Abstract
The pharmacokinetics and safety of extended-interval dosing of prophylactic liposomal amphotericin B (L-AMB) in peripheral stem cell transplant recipients were evaluated. The patients received L-AMB daily at 1 mg/kg of body weight or weekly at 7.5 mg/kg or received L-AMB as a single dose (15 mg/kg). The buccal mucosal tissue concentrations of L-AMB were measured. Of the 24 patients enrolled, 5 withdrew after the initial dose due to an infusion-related reaction (n = 2) or significant increases in the serum creatinine (Scr) levels (n = 3). Weekly L-AMB dosing (7.5 mg/kg) produced mean plasma concentrations of >0.300 μg/ml for the first 7 days and >0.220 μg/ml for 7 days after the second dose. A single L-AMB dose (15 mg/kg) produced mean plasma concentrations of >0.491 μg/ml for at least 7 seven days. These concentrations are within the range of the MICs reported in the literature for susceptible strains of Candida and are at the lower limits of the MICs for Aspergillus spp. Extended-interval dosing produced buccal mucosal tissue concentrations well in excess of the MICs reported in the literature for susceptible strains of Candida and Aspergillus spp. Infusion-related reactions occurred in 24% of the patients. Baseline and end-of-study Scr, electrolyte (K+, Mg2+, PO4), and serum transaminase levels were similar across the dosage groups. Five (31%) patients met the nephrotoxicity definition prior to completion of the study. Patients in the weekly or single-dose groups experienced nephrotoxicity significantly faster than the patients in the daily dosing cohort. A weekly L-AMB dose (7.5 mg/kg) or a single L-AMB dose (15 mg/kg) produced sufficient concentrations in plasma and highly vascular tissue to warrant further studies of the safety, efficacy, and practicality of the weekly prophylactic administration of L-AMB.
Amphotericin B (AMB) exhibits concentration-dependent fungicidal activity against many common opportunistic fungal pathogens (19, 20). A dose-fractionation study with a murine disseminated candidiasis model demonstrated that maximization of the maximum drug concentration in plasma (Cmax)/MIC ratio optimizes the efficacy of AMB (2). Theoretically, this should enable the intermittent administration of large amounts of AMB, which could make its use by ambulatory patients more practical. However, in practice, maximization of the concentration-dependent activity of AMB deoxycholate is difficult due to its dose-related nephrotoxicity. However, lipid AMB formulations are safer and demonstrate subtle pharmacokinetic differences in disposition from that of AMB deoxycholate, which allows the administration of higher doses. Lipid AMB formulations such as liposomal amphotericin B (L-AMB) should enable clinicians to optimize the pharmacodynamic properties of AMB and facilitate their use by ambulatory patients.
Data from studies with neutropenic animals suggest that the administration of a single dose or intermittent doses of L-AMB up to 20 mg/kg of body weight can be employed to prevent or manage infections due to yeasts or molds (1, 22). Higher L-AMB doses produce increased intravascular concentrations, which may facilitate its penetration into certain tissues and which may produce reductions in the fungal burdens in those tissues more marked than those achieved with other AMB formulations (17, 28). In mice, L-AMB administered at 15 mg/kg thrice weekly produced sufficiently high and sustained kidney and spleen concentrations to provide prophylactic efficacy against systemic challenge with a Candida sp. 3 and 6 weeks posttreatment (30). In a murine model, the administration of a single prophylactic L-AMB dose (1, 5, 10, or 20 mg/kg) 7 to 9 days prior to challenge with Candida albicans or Histoplasma capsulatum produced increased L-AMB kidney and spleen concentrations which correlated with dose-dependent increases in efficacy (14).
In humans, L-AMB has the pharmacokinetic profile of a small unilamellar vesicle. L-AMB is slowly cleared from the bloodstream; has a long circulation half-life (t1/2), and achieves a high Cmax and a high level of systemic drug exposure (area under the concentration-time curve [AUC]) but has a small volume of distribution (V) (4, 5). L-AMB exhibits a triphasic plasma concentration profile (5-7). The mononuclear phagocytic system (MPS) is primarily responsible for the tissue uptake and distribution of lipid AMB formulations (16, 29). L-AMB is not efficiently cleared by the MPS due to its small particle size distribution (diameter, <100 nm) (11, 12). The incorporation of AMB into a small unilamellar vesicle liposome changes its MPS uptake and alters its distribution and subsequent excretion (5).
A nonlinear relationship between the L-AMB dosage and the plasma AUC from time zero to 24 h (AUC0-24), the plasma AUC from time zero to infinity (AUC0-∞), and Cmax was observed in immunocompromised adults (32). The values of the pharmacokinetic parameters increased following the administration of up to 10 mg/kg/day but declined with subsequent increases in the dosage to 15 mg/kg/day, suggesting an alteration in elimination at higher doses (32). However, the disposition in tissue was not assessed in that study. Liposomal drugs sequestered within tissues of the MPS may not be able to diffuse freely back into the plasma prior to elimination and may be slowly eliminated from those tissue compartments (5, 12). Thus, whether changes in the elimination of L-AMB from the plasma reflect altered elimination or enhanced tissue penetration in humans is unknown.
Animal studies have demonstrated the importance of including tissue drug concentrations in the assessment of the pharmacokinetics of L-AMB in plasma (14, 17, 30). However, for technical and ethical reasons, such data from analyses of the pharmacokinetics in humans are often lacking (1, 4).
The purpose of the pilot study described here was to investigate the pharmacokinetics and safety of extended-interval dosing of prophylactic L-AMB in medically stable peripheral stem cell transplant (PSCT) recipients. Moreover, by using a readily accessible tissue (buccal mucosal tissue), this study sought to assess the persistence of L-AMB in the tissues of these patients.
(Preliminary findings of this work were presented at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, November 2004, Washington, DC [abstr. A-35].)
MATERIALS AND METHODS
This study was approved by the University of Arkansas for Medical Sciences Institutional Review Board, and the study was conducted in accordance with Good Clinical Practices and the Declaration of Helsinki. Adult patients (age, ≥18 years) who were of either gender, who had multiple myeloma, and who were undergoing either allogeneic or autologous PSCT were eligible for the study. Written informed consent was obtained from each patient who met the inclusion criteria prior to enrollment in this single-center study. Prior to enrollment, as part of their routine pretransplantation evaluation, female patients were given a urine pregnancy test, unless they were postmenopausal or surgically sterile. All patients were studied for 15 days. Patients were excluded from participating if they had clinical and laboratory evidence of veno-occlusive disease or aplastic anemia, moderate or severe liver disease (an aspartate transaminase [AST] or alanine aminotransferase [ALT] level >10 times the upper limit of normal [ULN], a total bilirubin concentration >5 times the ULN, or a serum alkaline phosphatase concentration >10 times the ULN), renal insufficiency or failure (a serum creatinine [Scr] level >3 times the ULN for age), hypokalemia (serum K+ concentration <3.0 meq/liter), or a history of anaphylaxis attributed to AMB. Patients with proven or probable invasive fungal infection were also excluded. Patients were also excluded if they had received any AMB formulation within the previous 30 days.
Patients were block randomized to receive the same total dose administered as either L-AMB as a daily dose (1 mg/kg/day for 15 days), a weekly dose (7.5 mg/kg/week for 2 weeks), or a single dose (15 mg/kg dose). The randomization scheme was originally designed to equally disperse autologous and allogeneic PSCT recipients to each regimen, but due to a significant decline in the number of allogeneic PSCTs performed at the Arkansas Cancer Research Center during the study period, more autologous PSCT recipients were ultimately enrolled.
Dosing regimen.
