Abstract
The mechanism underlying a dose-dependent, reversible increase in serum creatinine (SC) caused by the administration of PA-824, a novel nitroimidazo-oxazine, was evaluated in 47 healthy male and female volunteers. Subjects were administered either 800 or 1,000 mg PA-824 or matching placebo once daily for 8 days. The following renal function parameters were determined before and during dosing and after a 7-day washout: SC, glomerular filtration rate (GFR; measured as the iohexol clearance), effective renal plasma flow (ERPF; measured as the para-amino hippurate clearance), filtration fraction (FF), creatinine clearance (CrCl), extraglomerular creatinine excretion (EGCE; defined as CrCl minus GFR), blood urea nitrogen (BUN), and uric acid (UA) levels. Eight days' administration of 800 or 1,000 mg PA-824 was associated with increased SC and a trend toward decreased CrCl and EGCE. SC, CrCl, and EGCE values returned to normal/baseline within 1 week's washout. GFR, ERPF, FF, BUN, and UA values were similar across groups during treatment and washout. The reversible increase in SC observed in this and earlier trials of PA-824, thus, did not appear to be the result of a pathological effect on renal function (as measured by GFR, ERPF, FF, BUN, or UA). Pharmacokinetic analyses confirmed that PA-824 exposures were similar to those in previous healthy-volunteer clinical studies. That EGCE declined maximally when drug levels were highest suggests that PA-824 causes creatinine levels to rise by inhibiting renal tubular creatinine secretion. Such an effect, considered clinically benign, has been described for several marketed drugs.
PA-824 is a new chemical entity member of the class of compounds known as nitroimidazo-oxazines. PA-824 possesses significant antitubercular activity and a unique mechanism of action. Nonclinical studies of PA-824 highlighted important properties that may result in significant improvements in tuberculosis (TB) treatment. PA-824 demonstrated in vitro activity against both drug-sensitive and multidrug-resistant strains of TB and in vivo activity in a mouse model of TB (9, 11). In initial clinical studies to assess pharmacokinetics, safety, and tolerability in healthy volunteers, consistent, dose-dependent, and reversible elevations in serum creatinine (SC) were observed (4). Any one of several mechanisms could account for an increase in SC, including increased protein catabolism, reduced glomerular filtration rate (GFR), change in effective renal plasma flow (ERPF), and the inhibition of creatinine secretion by the renal proximal tubules. In subjects with normal renal function, the tubular secretion of creatinine can represent up to 40% of the total renal clearance of creatinine (10). A number of drugs, including pyrimethamine (1, 12), cimetidine (5, 8), and trimethoprim (7, 14), increase SC as a result of the clinically benign process of reducing tubular creatinine secretion, usually through competitive inhibition rather than through a pathological process that results in reduced GFR and/or effects on ERPF. Reliable markers of renal function, including monitoring iohexol and para-amino hippuric acid (PAH) clearance, have been developed for the determination of GFR and ERPF, respectively (2, 6).
The present study was designed to ascertain whether the observed effect of PA-824 on circulating creatinine is of clinical significance relative to renal function. To assess possible mechanisms, the study was designed to measure effects on GFR, ERPF, filtration fraction (FF), creatinine clearance (CrCl), extraglomerular creatinine excretion (EGCE), blood urea nitrogen (BUN), and uric acid (UA) levels that might result from the repeated administration of PA-824.
MATERIALS AND METHODS
Study design.
A phase I clinical study was conducted to evaluate effects on renal function of 8-day daily dosing of PA-824. Safety, tolerability, and pharmacokinetics (PK) of PA-824 also were evaluated. This study, conducted at the DaVita Clinical Research Unit in Minneapolis, MN, was a double-blind, placebo-controlled, ascending, multiple-dose study in healthy adult male and female volunteers. Renal function was assessed by determining the effects of PA-824 (compared to those of a placebo) on GFR, ERPF, FF, CrCl, EGCE, BUN, and UA.
The key indicators for renal function parameters were measured on days 5 and 8 of dosing and at the end of a 7-day washout period and were compared to baseline measures. To determine the effects of 8-day daily oral PA-824 dosing on GFR and ERPF, iohexol (3) and PAH (13) clearance, respectively, were determined during these specified time periods. The effect of 8-day daily oral PA-824 dosing on FF was determined by calculating the GFR/ERPF ratio for the specified time periods. CrCL was calculated from SC levels and 24-h urine creatinine (UC) excretion during the specified time periods by the equation CrCl = (UC × urine volume)/SC. A final renal function parameter was calculated as the difference between CrCl and GFR, which represents the proportion of excreted creatinine not removed from the blood by glomerular filtration; this is denoted here as EGCE. GFR, ERPF, CrCl, and EGCE then were adjusted (corrected) according to the subject's body surface area; data are expressed in nominal units of milliliters per minute per 1.73 m2 to reflect this adjustment.
