Abstract
DAS181 is a novel candidate therapeutic agent against influenza virus which functions via the mechanism of removing the virus receptor, sialic acid (Sia), from the adjacent glycan structures. DAS181 and its analogues have previously been shown to be potently active against multiple strains of seasonal and avian influenza virus strains in several experimental models, including cell lines, mice, and ferrets. Here we demonstrate that DAS181 treatment leads to desialylation of both α2-6-linked and α2-3-linked Sia in ex vivo human lung tissue culture and primary pneumocytes. DAS181 treatment also effectively protects human lung tissue and pneumocytes against the highly pathogenic avian influenza virus H5N1 (A/Vietnam/3046/2004). Two doses of DAS181 treatment given 12 h apart were sufficient to block H5N1 infection in the ex vivo lung tissue culture. These findings support the potential value of DAS181 as a broad-spectrum therapeutic agent against influenza viruses, especially H5N1.
Since 1997, the highly pathogenic avian influenza virus H5N1 subtype has been causing epidemics in wild and domesticated birds. From 2003 to March 2009, 411 cases of human infections with the avian H5N1 virus have been confirmed and over 60% of the cases were lethal (http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_03_11/en/index.html). These events have heightened public awareness of an impending influenza pandemic. However, investigation of H5N1 virus pathogenesis has been hampered not only by the strict biosafety requirement for handling the virus but also by the lack of a readily available experimental model that faithfully replicates the human respiratory system, as animal models differ in their similarity of binding and infection for human and avian influenza viruses (18, 19) Several experimental models have been used to study influenza virus infection in humans, including human cell lines; short-term cultures of in vitro differentiated epithelia, e.g., well-differentiated human airway epithelia (5, 16); and ex vivo cultures of human tissues (8, 11). Each of these has its advantages and disadvantages. We (8) and others (11) have demonstrated that short-term cultures of normal nasopharyngeal tissue, primary pneumocytes, and lung tissue sections can support H5N1 virus infection. These ex vivo human airway tissues or primary cells can potentially be more relevant models for investigating viral tropism and new antiviral agents since they are directly derived from normal human airway epithelia with little in vitro manipulation.
The existing influenza virus therapeutic agents recognize various viral components as molecular targets. In recent years, modifying host cells as a way to interrupt the life cycle of a pathogen has emerged as a novel approach to tackle infectious diseases, and DAS181 is the first anti-influenza virus therapeutic candidate that targets the host cells rather than the virus. Evidence accumulated since the 1940s has indisputably demonstrated the key role of sialic acid (Sia) as the receptor for influenza virus infection (2, 10, 12, 14). DAS181 is designed to inhibit influenza virus infection by inactivating the viral receptor, Sia. It is a recombinant sialidase fusion protein composed of the active domain of Actinomyces viscosus sialidase and the heparin binding sequence derived from the human protein amphiregulin for anchoring to epithelial surfaces (4). The A. viscosus sialidase domain selectively cleaves Sia from host cells, rendering them inaccessible to influenza viral particles. By binding to the negatively charged glycosaminoglycans on the airway epithelial cell surface, the cationic C-terminal amphiregulin tag anchors DAS181 on the respiratory epithelium, thereby improving the potency of the molecule.
We previously reported potent in vitro and in vivo efficacy of DAS181 against multiple influenza virus A and B strains (4), including the highly pathogenic H5N1 influenza virus infection of mice (A/Vietnam/1203/2004 or VN/1203) (1). Daily DAS181 treatment of mice at 1 mg/kg/day beginning 1 day preinfection with VN/1203 has been shown to protect 100% of the mice tested from this fatal disease, prevented viral dissemination to the brain, and effectively blocked infection in 70% of the treated mice. DAS181 at 1 mg/kg/day was also therapeutically effective, conferring enhanced survival of H5N1 virus-challenged mice when treatment began 72 h postinfection (1). To further evaluate DAS181 efficacy against the highly pathogenic H5N1 virus in model systems that closely mimic the human respiratory tract in vivo, we conducted studies with ex vivo cultures of human lung tissue and primary pneumocytes. The study results demonstrate that DAS181 effectively removes the influenza virus Sia receptors and inhibits H5N1 infection within human lung tissue.
MATERIALS AND METHODS
Viruses.
Influenza virus strains A/Hong Kong/54/98 (H1N1) and A/Vietnam/3046/04 (H5N1) were isolated in Madin-Darby canine kidney cells. They were cloned by limiting dilution, and seed virus stocks were prepared with Madin-Darby canine kidney cells as previously described (8). All H5N1 virus experiments were carried out in a biosafety level 3 biocontainment certified facility in the Department of Microbiology of the University of Hong Kong.