All dosages were calculated by using each patient's actual body weight on the first study day (study day 1). All dosages were prepared from commercially obtained vials containing 50 mg of L-AMB (AmBisome) manufactured by Gilead Sciences, (San Dimas, CA) for distribution by Astellas Inc. (Deerfield, IL). Pharmacy personnel prepared individual doses from refrigerated vials of L-AMB by reconstituting the L-AMB with sterile water for injection (USP). According to the manufacturer's recommendations, the dose was then filtered with the manufacturer-supplied 5-μm filter at the time of further dilution in 5% dextrose injection. All doses in 5% dextrose were prepared to a final concentration of 1 to 2 mg/ml. All L-AMB doses were administered through a central venous catheter (a Cook catheter), which is placed as the standard of care in all myeloma patients who consent to the treatment of their underlying malignancy.
Thirty minutes prior to each L-AMB dose, all patients received a standardized premedication regimen consisting of diphenhydramine (25 mg via intravenous push), hydrocortisone (25 mg via intravenous push), and acetaminophen (650 mg by mouth). Meperidine (25 mg via intravenous push) was given every hour as needed if a patient developed chills and/or rigors during the L-AMB infusion. For all dosage groups, L-AMB was administered over 2 h by using a controlled-infusion device. Intravenous hydration with normal saline (500 ml) over 1 h was administered to all patients prior to and after the L-AMB infusion. Patients could receive additional marketed nonpolyene antifungal agents at the discretion of their physicians.
Pharmacokinetic assessment.
Repeated blood sampling was performed as outlined in Table 1. In an effort to minimize the number of blood samples collected and on the basis of the findings of prior work, for patients randomized to the single-dose (15-mg/kg) group, no blood samples for pharmacokinetic analysis were obtained after study day 8. All blood samples were stored at −80°C until shipment.
TABLE 1.
Summary of pharmacokinetic blood sampling
| Study day | Sampling time(s) for the following L-AMB dosing groups:
|
||
|---|---|---|---|
| 1 mg/kg/day | 7.5 mg/kg/wk | 15 mg/kg, single dose | |
| 1 | Predosing, end of infusion (2 h), 4 h, and 8 h | Predosing, end of infusion (2 h), 4 h, and 8 h | Predosing, end of infusion (2 h), 4 h, and 8 h |
| 2 | Prior to dosing (24 h) | 24 h postdosing | 24 h postdosing |
| 3-6 | Prior to dosing on days 4 and 6 | Single samples at 72 and 120 h postdosing | Single samples at 72 and 120 h postdosing |
| 7 | Predosing, end of infusion (2 h), 4 h, and 8 h | Predosing, end of infusion (2 h), 4 h, and 8 h | Single sample 144 h postdosing |
| 8 | Prior to dosing (24 h) | 24 h postdosing | Single sample 168 h postdosing |
| 9-15 | Single samples prior to dosing on days 10, 12, 14, and 15 | Single samples at 72, 120, 168, and 192 h postinfusion | No samples collected |
| 16 | 24 h after day 15 dose | No samples collected | No samples collected |
Tissue sampling.
A member of the University of Arkansas for Medical Sciences Consultant Dentistry Service obtained a biopsy specimen of the lateral buccal mucosa for determination of the AMB tissue concentration on study days 7 and 15. On study day 7, the biopsy specimen was obtained from patients randomized to receive daily dosing (1 mg/kg) for 15 days or weekly dosing (7.5 mg/kg) for 2 weeks prior to dosing and prior to collection of the 168-h blood sample for patients randomized to receive the single dose (15 mg/kg). On study day 15, the biopsy specimen was obtained from patients receiving the daily dose (1 mg/kg) prior to dosing and prior to collection of the 168-h blood sample for patients randomized to receive weekly dosing (7.5 mg/kg). For patients randomized to receive the single dose (15 mg/kg), the study day 15 biopsy was performed approximately 360 h after dosing. All biopsy specimens were obtained from the lateral buccal mucosa and were approximately 5 mm in diameter. On study day 15, the biopsy specimen was collected from a location on the lateral buccal mucosa as close as possible to the day 7 biopsy site. All biopsy specimens were placed in cryogenic vials and were frozen at −20°C until shipment.
Plasma sample analysis.
Plasma AMB concentrations were measured by a proprietary validated analytical method by MDS Pharma Services (data on file at MDS Pharma Services, Lincoln, NE). Calibration standards and quality control (QC) samples were prepared with human plasma in sodium heparin as the anticoagulant. The sodium heparin did not present significant interference. To generate standard curves for concentrations between 0.100 to 20.0 μg/ml, AMB (USP reference standard) was added to an appropriate aliquot of the human plasma, and the analyte was extracted by a protein precipitation procedure. The extracted samples were analyzed by high-pressure liquid chromatography with a chromatograph equipped with a Waters model 2487 UV detector.
Quantitation was performed by measurement of the peak height. A linear equation (weighted 1/concentration2) was determined to best represent the concentration for AMB in human plasma by minimization of the back-calculated percent error. Assay parameters were acceptable (all coefficients of variation were ≤5.2%, and R2 values ranged from 0.996 to 0.998). The lower limit of reliable quantitation (LLOQ) for AMB was 0.100 μg/ml. For the samples used for validation of the LLOQ, the inter-and intrabatch precisions were 8.8% and 2.9%, respectively, and the inter-and intrabatch accuracies were 107% and 108%, respectively. The inter- and intrabatch precision and accuracy results for the QC samples for AMB in human plasma prepared with low, medium, and high concentrations met the acceptance criteria (data on file at MDS PS). The high-pressure liquid chromatography method used to determine the AMB concentrations in human plasma met the validation requirements. This assay was validated by using the standard operating procedures of MDS PS for all criteria with the exception of the acceptance criteria. The adjustments for this method allowed expansion of the acceptance criteria to 30% for both the coefficient of variation and the relative error. A negative trend was observed for the QC samples for this validation, which required the use of the expanded acceptance criteria. A 30% acceptance criterion has been applied to AMB validations when AMB adsorption to the QC sample storage tubes was observed (data on file at MDS Pharma Services).
Tissue sample analysis.
The proprietary method for tissue sample analysis was developed at MDS Pharma Services (data on file at MDS Pharma Services). Calibration standards and QC samples were prepared by using aliquots of human buccal mucosa homogenates containing AMB (lot nos. 4499F and 8568F; ICN Biomedicals, Inc.) prepared by a dilution sample processing procedure. Blank control human buccal mucosa homogenate, working standards, QC, and analytical samples were prepared and analyzed. The analysis was performed with an AB/MDS SCIEX API 4000 liquid chromatograph-tandem mass spectrometer. The mobile phase, which consisted of methanol-water-acetic acid (68.6:29.4:1.96, by percent vol/vol/vol), was pumped at a flow rate of 0.5 ml/min with a Jasco PU-980 solvent delivery unit onto a Waters Symmetry C18 analytical column (150 by 3 mm; particle size, 5 μm). AMB was monitored at m/z 924.5 → 906.5 (400 ms). Positive ions were monitored in the multiple-reaction-monitoring mode.
Quantitation was by peak area analysis. No significant interference from endogenous components was observed at the retention time of AMB for any of the six human buccal mucosal sample lots screened. The limit of reliable quantitation was set at the concentration of the lowest nonzero standard, 2.00 ng/ml, for AMB. The interbatch and intrabatch precision and accuracy results for LLOQ validation samples met the proposed acceptance criteria of 30%. No significant matrix effect was observed for any of the six human buccal mucosal sample lots that were spiked with AMB at a concentration near the concentration of the LLOQ and near the upper limit of the analytical range. The interbatch and intrabatch precision and accuracy results for QC samples for AMB in human buccal mucosal homogenate prepared with low, medium, and high concentrations of AMB met acceptance criteria. The liquid chromatography-tandem mass spectrometry method for determination of the AMB concentration in human buccal mucosa met the validation requirements. To calculate the AMB concentrations in buccal mucosal tissue, the volume of the tissue was assumed to be the observed weight of the tissue. Therefore, the assumed volume of each sample was the product of the observed tissue weight and the volume of dimethyl sulfoxide added to each sample. The measured tissue AMB concentration was then converted from ng/ml solution to μg/g tissue by use of the following formula: (measured amphotericin B concentration [ng/ml]/(sample weight [g tissue]/assumed volume [ml]) = ng/g tissue × 1 μg/1,000 ng = μg/g tissue.