A total of 47 subjects were enrolled in three cohorts staggered over time and randomized within each cohort to either PA-824 or matching placebo in an approximate 2:1 ratio. The first two cohorts of subjects (n = 16 for cohort 1; n = 15 for cohort 2) were dosed with 800 mg/day PA-824 or matching placebo; the third cohort (n = 16) was dosed with 1,000 mg/day PA-824 or matching placebo. Subjects were administered oral doses of PA-824 once daily, after an overnight fast, for 8 days. Subjects were housed in the clinic facility from the evening of day −3 (3 days before dosing) through day 9. Subjects returned on days 10, 11, and 12 for washout PK blood draws and on day 15 for a 24-h follow-up visit. Therefore, the total study duration for each subject from check-in through study termination spanned 19 days.
Subjects.
Healthy male and female volunteers were recruited for this study. Subjects were aged 19 to 50, and none was deemed by the principal investigator to have any clinically significant findings at study baseline in the medical history, clinical laboratory results, 12-lead electrocardiograms (ECGs), or physical examination. Entry criteria for this study were fundamentally the same as those for the studies summarized in the companion paper published in this issue (4). In brief, with the exception of hormonal contraceptives for women, subjects were excluded if they had taken any prescription or over-the-counter medication within 7 to 14 days of dosing or any of a variety of specific drugs with known significant side effects within 30 days of dosing. All study protocols and consent forms were reviewed and approved by an institutional review board constituted and operating per the U.S. Code of Federal Regulations. All subjects provided written informed consent prior to the initiation of the study. Subject safety was assessed by means of urinalysis and blood clinical laboratory tests (Table 1), 12-lead ECGs, physical exams and vital signs measurement, and the self reporting of adverse events (AEs) and regular direct AE query. All aspects of subject safety assessment were done at frequent intervals before, during, and after dosing.
TABLE 1.
Sampling schedule
| Sample and test type | Predose
|
Treatment
|
Postdose
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screening | Check-in (day −2) | Day −1 | Day 1 | Days 2-4 | Day 5 | Days 6-7 | Day 8 | Washout (day 9) | Follow-upd (day 15) | |
| Urine | ||||||||||
| Safety monitoring, urine drug screen, and/or storage | X | X | X | X | X | X | X | X | X | |
| CrCl (24 h) | X | X | X | X | X | X | ||||
| PAH clearance (0-4 h after injectiona) | X | X | X | X | ||||||
| Blood | ||||||||||
| Safety monitoring | X† | X | Xb | Xb | X | Xb | X | Xb | X | |
| Pharmacokinetics | X | X | X | X | X | Xc | X | |||
| Iohexol clearance (1-4 h after injectiona) | X | X | X | X | X | |||||
| PAH clearance (1-4 h after injectiona) | X | X | X | X | ||||||
Iohexol and PAH infusion times were standardized during the study to occur 3 h after dosing would occur on days 5 and 8.
Only BUN and SC were assessed on these days.
Washout PK draws were taken on each of days 9 to 12.
Or early withdrawal.
PK and pharmacodynamic (PD) sampling.
Plasma samples for PK analysis were collected before dosing each day during the treatment period (i.e., days 1 through 8) on the following schedule: 1, 2, 3, 4, 5, 6, 7, 8, 12, and 16 h after dosing on days 1 and 8; 24, 30, and 36 h after the day 8 dosing (i.e., during day 9); approximately 48, 72, and 96 h after the day 8 dosing (i.e., on days 10, 11, and 12); and during the follow-up visit on day 15 or on early withdrawal from the study. Additionally, for subjects in all cohorts except cohort 1, blood samples for PK were drawn on day 5 at 1, 2, 3, 4, 5, 6, 7, 8, 12, and 16 h after dosing. Blood and urine samples also were collected for measurement of iohexol, PAH, and CrCL before dosing and on days 5, 8, and 15 (Table 1).
Bioanalytical methods.
Blood samples were processed, stored, and analyzed as described in the companion paper (4).
PK and PD analyses.
