Abstract
A series of 44 4-aminopiperidine derivatives was screened in vitro against four protozoan parasites (Trypanosoma brucei rhodesiense, Trypanosoma cruzi, Leishmania donovani, and Plasmodium falciparum). This screening identified 29 molecules selectively active against bloodstream-form T. b. rhodesiense trypomastigotes, with 50% inhibitory concentrations (IC50) ranging from 0.12 to 10 μM, and 33 compounds active against the chloroquine- and pyrimethamine-resistant K1 strain of P. falciparum (IC50 range, 0.17 to 5 μM). In addition, seven compounds displayed activity against intracellular T. cruzi amastigotes in the same range as the reference drug benznidazole (IC50, 1.97 μM) but were also cytotoxic to L-6 cells, showing little selectivity for T. cruzi. None of the molecules tested showed interesting antileishmanial activity against axenic amastigotes of L. donovani. To our knowledge, this is the first report of the antitrypanosomal activity of molecules bearing the 4-aminopiperidine skeleton.
Tropical diseases due to parasitic protozoa cause great mortality and disability in the less developed world. Malaria and kinetoplastid diseases such as human African trypanosomiasis (HAT, or sleeping sickness), Chagas' disease, and leishmaniases are among the main neglected parasitic diseases, affecting hundreds of millions of people worldwide (http://www.dndi.org/index.php/diseases.html?ids=2). However, the low income of the affected populations does not make it financially attractive for pharmaceutical companies to invest in the development of new drugs against these diseases. The number of antiprotozoal drugs available is limited, and they are old, toxic, and losing efficacy due to the emergence of resistant parasites. New drugs are thus urgently needed (37).
The landscape of drug discovery and development for new antiparasitic drugs has changed in the past few years thanks to financial backing from not-for-profit organizations and the involvement of public-private partnerships. The collaboration of various pharmaceutical companies with the Special Programme for Research and Training in Tropical Diseases (TDR), giving access to their compound libraries to help scientists search for new antiparasitic drugs, is a good example of the pragmatic approach required for the development of new medicaments for those neglected diseases (15, 32, 40). The screening of compound libraries to discover new hit and lead compounds against protozoan parasites has been used very efficiently by our group for the past few years. Very potent antiprotozoal compounds, active in vitro and in vivo against Trypanosoma brucei rhodesiense and Plasmodium falciparum, were identified by our group (16, 17, 38). These lead compounds were discovered by screening against T. brucei and P. falciparum a small library (<100 molecules) of dicationic compounds structurally related to known antiparasitic drugs (e.g., pentamidine, diminazene) but primarily synthesized by us for different medicinal targets.
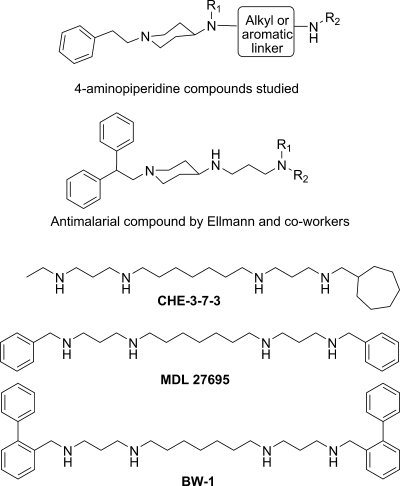
Following this successful approach, we have now selected from our in-house compound collection another series of polyamine molecules that possess the 1-phenethyl-4-aminopiperidine skeleton (Fig. 1). These molecules were anticipated to display antiprotozoal activity because they are structurally related to the known antitrypanosomal alkylpolyamines MDL27695 (7, 22), CHE-3-7-3 (5), and BW-1 (43) and particularly to the 4-aminopiperidine-based antimalarial agents described recently by Ellman and coworkers (8, 9). To test this hypothesis, 44 1-phenethyl-4-aminopiperidine derivatives were tested in vitro against four protozoan parasites responsible for HAT (T. brucei rhodesiense), Chagas' disease (Trypanosoma cruzi), visceral leishmaniasis (Leishmania donovani), and malaria (P. falciparum). Two compounds found to be active in vitro (50% inhibitory concentrations [IC50], <0.2 μM) and displaying acceptable selectivity toward the parasites (selectivity index [SI], >100) were subjected to in vivo assays in mouse models of acute HAT (T. b. brucei) or malaria (Plasmodium berghei). SI is defined as (50% cytotoxic concentration [CC50] for L-6 cells)/(IC50 for the parasite).
FIG. 1.
General structures of 1-phenethyl-4-aminopiperidine derivatives screened in this study and of known alkylpolyamine analogues with antiprotozoal activity.
MATERIALS AND METHODS
Chemistry.