Pharmacokinetic analysis.
The values of the pharmacokinetic parameters for AMB were determined by noncompartmental analysis with the WinNonlin Professional computer program (version 4.1; Pharsight, Mountain View, CA). Cmax and the time to reach Cmax were obtained directly by visual inspection of the concentration-time profiles. The elimination rate constant (λz) was estimated as the absolute value of the slope of a linear regression of the natural logarithm of the concentration versus time. The t1/2 was calculated as ln 2/λz The total AUC was calculated by use of the linear trapezoidal rule. AUC values were extrapolated to infinity as C/λz, in which C is the last AMB concentration measured. Log-linear plots of the data were visually inspected to ensure that the slopes were in the terminal elimination phase. The total body clearance (CL) was calculated by dividing the L-AMB dose by AUC0-∞. The resulting values for CL were adjusted for actual body weight by dividing CL by each subject's body weight on the first day of drug administration. Geometric means with 95% confidence intervals were calculated for the parameters.
Safety assessment.
To assess the safety and tolerance of L-AMB, the L-AMB infusions were monitored prospectively, particularly with regard to infusion-related reactions and nephrotoxicity. Premedications were administered as described above prior to the administration of each dose. Infusion-related reactions were defined as any adverse event whose onset occurred during or within 1 h of completion of the L-AMB infusion. Serial vital signs during and after infusion, as well as signs and symptoms of infusion-related toxicity or any adverse effect that were associated with the drug infusion or that occurred at any time during the study period, were documented and assessed for their relationship to L-AMB.
The following laboratory examinations for the assessment of safety were performed daily as part of routine care during the course of the study: determination of hemoglobin and hematocrit concentrations; total white count with automated differential; platelet count; and blood urea nitrogen, Scr, ionized calcium, sodium, potassium, chloride, magnesium, phosphorus, and serum bicarbonate concentrations. The levels of liver transaminases (AST, ALT), lactate dehydrogenase, total bilirubin, and alkaline phosphatase were determined at least every 4 days or more frequently, at the discretion of the treating physician.
Two degrees of nephrotoxicity were assessed. Moderate nephrotoxicity was defined as an Scr level 1.5 times the patient's baseline (study day 0) level and greater than 1.2 mg/dl. Severe nephrotoxicity was defined as an Scr level two times the patient's baseline (study day 0) level and greater than 1.2 mg/dl. Several degrees of hypokalemia were assessed. Severe hypokalemia was defined as a serum potassium level less than or equal to 2.5 mmol/liter, and mild hypokalemia was defined as a serum potassium level less than 3 mmol/liter. Mild hepatotoxicity was defined as either an AST (serum glutamic oxalacetic transaminase [SGOT]) or an ALT (serum glutamic pyruvic transaminase [SGPT]) level greater than 10 times the ULN when the baseline level was less than two times the ULN. Moderate hepatotoxicity was defined as either an AST (SGOT) or an ALT (SGPT) level greater than 15 times the ULN when the baseline level was two to five times the ULN. Severe hepatotoxicity was defined as either an AST (SGOT) or ALT (SGPT) level of at least 20 times the ULN when the baseline level was 5 to 10 times the ULN. Anemia was defined as a hemoglobin level of less than 8 g/dl.
Statistical analysis.
Statistical analysis was performed with the NCSS2000 statistical software package (Number Cruncher Statistical Systems, Kaysville, UT). Discrete demographic data were analyzed by the chi-square test, and continuous demographic data and data on the minimum concentration in plasma (Cmin) for patients receiving daily dosing (1 mg/kg) were analyzed by the Kruskal-Wallis multiple-comparison Z-value test. The Cmin data between days 7 and 15 for patients who received weekly dosing (7.5 mg/kg) were compared by a paired t test. Meaningful statistical comparisons of the plasma and tissue pharmacokinetic parameters were not performed between all groups because of the small number of patients in each group and because of the different total doses received by each group on study days 1 and 7. Because the total doses were similar, the day 7 buccal mucosal concentrations between patients who received daily dosing (1 mg/kg) and those who received weekly dosing (7.5 mg/kg) were compared by a two-sample t test. Similarly, a one-way analysis of variance (ANOVA) for normally distributed data with equal variances or the Kruskal-Wallis one-way ANOVA on ranks for nonnormality were used to compare the study day 15 buccal mucosal AMB concentrations in all groups and to determine the changes in serum electrolyte and creatinine values. When differences were detected by the ANOVA, Scheffe's test for multiple comparisons was used to pinpoint which difference among the groups was statistically significant. To account for unequal variances, the square root of the number of days to the maximum Scr level was used as a stabilizing transformation prior to use of the one-way ANOVA. Buccal mucosal tissue is highly vascular; therefore, to account for contamination from the plasma compartment, tissue concentration/plasma concentration ratios were calculated for each group. The day 15 tissue concentration/plasma concentration ratios for patients who received the single dose (15 mg/kg) were not calculated because plasma samples were not obtained from this group after day 7. For all comparisons, differences were considered significant when the P value was <0.05, which was established a priori.
RESULTS
Twenty-four patients were enrolled in the study. Eight patients either dropped out or were removed from the study. One patient in the daily dosing (1-mg/kg) group withdrew on day 1 due to an infusion-related adverse event (sternal pain). Among the patients in the weekly dosing (7.5-mg/kg) group, one patient was withdrawn due an infusion-related event (bronchospam) after the first dose, two patients were withdrawn due to significant increases in Scr levels before day 7, and two patients consented but voluntarily withdrew prior to dosing. Among the patients in the single-dose (15-mg/kg) group, one patient developed acute renal failure on day 3 and one patient consented but voluntarily withdrew prior to dosing. The results that follow are for the 16 patients who completed the study Table 2. summarizes the baseline demographics of the patients in this study.
TABLE 2.
Baseline patient demographic characteristics
| Characteristic | Value for the following L-AMB dosing group:
|
P value | ||
|---|---|---|---|---|
| 1 mg/kg/day | 7.5 mg/kg/wk | 15 mg/kg, single dose | ||
| Gender (no. of patients) | ||||
| Male | 3 | 3 | 4 | 0.70 |
| Female | 3 | 1 | 2 | |
| Mean (SD) age (yr) | 57.5 (12.9) | 61 (7.7) | 56.7 (7.1) | 0.81 |
| No. of patients with the following PSCT type: | ||||
| Allogeneic | 2 | 1 | 2 | 0.097 |
| Autologous | 4 | 3 | 4 | |
| Mean (SD) wt (kg) | 71.7 (13.3) | 83.9 (26.1) | 87.5 (27.1) | 0.47 |
| No. of patients with values above ULN fora: | ||||
| Scr | 1 | 2 | 0 | |
| ALT | 1 | 0 | 1 | |
| AST | 0 | 0 | 2 | |
| Total bilirubin | 1 | 1 | 0 | |
| Alkaline phosphatasae | 1 | 0 | 0 | |
| No. of patients with values below those at baseline forb: | ||||
| Potassium (<3.5 mM) | 0 | 0 | 0 | |
| Phosphorous (<2.5 mg/dl) | 1 | 1 | 0 | |
| Magnesium (<1.5 mg/dl) | 0 | 0 | 0 | |
ULNs are >1.1 mg/dl for Scr, >40 IU/liter for ALT, >40 IU/liter for AST, >1.2 mg/dl for total bilirubin, and >120 IU/liter for alkaline phosphatase.
The values in parentheses are lower limits of normal value ranges.
