loads in sputum among patients with CF infected with P. aeruginosa (28). Aztreonam, a monbactam antibiotic, is currently in phase III clinical development for the treatment of patients with CF infected with P. aeruginosa. While fluoroquinolones (MP-376, levofloxacin) and aminoglycosides (tobramycin) have concentration-dependent antibacterial effects (Cmax/MIC or AUC/MIC), monobactams like aztreonam have time-dependent antibacterial effects; thus, the percentage of the dosing interval during which the concentrations exceed the MIC is linked to the antibacterial effects (1, 4); as such, more frequent dosing of these compounds is required to achieve optimal efficacy.
In comparisons of the antibacterial activity of MP-376 with the activities of tobramycin and aztreonam, MP-376 reduced the lung bacterial load to a similar or greater extent than that observed with aerosol tobramycin and aztreonam when they were used at similar or higher doses (Fig. 3 and 5). This greater reduction in bacterial load in the lungs translated to improved survival (Fig. 4).
Current aerosol dosing regimens for the treatment of chronic lung infection require multiple daily administrations, resulting in inconvenience for patients and poor adherence to treatment (31). As noted above, the efficacy of fluoroquinolones in vivo is linked to high Cmax/MIC and AUC/MIC ratios and not the duration of time during which the concentration remains above the MIC. Thus, drugs like MP-376 (levofloxacin) would be ideal for use for prolonged dosing intervals (e.g., once daily). Comparisons of single daily dosing versus BID dosing of MP-376 showed that they resulted in comparable levels of bacterial killing and survival. These data suggest that once-daily treatment with MP-376 may be possible in humans. The clinical evaluation of aerosol MP-376 for the treatment of chronic pulmonary infections in patients with CF and COPD is in progress.
Acknowledgments
We are grateful to Niels Hoiby for providing P. aeruginosa NH75388A, Nadine Hoffmann for technical assistance, and Dana Johnson for the animal care.
Footnotes
Published ahead of print on 15 June 2009.
REFERENCES
- 1.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2006. Methods for dilution of antimicrobial susceptibility test for bacteria that grow aerobically, 7th ed. CLSI document M7-A7. Clinical and Laboratory Standard Institute, Wayne, PA.
- 3.Conway, S. P. 2005. Nebulized antibiotic therapy: the evidence. Chron. Respir. Dis. 2:35-41. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 5.Cystic Fibrosis Foundation. 2000. Cystic Fibrosis Foundation patient registry annual data report, 2000. Cystic Fibrosis Foundation, Bethesda, MD.
- 6.Dubus, J. C., E. Bosdure, L. Bakuridze, and V. Andrieu. 2007. Nebulized drugs: the evolution? Arch. Pediatr. 14:504-506. (In French.) [DOI] [PubMed] [Google Scholar]
- 7.Emerson, J., M. Rosenfeld, S. McNamara, B. Ramsey, and R. L. Gibson. 2002. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 34:91-100. [DOI] [PubMed] [Google Scholar]
- 8.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith, D. C., E. Corcoran, D. Lofland, A. Lee, D. Cho, O. Lomovskaya, and M. N. Dudley. 2006. Pharmacodynamics of levofloxacin against Pseudomonas aeruginosa with reduced susceptibility due to different efflux pumps: do elevated MICs always predict reduced in vivo efficacy? Antimicrob. Agents Chemother. 50:1628-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann, N., T. B. Rasmussen, P. O. Jensen, C. Stub, M. Hentzer, S. Molin, O. Ciofu, M. Givskov, H. K. Johansen, and N. Hoiby. 2005. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect. Immun. 73:2504-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoiby, N., E. W. Flensborg, B. Beck, B. Friis, S. V. Jacobsen, and L. Jacobsen. 1977. Pseudomonas aeruginosa infection in cystic fibrosis. Diagnostic and prognostic significance of Pseudomonas aeruginosa precipitins determined by means of crossed immunoelectrophoresis. Scand. J. Respir. Dis. 58:65-79. [PubMed] [Google Scholar]
- 12.Jumbe, N., A. Louie, R. Leary, W. Liu, M. R. Deziel, V. H. Tam, R. Bachhawat, C. Freeman, J. B. Kahn, K. Bush, M. N. Dudley, M. H. Miller, and G. L. Drusano. 2003. Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J. Clin. Investig. 112:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King, P., O. Lomovskaya, D. C. Griffith, D. C. Citron, J. Burns, and M. N. Dudley. 2008. Antibacterial activity of levofloxacin (MP-376) under varying in vitro conditions relevant to chronic pulmonary infection in CF patients, abstr. 358. Abstr. 22nd Annu. N. Am. Cystic Fibrosis Conf.
