Abstract
Probenecid interacts with transport processes of drugs at several sites in the body. For most quinolones, renal clearance is reduced by concomitant administration of probenecid. The interaction between gemifloxacin and probenecid has not yet been studied. We studied the extent, time course, site(s), and mechanism of this interaction. Seventeen healthy volunteers participated in a randomized, two-way crossover study. Subjects received 320 mg gemifloxacin as an oral tablet without and with 4.5 g probenecid divided in eight oral doses. Drug concentrations in plasma and urine were analyzed by liquid chromatography-tandem mass spectrometry. WinNonlin was used for noncompartmental analysis, compartmental modeling, and statistics, and NONMEM was used for visual predictive checks. Concomitant administration of probenecid increased plasma gemifloxacin concentrations and amounts excreted in urine compared to baseline amounts. Data are average estimates (percent coefficients of variation). Modeling showed a competitive inhibition of the renal tubular secretion of gemifloxacin by probenecid as the most likely mechanism of the interaction. The estimated Km and Vmax for the saturable part of renal elimination were 9.16 mg/liter (20%) and 113 mg/h (21%), respectively. Based on the molar ratio, the affinity for the renal transporter was 10-fold higher for gemifloxacin than for probenecid. Since probenecid reached an ∼200-times-higher area under the molar concentration-time curve from 0 to 24 h than gemifloxacin, probenecid inhibited the active tubular secretion of gemifloxacin. Probenecid also reduced the nonrenal clearance of gemifloxacin from 25.2 (26%) to 21.0 (23%) liters/h. Probenecid inhibited the renal tubular secretion of gemifloxacin, most likely by a competitive mechanism, and slightly decreased nonrenal clearance of gemifloxacin.
Gemifloxacin is a fluoronaphthyridone antimicrobial with an enhanced activity against gram-positive pathogens (7). It is approved by the FDA for treatment of acute bacterial exacerbations of chronic bronchitis and mild to moderate community-acquired pneumonia. Renal clearance of gemifloxacin exceeds the glomerular filtration rate, which indicates net tubular secretion (2). As gemifloxacin exists primarily as a zwitterion at physiological pH, it is likely to interact with organic anion transporters (OATs) and organic cation transporters (OCTs) in renal tubular cells (31). Probenecid is known to inhibit active transport processes of anionic and cationic drug molecules at several sites in the body (13, 15, 16). Probenecid is well documented to decrease the renal secretion of many quinolones, e.g., gatifloxacin (21), levofloxacin (10, 11), and ciprofloxacin (14). However, there are no reports on the interaction between probenecid and gemifloxacin.
For studies of the time course and mechanism of a drug-drug interaction, compartmental modeling is superior to standard noncompartmental analysis (NCA). In addition to having other limitations, standard NCA does not allow one to predict the interaction for other dosage regimens of the drug plus inhibitor which might be more relevant for clinical practice. We are not aware of any studies of the time course and mechanism of interaction between probenecid and quinolones via compartmental modeling with humans or animals.
Our primary objective was to describe the extent of the interaction between gemifloxacin and probenecid using the administration of multiple doses of probenecid. Our secondary objective was to describe the time course and plausible mechanisms for the interaction between gemifloxacin and probenecid at the renal and nonrenal sites by compartmental modeling.
MATERIALS AND METHODS
Study design and drug administration.
Seventeen healthy Caucasian subjects (nine males and eight females) participated in the study. General clinical procedures were as previously described (18). The study was a randomized, controlled, two-way crossover analysis. Subjects fasted from 12 h before until 3 h after administration of gemifloxacin. In each of the two study periods, each subject received a single oral dose of 320 mg gemifloxacin (Factive, 320-mg tablets) either alone or with 4.5 g of probenecid (Probenecid Weimer, 500-mg tablets) divided into eight oral doses. We intended to study the maximum extent of interaction between probenecid and gemifloxacin. Therefore, we administered relatively high doses of probenecid throughout the whole plasma gemifloxacin concentration-time profile. Probenecid dosing was 1,000 mg at 10 h and 2 h before the administration of gemifloxacin, followed by 250 mg at 6 h and 14 h after the administration of gemifloxacin and 500 mg at 24 h, 36 h, 48 h, and 60 h after the administration of gemifloxacin. Doses of all drugs were given with 240 ml of low-carbonation, calcium-poor mineral water. Food and fluid intakes were strictly standardized on each study day. The treatment periods were separated by a washout period of at least 7 days. Subjects were requested to abstain from caffeine-containing foods and beverages, grapefruit products, orange juice, alcohol, and excessive exposure to sunlight during the study periods.