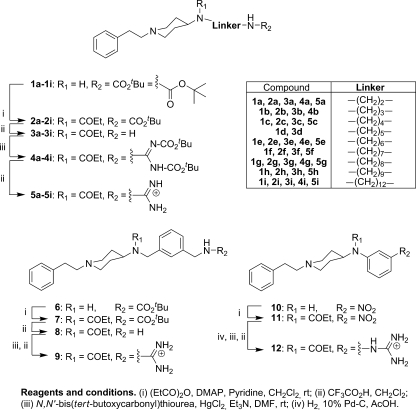
All of the compounds were synthesized by following the procedures previously reported by us (18-20) (Fig. 2) and were tested against the clinically relevant stages of four different parasites: T. b. rhodesiense trypomastigotes, T. cruzi amastigotes, L. donovani amastigotes, and P. falciparum erythrocytic stages. Compounds 3a to 3i, 5a to 5i, 8, 9, and 12 were tested in vitro as their trifluoroacetate salts. Compound 5h was administered in vivo as its dihydrochloride salt (molar mass, 516.59 g/mol), which was obtained at a 72% yield from the trifluoroacetate salt derivative by using a strongly basic anion-exchange resin (Amberlite IRA400) in the HCl form.
FIG. 2.
Structure and synthesis protocol for the compounds screened against T. brucei, T. cruzi, L. donovani, and P. falciparum.
Biological assays. (i) Cultivation of parasites, in vitro activity, and cytotoxicity.
For all the susceptibility assays with parasites and cells, each drug was tested in duplicate and each assay was repeated at least once.
(a) Activity against Trypanosoma brucei rhodesiense STIB900.
The T. b. rhodesiense STIB900 stock was isolated in 1982 from a human patient in Tanzania and, after several mouse passages, was cloned and adapted to axenic culture conditions (4, 42). Minimum essential medium (50 μl) supplemented with 25 mM HEPES, 1g/liter additional glucose, 1% minimal essential medium nonessential amino acids (100×), 0.2 mM 2-mercaptoethanol, 1 mM sodium pyruvate, and 15% heat-inactivated horse serum was added to each well of a 96-well microtiter plate. Seven threefold dilutions were used, covering a range from 90 to 0.123 μg/ml. Then 104 bloodstream forms of T. b. rhodesiense STIB900 in 50 μl were added to each well, and the plate was incubated at 37°C under a 5% CO2 atmosphere for 72 h. A 10-μl volume of Alamar Blue (resazurin; 12.5 mg in 100 ml double-distilled water) was then added to each well, and incubation was continued for a further 2 to 4 h (35). Then the plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA) using an excitation wavelength of 536 nm and an emission wavelength of 588 nm. Data were analyzed using the microplate reader software Softmax Pro (Molecular Devices Cooperation, Sunnyvale, CA).
(b) Activity against T. cruzi.
Rat skeletal myoblasts (L-6 cells) were seeded in 96-well microtiter plates at 2,000 cells/well in 100 μl RPMI 1640 medium with 10% fetal bovine serum and 2 mM l-glutamine. After 24 h, the medium was removed and replaced with 100 μl per well containing 5,000 trypomastigote forms of T. cruzi Tulahuen strain C2C4 containing the β-galactosidase (LacZ) gene (10). After 48 h, the medium was removed from the wells and replaced with 100 μl fresh medium with or without a serial drug dilution. Seven threefold dilutions were used, covering a range from 90 to 0.123 μg/ml. After 96 h of incubation, the plates were inspected under an inverted microscope to ensure the growth of the controls and sterility. Then the substrate chlorophenyl red β-d-galactopyranoside (CPRG)-Nonidet (50 μl) was added to all wells. A color reaction developed within 2 to 6 h and could be read photometrically at 540 nm. Data were transferred to the graphic program Softmax Pro (Molecular Devices), which calculated IC50.
(c) Activity against L. donovani.
Amastigotes of L. donovani strain MHOM/ET/67/L82 were grown in axenic culture at 37°C in SM medium (14) at pH 5.4 supplemented with 10% heat-inactivated fetal bovine serum under an atmosphere of 5% CO2 in air. One hundred microliters of culture medium with 105 amastigotes from axenic culture, with or without a serial drug dilution, was seeded in 96-well microtiter plates. Sevenfold dilutions were used, covering a range from 30 to 0.041 μg/ml. After 72 h of incubation, the plates were inspected under an inverted microscope to ensure the growth of the controls and sterile conditions. A 10-μl volume of Alamar Blue (12.5 mg resazurin dissolved in 100 ml distilled water) (30) was then added to each well, and the plates were incubated for another 2 h. Then the plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA) using an excitation wavelength of 536 nm and an emission wavelength of 588 nm. Data were analyzed using Softmax Pro software (Molecular Devices Cooperation, Sunnyvale, CA). The decrease in fluorescence (indicating inhibition) was expressed as a percentage of the fluorescence of control cultures and plotted against the drug concentrations. From the sigmoidal inhibition curves, the IC50 were calculated.
(d) Activity against P. falciparum.