PK parameters were analyzed for treatment and gender effects by the analysis of variance of PK data following a single dose (on day 1) and multiple doses (on day 8). The model included factors to assess the comparative effects of the two treatment groups, gender, and the relationship between gender and each treatment group (treatment-gender).
PD parameters each were analyzed separately by using an analysis of covariance (ANCOVA) model with the baseline as a covariate and treatment visit (i.e., study day), the treatment-visit interaction, and gender as factors.
All subjects that received at least one dose of the study drug were included in the PD analyses: 46 subjects for day 5 and day 8 analyses and 45 subjects for day 15 analyses. All 31 PA-824 subjects were included in the PK analyses.
RESULTS
Demographics and subject disposition.
A total of 47 subjects were enrolled into the study in three separate cohorts enrolled sequentially. The PA-824 dose was 800 mg/day in the first two cohorts and 1,000 mg/day in the third cohort. Overall, 16, 21, and 10 subjects received placebo, 800 mg/day PA-824, and 1,000 mg/day PA-824, respectively. Of the 47 subjects enrolled, 45 subjects completed the study. One placebo female subject dropped out of the study on day 4 for personal reasons, and one female subject taking 1,000 mg/day was discontinued because of an AE (see the section on safety parameters). Further details are shown in Table 2.
TABLE 2.
Demographic summary
| Trait | No. (%) in PA-824 dosage group:
|
|||
|---|---|---|---|---|
| Placebo | 800 mg | 1,000 mg | Overall | |
| Gender | ||||
| Female | 7 (44) | 14 (67) | 7 (70) | 28 (60) |
| Male | 9 (56) | 7 (33) | 3 (30) | 19 (40) |
| Age | ||||
| Mean | 24.0 | 23.1 | 25.6 | 23.9 |
| SD | 7.1 | 3.9 | 7.0 | 5.8 |
| Disposition | ||||
| Enrolled and dosed | 16 | 21 | 10 | 47 |
| Completed | 15 | 21 | 9 | 45 |
| Withdrawn early | 1 | 0 | 1 | 2 |
PK parameters.
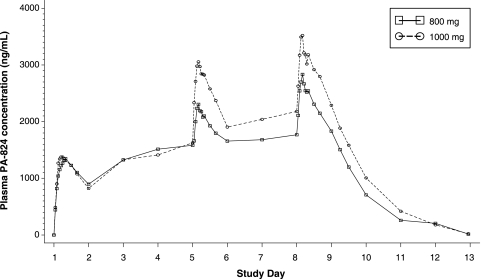
The daily administration of 800-mg and 1,000-mg tablets of PA-824 resulted in rising daily trough (Cmin) PA-824 levels until steady state was achieved (by day 7 or 8 for the majority of subjects), as shown in Fig. 1 and Table 3. Similar mean plasma concentrations were observed for each dose group following dosing through day 4. By day 8, the Cmin, Cmax (maximum concentration observed), and area under the concentration-time curve (AUC) all were 20 to 25% higher in the 1,000-mg/day group than in the 800-mg/day group (Table 3). However, the rates of absorption and elimination were similar in the two groups, as evidenced by comparable Tmax (time at which Cmax occurs) and t1/2 (half-life) values. PA-824 exposure was higher in females than in males after repeated administration. On day 8, Cmax values were 3.4 and 2.7 μg/ml (P = 0.0522) for females and males, respectively, and AUC values were 64.3 and 48.6 μg·h/ml (P = 0.0158) for females and males, respectively. Moreover, the median t1/2 was substantially lower for male subjects (14.9 h) than for female subjects (19.2 h) (P = 0.0034). This longer median half-life in female subjects may explain the greater PA-824 AUCs observed in females. Tmax values did not differ by gender.
FIG. 1.
Mean plasma concentrations of PA-824.
TABLE 3.