Sampling schedule.
Blood samples were collected from a forearm vein by use of an indwelling catheter immediately before the gemifloxacin dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 36, and 48 h postdose. Urine samples were collected before gemifloxacin administration and during the following time intervals: 0 to 4, 4 to 8, 8 to 12, 12 to 16, 16 to 24, 24 to 30, 30 to 36, 36 to 48, 48 to 60, and 60 to 72 h after the gemifloxacin dose. All samples were protected from daylight throughout sample preparation and analysis. All samples were immediately frozen and stored at −20°C until analysis.
Determination of plasma and urine drug concentrations.
For determination of plasma gemifloxacin and probenecid concentrations, 50 and 100 μl, respectively, of each sample were precipitated with 250 and 300 μl, respectively, acetonitrile containing the internal standard. After being thoroughly mixed, the samples were centrifuged for approximately 15 min at 11,000 × g for gemifloxacin samples or 5 min at 3,280 × g for probenecid samples. From each human urine sample, 50 μl of the supernatant was diluted by 400 μl of mobile phase containing the internal standard. For the prepared plasma and urine samples, 10 μl of each gemifloxacin sample or 15 μl of each probenecid sample was chromatographed on a reversed-phase column by isocratic elution. The samples were analyzed by liquid chromatography-tandem mass spectrometry, with a selected reaction-monitoring method. The reaction for gemifloxacin was precursor → product ion for gemifloxacin m/z 390 → m/z 313 and for the internal standard m/z 394 → m/z 313. Both analyses were in the positive mode. Gemifloxacin and the internal standard were eluted after approximately 1.1 min. Calibration was performed by weighted (1/y2) linear regression.
The reaction for probenecid was precursor → product ion for probenecid m/z 284 → m/z 240 and for the internal standard m/z 329 → m/z 205. Both analyses were in the negative mode. Probenecid and the internal standard were eluted after 1.4 and 0.8 min. Calibration was performed by weighted (1/x2) linear regression. The MacQuan software (version 1.6 [1991 to 1998]; PE Sciex, Thornhill, Ontario, Canada) was used for evaluation of chromatograms.
Calibration standards for plasma and urine were prepared by adding the appropriate volume of a standard solution of gemifloxacin or probenecid or of the more highly concentrated calibration standard solution to drug-free human plasma or urine. No interferences were observed in plasma and urine for gemifloxacin, probenecid, or the internal standard. Calibration curves in plasma and urine were linear between 0.0100 and 5.00 mg/liter for gemifloxacin and between 2.45 and 97.6 mg/liter for probenecid. The quantification limits were identical with the lowest calibration levels. The interday precision and the analytical recovery of the spiked quality control samples of gemifloxacin in human plasma (urine) ranged from 3.7 to 7.2% (5.7 to 7.7%) and from 100.9 to 101.4% (98.1 to 103.3%), respectively. The interday precision and the analytical recovery of the spiked quality control samples of probenecid in human plasma ranged from 5.1 to 5.8% and from 90.3 to 102.6%, respectively.
Computation.
WinNonlin (version 4.0.1; Pharsight Corporation) was used for NCA (as described previously [18]), compartmental modeling, and analysis of variance statistics. NONMEM version V release 1.1 (NONMEM Project Group, University of California, San Francisco) (4) was utilized for visual predictive checks (5) and Monte Carlo simulation.
Compartmental modeling.
