VIM-1-producing Klebsiella pneumoniae strains, increasingly isolated in Greece since 2002, constitute the majority of multiresistant clinical isolates of this species found in most Greek hospitals (9). Moreover, following reports on Klebsiella pneumoniae carbapenemase (KPC)-producing isolates from Greek patients transferred to other European countries (2, 10), a nationwide surveillance revealed a parallel epidemic of KPC-2 producers (4). Consequently, clinical laboratories were required to screen all K. pneumoniae isolates displaying reduced susceptibility to carbapenems (MIC of imipenem ≥ 1 μg/ml) by both EDTA- and boronic acid-imipenem combined disk tests (3, 8). Potential carbapenemase producers were sent to the Microbiology Laboratory, National School of Public Health (NSPH), Athens, Greece, for confirmation and further study.
Here, we report on the emergence of K. pneumoniae isolates coproducing KPC-2 and VIM-1.
Clinical isolates H-1406, A-1797, and T-1780 had been submitted to the reference laboratory (NSPH) as metallo-β-lactamase (MβL) positive (based on EDTA-imipenem synergy) from hospitals located in Crete (Heraklion), Athens, and Thessaly (Trikala), Greece, during December 2008 to April 2009. All three isolates were derived from severely ill patients who had been hospitalized for prolonged time periods (two were in intensive care units) and had received multiple courses of antibiotics, including carbapenems. MβL production was phenotypically confirmed, while boronic acid-based tests using either imipenem, meropenem, or ertapenem discs appeared negative (Table 1). PCR and sequencing showed carriage of blaVIM-1 by all three isolates. High-level resistance to penicillins, penicillin-inhibitor combinations, cefotaxime, and ceftazidime and decreased susceptibility or resistance to cefepime, imipenem, meropenem, and ertapenem determined by a microdilution technique were consistent with VIM-1 production (Table 1). To explain resistance to aztreonam, we applied isoelectric focusing of β-lactamase extracts; PCR assays specific for various class A and C bla genes, including the blaTEM, blaSHV, blaCTX-M, blaGES, blaVEB, blaKPC, blaCMY, blaDHA, and blaACC types (6, 7); and sequencing of PCR products. It was documented that isolates H-1406, A-1797, and T-1790 carry blaKPC-2 and produce the respective enzyme, with an isoelectric point of 6.7. To our knowledge, this is the first report of coproduction of VIM and KPC carbapenemases by clinical isolates.
TABLE 1.
β-Lactam resistance phenotypes and β-lactamase-related characteristics of carbapenemase-producing isolates of K. pneumoniae
| Isolate | β-Lactamase(s)b | MIC of β-lactam (μg/ml)c:
|
Carbapenemase activityd
|
EDTA test result | Increase in diam (mm) of boronic acid test zone (interpretation) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIP | PTZ | TIM | CTX | CAZ | FEP | ATM | IPM | MEM | ETP | Total (U) | Reduction by EDTA (%) | ||||
| H-1406 | VIM-1, KPC-2 | >256 | >256 | >256 | 256 | >256 | 128 | 64 | 32 | 8 | 64 | 55 | 57 | + | 3 (−) |
| A-1797 | VIM-1, KPC-2 | >256 | >256 | >256 | >256 | >256 | 16 | 64 | 2 | 2 | 8 | 42 | 80 | + | 2 (−) |
| T-1780 | VIM-1, KPC-2 | >256 | >256 | >256 | >256 | >256 | >256 | 64 | 32 | 64 | >128 | 76 | 64 | + | 3 (−) |
| A-1760a | VIM-1 | >256 | >256 | >256 | >256 | >256 | 256 | 0.12 | 32 | 64 | 64 | 80 | 100 | + | 0 (−) |
| T-1504a | KPC-2 | 128 | 64 | 256 | 8 | 16 | 16 | 32 | 2 | 16 | 32 | 31 | <2 | − | 9 (+) |
Isolate used for control purposes.