The majority of the patients were male and received an autologous PSCT. Six patients were assigned to both the daily dosing (1-mg/kg) group and the single-dose (15-mg/kg) group, whereas only four patients were assigned to the weekly dosing (7.5-mg/kg) group. The groups were comparably distributed by gender, weight, age, and type of PSCT. Although the patient weight on day 1 was similar across the groups, on average, patients randomized to the weekly dosing (7.5-mg/kg) group and the single-dose (15-mg/kg) group were approximately 20% heavier. However, in terms of individual doses on an mg/kg basis, patients in the weekly dosing (7.5-mg/kg) or the single-dose (15-mg/kg) group received 7.6 and 15.1 times the dose of L-AMB as patients in the daily dosing (1-mg/kg) group, respectively. Patients in the single-dose (15-mg/kg) group received twice the dose of L-AMB as those in the weekly dosing (7.5-mg/kg) group.
Plasma pharmacokinetics.
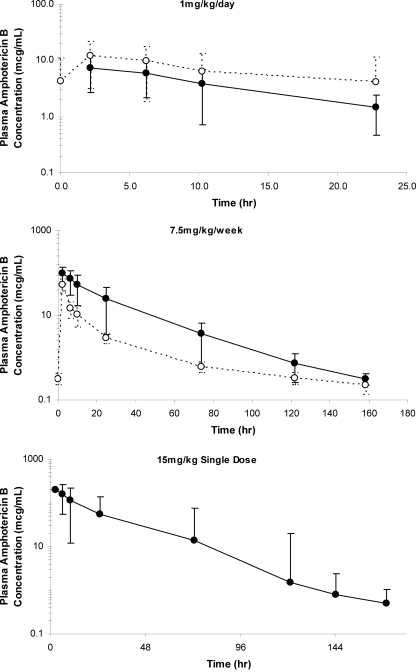
The noncompartmental pharmacokinetic parameter estimates for each group are summarized in Table 3. Figure 1 illustrates the plasma AMB concentration profiles obtained for each group during the study. Because of the sampling strategy employed and noncompartmental pharmacokinetic analysis, triphasic plasma AMB concentration profiles could not be characterized for any of the dosing regimens, although visual inspection of the plasma concentration curves for patients in the weekly dosing (7.5-mg/kg) group and those in the single-dose (15 mg/kg) group suggest multiphasic elimination.
TABLE 3.
Noncompartmental pharmacokinetic parameter values for L-AMB administered as a daily dose (1 mg/kg), weekly dose (7.5 mg/kg), and single dose (15 mg/kg)
| Group and day | Mean (SD) dose (mg) | Cmax (μg/ml)
|
AUC0-∞ (μg·h/ml)
|
CL (ml/h/kg)
|
t1/2 (h)
|
V (liter/kg)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | GM (95% CI)a | Mean (SD) | GM (95% CI) | Mean (SD) | GM (95% CI) | Mean (SD) | GM (95% CI) | Mean (SD) | GM (95% CI) | ||
| L-AMB at 1 mg/kg/day (n = 6) | |||||||||||
| Day 1 | 71 (13.3) | 8.1 (4.2) | 7.1 (3.6-12.5) | 112.2 (75.3) | 90.0 (33.2-191.2) | 15.6 (10.8) | 12.4 (4.2-27.0) | 9.7 (3.1) | 9.2 (6.4-12.9) | 0.19 (0.14) | 0.16 (0.05-0.34) |
| Day 7 | 13.5 (9.1) | 10.2 (3.9-23.1) | 333.7 (548.3) | 152.5 (−241.7-909.1) | 10.6 (10.6) | 6.6 (−0.5-21.7) | 13.0 (11.8) | 10.3 (0.7-25.4) | 0.16 (0.20) | 0.10 (−0.06-0.37) | |
| L-AMB at 7.5 mg/kg/wk (n = 4) | |||||||||||
| Days 1 to 7 | 629.3 (196.0) | 95.5 (39.9) | 87.6 (31.9-159) | 1,886.7 (1,344.1) | 1,369.7 (−252.0-4,025.5) | 8.9 (11.0) | 5.5 (−8.5-26.4) | 19.2 (1.8) | 19.2 (16.4-22.1) | 0.17 (0.20) | 0.11 (−0.15-0.49) |
| Days 7 to 15 | 52.3 (19.1) | 49.6 (21.8-82.7) | 384.7 (126.3) | 366.8 (183.7-585.7) | 21.6 (8.8) | 20.4 (7.6-35.6) | 36.4 (24.4) | 30.4 (−2.5-75.3) | 0.47 (0.22) | 0.43 (0.12-0.82) | |
| L-AMB at 15 mg/kg, single dose (n = 6), days 1 to 8 | 1,312.2 (407.2) | 206.3 (89.1) | 190.0 (112.7-299.8) | 5,019.3 (4,198.6) | 3,642.2 (613.2-9,425.4) | 5.6 (4.4) | 4.1 (0.9-10.2) | 32.8 (12.2) | 30.7 (20.0-45.6) | 0.28 (0.22) | 0.18 (0.04-0.50) |
GM, geometric mean; CI, confidence interval.
FIG. 1.
Mean plasma concentration-time profiles following administration of L-AMB. (Top panel) Daily dosing (1 mg/kg) (•, day 1; ○, day 7); (middle panel) weekly dosing (7.5 mg/kg) (•, days 1 to 7; ○, days 8 to 15); (bottom panel) single dose (15 mg/kg). Error bars represent standard deviations.
(i) L-AMB at 1 mg/kg/day.
The interpatient concentration-time data were highly variable. Dose proportionality for Cmax estimates was not observed between the daily dosing (1-mg/kg) group and the groups receiving the other two dosing regimens. Compared to the values for patients in the daily dosing (1-mg/kg) group, the day 1 Cmax estimates were approximately 12 and 25 times higher in patients who received a weekly dose (7.5 mg/kg) and patients who received a single dose (15 mg/kg), respectively. However, the values of Cmax, AUC0-∞, and t1/2 on day 1 were proportional to the values reported with the administration of a single L-AMB dose of 7.5 mg/kg by other investigators. V on day 1 in patients in the daily dosing (1-mg/kg) group exceeded the volume of the plasma compartment fourfold, but it was slightly less than the volume of extracellular water. Similarly, the t1/2 on day 1 likely reflects both the t1/2 at the distribution phase (t1/2α) and the t1/2 at the elimination phase (t1/2β) reported by other investigators. In patients who received a daily dose (1 mg/kg), the daily plasma AMB Cmins ranged from 1.1 to 4.5 μg/ml but did not differ significantly (P = 0.37) over the course of the study (data not shown).
(ii) L-AMB at 7.5 mg/kg/week.
Interpatient concentration-time data were highly variable. Dose proportionality for Cmax estimates was observed between the weekly dosing (7.5-mg/kg) group and the single-dose (15-mg/kg) group. Compared to the values for patients in the single-dose (15-mg/kg) group, Cmax estimates were approximately two times lower in the weekly dosing (7.5-mg/kg) group. The values of Cmax, AUC0-∞, and V on day 1 were similar to the values reported with the administration of a single L-AMB dose of 7.5 mg/kg by others. The mean plasma Cmin on day 7 (0.318 μg/ml) and day 15 (0.221 μg/ml) were similar (P = 0.46). The t1/2 associated with the first dose (days 1 to 7) likely reflects both the t1/2α and t1/2β values reported by others. Following administration of the second 7.5-mg/kg dose, the t1/2 nearly doubled. Similar to the results for patients in the daily dosing (1-mg/kg) group, V following administration of the first weekly L-AMB dose of 7.5 mg/kg exceeded the volume of the plasma compartment fourfold, but it was less than the volume of extracellular water. However, V following the second administration of the weekly L-AMB dose of 7.5 mg/kg nearly tripled and was nearly double the volume of extracellular water. The resulting increase in t1/2 and the reductions in Cmax and AUC0-∞ observed after administration of the second dose suggest that the changes in V may have offset the overall increase in plasma CL. Alternatively, it may also reflect the limitations of using the calculated values of V and CL to interpret the pharmacokinetic behavior of liposomal drugs.