All plasma and urine profiles for gemifloxacin (with and without probenecid) and probenecid were modeled simultaneously in order to derive the maximum amount of information from the data. Model discrimination, including visual predictive checks, was performed as reported previously (19).
Absorption and disposition of gemifloxacin.
One-, two-, and three-compartment disposition models with first-order absorption and with or without an absorption lag time were tested. The renal clearance of gemifloxacin was CLR=(fu·GFR)+{VmaxR/(KmR+[G])} where fu is the non-protein-bound fraction of gemifloxacin in plasma, GFR is the glomerular filtration rate, VmaxR is the maximum rate of the mixed-order renal elimination, KmR is the gemifloxacin concentration associated with a half-maximal rate for the mixed-order renal elimination of gemifloxacin, [G] is plasma gemifloxacin concentration, fu·GFR is filtration clearance, and VmaxR/(KmR + [G] describes net tubular secretion. Since the range of fu is between 0.3 and 0.4 (3) and all subjects had normal renal function, the renal filtration clearance of gemifloxacin, fu·GFR, is about 2 liters/h and accounts for only about 6% of the total body clearance. Therefore, the first-order component of renal clearance was fixed to 2 liters/h.
The nonrenal elimination of gemifloxacin was described as a first-order process. For models with a mechanism-based interaction at the nonrenal site, the nonrenal clearance was CLNR = VmaxNR/(KmNR+[G]) where VmaxNR is the maximum rate of the nonrenal elimination and KmNR is the gemifloxacin concentration associated with the half-maximal rate of the mixed-order nonrenal elimination.
Absorption and disposition of probenecid.
One- and two-compartment disposition models with parallel first-order and mixed-order elimination pathways were tested, since saturable elimination of probenecid has been reported previously (9, 34). The oral absorption was described as a first-order process with or without a lag time.
Interaction models.
It was assumed that the first-order renal elimination (glomerular filtration) of gemifloxacin was not influenced by probenecid and that probenecid interacts with the tubular secretion, with the nonrenal elimination, or with both. These interactions were described as a competitive, uncompetitive, or noncompetitive inhibition (Table 1). Alternatively, static interactions were expressed either as two different nonrenal clearances for gemifloxacin with and without probenecid or as two different intercompartmental clearances.
TABLE 1.
Mechanisms of inhibitiona
| Type of inhibition (inhibition constant) | Apparent Km | Apparent Vmax |
|---|---|---|
| Competitive (Kic) | Km·{1 + ([P]/Kic)} | Vmax |
| Uncompetitive (Kiu) | Km/{1 + ([P]/Kiu)} | Vmax/{1 + ([P]/Kiu)} |
| Noncompetitive (Ki) | Km | Vmax/{1 + ([P]/Ki)} |
[P], probenecid concentration. Kic describes the affinity of probenecid to the drug transporter without gemifloxacin. Kiu describes the affinity of probenecid to the transporter-gemifloxacin complex. Ki represents a special case in which probenecid binds to both the free transporter and the transporter-gemifloxacin complex with the same affinity (i.e., Ki = Kic = Kiu).
The models with different combinations of interactions at the renal and nonrenal sites are shown in Table 2. For the competitive interactions, the relative affinities (expressed as the ratio of the competitive inhibition constant [Kic] to the Km) of gemifloxacin and probenecid to the transporter were calculated (Table 1), with differences in molecular weight (389 g/mol for the gemifloxacin base and 285 g/mol for the probenecid base) being accounted for.
TABLE 2.
Interaction models
| Model | Inhibitiona of:
|
No. of subjects for which model had best AICb | ||
|---|---|---|---|---|
| Renal tubular secretion | Nonrenal clearance | Distributional clearance | ||
| 1 | C | S | None | 8 |
| 2 | NC | S | None | 2 |
| 3 | UC | S | None | 1 |
| 4 | C | C | None | 0 |
| 5 | NC | NC | None | 5 |
| 6 | C | S | S | 1 |
C, competitive inhibition; NC, noncompetitive inhibition; UC, uncompetitive inhibition (Table 1); S, static inhibition (two different parameters for treatment with and without probenecid).