Type I-like pneumocyte isolation.
Primary type II pneumocytes were isolated by using human nonmalignant lung tissue obtained from patients who underwent lung resection. We adopted a protocol that has previously been published (20). Briefly, after removing visible bronchi, the lung tissue was minced into pieces >0.5 mm in thickness with a tissue chopper and washed with a balanced salt solution containing Hanks' balanced salt solution (Gibco) with 0.7 mM sodium bicarbonate (Gibco) at pH 7.4 three times to partially remove macrophages and blood cells. The tissue was digested with a combination of 0.5% trypsin (GIBCO BRL, Gaithersburg, MD) and 4 U/ml elastase (Worthington Biochemical Corporation, Lakewood, NJ) for 40 min at 37°C in a shaking water bath. The digestion was stopped by adding Dulbecco modified Eagle medium/F12 (Gibco) with 40% fetal bovine serum and DNase I (350 U/ml) (Sigma). Cell clumps were dispersed by repeatedly pipetting the cell suspension for 10 min. A disposable cell strainer (BD Science) with a gauze size of 50 μm was used to filter the digested tissue fragments from the cell suspension. The single-cell suspension in the flowthrough was spun down and resuspended in a 1:1 mixture of Dulbecco modified Eagle medium/F12 and small airway basal medium (Lonza) supplemented with 0.5 ng/ml human epithelial growth factor, 500 ng/ml epinephrine, 10 μg/ml transferrin, 5 μg/ml insulin, 0.1 ng/ml retinoic acid, 6.5 ng/ml triiodothyronine, 0.5 μg/ml hydrocortisone, 30 μg/ml bovine pituitary extract, and 0.5 mg/ml bovine serum albumin together with 5% fetal bovine serum and 350 U/ml DNase I. The cell suspension was plated on a tissue culture grade plastic flask (Corning) and incubated in a 37°C water-jacketed incubator with a 5% CO2 supply for 90 min. Nonadherent cells were layered onto a discontinuous cold Percoll density gradient (densities, 1.089 and 1.040 g/ml) and centrifuged at 25 × g for 20 min without a brake. The cell layer at the interface of the two gradients was collected and washed four times with a balanced salt solution to remove the Percoll. The cell suspension was incubated with magnetic beads coated with anti-CD14 antibodies at room temperature for 20 min under constant mixing. After removal of the beads with a magnet (MACS CD14 MicroBeads), cell viability was assessed by trypan blue exclusion and flow cytometry determined that the pneumocyte population was 93% pure (data not shown). The purified type II pneumocyte suspension was resuspended in small-airway growth medium (SAGM) supplemented with 1% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin and plated at a density of 3 × 105 cells/cm2. The cells were maintained in a humidified atmosphere (5% CO2, 37°C) under liquid-covered conditions, and the growth medium was changed daily starting at 60 h after cell plating. When the cell layer approached 75% confluence, the type I-like pneumocytes were subcultured with Hanks' buffered saline solution-trypsin-EDTA and trypsin-neutralizing solution. Caveolin I was used to confirm pneumocyte differentiation.
Ex vivo lung tissues.
Nonmalignant lung tissue was obtained from lobectomy or pneumonectomy specimens from patients who underwent surgical lung tissue resection. The patients gave informed consent, and the study was approved by The Hong Kong University and Hospital Authority (Hong Kong West) Institutional Review Board. The tissue fragments were from macroscopically normal lung tissue as assessed at the time of surgical resection, and tissues were not sampled from areas of fibrosis, consolidation, or induration. Histological examination was performed in all cases after infection, and if there was any evidence of intrinsic lung disease, such as dysplasia, previous tuberculosis, or fibrosis, the tissues were not included in the analysis. The tissues measured approximately 4 mm3 and were placed into culture medium in 24-well tissue culture plates (F-12K nutrient mixture with l-glutamine and antibiotics) for subsequent desialylation or viral infection experiments.
Sia detection in ex vivo tissues and type I-like pneumocytes.
Detection of surface Sia on ex vivo tissue sections was carried out by lectin cytochemistry before and after DAS181 treatment. Tissue fragments were exposed to both 100 U/ml and 500 U/ml of DAS181 for 2 h, followed by washing and then culturing for up to 48 h in F-12K at 37°C to determine the effective length of desialylation, followed by fixation in 10% neutral buffered formalin and subsequent neo-fuchsin staining with the Roche DIG Glycan Detection Kit in accordance with the manufacturer's instructions. Lectins recognizing different penultimate structures, as well as the Sia linkages (α2-6 or α2-3 to either Galβ1-4GlcNAc or Galβ1-3GalNAc), were used: Peanut agglutinin (PNA) (which binds to Galβ1-3GalNAc), Maackia amurensis agglutinin (MAA) (which binds to a number of glycans, including α2-3-linked Sia), and Sambucus nigra agglutinin (SNA) (which binds to α2-6-linked Sia). For detection of Sia on a pneumocyte culture, additional lectin binding studies were also performed with biotinylated M. amurensis lectins MAL-I and MAL-II from Vector Laboratories, which identify Sia α2-3Gal linkages and horseradish peroxidase-conjugated SNA from EY Laboratories (7).