(iii) One dose of L-AMB at 15 mg/kg.
Interpatient concentration-time data were highly variable. The estimated values of Cmax and V on day 1 were comparable to those reported by others; however, differences in study design (single dose versus multiple doses) precluded comparisons with the values of the pharmacokinetic parameters reported in the literature. The mean plasma Cmin on day 7 was 0.491 μg/ml. The t1/2 was considerably less than that reported for the L-AMB t1/2γ reported by others, most likely because the calculated t1/2 likely incorporates the t1/2α, t1/2β, and t1/2γ (terminal half-life) characterized by others. V exceeded the volume of the plasma compartment and approximated the volume of extracellular water. The plasma CL among patients who received the single dose (15 mg/kg) was the lowest among the three dosing groups.
Buccal mucosal tissue concentrations.
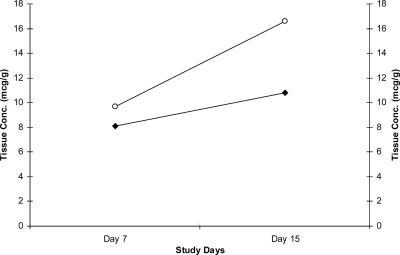
The mean buccal mucosal concentrations and tissue concentration-to-plasma concentration ratios on days 7 and 15 are summarized in Table 4. The interpatient buccal mucosal concentrations on days 7 and 15 were highly variable across all groups. The mean buccal mucosal AMB concentrations on day 7 were similar between the daily dosing (1-mg/kg) and the weekly dosing (7.5-mg/kg) groups (P = 0.47) (Fig. 2). Similarly, the buccal mucosal concentrations on day 15 were not significantly different between the dosing groups (P = 0.08) (Fig. 2). In the daily dosing (1-mg/kg) and single-dose (15 mg/kg) groups, the buccal mucosal concentrations increased 71% and 33%, respectively, from day 7 to day 15. The buccal mucosal concentrations declined 74% in the weekly dosing (7.5 mg/kg) group, which may be an artifact of assay variability and the small sample size (n = 3) on day 15.
TABLE 4.
Buccal mucosal tissue and plasma AMB Cmin data
| Dosing group | Day 7
|
Day 15
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma AMB
|
Buccal mucosal tissue AMB
|
Tissue concn/plasma concn ratio
|
Plasma AMB
|
Buccal mucosal tissue AMB
|
Tissue concn/plasma concn ratio
|
|||||||
| Cmin (μg/ml)a | No. of patients | Cmin (μg/g)a | No. of patients | Ratioa | No. of patients | Cmin (μg/ml)a | No. of patients | Cmin (μg/g)a | No. of patients | Ratioa | No. of patients | |
| 1 mg/kg/day | 4.3 (6.5) | 6 | 9.7 (9.2) | 5 | 6.2 (8.6) | 5 | 1.8 (1.2) | 6 | 16.6 (13.7) | 6 | 10.6 (6.3) | 6 |
| 7.5 mg/kg x 2 | 0.318 (0.09) | 4 | 16.8 (18.2) | 4 | 47.2 (48.5) | 4 | 0.221 (0.09) | 3 | 4.4 (3.2) | 3 | 22.8 (18.6) | 3 |
| 15 mg/kg, single dose x 1 | 0.491 (0.19) | 5 | 8.1 (3.3) | 4 | 16.3 (7.9) | 4 | 10.8 (8.0) | 4 | ||||
Values are means (standard deviations).
FIG. 2.
Mean day 7 and day 15 buccal mucosal L-AMB concentrations. ○, daily dose (1 mg/kg); ⧫, single dose (15 mg/kg). Data are not shown for the weekly dose (7.5 mg/kg).
Safety. (i) Infusion-related reactions.
Infusion-related reactions are summarized by dosage group in Table 5. Five of the 21 patients (24%) who received a dose of L-AMB experienced an infusion-related reaction. As discussed previously, in two patients (one each in the daily dosing [1-mg/kg] and the weekly dosing [7.5 mg/kg] groups), the infusion-related reaction was severe enough to warrant removal from the study. Shortly after the start of the infusion, the patient in the daily dosing (1-mg/kg) group complained of sternal pain. The infusion was immediately stopped and the pain subsided shortly thereafter. The patient was not rechallenged. The patient in the weekly dosing (7.5-mg/kg) group experienced bronchospasms shortly after the start of the infusion. The reaction subsided shortly after the infusion was stopped, and the patient was not rechallenged. The reactions in the remaining three patients were deemed mild and did not warrant removal of the patient from the study. One of the patients was in the weekly dosing (7.5-mg/kg) group and experienced rigors and an increase in heart rate. These symptoms were managed per protocol. The remaining two patients were in the single-dose (15 mg/kg) group. One experienced flushing near the end of the L-AMB infusion, but the flushing subsided within 30 min of the completion of the infusion. The other patient experienced rigors, which were managed per protocol.
TABLE 5.
Infusion-related events and laboratory data
| Parameter | Value for patients in the following L-AMB dosing group:
|
P value | ||
|---|---|---|---|---|
| 1 mg/kg/day | 7.5 mg/kg/wk | 15 mg/kg, single dose | ||
| Infusion-related events | 1 | 2 | 2 | 0.76 |
| Bronchospasm | 1 | |||
| Sternal pain | 1 | |||
| Rigors | 1 | 1 | ||
| Tachycardia | 1 | |||
| Flushing | 1 | |||
| Mean (SD) laboratory findings | ||||
| Creatinine concn (mg/dl) | ||||
| Baseline | 0.8 (0.3) | 1.0 (0.2) | 0.6 (0.15) | 0.064 |
| Highest | 1.0 (0.3) | 1.6 (0.9) | 1.6 (1.1) | 0.43 |
| Time to maximum Scr level (days) | 11.2 (4.3) | 4.3 (1.9) | 3 (1.3) | 0.0002 |
| Scr level (mg/dl) at end of therapy | 0.9 (0.4) | 0.9 (0.3) | 1.0 (0.4) | 0.83 |
| No. of patients with nephrotoxicitya | 1 | 1 | 3 | 0.43 |
| Mean (SD) day of nephrotoxicity | 16 | 3 | 4 (0.6) | 0.003 |
| Potassium concn (meq/liter) | ||||
| Baseline | 4.1 (0.4) | 3.9 (0.4) | 3.8 (0.4) | 0.76 |
| End of therapy | 3.3 (0.3) | 3.0 (0.1) | 4.3 (0.6) | 0.47 |
| No. of patients with hypokalemia | 1 | 1 | 3 | 0.44 |
| Magnesium concn (mg/dl) | ||||
| Baseline | 1.8 (0.1) | 1.9 (0.2) | 1.8 (0.5) | 0.82 |
| End of therapy | 1.8 (0.4) | 1.1 (0.6) | 1.7 (0.2) | 0.16 |
| No. of patients with hypomagnesemia | 5 | 4 | 6 | 0.41 |
| Phosphorus concn (mg/dl) | ||||
| Baseline | 3.0 (0.5) | 3.4 (0.9) | 3.2 (0.5) | 0.64 |
| End of therapy | 3.1 (0.8) | 3.0 (0.4) | 3.8 (1.5) | 0.13 |
| No. of patients with hypophosphatemia | 6 | 3 | 5 | 0.46 |
| AST concn (IU/liter) | ||||
| Baseline | 17.0 (1.4) | 19.0 (4.2) | 28.3 (28.6) | 0.88 |
| End of therapy | 15.0 (7.8) | 18.5 (10.6) | 20.3 (4.6) | 0.61 |
| ALT concn (IU/liter) | ||||
| Baseline | 17.5 (3.5) | 24 (4.2) | 33.0 (30.3) | 0.58 |
| End of therapy | 25.8 (15.0) | 27.5 (9.2) | 18.3 (10.0) | 0.72 |
| Alkaline phosphatase concn (IU/liter) | ||||
| Baseline | 61.5 (6.4) | 35.5 (14.8) | 53.7 (15.9) | 0.11 |
| End of therapy | 88.0 (31.8) | 115 (52.3) | 68.3 (35.8) | 0.90 |
| Total bilirubin level (mg/dl) | ||||
| Baseline | 0.6 (0.3) | 1.1 (0.4) | 0.9 (0.2) | 0.33 |
| End of therapy | 0.6 (0.4) | 0.7 (0.5) | 0.9 (0.1) | 0.11 |
(ii) Laboratory parameters.
