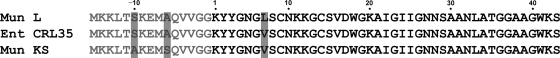Abstract
Enterococcus mundtii CUGF08, a lactic acid bacterium isolated from alfalfa sprouts, was found to produce mundticin L, a new class IIa bacteriocin that has a high level of inhibitory activity against the genus Listeria. The plasmid-associated operons containing genes for the mundticin L precursor, the ATP binding cassette (ABC) transporter, and immunity were cloned and sequenced. The fifth residue of the conservative consensus sequence YGNGX in the mature bacteriocin is leucine instead of valine in the sequences of the homologous molecules mundticin KS (ATO6) and enterocin CRL35. The primary structures of the ABC transporter and the immunity protein are homologous but unique.
Bacteriocins are ribosomally synthesized proteinaceous compounds that inhibit closely related bacteria (19). Due to consumer concerns with chemical and irradiation preservation methods and due to the rising demand for minimally processed food products, alternative methods for shelf life extension and enhanced safety are needed. Bacteriocins are considered “natural” antimicrobials since many bacteriocins are produced by food grade lactic acid bacteria, which are generally recognized as safe. Bacteriocins can be divided into three main classes: the class I lanthionine-containing lantibiotics, exemplified by nisin; the class II non-lanthionine-containing bacteriocins; and the class III heat-labile, large proteins (6). Class III bacteriocins have limited application due to their thermal instability and cytolytic activity against eukaryotic cells. Class II can be further divided into class IIa containing pediocin-like bacteriocins, class IIb containing two-peptide bacteriocins, and class IIc containing other bacteriocins (8). Class IIa bacteriocins have been extensively studied since pediocin PA-1 was first discovered (12) and characterized (20). Currently, only nisin in class I has been approved by the FDA as a natural food additive. Bacteriocins belonging to class IIa are promising alternative antimicrobials since they are more stable over a broader range of heating regimens and pH conditions. In addition, these bacteriocins exhibit stronger antimicrobial activity against the genus Listeria than nisin (17) but have a narrower antimicrobial spectrum.
The potential applications of class IIa bacteriocins in both meat and plant-based foods as a means to provide protection against potential food-borne pathogens and extend shelf life continue to expand. In an attempt to use biological methods for controlling food-borne pathogens on fresh sprouts, a number of food grade lactic acid bacteria were isolated from the indigenous microbiota on alfalfa sprouts. Some of these isolates were found to be bacteriocinogenic. This study describes a new class IIa bacteriocin, mundticin L produced by Enterococcus mundtii CUGF08 isolated from alfalfa sprouts.
Bacteriology.
The mundticin L producer E. mundtii CUGF08 was obtained from alfalfa sprouts. The genus and species were determined by BLAST homology analysis (http://blast.ncbi.nlm.nih.gov/blast.cgi) of the 16S rRNA gene sequence, which was amplified using primers 16S-F (5′-AGAGTTTGATCCTGGCTCAG−3′) and 16S-R (5′-AAGGAGGTGATCCAGCCGCA-3′) (10) at an annealing temperature of 37°C. On MRS medium, E. mundtii CUGF08 produced a typical yellow pigment that is distinct from those of other Enterococcus spp. (5). It is a homofermentative lactic acid bacterium obtained from plant-associated sources. E. mundtii CUGF08 and the mundticin ATO6 producer E. mundtii ATO6 (3) were grown in MRS broth (Difco, Becton Dickinson and Company, Sparks, MD; Criterion, Santa Maria, CA). The indicator strain Listeria ivanovii and the sensitive strain Listeria monocytogenes 10403S were grown in tryptic soy broth (Difco). Agar media contained 1.5% (wt/vol) agar (Difco). Escherichia coli DH5α with the cloning vector pUC19 was grown in LB medium (Difco) containing 100 μg/ml of ampicillin (Fisher Scientific, Fair Lawn, NJ). E. coli ElectroMAX DH5α electrocompetent cells (Invitrogen, Carlsbad, CA) were used for transformation of recombinant pUC19. LB agar containing ampicillin (100 μg/ml), 120 μg/ml of isopropyl-β-d-1-thiogalactopyranoside (IPTG) (Fisher Scientific), and 40 μg/ml of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Fisher Scientific) was used to screen transformed cells.
Characterization of bacteriocin.