Mean PK parameters for subjects treated with 800 and 1,000 mg PA-824
| Dosage group (n) | Arithmetic mean (SD; min, max) PK parameter on:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1
|
Day 5
|
Day 8
|
||||||||||
| Cmax (μg/ml) | Tmaxa (h) | AUC0-τd (μg·hr/ml) | Cmax (μg/ml) | Cmin (μg/ml) | Tmax (hr) | AUC0-τ (μg·h/ml) | Cmax (μg/ml) | Cmin (μg/ml) | Tmax (hr) | AUC0-τ (μg·h/ml) | t1/2 (h) | |
| 800 mg (21) | 1.46 (0.60; 0.94, 3.12) | 5.00 (3.00, 16.0) | 25.7 (8.67; 17.9, 47.1) | 2.35b (0.54; 1.54, 3.01) | 1.43b (0.41; 0.94, 2.04) | 4.00b (3.00, 8.00) | 44.9b (10.6; 29.8, 61.2) | 2.92 (0.76; 1.76, 4.37) | 1.74 (0.57; 0.87, 2.90) | 4.00 (2.00, 8.00) | 54.6 (15.0; 32.9, 82.4) | 17.9c (3.03; 12.2, 22.8) |
| 1,000 mg (10) | 1.45 (0.34; 0.74, 2.00) | 4.54 (3.00, 8.00) | 26.0 (6.52; 11.4, 36.6) | 3.13 (0.75; 1.49, 4.13) | 1.86 (0.64; 0.69, 2.80) | 4.00 (2.05, 6.00) | 59.6 (16.5; 25.9, 81.5) | 3.65 (0.85; 2.41, 4.86) | 2.11 (0.60; 1.03, 2.90) | 4.00 (3.00, 8.00) | 69.0 (17.2; 39.3, 96.7) | 18.4 (2.82; 12.0, 22.3) |
Tmax is presented as the median (minimum, maximum) value.
n = 10. Day 5 sampling was instituted partway through recruitment per a protocol amendment.
n = 20. One subject's data did not permit accurate parameter estimation.
AUC0-τ, area under the concentration-time curve during the dosing interval.
PD parameters.
Placebo subjects from the three cohorts were pooled prior to performing any statistical analysis between active and placebo groups. To determine whether there was a cohort effect among placebo subjects, an analysis of variance was performed to compare data for each PD parameter across the placebo subjects from the three cohorts. Intercohort differences were well within what would be expected by chance, and no temporal trends were observed among the three placebo groups. Therefore, the data from placebo subjects from all three cohorts were pooled for all PD analyses.
Each PD parameter was compared between the subjects treated with PA-824 and those treated with placebo. The principal questions of interest focused on the overall treatment effect, whether the temporal response pattern for each variable during the 15 days of study was similar for the three treatment groups, and whether there was any gender-treatment interaction. While other factors associated with gender also were included in the statistical model (gender-visit interaction and gender-treatment-visit interaction), no gender-related factor (with the exception of BUN and UA levels) suggested that gender was an important factor in interpreting the results of this study (P > 0.14 in all cases). The gender differences seen in BUN and UA levels reflect the known gender differences typically seen in these parameters.
The sections below discuss the results for each renal function parameter in terms of changes from the baseline value. Table 4 provides absolute baseline values for each renal parameter measured in this study from which the change-from-baseline data were computed. The baseline values shown in this table appear to be similar across treatment groups, except for FF and EGCE, both of which are computed from other parameters.
TABLE 4.
Mean baseline renal function parameters by treatment group
| Dosage group (n) | Arithmetic mean (SD) result for parameter:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SC (mg/dl) | GFR (ml/min/1.73 m2) | ERPF (ml/min/1.73 m2) | FF (%) | CrCL (ml/min/1.73 m2) | EGCE (ml/min/1.73 m2) | BUN (mg/dl) | Serum UA (mg/dl) | UC (mg/dl) | |
| Placeboa (15-16) | 0.98 (0.13) | 95.86 (8.81) | 521.60 (166.38) | 0.21 (0.05) | 109.58 (13.49) | 16.02 (12.83) | 12.66 (2.64) | 4.71 (1.29) | 86.48 (32.60) |
| 800 mga (20-21) | 0.95 (0.13) | 98.46 (9.56) | 515.44 (183.67) | 0.93 (2.05) | 106.42 (13.58) | 7.18 (9.85) | 11.35 (2.44) | 4.53 (1.10) | 85.06 (23.79) |
| 1,000 mg (10) | 0.91 (0.10) | 93.62 (12.22) | 486.24 (106.87) | 0.23 (0.06) | 107.82 (12.60) | 17.92 (13.12) | 11.88 (1.27) | 3.97 (0.86) | 80.00 (27.76) |
Data were not available for all subjects for some parameters in this dose group.