In vitro activity against erythrocytic stages of P. falciparum was determined by a [3H]hypoxanthine incorporation assay (21, 29) using the chloroquine- and pyrimethamine-resistant strain K1, which originated from Thailand (41), and the standard drug chloroquine (C6628; Sigma). Compounds were dissolved in dimethyl sulfoxide at 10 mg/ml and were added to parasite cultures incubated in RPMI 1640 medium without hypoxanthine, supplemented with HEPES (5.94 g/liter), NaHCO3 (2.1 g/liter), neomycin (100 U/ml), Albumax (5 g/liter), and washed A+ human red blood cells (RBC) at 2.5% hematocrit (0.3% parasitemia). Seven twofold dilutions were used, covering a range from 5 to 0.156 μg/ml. The 96-well plates were incubated under a humidified atmosphere comprising 4% CO2, 3% O2, and 93% N2 at 37°C. After 48 h, 50 μl of [3H]hypoxanthine (0.5 μCi) was added to each well of the plate. The plates were incubated for a further 24 h under the same conditions. The plates were then harvested with a Betaplate cell harvester (Wallac, Zurich, Switzerland), and the RBC were transferred to a glass fiber filter and then washed with distilled water. The dried filters were inserted into a plastic foil with 10 ml of scintillation fluid, and scintillation was counted in a Betaplate liquid scintillation counter (Wallac, Zurich, Switzerland). IC50 were calculated from sigmoidal inhibition curves using Microsoft Excel.
(e) In vitro cytotoxicity to L-6 cells.
Assays were performed in 96-well microtiter plates. Each well contained 100 μl of RPMI 1640 medium supplemented with 1% l-glutamine (200 mM) and 10% fetal bovine serum, as well as 4 × 10 4 L-6 cells (a primary cell line derived from rat skeletal myoblasts). Seven threefold dilutions were used, covering a range from 90 to 0.123 μg/ml. After 72 h of incubation, the plates were inspected under an inverted microscope to ensure the growth of the controls and sterile conditions. A 10-μl volume of Alamar Blue was then added to each well, and the plates were incubated for another 2 h. Then the plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA) using an excitation wavelength of 536 nm and an emission wavelength of 588 nm. Data were analyzed using the microplate reader software Softmax Pro (Molecular Devices Cooperation, Sunnyvale, CA).
(ii) In vivo activity. (a) In vivo antiplasmodial efficacy.
All efficacy studies were approved by the veterinary authorities of the local government (Canton Basel, Switzerland). In vivo antimalarial activity was assessed basically as described previously (34). Groups of three female NMRI mice (20 to 22 g) were intravenously infected on day zero with 2 × 107 erythrocytes parasitized with the green fluorescent protein-transfected P. berghei strain ANKA (24). Compound 5h was formulated in 100% dimethyl sulfoxide at 10 mg/ml (concentration, 19.36 mM), diluted 10-fold in distilled water, and administered intraperitoneally (i.p.) (on four consecutive days, 4, 24, 48, and 72 h postinfection) in a volume of 10 ml kg−1 (i.e., 10 mg kg−1). The level of parasitemia was determined on day 4 postinfection (24 h after the last treatment) by fluorescence-activated cell sorter analysis. Activity was calculated as the difference between the mean level of parasitemia for the control group of five mice (set at 100%) and the mean level for the treated group, expressed as a percentage of the level of parasitemia for the control group. The survival time in days was also recorded up to 30 days after infection. A compound was considered curative if the animal survived to day 30 after infection with no detectable parasites.
(b) In vivo efficacy against T. brucei.
Female NMRI mice weighing 22 to 25 g were infected with cryopreserved stabilates of Trypanosoma brucei brucei STIB795, a derivative of strain 427, originally isolated from a tsetse fly (Glossina pallidipes) in Uganda in 1960. After strain 427 was passaged in a sheep and a tsetse fly and several times in mice, a clone was adapted to axenic in vitro culture (42). Each mouse was injected i.p. with 1 × 105 STIB795 bloodstream forms. Groups of four mice were treated i.p. with the compound at 50 mg/kg of body weight on days 3, 4, 5, and 6 postinfection. A control group remained untreated. The parasitemia of all animals was checked every second day up to day 14 postinfection and twice a week thereafter until 60 days. Deaths of animals were recorded in order to calculate the mean survival time. Surviving and aparasitemic mice were considered cured at 60 days and were then euthanized.
RESULTS AND DISCUSSION
In vitro activity against T. brucei rhodesiense.
The results of the in vitro activity assay are shown in Table 1. Twenty nine of 44 molecules showed activity against trypomastigote bloodstream forms of T. b. rhodesiense, with IC50 of <10 μM. Seven derivatives in particular displayed IC50 of <1 μM and were selective for the parasite (compounds 1e, 1f, 1g, 1h, 1i, 5i, and 6). The best in vitro activity was observed for the tert-butoxycarbonyl-substituted 8-carbon chain derivative 1g (IC50, 0.119 μM; SI, 130).
TABLE 1.