The frequency of abnormal values for laboratory parameters of renal and hepatic function is presented in Table 5. As mentioned above, three patients (two in the weekly dosing [7.5-mg/kg] group and one in the single-dose [15-mg/kg] group) who received drug were withdrawn from the study due to significant increases in Scr levels. Overall, 31% (5 of 16) of the patients who completed the study had evidence of moderate nephrotoxicity (Scr levels that were ≥1.5 times greater than the baseline level and >1.2 mg/dl) at some time during the study. All of these patients also demonstrated evidence of severe nephrotoxicity (Scr levels that were ≥2.0 times greater than the baseline level and >1.2 mg/dl) at some time during the study. Three of the five patients were in the single-dose (15-mg/kg) group, and the remaining two patients consisted of one patient each from the other two dosing groups.
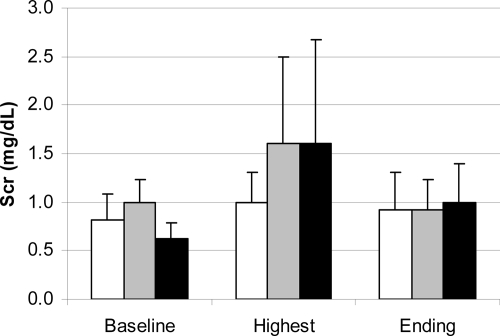
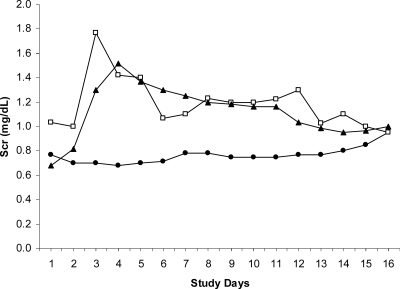
Mean baseline (study day 0) Scr levels for patients in the daily dosing (1-mg/kg) group (0.8 mg/dl), the weekly dosing (7.5 mg/kg) group (1.0 mg/dl), and the single-dose (15-mg/kg) group (0.6 mg/dl) were comparable (P = 0.06) (Fig. 3). Similarly, 1 day later, the Scr levels prior to administration of the first dose (study day 1) were comparable across the groups (P = 0.07). However, on both days, the Scr levels for patients in the weekly dosing (7.5-mg/kg) group tended to be higher. Excluding the two patients who were withdrawn from the study, peak Scr levels ranged from 0.6 mg/dl to 2.9 mg/dl. The mean maximum Scr levels for the daily dosing (1-mg/kg) group (1.0 mg/dl), the weekly dosing (7.5-mg/kg) group (1.6 mg/dl), and the single-dose (15-mg/kg) group (1.6 mg/dl) during therapy were comparable (P = 0.43) (Fig. 3). However, patients in the weekly dosing (7.5-mg/kg) and single-dose (15-mg/kg) groups experienced their maximum Scr levels significantly faster (P = 0.0002) than patients in the daily dosing (1-mg/kg) group (Fig. 4). On average, the patients in the weekly dosing (7.5-mg/kg) and single-dose (15-mg/kg) groups experienced maximum Scr levels on study days 3 and 4, respectively, whereas the patients in the daily dosing (1-mg/kg) group experienced maximum Scr levels on study day 11 (Fig. 4). The mean end-of-study (day 16) Scr levels for the daily dosing (1-mg/kg) group (0.9 mg/dl), the weekly dosing (7.5-mg/kg) group (0.9 mg/dl), and the single-dose (15-mg/kg) group (1.0 mg/dl) were comparable (P = 0.83) (Fig. 3). The end-of-study Scr levels were higher than the baseline levels in the daily dosing (1-mg/kg) and the single-dose (15-mg/kg) groups, but in all groups these values were lower than the maximum achieved during the course of therapy (Fig. 4).
FIG. 3.
Mean baseline, highest value, and end-of-treatment Scr concentrations for the daily dosing (1-mg/kg) group (white bars), weekly dosing (7.5-mg/kg) group (gray bars), and single-dose (15-mg/kg) group (black bars).
FIG. 4.
Mean daily Scr concentrations. •, daily dose (1 mg/kg); □, weekly dose (7.5 mg/kg); ▴, single dose (15 mg/kg).
Hypokalemia occurred in all groups. Five (31%) patients experienced mild hypokalemia (serum potassium level, <3 mmol/liter) during the course of treatment. The majority of the cases (n = 3) occurred in the single-dose (15-mg/kg) group, and one case each occurred in the two other dosing groups. One of the patients in the single-dose (15-mg/kg) group who experienced mild hypokalemia also developed the only case of severe hypokalemia (serum potassium level, ≤2.5 mmol/liter). All cases of hypokalemia resolved and did not necessitate the discontinuation of therapy. Hypomagnesemia (serum magnesium level, <1.5 mg/dl) was observed in all but one patient at some point during therapy, and hypophosphatemia was observed in all but two patients. Neither electrolyte disturbance persisted or showed a relation to the dosage administered. No patient fulfilled the criteria for hepatotoxicity. More than half of the patients (10 of 16 [62.5%]) were anemic at some time during the study.
DISCUSSION
Safer lipid AMB formulations have led investigators to explore alternative dosing regimens to optimize the pharmacokinetic and pharmacodynamic properties of AMB to facilitate its use in the outpatient or the prophylactic setting. Few studies with humans have examined the pharmacokinetics and distribution of extended-interval L-AMB dosing regimens. In pediatric PSCT patients administered prophylactic L-AMB at 10 mg/kg once weekly for 4 weeks, plasma nonliposomal AMB levels were detectable for 7 days (24). Furthermore, the Cmins following the first and last doses did not differ significantly, suggesting that there was no notable accumulation upon repeated dosing (24). That study did not measure the AMB concentrations in any tissue compartment.
Prior pharmacokinetic studies of high daily doses of L-AMB demonstrated that the values of pharmacokinetic parameters for the drug in plasma (AUC, t1/2, V, and CL) consistently increased during a course of therapy and with increasing daily dosages up to 5.0 mg/kg but declined with daily dosages in excess of 7.5 to 10 mg/kg (32, 33). These findings suggest that altered mechanisms of elimination may be activated at daily dosage levels exceeding 10 mg/kg.
AMB is cleared from the circulation by processes involving the MPS, the biliary system, or the renal elimination system (21, 31). Higher L-AMB concentrations may induce a concentration-driven clearance mechanism for AMB (32, 33). The findings of the prior pharmacokinetic studies with a high daily dose of L-AMB may reflect dose-dependent uptake or a high-dose, first-pass effect in tissue compartments such as the lung, or both (23). For example, investigators reported enhanced L-AMB distribution to lung tissue when L-AMB was administered at 10 mg/kg/day compared with that when it was administered at 5 mg/kg/day in a murine model of invasive pulmonary aspergillosis (23). The disposition in tissue was not assessed in prior pharmacokinetic studies of a high daily dose of L-AMB; therefore, it is unclear whether the observed nonlinear reductions in the values of the plasma pharmacokinetic parameters resulted from enhanced tissue penetration and distribution with increasing dosage or the alteration of other elimination mechanisms, or both (32, 33).