Bacteriocin production was confirmed by performing a deferred inhibition assay as described by Schillinger and Lücke (27), with the following modifications. A pure culture of each isolate was spotted onto MRS agar and incubated at 30°C for 24 h. Proteolytic enzymes were used to confirm the proteinaceous nature of bacteriocins. Ten-microliter aliquots of different proteolytic enzymes, including proteinase K (20 mg/ml; Fisher Scientific) and pronase E (10 mg/ml; Sigma, St. Louis, MO) were added close to the 24-h colonies. After drying, 8 ml of soft agar (0.75%, wt/vol) medium containing 0.05 ml of an 18-h culture of an indicator strain was overlaid. To eliminate the antimicrobial effect of acids produced by lactic acid bacteria, 10 μl of Tris buffer (2 M Tris-HCl, pH 8.0) was added as described above for proteolytic enzymes. The plates were incubated for an additional 24 h. Loss of clear inhibition zones, attributable to inactivation by any of the proteolytic enzymes tested but not by the Tris buffer, were considered an indication of bacteriocin production by the isolates.
The bacteriocin was extracted (as described below) and subjected to Tricine- sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (25, 26) using a Mini-Protean II electrophoresis system (Bio-Rad, Hercules, CA). Duplicate gels were run at 120 V for 1.5 h before they were stained with Coomassie blue G-250 (Bio-Rad). Ultra-low-range molecular weight markers (molecular weights, 1,060 to 26,600; Sigma) were used to estimate the molecular weight of the unknown bacteriocin. Following fixation with methanol (30%, vol/vol), one of the duplicate gels was overlaid with 8 ml of soft agar containing 50 μl of the late-log-phase sensitive indicator strain.
The putative bacteriocin produced by E. mundtii CUGF08 was inactivated by both pronase E and proteinase K and was not affected by Tris buffer, indicating that the putative antimicrobial compound produced by E. mundtii CUGF08 was proteinaceous. Tricine-SDS-PAGE analysis of the extracted bacteriocin and overlay of the Tricine-SDS-PAGE gel with the L. ivanovii indicator strain showed that the molecular weight was approximately 4,000. The bacteriocin was designated mundticin L.
Isolation, cloning, and sequencing of the bacteriocin genetic determinants.
Standard molecular biology methods described by Sambrook and Russell (24) were used, unless otherwise noted. E. mundtii CUGF08 chromosomal DNA was purified from a 30-ml overnight culture using a method described by Mengaud et al. (21), with modifications. The cloning vector pUC19 and subsequent recombinant plasmids from E. coli DH5α were purified using a plasmid mini kit (Qiagen) according to the procedures described by the manufacturer. Plasmid isolation from E. mundtii CUGF08 was performed by using the method described by Anderson and McKay (1). PCR products were purified with a QIAquick gel extraction kit. The DNA concentration was determined with a NanoDrop ND 1000 (Thermo Scientific, Waltham, MA). Restriction enzymes were obtained from Fisher Scientific or Promega, Madison, WI. Shrimp alkaline phosphatase (Boehringer Mannheim, Germany) was used to dephosphorylate cloning vector pUC19 digested by single restriction enzymes. T4 DNA ligase was obtained from Invitrogen. Genomic DNA from E. mundtii CUGF08 was digested with KpnI and HindIII, which was followed by electrophoresis, and then the DNA was alkaline transferred onto a positively charged Hybond-N+ nylon membrane (Amersham, United Kingdom), fixed by UV cross-linking, and hybridized with the bacteriocin structural gene amplified with primers Mnt-1F (5′-TGAGAGAAGGTTTAAGTTTTGAAGAA-3′) and Mnt-1R (5′-TCCACTGAAATCCATGAATGA-3′) using conditions described by Zendo et al. (33). DNA digested with HindIII was also hybridized with the immunity gene amplified at an annealing temperature of 37°C with primers IM-F (5′-GACAAGTGTGACATAATCATTG-3′) and IM-R (5′-CAGCATTTTTAAAGATACCAAC-3′), which were designed based on the reported sequence of the mundticin KS gene cluster (18). All probes were labeled with thermostable alkaline phosphatase and detected by using GE Amersham AlkPhos direct labeling and CDP Star detection systems. Chemiluminescent film and developing and fixing solutions (Kodak, Rochester, NY) were used to visualize the hybridization patterns.