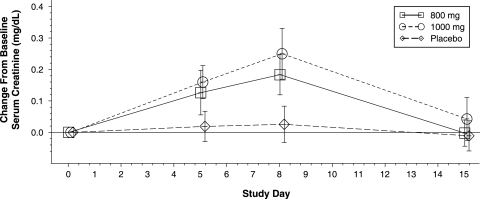
SC levels in the placebo group remained constant throughout the 15-day study period, as shown in Fig. 2 and Table 5. In contrast, the mean SC levels for both PA-824 dose groups progressively increased from baseline to day 5 to the last day of dosing (day 8). Between baseline and day 8, levels rose on average by 0.18 mg/dl (19%) and 0.25 mg/dl (27%) in the 800- and 1,000-mg/day groups, respectively; the largest individual increase was approximately 40% above the baseline level. After dosing was completed on day 8, SC levels returned to baseline by day 15. An ANCOVA model was used to compare results across visit and treatment groups, with the results confirming clear effects for treatment, visit, and the treatment-visit interaction (P < 0.0001 for each).
FIG. 2.
Mean (± standard deviations) changes from baseline values against time for SC levels.
TABLE 5.
Mean day 8 renal function parameter change-from-baseline values by treatment group
| Dosage group (n) | Day 8 change-from-baseline arithmetic mean (SD) result for parameter:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SC (mg/dl) | GFR (ml/min/1.73 m2) | ERPF (ml/min/1.73 m2) | FF (%) | CrCL (ml/min/1.73 m2) | EGCE (ml/min/1.73 m2) | BUN (mg/dl) | Serum UA (mg/dl) | UC (mg/dl) | |
| Placebo (15) | 0.02 (0.05) | −6.43 (6.69) | 102.38 (218.28) | −0.04 (0.05) | −13.17 (18.72) | −9.56 (19.90) | 1.10 (2.05) | 0.05 (0.40) | −28.50 (20.30) |
| PA-824a (800 mg) (20-21) | 0.18d (0.06) | −6.11 (6.36) | 130.27 (484.36) | −0.77 (2.10) | −21.78b (10.67) | −15.80c (13.06) | 1.53 (2.33) | −0.13 (0.45) | −15.15 (40.02) |
| PA-824 (1,000 mg) (10) | 0.25d (0.07) | −2.88 (10.16) | 2.80 (90.62) | −0.03 (0.06) | −21.60b (8.53) | −22.44b (13.88) | 0.73 (0.88) | −0.39b (0.50) | −17.70 (15.78) |
Data were not available for all subjects for some parameters in this dosage group.
P < 0.10.
P < 0.05.
P < 0.0001.
Taken together, these findings confirm the PA-824-induced SC elevation seen in previous phase I clinical studies (4). In the current study, data for each PD parameter (i.e., GFR, ERPF, FF, CrCl, EGCE, and BUN and UA levels) were examined using an ANCOVA model analogous to that used to analyze the SC data. For each PD parameter, the mean change-from-baseline values on day 8, when PA-824 plasma levels were maximal, were not dramatically different for subjects treated with PA-824 compared to those treated with placebo, as shown in Table 5. A similar pattern was noted after 5 days of dosing (not shown).
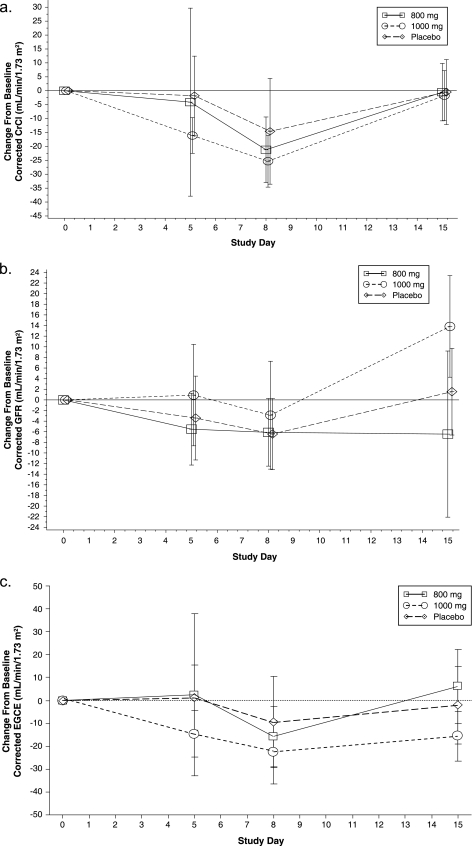
Minor statistical differences between the groups treated with PA-824 and the groups treated with placebo were seen for CrCl at days 5 and 8, GFR and FF at day 15, and EGCE at day 8. Mean CrCl values for each treatment group dropped somewhat by day 5, continued to drop through day 8, and returned to baseline at day 15 (Fig. 3a). These changes are consistent with the demonstrated effect of PA-824 on circulating creatinine levels and the fact that UC values did not change over time between the dose groups. The lack of change in UC values indicates overall appropriate 24-h urine collection procedures, which are essential for interpreting the CrCl findings.