DAS181 concentration.
We initially used DAS181 from NexBio at a concentration of 500 U/ml for desialylation and lectin binding experiments (1 unit of DAS181 = approximately 1.427 μg). This concentration was based on previous results of bronchial desialylation (6). In later experiments determining the prophylactic effect of DAS181, we used two concentrations of DAS181, 500 U/ml and 100 U/ml, as the latter was the lowest concentration that would result in desialylation as determined by lectin binding experiments with human airway epithelium.
MS analysis of lung tissues.
Mass spectrometry (MS) analysis was also performed to detect sialylated N-glycans in ex vivo lung tissues before and after DAS181 treatment. Normal lung tissues were incubated with DAS181 at 500 U/ml for 2 h at 37°C. Half of the tissue was frozen for MS analysis (the other half was fixed for lectin binding analysis as described above) as previously described (9). Briefly, lung tissues (treated with DAS181 or left untreated) were homogenized and subsequently sonicated in 1 ml cold water prior to the separation of glycoproteins (GPs) from glycolipids by chloroform-methanol extraction. The GP-containing pellet was reduced with dithiothreitol and carboxymethylated with iodoacetic acid. After lyophilization, the GPs were digested with 2.5 mg trypsin and purified. The N-glycans were released from the GP and glycopeptides by peptide:N-glycosidase F digestion and purified. The N-glycans were permethylated (methyl iodide-dimethyl sulfoxide-NaOH procedure) and purified by C18 Sep-Pak chromatography. The samples were analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) MS to obtain molecular ion profiles of the glycans, and selected ions were further characterized by tandem MS (MS/MS) analysis. MALDI-TOF MS and MALDI-TOF/TOF MS/MS analyses were performed with a 4800 MALDI TOF/TOF analyzer (Applied Biosystems, Foster City, CA). MS experiments were acquired with the reflectron settings in the positive mode. MS/MS data were obtained in the 1-kV mode with nitrogen as the collision gas (collision-induced dissociation cell gas pressure, 3.5 × 10−6 torr) (9).
Detection of virus-infected pneumocytes and cells in lung tissues by immunohistochemistry.
The pneumocyte cultures in a 24-well tissue culture plate were exposed to DAS181 for 2 h at 500 U/ml, followed by washing and H5N1 infection (1 × 106 50% tissue culture infective doses [TCID50]/ml). The influenza virus-infected pneumocytes were detected at 18 h postinfection with a mouse anti-influenza virus nucleoprotein (NP) antibody (HB65; EVL Laboratories, The Netherlands) as described previously (8). Ex vivo lung tissues were infected with 1 × 106 TCID50/ml of A/Vietnam/3046/04 (H5N1). We used three protocols, a single 2-h incubation with 500 U/ml DAS181, followed by infection for 30 min and then replenishment with fresh medium; a 2-h incubation with 500 U/ml of DAS181, followed by continuous exposure to DAS181 after the 30-min infection; and control tissues where there was infection but no DAS181 exposure. After 24 and 48 h of infection, the tissues were fixed and the number of influenza virus NP-positive cells was determined by counting red-to-brown nuclei over the total tissue section and then dividing this by the total number of high-power fields in the section (Nikon Eclipse 80i 40× objective).
Determination of viral replication by quantitative reverse transcription (RT)-PCR in ex vivo lung tissues after DAS181 treatment.
To determine the inhibition of H5N1 replication in lung tissues, two different DAS181 treatment protocols were used to evaluate anti-H5N1 activity: (i) 2-h preincubation with DAS181 at 100 or 500 U/ml, followed by phosphate-buffered saline washing and then influenza virus infection, and (ii) 2-h preincubation with DAS181 at 100 U/ml, followed by influenza virus infection and then continued postexposure incubation with DAS181 at 100 U/ml. These postinfection incubations either had replenishment of DAS181 with washing at 12-h intervals with fixing of the lung tissue samples at 72 h or continuous exposure to DAS181. H5N1 virus at 1 × 106 TCID50/ml (a titer similar to that used previously by us and other investigators [8, 11]) was used in this study.