The intrinsic penicillinase produced by all isolates is not indicated.
PIP, piperacillin; PTZ, piperacillin plus 4 μg/ml tazobactam; TIM, ticarcillin plus 2 μg/ml clavulanic acid; CTX, cefotaxime; CAZ, ceftazidime, FEP, cefepime; ATM, aztreonam; IPM, imipenem; MEM, meropenem; ETP, ertapenem.
Determined as described in reference 5. EDTA was used at a final concentration of 100 μM. One unit was the amount of enzyme hydrolyzing 1 nmol of imipenem per min per mg of protein. Values are the means of three measurements not differing more than 10%.
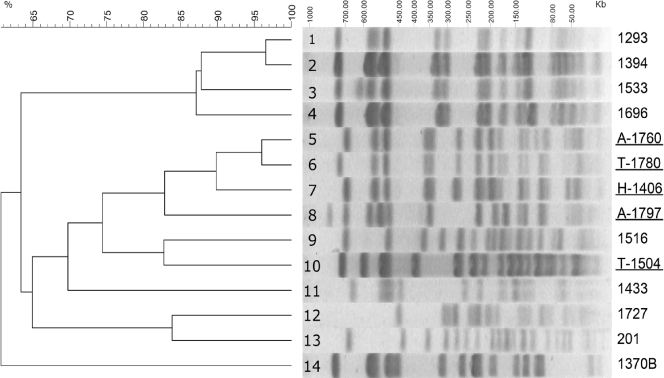
Pulsed-field gel electrophoresis of XbaI-digested genomic material from H-1406, A-1797, and T-1780 produced patterns with significant similarity (>80%), suggesting a genetic relationship. These patterns were distinct from those of four KPC-2-producing strains identified so far, including the prevalent one (represented by T-1504) (Table 1 and Fig. 1). On the other hand, the genomic type of a VIM-1-producing K. pneumoniae strain occurring sporadically since 2003 in Athens hospitals (represented by isolate A-1760) was highly similar (>95%) to that of the K. pneumoniae VIM-1 and KPC-2 coproducer T-1780 (Fig. 1). Notwithstanding the small number of isolates and the potentially frequent changes in plasmid content, a plausible hypothesis is that the K. pneumoniae VIM-1 and KPC-2 coproducer evolved through acquisition of a blaKPC-2-carrying plasmid by an established VIM-1-producing K. pneumoniae strain. Plasmid content analysis did not contradict the clonal and evolutionary hypotheses regarding K. pneumoniae VIM-1 and KPC-2 coproducer isolates. The S1 nuclease method (1) showed that H-1406, A-1797, and T-1780, as well as the KPC-2-producing K. pneumoniae isolate T-1504, all harbored similarly sized plasmids (approximately 100 kb) that hybridized strongly with a blaKPC-2-specific probe (data not shown). Conjugation experiments by a mixed broth culture method using a rifampin (rifampicin)-resistant Escherichia coli K-12 laboratory strain as a recipient and isolates H-1406 and A-1780 as donors were successful. KPC-2-producing E. coli transconjugant clones were obtained in both cases at low frequencies (approximately 5 × 10−10 transconjugants per donor cell). Moreover, the VIM-1 MβL in the K. pneumoniae VIM-1 and KPC-2 coproducer isolates and the related VIM-1-producing K. pneumoniae isolate A-1760 was probably mediated by plasmids; these isolates were unable to transfer VIM-1 production by conjugation under the in vitro conditions employed. However, results of hybridization of S1 nuclease-treated plasmid preparations with a blaVIM-1-specific probe indicated the existence of large (approximately 350-kb) VIM-1-encoding plasmids (data not shown).
FIG. 1.