FIG. 3.
Mean (± standard deviations) change from baseline values against time for CrCl (a), GFR (b), and EGCE (c) levels.
For GFR, the mean day 15 change-from-baseline values were different between the 800-mg PA-824 and placebo groups (−6.44 and 1.54 ml/min/1.73 m2, respectively; P = 0.0523) and between the 1,000-mg PA-824 and placebo groups (13.83 and 1.54 ml/min/1.73 m2, respectively; P = 0.0047); the lack of a dose response likely reflects variability in the measure as opposed to any effects of PA-824. Change-from-baseline values for GFR for the three treatment groups on days 5, 8, and 15 are shown in Fig. 3b. The FF differences from baseline seen at day 15 reflect the noted GFR differences, since FF is calculated as GFR/ERPF (data not shown). None of these changes is believed to be clinically meaningful.
EGCE values (calculated as the difference between CrCl and GFR) decreased modestly during the treatment, showing a maximal decrease at day 8 that reversed essentially to baseline by day 15, consistently with changes in CrCl levels (Fig. 3c). This pattern was similar for all treatment groups, although group comparisons showed modest differences between the PA-824 groups and placebo groups on day 8 (Table 5).
To further probe the possible impact of PA-824 treatment on renal function, PA-824 subjects were grouped not by dose level but by the degree of creatinine elevation. This approach was based on the logic that the subset of subjects with the greatest elevation in SC provides the best opportunity to rigorously query whether PA-824 affects other parameters of renal function. A ≥15% change in SC level from baseline was selected as potentially clinically meaningful. Therefore, data for PA-824-treated subjects were grouped for further analysis for each PD parameter according to whether their SC had or had not increased by 15% or more compared to the baseline level by the time PA-824 steady-state levels had been reached (day 8 or 9).
Using this analysis, change-from-baseline values for GFR, ERPF, FF, BUN, and UA generally all were similar for the PA-824 and placebo groups (data not shown). In the subset of PA-824 subjects whose SC values rose ≥15%, the mean CrCl and EGCE change-from-baseline values were modestly decreased at day 8 compared to those of the placebo group (−22.89 and −13.17 [P = 0.0226] and −19.73 and −9.56 ml/min/1.73 m2 [P = 0.0238], respectively); by day 15, the differences in change-from-baseline values between the PA-824 and placebo groups had disappeared.
Safety parameters.
No serious AEs were reported in this study. A greater percentage of 1,000-mg-dose subjects (90% [9 of 10 subjects]) reported at least one AE than did 800-mg-dose subjects (52% [11 of 21 subjects]) or placebo subjects (38% [6 of 16 subjects]). Headache was the most common AE, with 19% of the placebo subjects, 33% of the 800-mg-dose subjects, and 80% of the 1,000-mg-dose subjects reporting at least one headache. The principal investigator identified nine instances of prolonged corrected QT (QTc) intervals (by Bazett's formula), as summarized in Table 6. Each of these was flagged as an AE; all were deemed mild in severity, did not require any action, and resolved without sequelae. The prolongations detected at day 8 were judged possibly to be related to the study drug, whereas those detected at checkout were noted as unlikely to be related to the drug. All other ECG abnormalities were considered by the principal investigator to be clinically insignificant.
TABLE 6.
Summary of QTc prolongation AEs by treatment group
| Dosage group (n) | No. of events | No. with mild severity | No. with resolved outcome | No. detected on day 8 | No. detected at checkout | Mean absolute QTc (ms) | Mean QTc prolongation (ms) |
|---|---|---|---|---|---|---|---|
| Placebo (16) | 3 | 3 | 3 | 1 | 2 | 455 | 42 |
| PA-824 (800 mg) (21) | 5 | 5 | 5 | 4 | 1 | 463 | 42 |
| PA-824 (1,000 mg) (10) | 1 | 1 | 1 | 1 | 0 | 452 | 11 |
The principal investigator discontinued one subject (a 20-year-old female in the 1,000-mg-dose group) because of a severe generalized rash that occurred on day 9, approximately 32 h following the final dose administration. No other symptoms were observed. The subject was evaluated in the emergency room, and diphenhydramine, prednisone, and hydroxyzine were administered as concomitant therapy. The rash resolved approximately 11 days after onset. No other subjects were discontinued because of AEs.