In our study, the interpatient plasma concentration-time data were highly variable among all groups. This variability is consistent with the findings of others and likely reflects interpatient differences in the action of the MPS (6) Our study demonstrated that weekly L-AMB dosing (7.5 mg/kg) produced mean plasma concentrations in excess of 0.300 μg/ml for the first 7 days and 0.220 μg/ml for 7 days after the second dose. Furthermore, a single L-AMB dose (15 mg/kg) produced mean plasma concentrations in excess of 0.491 μg/ml for at least 7 days. These concentrations are comparable to the concentrations 1 week following the administration of an L-AMB dose of 10 mg/kg dose to pediatric patients undergoing hematopoietic stem cell transplantation.
Our study was not designed to measure prophylactic efficacy. However, the day 7 total plasma L-AMB concentrations were within the range of MICs for susceptible strains of Candida (0.25 to 1 mg/liter) and at the lower limits for Aspergillus (0.5 to 2 mg/liter) (3). Measurement of the plasma drug concentration in the single-dose (15-mg/kg) group was not carried out for the full 2-week dosing interval. Nonetheless, while extended-interval L-AMB dosing may not be suitable for the treatment of established fungal infections such as invasive pulmonary aspergillosis, our data suggest that on the basis of the total plasma drug concentrations, such dosing may be practical in the prophylactic setting, particularly if the dosing interval is weekly (23).
On the basis of the calculated V, regardless of the dosing regimen, our study demonstrates that L-AMB distributes extravascularly (10). Furthermore, the decline in the calculated values of V and CL observed with daily dosing (1 mg/kg) may represent a saturable distribution, whereby continual L-AMB administration saturates drug uptake by the MPS and leads to sustained plasma concentrations and reduced CL (33). The increases in V observed with weekly dosing (7.5 mg/kg) during the study suggests that the extravascular distribution continued to increase. However, there are limitations to the use of calculated V and CL values to interpret the pharmacokinetic behavior of liposomal drugs such as L-AMB. The conventional means of calculation of V assumes that plasma and tissue concentrations decline in parallel during the postdistributional elimination phase (8, 12). However, liposomes may not undergo equilibrium distributional processes. Liposomal uptake into tissues may be unidirectional, or after uptake, liposomes may be sequestered in tissues for a period of time before they release their contents (12). These processes also affect plasma-derived CL calculations (12). Therefore, unless it is known when the distributional equilibrium is achieved (i.e., when the liposome can freely rapidly diffuse back into the circulation), plasma concentrations and values derived from them may not accurately approximate tissue concentrations and pharmacokinetic values (9, 12, 13, 27).
Invasive fungal infections predominately afflict extravascular tissue sites. A significant amount of data from studies with animals describing the tissue concentrations of L-AMB exist, but there are few data describing the distribution of L-AMB in human tissues. We measured the L-AMB concentrations in the buccal mucosa, which is a tissue that is readily accessible via a minimally invasive means that posed a minimal risk to the study participants. Although our study design precludes a full characterization of the dynamic movement of drug between the plasma and the tissue compartments, measurement of the L-AMB concentrations in this tissue does allow several basic observations to be made. First, with daily dosing (1 mg/kg), the mean plasma Cmins declined nearly 60% between days 7 and 16, yet the mean buccal mucosal concentrations increased approximately 64% during that period. Second, in the single-dose (15-mg/kg) group, the mean plasma Cmin on day 7 was approximately 0.5 μg/ml and had declined nearly 48% during the preceding 24 h. Although no samples were obtained, plasma concentrations likely declined to undetectable levels from day 7 to 15. However, during that period the mean buccal mucosal tissue concentrations continued to increase approximately 50%. With weekly dosing (7.5 mg/kg), the mean plasma Cmins were comparable on day 7 and day 15, but buccal mucosal tissue concentrations declined nearly 74% during that period. The latter finding may be an artifact of assay variability and the small sample size (n = 3) on day 15. Lastly, regardless of whether the tissue concentrations declined or increased, extended-interval L-AMB dosing produced concentrations in a highly vascular tissue that were well in excess of the MICs for susceptible strains of Candida (0.25 to 1 mg/liter) and Aspergillus (0.5 to 2 mg/liter) (3). Furthermore, when equivalent total doses were compared, with the exception of the buccal mucosal concentrations achieved on day 15 with weekly dosing, all other buccal mucosal tissue concentrations achieved by extended-interval L-AMB dosing were comparable to those produced by daily dosing (1 mg/kg). These data provide a crude illustration of the differences between the intravascular and the extravascular disposition of L-AMB in humans and lend further credence to the belief that extended-interval L-AMB dosing may be practical in the prophylactic setting (23).
The limitations of tissue concentration data are well described, and these data should be interpreted cautiously (25, 26). The transfer of drug from plasma to tissues is heterogeneous and tissue specific throughout the body (25). Therefore, the L-AMB concentrations in buccal mucosal tissue cannot be extrapolated to the concentrations in tissues at other anatomical sites. Whole biopsy tissue concentrations, as measured in this study, were obtained by homogenizing the biopsy sample and determining the concentration of drug in the tissue homogenate. Tissues are comprised of several distinct compartments, and the homogenization process destroys these distinct compartments, yielding a uniform concentration in solution. However, in intact tissue the drug may not be homogeneously distributed among the distinct compartments (25). Therefore, the concentrations represent the overall concentration in the tissue homogenate and do not necessarily represent the active concentration at the infection site (25).
Moreover, we report the entire drug fraction present in the buccal mucosal tissue and did not distinguish between liposome-associated and non-liposome-associated AMB. Therefore, we cannot characterize or extrapolate the antifungal activity represented by the concentrations that we measured. Lastly, the barrier properties of the capillaries supplying the buccal mucosa could not be characterized. Thus, because buccal mucosal tissue is highly vascular, whether the high tissue concentrations represented truly tissue-bound drug or were due to high drug concentrations in the microcirculation cannot be determined. However, the increase in buccal mucosal tissue concentrations between days 7 and 15 observed in the single-dose (15-mg/kg) group suggest that the drug buccal mucosal tissue concentrations measured on day 15 were likely representative of tissue-bound drug rather than high drug concentrations in the microcirculation of the tissue.
A slightly higher rate of infusion-related reactions was observed in this study than in others (32). This illustrates the unpredictable efficacy of premedication regimens with AMB formulations (15). The use of L-AMB results in a lower rate of infusion-related reactions than the rates achieved with other AMB formulations (33). In contrast to the findings presented in other reports, we observed the idiosyncratic reaction associated with severe chest discomfort with the standard daily L-AMB dose (1 mg/kg) rather than higher doses (7.5 to 15 mg/kg) (32). Since the reaction occurred very shortly after the start of the infusion, it is unlikely that the absolute amount of L-AMB contributed to this toxicity. The reaction is postulated to be related to several interactions between the lipid material and host factors (32).
Overall, a high percentage of patients had characteristics that met the definition of severe nephrotoxicity. However, in most patients the elevations were transient and the Scr concentrations returned to levels approximating those at the baseline by the end of the study. Furthermore, although L-AMB likely contributed to the increases in the Scr concentrations, renal dysfunction is a manifestation of multiple myeloma, and the patients in this study also concomitantly received nephrotoxic agents, which may also have contributed to the elevation in the Scr concentrations. Nonetheless, the Scr levels rose significantly more rapidly with weekly dosing (7.5 mg/kg) and the single dose (15 mg/kg) than with daily dosing (1 mg/kg). The rapid rise observed with weekly L-AMB dosing (7.5 mg/kg) or the single dose (15 mg/kg) is similar to the time course of increased Scr levels that we previously observed in this population with the daily administration of lower doses of AMB deoxycholate (18). In this study, the serum potassium, magnesium, and phosphorus levels were not significantly changed from those at the baseline (33) Furthermore, there were no significant increases in hepatic transaminase, serum bilirubin, or alkaline phosphatase levels in any of the dosage groups.