In vitro antiprotozoal activities of 4-aminopiperidine derivatives
| Compound | IC50 (μM) (SIa) for the following parasiteb:
|
Cytotoxicity (CC50 [μM]) for L-6 cellsc | |||
|---|---|---|---|---|---|
| T. b. rhodesiense | T. cruzi | L. donovani | P. falciparum | ||
| Reference drugd | 0.0075 | 1.97 | 0.205 | 0.172 | |
| 1a | 25.637 | 55.48 | 81.20 | 2.06 | 173.72 |
| 1b | 8.643 | 55.37 | 68.94 | 2.08 | 128.55 |
| 1c | 10.933 | 56.27 | >80 | 1.28 | 154.81 |
| 1d | 8.733 | 68.97 | >77 | 1.53 | 95.60 |
| 1e | 0.790 (36) | 28.59 | >74 | 0.90 (43) | 39.34 |
| 1f | 0.289 (41) | 11.94 | >72 | 0.77 (42) | 32.87 |
| 1g | 0.119 (130) | 5.72 | >69 | 0.62 (25) | 15.53 |
| 1h | 0.126 (48) | 3.73 | >67 | 0.36 (16) | 6.05 |
| 1i | 0.156 (52) | 3.16 | 17.82 | 0.32 (25) | 8.20 |
| 2a | 65.643 | >68 | >68 | 5.07 | >204 |
| 2b | 52.084 | >66 | >66 | 2.83 | >198 |
| 2c | 16.659 | >69 | >69 | 2.54 | 156.63 |
| 2e | 6.427 | >65 | 47.31 | 3.20 | 130.01 |
| 2f | 3.742 | 9.93 | 19.28 | 1.00 | 33.74 |
| 2g | 3.943 | 24.17 | 23.37 | 1.54 | 30.55 |
| 2h | 5.968 | 11.68 | 34.83 | 1.43 | 16.01 |
| 2i | 4.070 | 8.07 | 6.20 | 1.32 | 13.75 |
| 3a | 61.507 | >56 | >56 | >9.42 | >204 |
| 3b | 34.881 | >55 | >55 | >9.17 | >165 |
| 3c | 76.977 | >53 | >53 | >8.94 | >161 |
| 3d | 21.239 | >52 | 6.49 | >8.73 | >157 |
| 3e | 6.320 | >51 | >51 | >8.52 | >153 |
| 3f | 4.775 | >49 | >49 | >8.32 | >149 |
| 3g | 4.081 | >48 | >48 | >8.13 | 138.13 |
| 3h | 3.046 | >47 | >47 | >7.97 | 129.94 |
| 3i | 1.247 | 29.55 | 13.68 | 3.64 | 20.87 |
| 4a | 4.092 | 36.77 | 19.10 | 1.85 | 35.63 |
| 4b | 7.603 | >53 | 31.04 | 1.35 | 80.67 |
| 4e | 2.379 | 23.16 | 13.23 | 0.85 (29) | 24.89 |
| 4g | 1.590 | 12.29 | 14.81 | 0.60 (35) | 21.51 |
| 4i | 1.257 | 5.20 | 5.78 | 0.41 (18) | 7.54 |
| 5a | 54.677 | >52 | 22.76 | 3.91 | >157 |
| 5c | 129.667 | >49 | 23.02 | 3.18 | >149 |
| 5e | 2.925 | >47 | 39.66 | 0.80 (83) | 104.76 |
| 5f | 14.068 | >46 | 32.15 | 0.91 (>152) | >139 |
| 5g | 6.836 | >45 | 18.74 | 0.31 (222) | 69.09 |
| 5h | 3.748 | >44 | 14.85 | 0.17 (558) | 94.87 |
| 5i | 0.431 (24) | 6.90 | 10.37 | 0.21 (50) | 10.61 |
| 6 | 0.503 (219) | 17.30 | 78.11 | 1.66 | 110.62 |
| 7 | 15.140 | 41.03 | >79 | 5.87 | 128.07 |
| 8 | 9.270 | >49 | >49 | >8.24 | >148 |
| 9 | 98.813 | >56 | >56 | 2.49 | >168 |
| 10 | 21.280 | 46.15 | 23.25 | 10.55 | 256.68 |
| 11 | 38.624 | 49.09 | 21.45 | 2.44 | >191 |
| 12 | 4.238 | >62 | 73.77 | 2.17 | 102.34 |
Calculated as (CC50 for L6 cells)/(IC50 for parasites).
T. b. rhodesiense STIB900 trypomastigotes, T. cruzi Tulahuen strain C2C4 trypomastigotes, axenically grown L. donovani strain MHOM/ET/67/L82 amastigotes, and P. falciparum K1 erythrocytic stages were used. Boldface highlights IC50 of <1 μM.
Rat skeletal myoblast L-6 cells.
Reference drugs were melarsoprol for T. b. rhodesiense, benznidazole for T. cruzi, miltefosine for L. donovani, and chloroquine for P. falciparum. The IC50 of chloroquine for the chloroquine-sensitive strain of P. falciparum, NF54, is 0.0077 μM in this assay.
Interestingly, the most active compounds, except 5i, had the alkane structure 1, where the R1 position on the piperidine 4-amino group remains unsubstituted (R1 = H). This suggests that the substitution of this amine by a propionyl group (R1 = COEt) is detrimental to the antitrypanosomal activity for this series. The same trend was observed with the aromatic derivative compound 6, which was 30-fold more active than its propionamide derivative compound 7. This may indicate that the presence of an H-bond-donating group or a basic nitrogen atom in this position is critical for anti-T. brucei activity.