One subject (a 23-year-old female in the 800-mg-dose group) completed the study with three ongoing AEs (mild proteinuria in the nephrotic range, mild hypoalbuminemia, and moderate iron deficiency). The subject's proteinuria decreased after the end of treatment and is now in the nonnephrotic range; the subject is being monitored periodically by a nephrologist and by trial site staff. A renal biopsy performed 20 months poststudy revealed focal segmental glomerulosclerosis, likely secondary type, although the subject remains fundamentally healthy, with normal renal function indices and no signs of peripheral edema or hypertension. A complete review of her screening and check-in laboratory values suggests that she had a preexisting undiagnosed clinical condition, including an atypical lipid profile, a BUN level below the lower limit of the normal range, and alanine aminotransferase and aspartate aminotransferase levels above the upper limit of the normal range at some baseline and/or screening evaluations. Furthermore, her eosinophil count was above normal at screening, at 6.7%, and progressively rose during the study to 8.9% by day 15, and she reported a personal and family history of allergies and rhinorrea.
DISCUSSION
This phase I, placebo-controlled, double-blind study was designed to characterize the renal effects of PA-824 at doses of 800 and 1,000 mg per day for 8 days in normal volunteers, as measured by changes in SC, GFR, ERPF, FF, CrCl, EGCE, BUN, and UA to determine the likely mechanism underlying the previously observed effect of PA-824 on circulating creatinine levels. The PK results of this study show that the Cmax and AUC values following the 800- and 1,000-mg PA-824 doses were similar on day 1 but significantly lower following an 800-mg dose than a 1,000-mg dose on day 8. The difference in exposure as measured by these two parameters was approximately 23%. Following the multiple daily administration of 800 and 1,000 mg PA-824, the median Tmax (4 h) and mean t1/2 (approximately 18 h) were similar between the 800- and 1,000-mg/day doses and similar to levels seen in previous phase I studies of PA-824 with similar dosing regimens (4).
Prior clinical studies of PA-824 demonstrated a reversible, but moderate, isolated increase in SC following single doses and multiple daily doses of PA-824 (4). In the present study, it was anticipated that healthy volunteers administered 800 or 1,000 mg PA-824 for 8 days, but not those who received placebo, would show a reversible increase in circulating creatinine levels compared to the baseline, and that circulating creatinine levels in these subjects would return to prestudy levels at the end of a 7-day posttreatment washout period. As expected, SC values rose and declined in parallel with rising and falling plasma drug levels.
The statistical evaluation of changes from the baseline for PD measures of renal function (GFR, ERPF, FF, CrCl, SC, BUN, UA, and EGCE) showed that other than creatinine elevations, 800- and 1,000-mg PA-824 multiday dosing were not associated with clinically meaningful alterations in any of the other renal function parameters examined. Mean CrCl and EGCE values for each treatment group dropped transiently during the study, with a marginally larger effect in PA-824 groups than in the placebo group. The changes noted in CrCl are consistent with the increased levels of circulating creatinine observed in subjects treated with 800 or 1,000 mg/day PA-824. Similar changes during time in EGCE among PA-824 subjects are to be expected based on these CrCl effects and an overall lack of effect of PA-824 on GFR. Any changes noted over time for the remaining parameters were comparable to those seen in subjects administered placebo. The fact that the BUN and UA data were similar in the PA-824 and placebo groups further underscores the conclusion that PA-824 selectively elevates circulating creatinine levels without causing a clinically significant change in renal physiology.
A further rigorous analysis for possible effects of PA-824 on renal function was performed by comparing the changes from baseline in PD measures of renal function between the subset of PA-824-administered subjects exhibiting a potentially clinically meaningful increase of ≥15% (from baseline) in SC by either day 8 or 9 and those of subjects receiving placebo. This analysis further confirmed that there was not a clinically meaningful difference in renal function as measured by GFR, ERPF, FF, CrCl, BUN, UA, and EGCE between subjects receiving PA-824 and those receiving placebo.