AIC, Akaike information criterion.
Ethics.
The study was approved by the local ethics committee, and all subjects gave their written informed consent prior to starting the study. The study was conducted according to the revised version of the Declaration of Helsinki.
RESULTS
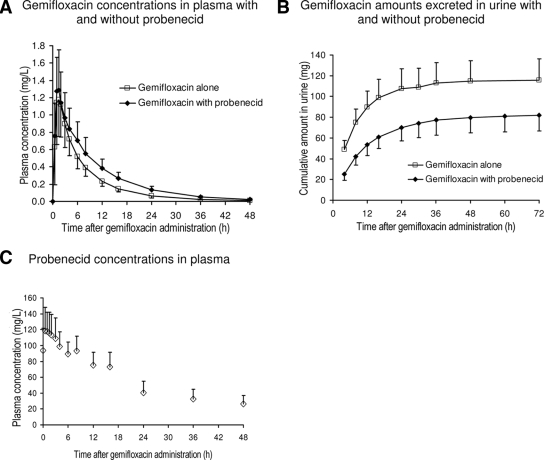
All 17 subjects completed the study. The average weight ± standard deviation was 69.1 ± 13 kg, and the average height was 173 ± 10 cm. Gemifloxacin concentrations in plasma were slightly higher for the treatment with probenecid (Fig. 1A), and the amount of gemifloxacin excreted in urine (Fig. 1B) was reduced by probenecid concentrations (Fig. 1C).
FIG. 1.
Gemifloxacin and probenecid concentrations in plasma and amounts in urine (averages ± standard deviations).
NCA.
Addition of probenecid to gemifloxacin reduced the median renal clearance from 13.1 to 6.49 liters/h (reduction by 51%, P < 0.01) and the median nonrenal clearance from 24.2 to 19.0 liters/h (reduction by 19%, P < 0.01). Therefore, total body clearance was decreased by 31% (P < 0.01). The median terminal half-life of gemifloxacin in plasma was increased from 8.09 to 9.49 h (+22%, P < 0.01) with probenecid.
Compartmental modeling.
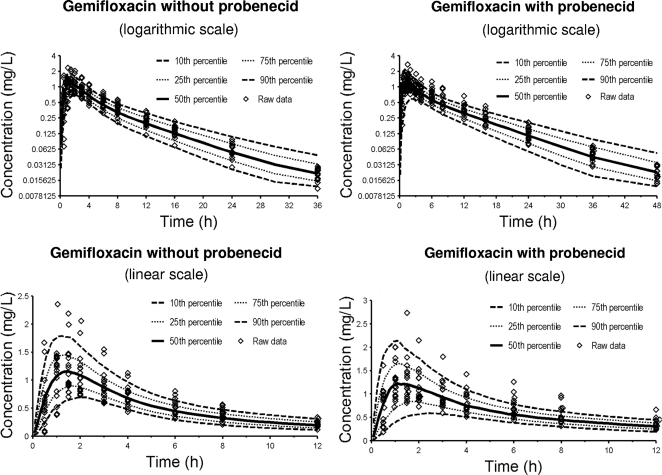
A two-compartment disposition model with a lag time was selected for gemifloxacin, and a one-compartment model with a lag time was selected for probenecid. The Akaike information criterion (data not shown) and the visual predictive checks showed that model 1 had the best predictive performance among the tested interaction models (Table 2 and Fig. 2). This suggested a competitive inhibition of the renal tubular secretion of gemifloxacin by probenecid as the most likely mechanism. Table 3 lists the average pharmacokinetic parameters of gemifloxacin for model 1.
FIG. 2.
Visual predictive check for plasma concentrations of gemifloxacin with and without probenecid for model 1 (Table 2). The plots show the observed data, the 80% prediction intervals (10th to 90th percentiles), and the interquartile range (25th to 75th percentiles). Ideally, 50% of the observed data points should fall inside the interquartile range and 80% of the observed data should fall inside the 80% prediction interval at each time point.