Though two methods to detect viral replication can be used (TCID50 and influenza virus matrix [M] gene production measurements), we chose to use M gene analysis, as in the ex vivo lung model, this correlated more with influenza virus protein production than TCID50, the reason being that TCID50 is preferable when a cell monolayer is used but is less accurate when dealing with a more three-dimensional compartmentalized tissue, which lung tissue represents. Viral replication was determined by quantifying viral M gene RNA by quantitative RT-PCR. Briefly, DNase-treated mRNA was extracted from infected cells or lung fragments with an RNeasy Mini kit (Qiagen, Hilden, Germany). Lung fragments were homogenized with a Tissueruptor (Qiagen, Hilden, Germany). The cDNA was synthesized from mRNA with oligo(dT) primers and Superscript III reverse transcriptase (Invitrogen), and the viral M gene copy number was quantified by real-time quantitative PCR analysis with a LightCycler (Roche, Mannheim, Germany) by using previously published protocols (3). The viral M gene RNA was quantified and normalized to the housekeeping gene product β-actin mRNA.
RESULTS
Desialylation of human lung tissue and primary pneumocytes with DAS181.
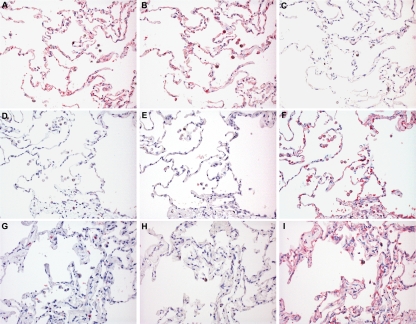
In keeping with previous observations (8), untreated human lung tissue sections bound both SNA and MAA, which are lectins that specifically recognize α2-6-linked or α2-3-linked Sia, respectively (Fig. 1A and B). Following incubation with DAS181 for 2 h, binding by both SNA and MAA was greatly diminished, demonstrating effective desialylation by DAS181 treatment (Fig. 1D and E). Consistently, DAS181 treatment led to increased exposure of the subterminal glycan structures (such as Gal and Galβ1-3GalNAc) and thus increased binding with PNA, which binds to Galβ1-3GalNAc, compared to the untreated tissue (Fig. 1C and F). These results confirmed that DAS181 effectively removes terminal Sia of both α2-6-linked and α2-3-linked types (Sia α2-3Gal and Siaα2-6Gal) from the human lung tissue surface and thereby exposes the subterminal glycan sequences (Gal and Galβ1-3GalNAc). This desialylation effect was present up to 48 h after the 2-h DAS181 incubation (Fig. 1G, H, and I).
FIG. 1.
Lectin binding to lung tissues with and without DAS181 treatment. Lectin binding of SNA (A, D, and G), MAA (B, E, and H), and PNA (C, F, and I) on normal lung tissue with no incubation with DAS181 (A to C), after 2 h of incubation with 500 U/ml DAS181 (D to F), and after 2 h of incubation with 500 U/ml, followed by removal of DAS181 and then incubation in medium for 48 h (G to I). Neo-fuchsin staining with hematoxylin counterstaining was used. Magnification, ×200.
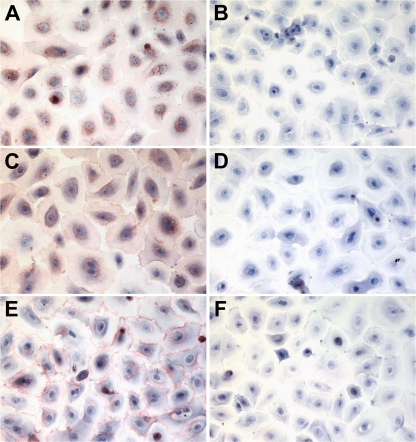
To confirm the desialylation of human pneumocytes by DAS181, we isolated type I-like pneumocytes from human lung tissues and established a primary culture as shown in Fig. 2. Cultures of type I-like pneumocytes were treated with DAS181 and stained with the lectins MAL-II (binds to Siaα2-3Galβ1,3GalNAc), MAL-I (binds to Siaα2-3Galβ1,4GlcNAc), and SNA (Fig. 2). The result showed that DAS181 indeed completely removed the Sia on type I-like pneumocytes (Fig. 2B, D, and F), thus establishing the pneumocyte model as a good comparison with the ex vivo system.
FIG. 2.
Sia expression on type I-like pneumocytes in vitro. Primary human type I-like pneumocytes were incubated with control SAGM (left panels) or 500 U/ml of DAS181 (right panels) for 2 h at 37°C and fixed. Sia in α2-3 linkages was stained reddish brown by biotinylated MAL-I (A and B) and MAL-II (C and D) lectins, while Sia in α2-6 linkages was stained red by horseradish peroxidase-conjugated SNA (E and F). Magnification, ×400.