Pulsed-field gel electrophoresis of XbaI-generated macrorestriction fragments of three VIM-1- and KPC-2-coproducing K. pneumoniae (lanes 6 to 8), five VIM-1-producing K. pneumoniae (lanes 1 to 5), four KPC-2-producing K. pneumoniae (lanes 9 to 11 and 14), and two non-carbapenemase-producing K. pneumoniae (lanes 12 and 13) isolates used for comparison. Underlined designations indicate isolates included also in Table 1. Preparations were run in 1% agarose under 6.0 V/cm for 20 h with a pulsing time linearly ramped from 5 to 55 s. The dendrogram was constructed using the GelCompar II v4.1 software (Applied Maths). The percentages of similarity were calculated with the Dice coefficient (position tolerance, 2.5%) and are represented by the results of the unweighted-pair group method using average linkages.
The false-negative results of the boronic acid-based test must be attributed to a masking effect of the coproduced VIM-1. Activities of β-lactamase extracts with and without EDTA against imipenem indicated the production of meaningful relative amounts of KPC-2 (Table 1), which, nevertheless, were not adequate to produce a positive synergy image under the conditions employed in the boronic acid-based test. Identification of K. pneumoniae VIM-1 and KPC-2 coproducer isolates in diverse locations within a short time period along with detection difficulties may suggest a thus far unnoticed yet significant spread. Studies are under way to asses the actual prevalence of K. pneumoniae VIM-1 and KPC-2 coproducer isolates and the potential therapeutic impact of the coproduction of VIM-1 and KPC-2 in K. pneumoniae.
Acknowledgments
This work was supported by funding from the European Community (TROCAR contract HEALTH-F3-2008-223031).
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Barton, B. M., G. P. Harding, and A. J. Zuccarelli. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235-240. [DOI] [PubMed] [Google Scholar]
- 2.Cuzon, G., T. Naas, M. C. Demachy, and P. Nordmann. 2008. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC-2 in Klebsiella pneumoniae isolate from Greece. Antimicrob. Agents Chemother. 52:796-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galani, I., P. D. Rekatsina, D. Hatzaki, D. Plachouras, M. Souli, and H. Giamarellou. 2008. Evaluation of different laboratory tests for the detection of metallo-β-lactamase production in Enterobacteriaceae. J. Antimicrob. Chemother. 61:548-553. [DOI] [PubMed] [Google Scholar]
- 4.Giakoupi, P., H. Maltezou, M. Polemis, O. Pappa, G. Saroglou, A. Vatopoulos, and the Greek System for the Surveillance of Antimicrobial Resistance. 2009. KPC-2-producing Klebsiella pneumoniae infections in Greek hospitals are mainly due to a hyperepidemic clone. Euro Surveill. 14(21):pii=19218. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19218. [DOI] [PubMed]
- 5.Loli, A., L. S. Tzouvelekis, E. Tzelepi, A. Carattoli, A. C. Vatopoulos, P. T. Tassios, and V. Miriagou. 2006. Sources of diversity of carbapenem resistance levels in Klebsiella pneumoniae carrying blaVIM-1. J. Antimicrob. Chemother. 58:669-672. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Perez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated ampC β-lactamase genes in clinical isolates by using multiplex PCR. J. Antimicrob. Chemother. 40:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsakris, A., I. Kristo, A. Poulou, F. Markou, A. Iconomidis, and S. Pournaras. 2008. First occurrence of KPC-2-possessing Klebsiella pneumoniae in a Greek hospital and recommendation for detection with boronic acid disc tests. J. Antimicrob. Chemother. 62:1257-1260. [DOI] [PubMed] [Google Scholar]
- 9.Vatopoulos, A. C. 2008. High rates of metallo-β-lactamase-producing Klebsiella pneumoniae in Greece. A review of the current evidence. Euro Surveill. 13:pii=8023. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=8023. [PubMed]
- 10.Wisell, K. T., S. Haeggman, L. Gezelius, O. Thompson, I. Gustafsson, T. Ripa, and B. Olsson-Liljequist. 2007. Identification of Klebsiella pneumoniae carbapenemase (KPC) in Sweden. Euro Surveill. 12(51):pii=3333. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3333. [DOI] [PubMed]