The polyamine metabolic pathway, which is present in most eukaryotes, including the majority of protozoa, has been explored as a possible target for chemotherapy in the past 25 years because endogenous polyamines (i.e., putrescine, spermidine, and spermine) are necessary for cellular growth and proliferation (36). The most successful example of this approach is represented by α-difluoromethylornithine, a suicide inhibitor of ornithine decarboxylase that was registered for the treatment of late-stage Trypanosoma brucei gambiense sleeping sickness in the 1990s (13). S-Adenosylmethionine decarboxylase and spermidine synthase, the other enzymes of the T. brucei polyamine biosynthetic pathway (31), have also been proposed as potential therapeutic targets (25, 26). On the other hand, enzymes of trypanothione metabolism, such as trypanothione reductase and trypanothione synthase, have also been validated as drug targets against trypanosomes (3, 12, 39). Trypanothione is a distinctive spermidine-glutathione conjugate synthesized by trypanosomes and used as an antioxidant mechanism similarly to glutathione in mammalian cells (23). The higher activity observed with the derivatives (compounds 1e to 1i) where the R1 position on the piperidine 4-amino group remains unsubstituted (i.e., secondary amino group, R1 = H) is compatible with a mechanism of action involving compounds that are able to mimic spermidine. In fact, the secondary amino group of these compounds may be protonated at physiological pH and may mimic the positively charged central NH group of spermidine. Accordingly, reduced activity was observed with the propionamide derivatives, which would not be protonated at physiological pH due to the reduced basicity of the propionamide nitrogen. Thus, it is possible that the antitrypanosomal activity of compounds 1e to 1i relates to their inhibition of trypanothione reductase, the enzyme responsible for maintaining the intracellular redox balance in trypanosomes by regenerating trypanothione disulfide [T(S)2] to its reduced state [T(SH)2] (28). Work to test this hypothesis is currently ongoing and will be reported in due course.
In vitro activity against P. falciparum.
The compounds were tested against the K1 strain of P. falciparum, which is resistant to the malaria drugs chloroquine and pyrimethamine (Table 1). Thirty-three compounds showed some activity against P. falciparum (IC50, <5 μM); among these, 13 (compounds 1e to 1i, 4e, 4g, 4i, and 5e to 5i) displayed low micromolar IC50 in the range of 0.17 to 0.91 μM. Most of these compounds were not very specific, showing low SI values from 16 to 83. However, two compounds, 5g and 5h, were fairly selective for the parasite, and 5h was the most active and selective compound of the series (IC50, 0.17 μM; SI, 558). Its IC50 was similar to that of the reference drug chloroquine in this assay (0.172 μM). Interestingly, six of the molecules showing the best activities against P. falciparum (compounds 1e, 1f, 1g, 1h, and 1i) also displayed the best anti-T. brucei action (Table 1).
Overall, the safety profile of the antimalarial hit compounds presented here (SI range, 16 to 558) was superior to that of the 4-aminopiperidine-based compounds described by Ellman and coworkers for the same range of activity against chloroquine-resistant strains of P. falciparum (9). The hit compound 5h showed the same potency as chloroquine (IC50, 0.17 μM) against the chloroquine- and pyrimethamine-resistant strain K1, with a SI of 558. As a whole, the antiplasmodial activity results were consistent with the structure-activity relationship observed by Ellman and colleagues (8, 9); thus, the presence of a basic amine in the linker was important, and the acylation of this group resulted in a drop in potency (compare compounds 1a to 1i with compounds 2a to 2i). Nevertheless, the most potent and selective compounds (5g, 5h, and 5i) bear acylated amines. This apparent contradiction may be explained by the presence of the guanidinium moiety, since basic groups, potentially protonated at intracellular pH, are thought to be important for the uptake and concentration of this kind of antimalarial compound in the acidic food vacuole (27).
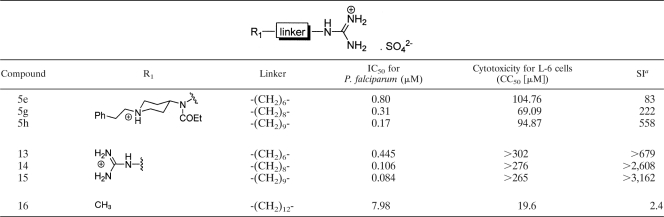
Another interesting feature of the structure-activity relationships of these polyamine compounds was the correlation observed between antimalarial activity and increasing methylene chain length for compounds 1d to 1i, 4a to 4i, and 5a to 5i. A series of methylene chain bisguanidinium analogues, compounds 13 to 15, previously synthesized by us (20) and structurally related to compounds 5e, 5g, and 5h, respectively, were also evaluated against P. falciparum K1, revealing a similar trend (Table 2). In consequence, the antimalarial activity of the compounds may be related to their lipophilicity and the selective uptake by infected RBC. A similar pattern was observed previously by Edwards et al. (22) for a series of α,ω-dibenzyltetraamine analogues of MDL27695 (Fig. 1), the activity of which correlated with selective uptake by RBC. The authors postulated that accumulation in RBC may be related to the lipophilicity of the compounds (22). On the other hand, Calas and colleagues have shown a positive relationship between the abilities of long-methylene-chain mono- and bisquaternary ammonium salts to inhibit P. falciparum growth in vitro and their inhibition of phosphatidylcholine biosynthesis (1, 2). A recent report by that group on the antimalarial activity of 12- and 16-methylene chain biscationic compounds mimicking the structure of choline and structurally related to compounds 13 to 15 (e.g., bisamidines, bisguanidines) also points to a similar mechanism of action involving the inhibition of the phospholipid-related choline carrier (11). Compounds that mimic the structure of choline, the major malarial phospholipid necessary for the de novo biosynthesis of phosphatidylcholine, are potential substrates for the parasite choline carrier (6). Thus, the methylene (di)cationic lead compounds (compounds 13 to 15) and the piperidine derivatives compounds 5e to 5i (assuming that the piperidinyl nitrogen atom is charged at physiological pH) might possibly interfere with the parasite choline transporter, as reported previously for structurally related compounds bearing two polar heads separated by a methylene spacer (11). However, this hypothesis would need experimental confirmation.