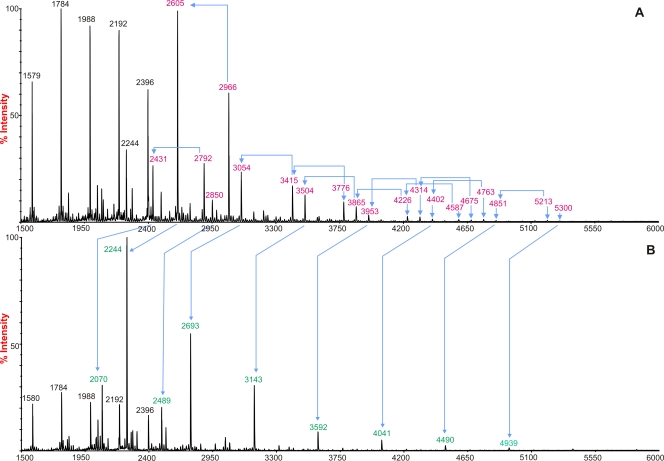
By MS analysis, we also compared the glycan patterns in human lung tissue sections with or without DAS181 treatment. There was extensive desialylation of Sia compared with that in control untreated tissue. (Fig. 3). For example, for glycans in the m/z range of 2,000 to 3,100, peaks at m/z 2,431 and 2,605 (NeuAc1Hex5HexNAc4Fuc0-1), which are consistent with monosialylated biantennary complex structures with and without core fucosylation; peaks at m/z 2,792 and 2,966 (NeuAc2Hex5HexNAc4Fuc0-1), which are consistent with desialylated biantennary complex structures with and without core fucosylation; and the peak at m/z 3,054 (NeuAc1Hex6HexNAc5Fuc1), which is consistent with monosialylated triantennary complex structures with core fucosylation, all decreased in abundance after DAS181 treatment. Coherent with these observations, the glycans at m/z 2,070 and 2,244 (Hex5HexNAc4Fuc0-1) and m/z 2,693 (Hex6HexNAc5Fuc1), which would be produced by desialylating the above glycans, increased in abundance after DAS181 treatment. In other words, in the untreated sample, the sialylated biantennary glycan with a mass of 2,605 was the most abundant glycan present (intensity, 100%) but after DAS181 treatment, this peak was reduced to less than 2% and the desialylated glycan with a mass of 2,244 now occupied the highest intensity (100%). Furthermore, in untreated normal lung tissue, there were abundant Gal-GlcNAc moieties present which are recognized by pneumococci, similar to those seen in the tracheal and bronchial models (6).
FIG. 3.
MALDI-TOF mass spectra of permethylated N-glycans from human ex vivo lung biopsy material. Results obtained without DAS181 treatment (A) and after 2 h of DAS181 (500 U/ml) treatment (B) are shown. The red peaks represent the abundance of a specific sialylated glycan present in the lung tissue, and the green peaks represent the desialylated glycans. All molecular ions are [M+Na]+. Structural assignments are based on monosaccharide composition, MALDI-TOF/TOF MS/MS analysis, and knowledge of biosynthetic pathways.
Inhibition of H5N1 infection of human primary pneumocytes and human lung tissue by DAS181.
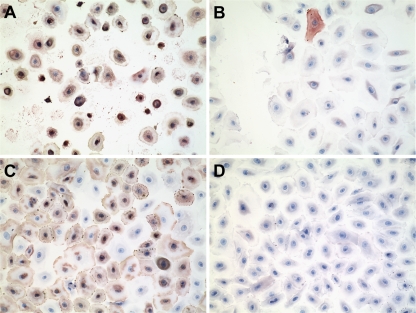
Primary cultures of human type I-like pneumocytes were incubated with DAS181 for 2 h, followed by infection with A/HK/54/98 (H1N1) or A/VN/3046/04 (H5N1), removal of the virus, and then incubation for 18 h before fixing and staining for influenza virus NP antigen. As shown in Fig. 4, DAS181 treatment completely protected pneumocytes from infection and cytotoxic effects caused by both influenza virus subtypes (Fig. 4B and D). To mimic this H5N1 virus infection in vivo, we incubated ex vivo cultures of human lung tissue with DAS181 for 2 h, followed by inoculation with A/VN/3046/04 (H5N1) and then fresh medium, or continued with DAS181 for 24 h after infection. The lung tissue fragments were then fixed, sectioned, and examined for evidence of viral replication as determined by positive nuclear staining for influenza virus NP. The results showed that continuous DAS181 treatment completely blocked H5N1 virus infection of the lung tissue (Fig. 5) (P = 0.0119, Student one-tailed t test). A shorter, 2-h treatment with DAS181 also reduced the level of H5N1 infection, although this was not statistically significant.