TABLE 3.
Pharmacokinetic parameter estimates and between-subject variability for gemifloxacin for model 1d
| With or without probenecid | Avg (% coefficient of variation) for gemifloxacina
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ka (h−1) | Tlag (h) | CLNR (liters h−1) | KmR (mg liter−1) | VmaxR (mg h−1) | CLR (liters h−1) | Kic (mg liter−1) | V1 (liters) | V2 (liters) | CLIC (liters h−1) | |
| With probenecid | 0.897 (104) | 0.129 (104) | 21.0 (23)b | |||||||
| 9.16 (20) | 113 (21) | 2c | 69.3 (26) | 89.5 (62) | 160 (31) | 39.8 (58) | ||||
| Without probenecid | 0.839 (62) | 0.223 (104) | 25.2 (26)b | |||||||
Ka, absorption rate constant; Tlag, absorption lag time; CLNR, apparent nonrenal clearance; V1, apparent volume of distribution of central compartment; V2, apparent volume of distribution of peripheral compartment; CLIC, apparent intercompartmental clearance. Only for Ka, Tlag, and CLNR, two parameters were allowed within subjects for gemifloxacin with and without probenecid.
Values are significantly different (P < 0.01).
Fixed, not estimated.
See Table 2 for model 1.
After we accounted for differences in molecular weight, the affinity of gemifloxacin to the renal tubular transporter was 10-fold higher than that of probenecid. Since probenecid reached an ∼200-fold-higher area under the molar concentration-time curve from 0 to 24 h than probenecid (Fig. 1), probenecid inhibited the secretion of gemifloxacin at the renal transporter.
Monte Carlo simulations suggested that the gemifloxacin area under the concentration-time curve (0 to 24 h) is increased by 20% (median) when gemifloxacin is given together with clinically relevant doses of probenecid (500 mg twice daily).
DISCUSSION
The interaction with probenecid has been studied for a long time and for many quinolones and beta-lactams. The extent of interaction with probenecid may reach clinical significance for drugs which display active tubular secretion (12, 21, 33). Reduced renal clearance with probenecid has been reported for several quinolones, e.g., norfloxacin (25), fleroxacin (23), enoxacin (35), ciprofloxacin (14), levofloxacin (10, 11), and gatifloxacin (21). Moxifloxacin and sparfloxacin were not affected by probenecid (24, 28).
In the vast majority of studies, the interaction with probenecid is studied at the renal site. Less is known about the interaction of probenecid with drugs at other sites in the body. Probenecid interacts with both OATs and OCTs that are involved in the active renal secretion of drug molecules (13), and they have been found at various other sites in the body (20, 26). Recently, probenecid was shown to affect active transport processes at the blood-brain barrier (6). The importance of drug transporters in hepatocytes has been highlighted by Cummins et al. (8). Through the influence of probenecid on drug transporters in the hepatocytes or enterocytes, an influence of probenecid on the nonrenal elimination and metabolism of drugs seems likely. In humans, probenecid decreases the renal excretion of paracetamol glucuronide by 79% (15) and also increases the biotransformation of carbamazepine (17).
The renal elimination of unchanged gemifloxacin accounts for 20 to 40% of the dose. Most of the dose is eliminated via other routes, which offers various possibilities for an interaction with probenecid at the nonrenal site(s). As a zwitterion at physiological pH values (pKa1 = 6.5, pKa2 = 8.9), gemifloxacin might be able to interact with both OATs and OCTs (27). Cimetidine, an inhibitor of the organic cationic renal transport, decreases the renal clearance of gemifloxacin by 28% (1).
We studied the interaction between gemifloxacin and probenecid. Compartmental modeling of plasma and urine profiles of gemifloxacin and probenecid simultaneously showed that a model with competitive interaction at the renal site and static interaction at the nonrenal site had the best predictive performance of the tested models. As the extent of inhibition at the nonrenal site was much smaller than at the renal site, a specific mechanism could not be identified for the nonrenal interaction. Therefore, our final model had a competitive interaction at the renal site and a static interaction at the nonrenal site. Also, from the physiological point of view, a competitive mechanism seems the most reasonable, as it describes a situation in which gemifloxacin and probenecid compete for the same active site of the renal tubular transporter.