TABLE 2.
In vitro activities of alkyl mono- and bis-guanidinium compounds against P. falciparum K1
a Calculated as (CC50 for L6 cells)/(IC50 for P. falciparum).
In vitro activities against L. donovani and T. cruzi.
None of the 1-phenethyl-4-aminopiperidine derivatives screened in this study showed significant activity in vitro against axenic L. donovani amastigotes. There is no clear explanation for the lack of activity against axenic L. donovani. However, the low pH of the culture medium (pH 5.4), which corresponds to the pH of the phagolysosome, may reduce the diffusion across the parasite's membrane of these basic amino compounds that would be charged at this pH (33) and therefore would not reach their intracellular target.
On the other hand, seven compounds (compounds 1g to 1i, 2f, 2i, 4i, and 5i) showed notable activity (IC50, <10 μM) against intracellular T. cruzi amastigotes in the range of the reference drug benznidazole (1.97 μM). However, these compounds were not selective (SI, <3), since they were cytotoxic to L-6 cells.
In vivo activity.
Two compounds, 1g and 5h, were subjected to in vivo assays in the mouse models of acute HAT (T. b. brucei) and malaria (P. berghei) infection, respectively. Both compounds were chosen because they fulfilled the “hit criteria” of activity and selectivity against P. falciparum K1 and T. b. rhodesiense STIB900 (IC50, <0.2 μg/ml; SI, >100) as defined by the drug-screening network of TDR (32).
To detect a possible in vivo effect of compound 1g, the compound was first tested against the STIB795 mouse model, in which the disease has proven to be relatively easy to cure by trypanocides such as pentamidine or diminazene. However, since the i.p. injection of compound 1g (50 mg/kg given four times) into mice infected with T. b. brucei STIB795 did not extend the survival of the animals, the compound was not tested further in the more-difficult-to-cure STIB900 mouse model. On the other hand, compound 5h appeared to be toxic after the first application of 50 mg/kg to mice infected with P. berghei. Application of a lower dose of compound 5h (10 mg/kg given four times) produced no activity in this murine model of malaria infection. The lack of in vivo activity of compounds 1g and 5h may possibly be explained by unfavorable pharmacokinetic properties of the compounds.
Conclusion.
The screening of an in-house collection of 4-aminopiperidine analogues allowed the discovery of several compounds with submicromolar activity in vitro against a chloroquine- and pyrimethamine-resistant strain of P. falciparum and against T. b. rhodesiense. Interestingly, these compounds displayed higher SI values than the antimalarial 4-aminopiperidine derivatives described by Ellman and coworkers (8, 9). However, compounds 1g and 5h, the two compounds that were selected and tested in murine models of sleeping sickness and malaria, respectively, showed no activity in vivo. Poor pharmacokinetics may possibly account for the lack of in vivo efficacy of these compounds. Further investigations to determine the possible targets of the antitrypanosomal hit compounds are ongoing.
Acknowledgments
C.D. was the recipient of an I3P postdoctoral fellowship from the CSIC (I3P-DOC2004). C.F.-F. is the recipient of a postgraduate fellowship from the CSIC (I3P-BPD2004). This work was supported by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (to R.B.), “Proyecto Intramural Especial” grant 200680I121 from the CSIC (to L.N.), and “Programa Nacional de Biomedicina” grants SAF2006-04698 and SAF2006-13391-C03-02 from the Spanish “Ministerio de Educación y Ciencia.”