FIG. 4.
Effective inhibition of influenza virus infection of primary human pneumocytes by DAS181. Cultures were incubated with medium (vehicle) (A and C) or 500 U/ml DAS181 (B and D) for 2 h, followed by infection with influenza virus A/HK/54/98 (H1N1) (A and B) or A/VN/3046/04 (H5N1) (C and D). After being washed, the cultures were incubated for 18 h at 37°C before fixation and staining with an antibody against influenza virus antigen. Brown staining represents sites of antibody binding.
FIG. 5.
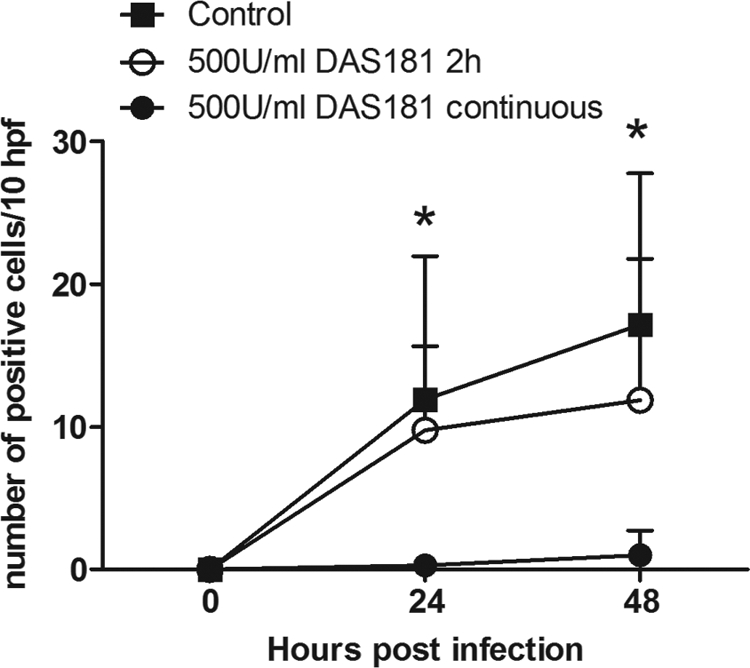
Immunohistochemical analysis of lung tissue for influenza virus NP. The number of positive cells detected in infected lung biopsy samples after a single 2-h incubation with 500 U/ml DAS181 before A/VN/3046/04 (H5N1) infection (open circles) and with continuous exposure to DAS181 (closed circles) compared to that in the control treatment (closed squares). Error bars represent standard deviations of three experiments, and an asterisk indicates a P value of <0.05 with the Student one-tailed t test. hpf is high-power fields.
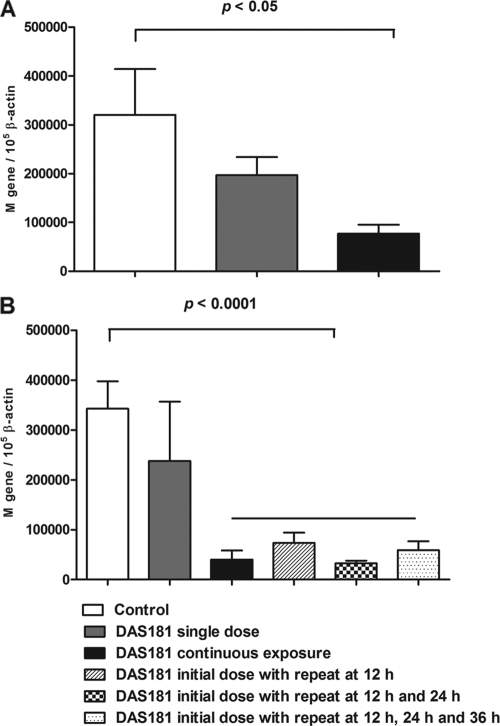
With the ex vivo model of human lung tissue, we further quantitatively evaluated the anti-H5N1 effects of various DAS181 treatment regimens by measuring the viral M gene RNA level by RT-PCR. Similar to the previous experiment, at 24 h postinfection, continuous treatment with DAS181 significantly inhibited H5N1 infection (P < 0.05) whereas a single 2-h treatment was less effective (Fig. 6A). However, judged by the viral M gene level at 48 h postinfection, multiple 2-h treatments with DAS181 at 12-h intervals were very effective and eliminated H5N1 infection (P < 0.0001) (Fig. 6B) and there was a good correlation of this M gene reduction with TCID50 measurements (data not shown). In fact, H5N1 infection was extensively inhibited with only two doses of DAS181 given 12 h apart (Fig. 6B), and additionally, the two-dose regimen was just as effective as continuous exposure to DAS181.