Extended-interval L-AMB dosing with weekly dosing (7.5 mg/kg) or a single dose (15 mg/kg) produced sufficient total plasma drug concentrations for 7 days to warrant further studies of the safety, efficacy, and practicality of the use of weekly L-AMB regimens for prophylaxis in the ambulatory care setting. Furthermore, extended-interval L-AMB dosing produced variable but high concentrations in highly vascular tissue. Weekly dosing with an equivalent total dose produced concentrations in highly vascular buccal mucosal tissue that were comparable to those produced by daily dosing (1 mg/kg).
Acknowledgments
This work was funded by a grant from Astellas Pharma US, Inc.
We acknowledge and thank Donald N. Buell for his thoughtful review of the draft manuscript.
Footnotes
Published ahead of print on 22 June 2009.
REFERENCES
- 1.Adler-Moore, J. P., J. A. Olsen, and R. T. Proffitt. 2004. Alternative dosing regimens of liposomal amphotericin B (AmBisome) effective in treating murine systemic candidiasis. J. Antimicrob. Chemother. 54:1096-1102. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D., T. Stamsted, and R. Conklin. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D. 2004. Clinical utility of antifungal pharmacokinetics and pharmacodynamics. Curr. Opin. Infect. Dis. 17:533-540. [DOI] [PubMed] [Google Scholar]
- 4.Andes, D., N. Safdar, K. Marchillo, and R. Conklin. 2006. Pharmacokinetic-pharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob. Agents Chemother. 50:674-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekersky, I., R. M. Fielding, D. E. Dressler, S. Kline, D. N. Buell, and T. J. Walsh. 2001. Pharmacokinetics, excretion, and mass balance of 14C after administration of 14C-cholesterol-labeled AmBisome to healthy volunteers. J. Clin. Pharmacol. 41:963-971. [DOI] [PubMed] [Google Scholar]
- 8.Boswell, G. W., I. Bekersky, D. Buell, R. Hiles, and T. J. Walsh. 1998. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob. Agents Chemother. 42:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, C. W., L. Barber, C. Ouyang, M. B. Bally, and T. D. Madden. 1997. Plasma clearance, biodistribution and therapeutic properties of mitoxantrone encapsulated in conventional and sterically stabilized liposomes after intravenous administration in BDF1 mice. Br. J. Cancer 75:169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 11.de Marie, S., R. Janknegt, and I. A. Bakker-Woudenberg. 1994. Clinical use of liposomal and lipid-complexed amphotericin B. J. Antimicrob. Chemother. 33:907-916. [DOI] [PubMed] [Google Scholar]
- 12.Fielding, R. M. 2001. Relationship of pharmacokinetically-calculated volumes of distribution to the physiologic distribution of liposomal drugs in tissues: implications for the characterization of liposomal formulations. Pharm. Res. 18:238-242. [DOI] [PubMed] [Google Scholar]
- 13.Fielding, R. M., R. O. Lewis, and L. Moon-McDermott. 1998. Altered tissue distribution and elimination of amikacin encapsulated in unilamellar, low-clearance liposomes (MiKasome). Pharm. Res. 15:1775-1781. [DOI] [PubMed] [Google Scholar]
- 14.Garcia, A., J. P. Adler-Moore, and R. Proffitt. 2000. Single-dose AmBisome (liposomal amphotericin B) as prophylaxis for murine systemic candidiasis and histoplasmosis. Antimicrob. Agents Chemother. 44:2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwin S. D., J. D. Cleary, C. A. Walawander, J. W. Taylor, and T. H. Grassela, Jr. 1995. Pretreatment regimens for adverse effects related to infusion of amphotericin B. Clin. Infect. Dis. 20:755-761. [DOI] [PubMed] [Google Scholar]
- 16.Groll, A., S. C. Piscitelli, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343-500. [DOI] [PubMed] [Google Scholar]
- 17.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 18.Gubbins, P. O., S. R. Penzak, S. Polston, S. A. McConnell, and E. J. Anaissie. 2002. Characterizing and predicting amphotericin B-associated nephrotoxicity in bone marrow or peripheral blood stem cell transplant recipients. Pharmacotherapy 22:961-971. [DOI] [PubMed] [Google Scholar]
- 19.Klepser, M. E., E. J. Wolfe, R. N. Jones, C. H. Nightingale, and M. A. Pfaller. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klepser, M. E., E. J. Wolfe, and M. A. Pfaller. 1998. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B against Cryptococcus neoformans. J. Antimicrob. Chemother. 41:397-401. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence, R. M., P. D. Hoeprich, F. A. Jagdis, N. Monji, A. C. Huston, and C. P. Schaffner. 1980. Distribution of doubly radiolabelled amphotericin B methyl ester and amphotericin B in the non-human primate Maccaca mulatta. J. Antimicrob. Chemother. 6:241-249. [DOI] [PubMed] [Google Scholar]
- 22.Lewis, R. E., N. D. Albert, and D. P. Kontoyiannis. 2008. Efficacy of single-dose liposomal amphotericin B or micafungin prophylaxis in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 52:4178-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis, R. E., L. Guangling, J. Hou, G. Chamilos, R. A. Prince, and D. P. Kontoyiannis. 2007. Comparative analysis of amphotericin B lipid complex and liposomal amphotericin B kinetics of lung activation and fungal clearance in a murine model of acute invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 51:1253-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta, P., A. Vinks, A. Flipovich, G. Vaughn, D. Fearing, C. Sper, and S. Daives. 2006. High-dose weekly AmBisome antifungal prophylaxis in pediatric patients undergoing hematopoietic stem cell transplantation: a pharmacokinetic study. Biol. Blood Marrow Transplant. 12:235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouton, J. W., U. Theuretzbacher, W. A. Craig, P. M. Tulkens, H. Derendorf, and O. Cars. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235-237. [DOI] [PubMed] [Google Scholar]
- 26.Müller, M., A. dela Pena, and H. Derendorf. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 48:1441-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman, M. S., G. T. Colbern, P. K. Working, C. Engbers, and M. A. Amantea. 1999. Comparative pharmacokinetics, tissue distribution, and therapeutic effectiveness of cisplatin encapsulated in long circulating, pegylated liposomes (SPI-077) in tumor-bearing mice. Cancer Chemother. Pharmacol. 43:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Olson, J. A., J. P. Adler-Moore, J. Schwartz, G. M. Jensen, and R. T. Proffitt. 2006. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob. Agents Chemother. 50:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostro, M. J., and P. R. Cullis. 1989. Use of liposomes as injectable drug delivery systems. Am. J. Hosp. Pharm. 46:1576-1587. [PubMed] [Google Scholar]
- 30.Smith, P. J., J. A. Olson, D. Constable, J. Schwartz, R. T. Profitt, and J. P. Adler-Moore. 2007. Effects of dosing regimen on accumulation, retention and prophylactic efficacy of liposomal amphotericin B. J. Antimicrob. Chemother. 59:941-951. [DOI] [PubMed] [Google Scholar]
- 31.van Etten, E. W., S. V. Snijders, W. van Vianen, and I. A. Bakker-Woudenberg. 1998. Superior efficacy of liposomal amphotericin B with prolonged circulation in blood in the treatment of severe candidiasis in leukopenic mice. Antimicrob. Agents Chemother. 42:2431-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh, T. J., V. Yeldandi, M. McEvoy, C. Gonzalez, S. Chanock, A. Freifeld, N. I. Seibel, P. O. Whitcomb, P. Jarosinski, G. Boswell, I. Bekersky, A. Alak, D. Buell, J. Barret, and W. Wilson. 1998. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob. Agents Chemother. 42:2391-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]