It was recently pointed out that information about renal transport processes of quinolones is important, e.g., to explore potential toxicities due to administration of quinolones with other drugs, such as methotrexate (32). While from our modeling no conclusions can be drawn about which particular transporters are involved in the studied interaction, the model proposed here can predict the extent of the interaction for dosage regimens other than those studied. Drug interactions at the tubular secretion site are frequently reported, and our model can be utilized as the basis for models for other drugs and drug groups.
In a recent overview of the quinolone renal transport literature, studies were cited suggesting the involvement of both OATs and OCTs for ciprofloxacin, norfloxacin, ofloxacin, enoxacin, enrofloxacin, fleroxacin, pefloxacin, and levofloxacin (32). Both OAT1 and OAT3 were previously identified for renal uptake of carboxyfluoroquinolones (29, 30, 32). Levofloxacin was shown to inhibit OCT2 (22). It was suggested that zwitterionic quinolones such as ofloxacin, levofloxacin, and gemifloxacin can interact with both OATs and OCTs. VanWert et al. (32) found that ciprofloxacin was transported by OAT3 and that ciprofloxacin, norfloxacin, ofloxacin, and gatifloxacin inhibited OAT3. Ciprofloxacin did not interact with OAT1, while probenecid inhibited both OAT1 and OAT3. In the absence of information about specific transporters interacting with gemifloxacin, it seems likely that OATs, OCTs, or both are involved in the interaction between gemifloxacin and probenecid. For probenecid and also several quinolones, interactions have been reported with OAT1, OAT3, and OCTs.
We are not aware of any reports of a mechanism-based model for the interaction of quinolones and probenecid in humans or animals. As most quinolones are eliminated via tubular secretion, the competitive inhibition of renal tubular secretion that we found for gemifloxacin with probenecid could also apply to other quinolones.
In conclusion, modeling suggested a competitive inhibition of the renal tubular secretion of gemifloxacin by probenecid. Based on the molar ratio, the affinity to the renal transporter was 10-fold higher for gemifloxacin than for probenecid, but probenecid inhibited the secretion of gemifloxacin because probenecid reached an ∼200 times-higher average area under the molar concentration-time curve from 0 to 24 h than gemifloxacin. Future mechanistic studies for other quinolones are required to further explore this mechanism of interaction.
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Allen, A., N. Bird, R. Dixon, F. Hickmott, V. Pay, A. Smith, and M. Stahl. 2001. Effect of cimetidine on the pharmacokinetics of oral gemifloxacin in healthy volunteers. Clin. Drug Investig. 21:519-526. [Google Scholar]
- 2.Allen, A., E. Bygate, S. Oliver, M. Johnson, C. Ward, A.-J. Cheon, Y. S. Choo, and I.-C. Kim. 2000. Pharmacokinetics and tolerability of gemifloxacin (SB-265805) after administration of single oral doses to healthy volunteers. Antimicrob. Agents Chemother. 44:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. 1999. FACTIVE investigator brochure, 4th ed. SmithKlineBeecham, Herts, United Kingdom.
- 4.Beal, S. L., A. J. Boeckmann, L. B. Sheiner, and the NONMEM Project Group. 1999. NONMEM users guides, version 5. University of California at San Francisco, San Francisco.