FIG. 6.
Influenza virus M gene analysis of normal human lung tissue with and without DAS181 treatment. Lung tissue was infected with A/VN/3046/04 (H5N1) and sampled 24 h (A) and 48 h (B) after treatment with DAS181 (100 U/ml) at different dosage regimens. Error bars indicate the standard deviations of three experiments. The indicated P values were calculated with the post-hoc Bonferroni test. M gene/105 ß-actin represents the number of influenza virus M gene copies/105 copies of the gene for ß-actin.
DISCUSSION
We have presented the first evidence of inhibition of the highly pathogenic avian influenza virus H5N1 subtype in both primary type I-like pneumocyte cultures and ex vivo lung fragment cultures by a novel influenza virus receptor inactivator, DAS181. The results of lectin binding, as well as MS, analyses have demonstrated that DAS181 effectively desialylates the surface of the human lower respiratory tract and that, interestingly, two doses of DAS181 given 12 h apart were sufficient to significantly reduce H5N1 infection in an ex vivo lung tissue culture. These findings support the potential value of DAS181 as a prophylactic and therapeutic agent against H5N1 infection of the human lower respiratory tract. Alveolar epithelial cells and macrophages have been identified as major targets of highly pathogenic avian influenza virus subtype H5N1 in autopsy tissues and in experimental ex vivo cultures (8, 17). In corroboration of these studies, we have also observed strong efficacy of DAS181 against both seasonal influenza virus strains in a well-differentiated human airway epithelium system which mimics human airway epithelial tissue in the upper respiratory tract (4). Together, these reports indicate that DAS181 may potentially protect the human respiratory tract from infection with the H5N1 virus.
It is well established that seasonal influenza virus strains bind α2-6-linked Sia as a receptor while avian influenza virus strains recognize α2-3-linked Sia as a receptor (10). Recently, detailed analysis of influenza virus receptor specificity with glycan arrays has revealed that avian influenza virus H5 strains have a high affinity for α2-3-linked Sia with various subterminal glycan structures, including Galß1-3GalNAc and Galß1-4GlcNAc. Some viruses especially prefer subterminal structures which are sulfated at the 6 position of Gal or GlcNAc or fucosylated at the 3 position of GlcNAc (13). To assess if these types of H5N1 receptors can be equally inactivated by DAS181, we stained DAS181-treated human pneumocytes with both MAL-II, which binds mainly to O-linked Siaα2-3Galß1-3GalNAc, and MAL-I, which preferentially binds N-linked Siaα2-3Galß1-4GlcNAc (7). We also stained the cells with SNA, a lectin that broadly binds α2-6-linked Sia. Although the substrate specificity of DAS181 has yet to be confirmed in detail compared with its original characterization (15), our results of DAS181 desialylation and the anti-H5N1 effect in human lung tissue indicate that DAS181 broadly recognizes both α2-3-linked Sia with different subterminal glycans and α2-6-linked Sia as substrates.
We therefore see DAS181 as representing an additional approach to the regimen of antivirals, with the added benefit that the epithelial anchoring domain limits systemic absorption and thus reduces the potential of systemic toxicity. Thus, DAS181 may offer broad-spectrum protection against all influenza viruses, including seasonal and avian influenza virus strains. On the basis of lectin binding experiments, the desialylation effect may persist for up to 48 h. In this respect, phase 1a studies have been completed and these demonstrate that the study drug was tolerated at all dose levels, indicating that desialylation was not associated with serious adverse effects during the study period. In particular, there was no evidence of secondary bacterial infection. Further in vitro toxicity studies have not shown any adverse effects on cellular metabolism. Since A. viscosus is a natural commensal of the oral cavity, adverse immunogenicity is unlikely, though this is being examined in clinical studies.
Acknowledgments
This study was supported in part by NIH contracts HHSN266200600015C and 1U01AI070281, grant RFCID08070842 from the Hong Kong Government, and CERG773507M and the Area of Excellence program on influenza supported by the University Grants Committee of the Hong Kong Special Administrative Region, China (project AoE/M-12/06). The work at NexBio was supported in part by NIH NIAID contract HHSN266200600015C. S.M.H. and A.D. acknowledge the support of the Wellcome Trust (grant 082098) and the BBSRC.
Gallen Triana-Baltzer, Qixiang Li, and Fang Fang are employees of NexBio, a developer of DAS181. The nonemployees of NexBio have no vested interest.