- 14.Kulczycki, L. L., T. M. Murphy, and J. A. Bellanti. 1978. Pseudomonas colonization in cystic fibrosis. A study of 160 patients. JAMA 240:30-34. [PubMed] [Google Scholar]
- 15.Levy, J., A. L. Smith, M. A. Kenny, B. Ramsey, and F. D. Schoenknecht. 1983. Bioactivity of gentamicin in purulent sputum from patients with cystic fibrosis or bronchiectasis: comparison with activity in serum. J. Infect. Dis. 148:1069-1076. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Solano, L., M. D. Macia, A. Fajardo, A. Oliver, and J. L. Martinez. 2008. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin. Infect. Dis. 47:1526-1533. [DOI] [PubMed] [Google Scholar]
- 17.Mearns, M. B. 1972. Treatment and prevention of pulmonary complications of cystic fibrosis in infancy and early childhood. Arch. Dis. Child. 47:5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendelman, P. M., A. L. Smith, J. Levy, A. Weber, B. Ramsey, and R. L. Davis. 1985. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am. Rev. Respir. Dis. 132:761-765. [DOI] [PubMed] [Google Scholar]
- 19.Merlo, C. A., M. P. Boyle, M. Diener-West, B. C. Marshall, C. H. Goss, and N. Lechtzin. 2007. Incidence and risk factors for multiple antibiotic-resistant Pseudomonas aeruginosa in cystic fibrosis. Chest 132:562-568. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, T. F., A. L. Brauer, K. Eschberger, P. Lobbins, L. Grove, X. Cai, and S. Sethi. 2008. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 177:853-860. [DOI] [PubMed] [Google Scholar]
- 21.Pennington, J. E. 1981. Penetration of antibiotics into respiratory secretions. Rev. Infect. Dis. 3:67-73. [DOI] [PubMed] [Google Scholar]
- 22.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, F. A. Wong, and M. Corrado. 1998. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob. Agents Chemother. 42:1098-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey, B. W., M. S. Pepe, J. M. Quan, K. L. Otto, A. B. Montgomery, J. Williams-Warren, K. M. Vasiljev, D. Borowitz, C. M. Bowman, B. C. Marshall, S. Marshall, A. L. Smith, et al. 1999. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N. Engl. J. Med. 340:23-30. [DOI] [PubMed] [Google Scholar]
- 24.Remminghorst, U., and B. H. Rehm. 2006. In vitro alginate polymerization and the functional role of Alg8 in alginate production by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 72:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabet, M., C. E. Miller, M. N. Dudley, and D. C. Griffith. 2008. In vivo antibacterial activity of MP-376 after aerosol or systemic administration in a chronic mouse model of pulmonary infection, abstr. 287. Abstr. 22nd Annu. N. Am. Cystic Fibrosis Conf.
- 26.Sabet, M., B. Saechao, J. Nguyen, K. Effenberger, O. Lomovskaya, M. N. Dudley, and D. C. Griffith. 2007. In vivo antibacterial activity of aerosol MP-376 in mouse models of pulmonary infection, abstr. 288. Abstr. 21st Annu. N. Am. Cystic Fibrosis Conf.
- 27.Sermet-Gaudelus, I., Y. Le Cocguic, A. Ferroni, M. Clairicia, J. Barthe, J. P. Delaunay, V. Brousse, and G. Lenoir. 2002. Nebulized antibiotics in cystic fibrosis. Paediatr. Drugs 4:455-467. [DOI] [PubMed] [Google Scholar]
- 28.Smith, A. L., B. W. Ramsey, D. L. Hedges, B. Hack, J. Williams-Warren, A. Weber, E. J. Gore, and G. J. Redding. 1989. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr. Pulmonol. 7:265-271. [DOI] [PubMed] [Google Scholar]
- 29.Valcke, Y., R. Pauwels, and M. Van der Straeten. 1990. The penetration of aminoglycosides into the alveolar lining fluid of rats. The effect of airway inflammation. Am. Rev. Respir. Dis. 142:1099-1103. [DOI] [PubMed] [Google Scholar]
- 30.Valcke, Y., R. Pauwels, and M. Van der Straeten. 1990. Pharmacokinetics of antibiotics in the lungs. Eur. Respir. J. 3:715-722. [PubMed] [Google Scholar]
- 31.Zindani, G. N., D. D. Streetman, D. S. Streetman, and S. Z. Nasr. 2006. Adherence to treatment in children and adolescent patients with cystic fibrosis. J. Adolesc. Health 38:13-17. [DOI] [PubMed] [Google Scholar]