Footnotes
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Ancelin, M. L., M. Calas, A. Bonhoure, S. Herbute, and H. J. Vial. 2003. In vivo antimalarial activities of mono- and bis quaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 47:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancelin, M. L., M. Calas, V. Vidal-Sailhan, S. Herbute, P. Ringwald, and H. J. Vial. 2003. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob. Agents Chemother. 47:2590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustyns, K., K. Amssoms, A. Yamani, P. K. Rajan, and A. Haemers. 2001. Trypanothione as a target in the design of antitrypanosomal and antileishmanial agents. Curr. Pharm. Des. 7:1117-1141. [DOI] [PubMed] [Google Scholar]
- 4.Baltz, T., D. Baltz, C. Giroud, and J. Crockett. 1985. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 4:1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellevue, F. H., III, M. Boahbedason, R. Wu, P. M. Woster, J. Casero, A. Robert, D. Rattendi, S. Lane, and C. J. Bacchi. 1996. Structural comparison of alkylpolyamine analogues with potent in vitro antitumor or antiparasitic activity. Bioorg. Med. Chem. Lett. 6:2765-2770. [Google Scholar]
- 6.Biagini, G. A., E. M. Pasini, R. Hughes, H. P. De Koning, H. J. Vial, P. M. O'Neill, S. A. Ward, and P. G. Bray. 2004. Characterization of the choline carrier of Plasmodium falciparum: a route for the selective delivery of novel antimalarial drugs. Blood 104:3372-3377. [DOI] [PubMed] [Google Scholar]
- 7.Bitonti, A. J., J. A. Dumont, T. L. Bush, M. L. Edwards, D. M. Stemerick, P. P. McCann, and A. Sjoerdsma. 1989. Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with alpha-difluoromethylornithine cure murine malaria. Proc. Natl. Acad. Sci. USA 86:651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinner, K. M., J. Mi Kim, H. Habashita, I. Y. Gluzman, D. E. Goldberg, and J. A. Ellman. 2002. Novel and potent anti-malarial agents. Bioorg. Med. Chem. 10:3649-3661. [DOI] [PubMed] [Google Scholar]
- 9.Brinner, K. M., M. A. Powles, D. M. Schmatz, and J. A. Ellman. 2005. Potent 4-aminopiperidine based antimalarial agents. Bioorg. Med. Chem. Lett. 15:345-348. [DOI] [PubMed] [Google Scholar]
- 10.Buckner, F. S., C. L. Verlinde, A. C. La Flamme, and W. C. Van Voorhis. 1996. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 40:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calas, M., M. Ouattara, G. Piquet, Z. Ziora, Y. Bordat, M. L. Ancelin, R. Escale, and H. Vial. 2007. Potent antimalarial activity of 2-aminopyridinium salts, amidines, and guanidines. J. Med. Chem. 50:6307-6315. [DOI] [PubMed] [Google Scholar]
- 12.Comini, M. A., S. A. Guerrero, S. Haile, U. Menge, H. Lünsdorf, and L. Flohé. 2004. Validation of Trypanosoma brucei trypanothione synthetase as drug target. Free Radic. Biol. Med. 36:1289-1302. [DOI] [PubMed] [Google Scholar]
- 13.Croft, S., J. Urbina, and R. Brun. 1997. Chemotherapy of human leishmaniasis and trypanosomiasis, p. 245-257. In G. Hide, J. C. Mottram, G. H. Coombs, and P. H. Holmes (ed.), Trypanosomiasis and leishmaniasis: biology and control. CAB International, Oxon, United Kingdom.
- 14.Cunningham, I. 1977. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J. Protozool. 24:325-329. [DOI] [PubMed] [Google Scholar]
- 15.Dardonville, C. 2005. Recent advances in antitrypanosomal chemotherapy: patent literature 2002-2004. Expert Opin. Ther. Patents 15:1241-1257. [Google Scholar]
- 16.Dardonville, C., M. P. Barrett, R. Brun, M. Kaiser, F. Tanious, and W. D. Wilson. 2006. DNA binding affinity of bisguanidine and bis(2-aminoimidazoline) derivatives with in vivo antitrypanosomal activity. J. Med. Chem. 49:3748-3752. [DOI] [PubMed] [Google Scholar]
- 17.Dardonville, C., and R. Brun. 2004. Bisguanidine, bis(2-aminoimidazoline), and polyamine derivatives as potent and selective chemotherapeutic agents against Trypanosoma brucei rhodesiense. Synthesis and in vitro evaluation. J. Med. Chem. 47:2296-2307. [DOI] [PubMed] [Google Scholar]
- 18.Dardonville, C., C. Fernandez-Fernandez, S. L. Gibbons, G. J. Ryan, N. Jagerovic, A. M. Gabilondo, J. J. Meana, and L. F. Callado. 2006. Synthesis and pharmacological studies of new hybrid derivatives of fentanyl active at the μ-opioid receptor and I2-imidazoline binding sites. Bioorg Med. Chem. 14:6570-6580. [DOI] [PubMed] [Google Scholar]
- 19.Dardonville, C., N. Jagerovic, L. F. Callado, and J. J. Meana. 2004. Fentanyl derivatives bearing aliphatic alkaneguanidinium moieties: a new series of hybrid molecules with significant binding affinity for μ-opioid receptors and I2-imidazoline binding sites. Bioorg Med. Chem. Lett. 14:491-493. [DOI] [PubMed] [Google Scholar]
- 20.Dardonville, C., I. Rozas, L. F. Callado, and J. J. Meana. 2002. I2-imidazoline binding site affinity of a structurally different type of ligands. Bioorg. Med. Chem. 10:1525-1533. [DOI] [PubMed] [Google Scholar]
- 21.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards, M. L., D. M. Stemerick, A. J. Bitonti, J. A. Dumont, P. P. McCann, P. Bey, and A. Sjoerdsma. 1991. Antimalarial polyamine analogs. J. Med. Chem. 34:569-574. [DOI] [PubMed] [Google Scholar]
- 23.Fairlamb, A. H., and A. Cerami. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46:695-729. [DOI] [PubMed] [Google Scholar]
- 24.Franke-Fayard, B., H. Trueman, J. Ramesar, J. Mendoza, M. van der Keur, R. van der Linden, R. E. Sinden, A. P. Waters, and C. J. Janse. 2004. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137:23-33. [DOI] [PubMed] [Google Scholar]
- 25.Heby, O., L. Persson, and M. Rentala. 2007. Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas' disease, and leishmaniasis. Amino Acids 33:359-366. [DOI] [PubMed] [Google Scholar]
- 26.Heby, O., S. C. Roberts, and B. Ullman. 2003. Polyamine biosynthetic enzymes as drug targets in parasitic protozoa. Biochem. Soc. Trans. 31:415-419. [DOI] [PubMed] [Google Scholar]
- 27.Kumar, S., M. Guha, V. Choubey, P. Maity, and U. Bandyopadhyay. 2007. Antimalarial drugs inhibiting hemozoin (β-hematin) formation: a mechanistic update. Life Sci. 80:813-828. [DOI] [PubMed] [Google Scholar]
- 28.Martyn, D. C., D. C. Jones, A. H. Fairlamb, and J. Clardy. 2007. High-throughput screening affords novel and selective trypanothione reductase inhibitors with anti-trypanosomal activity. Bioorg Med. Chem. Lett. 17:1280-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matile, H., and J. R. L. Pink. 1990. Plasmodium falciparum malaria parasite cultures and their use in immunology, p. 221. In I. Lefkovits and B. Pernis (ed.), Immunological methods. Academic Press, San Diego, CA.