Kevin Fung is thanked for excellent technical assistance.
Footnotes
Published ahead of print on 13 July 2009.
REFERENCES
- 1.Belser, J. A., X. Lu, K. J. Szretter, X. Jin, L. M. Aschenbrenner, A. Lee, S. Hawley, H. Kim do, M. P. Malakhov, M. Yu, F. Fang, and J. M. Katz. 2007. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J. Infect. Dis. 196:1493-1499. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekaran, A., A. Srinivasan, R. Raman, K. Viswanathan, S. Raguram, T. M. Tumpey, V. Sasisekharan, and R. Sasisekharan. 2008. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat. Biotechnol. 26:107-113. [DOI] [PubMed] [Google Scholar]
- 3.Cowling, B. J., R. O. Fung, C. K. Cheng, V. J. Fang, K. H. Chan, W. H. Seto, R. Yung, B. Chiu, P. Lee, T. M. Uyeki, P. M. Houck, J. S. Peiris, and G. M. Leung. 2008. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS ONE 3:e2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malakhov, M. P., L. M. Aschenbrenner, D. F. Smee, M. K. Wandersee, R. W. Sidwell, L. V. Gubareva, V. P. Mishin, F. G. Hayden, D. H. Kim, A. Ing, E. R. Campbell, M. Yu, and F. Fang. 2006. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob. Agents Chemother. 50:1470-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 101:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls, J. M., L. M. Aschenbrenner, J. C. Paulson, E. R. Campbell, M. P. Malakhov, D. F. Wurtman, M. Yu, and F. Fang. 2008. Comment on: concerns of using sialidase fusion protein as an experimental drug to combat seasonal and pandemic influenza. J. Antimicrob. Chemother. 62:426-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholls, J. M., A. J. Bourne, H. Chen, Y. Guan, and J. S. Peiris. 2007. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir. Res. 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholls, J. M., M. C. Chan, W. Y. Chan, H. K. Wong, C. Y. Cheung, D. L. Kwong, M. P. Wong, W. H. Chui, L. L. Poon, S. W. Tsao, Y. Guan, and J. S. Peiris. 2007. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat. Med. 13:147-149. [DOI] [PubMed] [Google Scholar]
- 9.Parry, S., V. Ledger, B. Tissot, S. M. Haslam, J. Scott, H. R. Morris, and A. Dell. 2007. Integrated mass spectrometric strategy for characterizing the glycans from glycosphingolipids and glycoproteins: direct identification of sialyl Lex in mice. Glycobiology 17:646-654. [DOI] [PubMed] [Google Scholar]
- 10.Rogers, G. N., and J. C. Paulson. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127:361-373. [DOI] [PubMed] [Google Scholar]
- 11.Shinya, K., M. Ebina, S. Yamada, M. Ono, N. Kasai, and Y. Kawaoka. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440:435-436. [DOI] [PubMed] [Google Scholar]
- 12.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 13.Stevens, J., O. Blixt, L. M. Chen, R. O. Donis, J. C. Paulson, and I. A. Wilson. 2008. Recent avian H5N1 viruses exhibit increased propensity for acquiring human receptor specificity. J. Mol. Biol. 381:1382-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stray, S. J., R. D. Cummings, and G. M. Air. 2000. Influenza virus infection of desialylated cells. Glycobiology 10:649-658. [DOI] [PubMed] [Google Scholar]
- 15.Teufel, M., P. Roggentin, and R. Schauer. 1989. Properties of sialidase isolated from Actinomyces viscosus DSM 43798. Biol. Chem. Hoppe-Seyler 370:435-443. [DOI] [PubMed] [Google Scholar]
- 16.Thompson, C. I., W. S. Barclay, M. C. Zambon, and R. J. Pickles. 2006. Infection of human airway epithelium by human and avian strains of influenza A virus. J. Virol. 80:8060-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uiprasertkul, M., P. Puthavathana, K. Sangsiriwut, P. Pooruk, K. Srisook, M. Peiris, J. M. Nicholls, K. Chokephaibulkit, N. Vanprapar, and P. Auewarakul. 2005. Influenza A H5N1 replication sites in humans. Emerg. Infect. Dis. 11:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2006. H5N1 virus attachment to lower respiratory tract. Science 312:399. [DOI] [PubMed] [Google Scholar]
- 19.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am. J. Pathol. 171:1215-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, J., K. Edeen, R. Manzer, Y. Chang, S. Wang, X. Chen, C. J. Funk, G. P. Cosgrove, X. Fang, and R. J. Mason. 2007. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am. J. Respir. Cell Mol. Biol. 36:661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]