These results demonstrate that PA-824-related increases in circulating creatinine observed with multiday dosing at 800 and 1,000 mg/day are reversible after drug administration is terminated and are not reflective of an effect on GFR, ERPF, or any of the other measures of renal function examined in this study. The increases in SC are explained most readily by the clinically benign phenomenon of the inhibition of the tubular secretion of creatinine, which manifests in the present study's data as a decrease in EGCE, a parameter that represents CrCL not attributable to glomerular filtration. The inhibition of tubular secretion has been described as an effect of several marketed drugs, including pyrimethamine, cimetidine, and trimethoprim (1, 5, 7, 8, 12, 14).
Overall, the study findings indicate that, at a dose of up to 1,000 mg/day for 8 days, PA-824 is safe and well tolerated in healthy volunteers, and that the isolated and reversible increase in circulating creatinine observed after daily dosing with 800 or 1,000 mg PA-824 does not reflect a clinically meaningful impact on renal physiology.
Acknowledgments
This work was supported by grants from the Bill and Melinda Gates Foundation and the U.S. Agency for International Development.
We also thank Bruce Rodda, George Bakris, Ronald Portman, Massimo Cirillo, Elliott Pauli, the staff of DaVita Clinical Research, and the volunteers who participated in this study.
RTI International is a trade name of the Research Triangle Institute.
Footnotes
Published ahead of print on 15 June 2009.
REFERENCES
- 1.Andreev, E., M. Koopman, and L. Arisz. 1999. A rise in plasma creatinine that is not a sign of renal failure: which drugs can be responsible? J. Intern. Med. 246:247-252. [DOI] [PubMed] [Google Scholar]
- 2.Gabel, R. A., R. A. Ranaei, and S. D. Kivlighn. 1996. A new method of measuring renal function in conscious rats without the use of radioisotopes. J. Pharmacol. Toxicol. Methods 36:189-197. [DOI] [PubMed] [Google Scholar]
- 3.Gaspari, F., N. Perico, P. Ruggenenti, L. Mosconi, C. S. Amuchastegui, E. Guerini, E. Daina, and G. Remuzzi. 1995. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J. Am. Soc. Nephrol. 6:257-263. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg, A. M., M. W. Laurenzi, D. J. Rouse, K. D. Whitney, and M. K. Spigelman. 2009. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob. Agents Chemother. 53:3720-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gisclon, L. G., and K. M. Giacomini. 1988. Inhibition of cimetidine transport by creatinine in luminal membrane vesicles prepared from rabbit kidney. Drug Metab. Dispos. 16:331-332. [PubMed] [Google Scholar]
- 6.James, T. J., A. V. Lewis, G. D. Tan, P. Altmann, R. P. Taylor, and J. C. Levy. 2007. Validity of simplified protocols to estimate glomerular filtration rate using iohexol clearance. Ann. Clin. Biochem. 44:369-376. [DOI] [PubMed] [Google Scholar]
- 7.Kastrup, J., P. Petersen, R. Bartram, and J. M. Hansen. 1985. The effect of trimethoprim on serum creatinine. Br. J. Urol. 57:265-268. [DOI] [PubMed] [Google Scholar]
- 8.Larsson, R., G. Bodemar, B. Kagedal, and A. Walan. 1980. The effects of cimetidine (Tagamet) on renal function in patients with renal failure. Acta Med. Scand. 208:27-31. [DOI] [PubMed] [Google Scholar]
- 9.Lenaerts, A. J., V. Gruppo, K. S. Marietta, C. M. Johnson, D. K. Driscoll, N. M. Tompkins, J. D. Rose, R. C. Reynolds, and I. M. Orme. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey, A., R. Perrone, and N. Madias. 1988. Serum creatinine and renal function. Annu. Rev. Med. 39:465-490. [DOI] [PubMed] [Google Scholar]
- 11.Nuermberger, E., I. Rosenthal, S. Tyagi, K. N. Williams, D. Almeida, C. A. Peloquin, W. R. Bishai, and J. H. Grosset. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50:2621-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opravil, M., G. Keusch, and R. Luthy. 1993. Pyrimethamine inhibits renal secretion of creatinine. Antimicrob. Agents Chemother. 37:1056-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiser, I. W., and J. G. Porush. 2001. Evaluation of renal function, p. 1793-1801. In S. G. Massry and R. J. Glassock (ed.), Massry & Glassock's textbook of nephrology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 14.Roy, M. T., M. R. First, S. A. Myre, and W. Cacini. 1982. Effect of trimethoprim and sulphamethoxazole on serum creatinine in normal subjects. Ther. Drug Monit. 4:77-79. [DOI] [PubMed] [Google Scholar]