- 30.Mikus, J., and D. Steverding. 2000. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 48:265-269. [DOI] [PubMed] [Google Scholar]
- 31.Müller, S., G. H. Coombs, and R. D. Walter. 2001. Targeting polyamines of parasitic protozoa in chemotherapy. Trends Parasitol. 17:242-249. [DOI] [PubMed] [Google Scholar]
- 32.Nwaka, S., and A. Hudson. 2006. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 5:941-955. [DOI] [PubMed] [Google Scholar]
- 33.Orenes Lorente, S., R. Gomez, C. Jimenez, S. Cammerer, V. Yardley, K. de Luca-Fradley, S. L. Croft, L. M. Ruiz Perez, J. Urbina, D. Gonzalez Pacanowska, and I. H. Gilbert. 2005. Biphenylquinuclidines as inhibitors of squalene synthase and growth of parasitic protozoa. Bioorg. Med. Chem. 13:3519-3529. [DOI] [PubMed] [Google Scholar]
- 34.Peters, W. 1987. Chemotherapy and drug resistance in malaria, vol. 1. Academic Press, London, United Kingdom.
- 35.Raz, B., M. Iten, Y. Grether-Buhler, R. Kaminsky, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 36.Reguera, R. M., B. L. Tekwani, and R. Balana-Fouce. 2005. Polyamine transport in parasites: a potential target for new antiparasitic drug development. Comp. Biochem. Physiol. C 140:151-164. [DOI] [PubMed] [Google Scholar]
- 37.Renslo, A. R., and J. H. McKerrow. 2006. Drug discovery and development for neglected parasitic diseases. Nat. Chem. Biol. 2:701-710. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez, F., I. Rozas, M. Kaiser, R. Brun, B. Nguyen, W. D. Wilson, R. N. García, and C. Dardonville. 2008. New bis(2-aminoimidazoline) and bisguanidine DNA minor groove binders with potent in vivo antitrypanosomal and antiplasmodial activity. J. Med. Chem. 51:909-923. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt, A., and R. L. Krauth-Siegel. 2002. Enzymes of the trypanothione metabolism as targets for antitrypanosomal drug development. Curr. Top. Med. Chem. 2:1239-1259. [DOI] [PubMed] [Google Scholar]
- 40.Special Programme for Research and Training in Tropical Diseases (TDR). November 2008. Novo nordisk licences compound library to TDR-supported researchers. TDRnews 2008(18):6. http://apps.who.int/tdr/publications/tdrnews/pdf/TDRnews_issue_81.pdf. [Google Scholar]
- 41.Thaithong, S., G. H. Beale, and M. Chutmongkonkul. 1983. Susceptibility of Plasmodium falciparum to five drugs: an in vitro study of isolates mainly from Thailand. Trans. R. Soc. Trop. Med. Hyg. 77:228-231. [DOI] [PubMed] [Google Scholar]
- 42.Thuita, J. K., S. M. Karanja, T. Wenzler, R. E. Mdachi, J. M. Ngotho, J. M. Kagira, R. Tidwell, and R. Brun. 2008. Efficacy of the diamidine DB75 and its prodrug DB289, against murine models of human African trypanosomiasis. Acta Trop. 108:6-10. [DOI] [PubMed] [Google Scholar]
- 43.Zou, Y., Z. Wu, N. Sirisoma, P. M. Woster, R. A. Casero, Jr., L. M. Weiss, D. Rattendi, S. Lane, and C. J. Bacchi. 2001. Novel alkylpolyamine analogues that possess both antitrypanosomal and antimicrosporidial activity. Bioorg. Med. Chem. Lett. 11:1613-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]