- 5.Bulitta, J. B., S. B. Duffull, M. Kinzig-Schippers, U. Holzgrabe, U. Stephan, G. L. Drusano, and F. Sörgel. 2007. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 51:2497-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinckers, R., I. Smolders, A. Meurs, G. Ebinger, and Y. Michotte. 2005. Quantitative in vivo microdialysis study on the influence of multidrug transporters on the blood-brain barrier passage of oxcarbazepine: concomitant use of hippocampal monoamines as pharmacodynamic markers for the anticonvulsant activity. J. Pharmacol. Exp. Ther. 314:725-731. [DOI] [PubMed] [Google Scholar]
- 7.Cormican, M. G., and R. N. Jones. 1997. Antimicrobial activity and spectrum of LB20304, a novel fluoronaphthyridone. Antimicrob. Agents Chemother. 41:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummins, C. L., C. Y. Wu, and L. Z. Benet. 2002. Sex-related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P-glycoprotein. Clin. Pharmacol. Ther. 72:474-489. [DOI] [PubMed] [Google Scholar]
- 9.Emanuelsson, B. M., B. Beermann, and L. K. Paalzow. 1987. Non-linear elimination and protein binding of probenecid. Eur. J. Clin. Pharmacol. 32:395-401. [DOI] [PubMed] [Google Scholar]
- 10.Fish, D. N., and A. T. Chow. 1997. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 32:101-119. [DOI] [PubMed] [Google Scholar]
- 11.Gaitonde, M. D., P. Mendes, E. S. A. House, and K. H. Lehr. 1995. The effects of cimetidine and probenecid on the pharmacokinetics of levofloxacin (LVFX), abstr. A41, p. 8. Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA.
- 12.Garton, A. M., R. P. Rennie, J. Gilpin, M. Marrelli, and S. D. Shafran. 1997. Comparison of dose doubling with probenecid for sustaining serum cefuroxime levels. J. Antimicrob. Chemother. 40:903-906. [DOI] [PubMed] [Google Scholar]
- 13.Gisclon, L. G., R. A. Boyd, R. L. Williams, and K. M. Giacomini. 1989. The effect of probenecid on the renal elimination of cimetidine. Clin. Pharmacol. Ther. 45:444-452. [DOI] [PubMed] [Google Scholar]
- 14.Jaehde, U., F. Sorgel, A. Reiter, G. Sigl, K. G. Naber, and W. Schunack. 1995. Effect of probenecid on the distribution and elimination of ciprofloxacin in humans. Clin. Pharmacol. Ther. 58:532-541. [DOI] [PubMed] [Google Scholar]
- 15.Kamali, F. 1993. The effect of probenecid on paracetamol metabolism and pharmacokinetics. Eur. J. Clin. Pharmacol. 45:551-553. [DOI] [PubMed] [Google Scholar]
- 16.Kamali, F., and M. D. Rawlins. 1992. Influence of probenecid and paracetamol (acetaminophen) on zidovudine glucuronidation in human liver in vitro. Biopharm. Drug Dispos. 13:403-409. [DOI] [PubMed] [Google Scholar]
- 17.Kim, K. A., S. O. Oh, P. W. Park, and J. Y. Park. 2005. Effect of probenecid on the pharmacokinetics of carbamazepine in healthy subjects. Eur. J. Clin. Pharmacol. 61:275-280. [DOI] [PubMed] [Google Scholar]
- 18.Landersdorfer, C. B., C. M. Kirkpatrick, M. Kinzig-Schippers, J. B. Bulitta, U. Holzgrabe, G. L. Drusano, and F. Sörgel. 2007. Population pharmacokinetics at two dose levels and pharmacodynamic profiling of flucloxacillin. Antimicrob. Agents Chemother. 51:3290-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landersdorfer, C. B., C. M. Kirkpatrick, M. Kinzig, J. B. Bulitta, U. Holzgrabe, and F. Sorgel. 2008. Inhibition of flucloxacillin tubular renal secretion by piperacillin. Br. J. Clin. Pharmacol. 66:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier, P. J., U. Eckhardt, A. Schroeder, B. Hagenbuch, and B. Stieger. 1997. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology 26:1667-1677. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima, M., T. Uematsu, K. Kosuge, H. Kusajima, T. Ooie, Y. Masuda, R. Ishida, and H. Uchida. 1995. Single- and multiple-dose pharmacokinetics of AM-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob. Agents Chemother. 39:2635-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuda, M., N. Kimura, and K. Inui. 2006. Interactions of fluoroquinolone antibacterials, DX-619 and levofloxacin, with creatinine transport by renal organic cation transporter hOCT2. Drug Metab. Pharmacokinet. 21:432-436. [DOI] [PubMed] [Google Scholar]
- 23.Shiba, K., A. Saito, J. Shimada, S. Hori, M. Kaji, T. Miyahara, H. Kusajima, S. Kaneko, S. Saito, T. Ooie, and H. Uchida. 1990. Renal handling of fleroxacin in rabbits, dogs, and humans. Antimicrob. Agents Chemother. 34:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimada, J., T. Nogita, and Y. Ishibashi. 1993. Clinical pharmacokinetics of sparfloxacin. Clin. Pharmacokinet. 25:358-369. [DOI] [PubMed] [Google Scholar]
- 25.Shimada, J., T. Yamaji, Y. Ueda, H. Uchida, H. Kusajima, and T. Irikura. 1983. Mechanism of renal excretion of AM-715, a new quinolonecarboxylic acid derivative, in rabbits, dogs, and humans. Antimicrob. Agents Chemother. 23:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shitara, Y., H. Sato, and Y. Sugiyama. 2005. Evaluation of drug-drug interaction in the hepatobiliary and renal transport of drugs. Annu. Rev. Pharmacol. Toxicol. 45:689-723. [DOI] [PubMed] [Google Scholar]
- 27.Sorgel, F., J. Bulitta, and M. Kinzig-Schippers. 2001. How well do gyrase inhibitors work? The pharmacokinetics of quinolones. Pharm. Unserer Zeit 30:418-427. [DOI] [PubMed] [Google Scholar]
- 28.Stass, H., and R. Sachse. 2001. Effect of probenecid on the kinetics of a single oral 400mg dose of moxifloxacin in healthy male volunteers. Clin. Pharmacokinet. 40(Suppl. 1):71-76. [DOI] [PubMed] [Google Scholar]
- 29.Sweet, D. H., L. M. Chan, R. Walden, X. P. Yang, D. S. Miller, and J. B. Pritchard. 2003. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am. J. Physiol. Renal Physiol. 284:F763-F769. [DOI] [PubMed] [Google Scholar]
- 30.Sweet, D. H., N. A. Wolff, and J. B. Pritchard. 1997. Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J. Biol. Chem. 272:30088-30095. [DOI] [PubMed] [Google Scholar]
- 31.Ullrich, K. J., G. Rumrich, C. David, and G. Fritzsch. 1993. Bisubstrates: substances that interact with both, renal contraluminal organic anion and organic cation transport systems. II. Zwitterionic substrates: dipeptides, cephalosporins, quinolone-carboxylate gyrase inhibitors and phosphamide thiazine carboxylates; nonionizable substrates: steroid hormones and cyclophosphamides. Pflugers Arch. 425:300-312. [DOI] [PubMed] [Google Scholar]
- 32.VanWert, A. L., C. Srimaroeng, and D. H. Sweet. 2008. Organic anion transporter 3 (oat3/slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin. Mol. Pharmacol. 74:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlasses, P. H., A. M. Holbrook, J. J. Schrogie, J. D. Rogers, R. K. Ferguson, and W. B. Abrams. 1980. Effect of orally administered probenecid on the pharmacokinetics of cefoxitin. Antimicrob. Agents Chemother. 17:847-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vree, T. B., E. W. Van Ewijk-Beneken Kolmer, E. W. Wuis, and Y. A. Hekster. 1992. Capacity-limited renal glucuronidation of probenecid by humans. A pilot Vmax-finding study. Pharm. Weekbl. Sci. 14:325-331. [DOI] [PubMed] [Google Scholar]
- 35.Wijnands, W. J., T. B. Vree, A. M. Baars, and C. L. van Herwaarden. 1988. Pharmacokinetics of enoxacin and its penetration into bronchial secretions and lung tissue. J. Antimicrob. Chemother. 21(Suppl. B):67-77. [DOI] [PubMed] [Google Scholar]