A 5.5-kb KpnI fragment and a 7.3-kb HindIII fragment, which contained the mundticin L structural gene, and an additional 1.8-kb HindIII fragment containing the immunity gene were isolated. DNA fragments of the corresponding sizes were recovered from agarose gels by electroelution and purified by chloroform-phenol extraction and standard ethanol precipitation. All three fragments were ligated into pUC19, which was digested by the corresponding restriction enzymes. The ligation mixture was electroporated into E. coli DH5α competent cells. Correct clones were screened by alpha complementation and were selected by colony hybridization and colony PCR using the probes and primers described above. The recombinant plasmids containing the correct inserts were sequenced with an Applied Biosystems (Foster City, CA) automated 3730 DNA analyzer at Cornell University Life Sciences Core Laboratories Center. Both DNA strands of the recombinant plasmids were sequenced using a series of primers designed based on the known nucleotide sequence of the flanking vector and the insert.
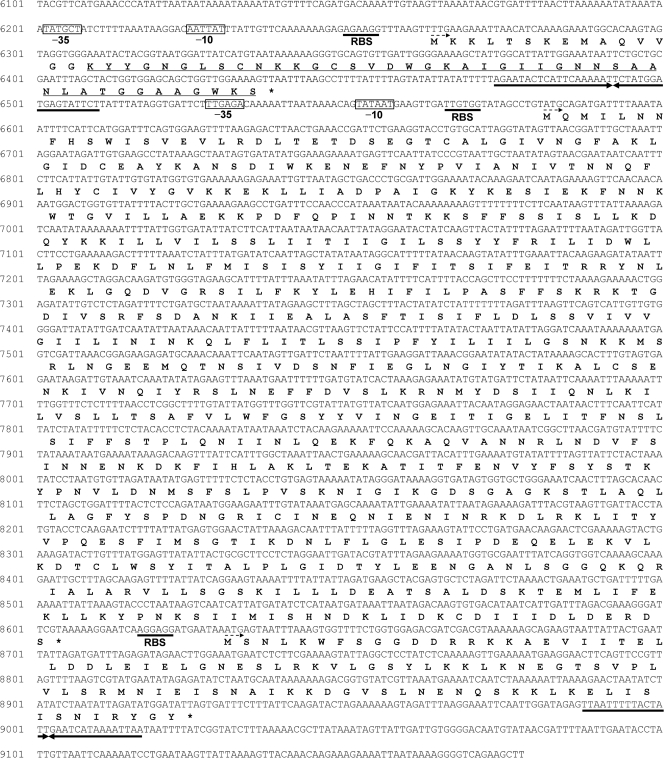
Three open reading frames (ORFs) of the relevant genetic determinants were identified (Fig. 1). The first ORF (ORF1) encodes the mundticin L prepeptide, which consists of 58 amino acids (Fig. 1). Following ORF1 is a putative rho-independent terminator. ORF2 encodes a putative ATP binding cassette (ABC) transporter with a separate set of transcriptional elements. ORF2 is followed closely by ORF3 encoding the putative immunity protein for mundticin L. ORF2 and ORF3 are in the same operon and share a common transcriptional start site and a rho-independent terminator. No additional sequence (9,178 bp) was found to be associated with the production of mundticin L.
FIG. 1.
Operons for mundticin L production, transport, and immunity. The first ORF encodes the bacteriocin precursor and is followed by the second ORF encoding the putative ABC transporter and the third ORF encoding the putative immunity protein. The amino acid residues are indicated under the nucleotide sequence, and the start codon is indicated by a dashed arrow. The underlined amino acid sequence is the mature mundticin L sequence. Boxes indicate nucleotides that are putative promoters. RBS, ribosome binding site. The sequence underlined by opposing arrows is a putative rho-independent terminator.
The mature peptide of mundticin L has 43 amino acids, and two cysteine residues in the carboxyl terminus form a putative disulfide bond (Fig. 2). The calculated molecular weight of mundticin L was determined to be 4,301.8, and the pI was 9.45. Mundticin L has a precursor cleavage site after the double glycine residues (Fig. 2) and the highly conserved YGNGX motif in the N terminus, both of which are typical of class IIa bacteriocins. However, in the YGNGX motif of mundticin L, the fifth residue was determined to be leucine instead of valine, the most common residue in this motif in class IIa bacteriocins. Therefore, mundticin L is a new bacteriocin with a single amino acid difference in the YGNGX motif compared to mundticin KS (18) and enterocin CRL35 (22). The electrostatic distribution along the molecule is highly polarized, with most of the cationic residues concentrated in the N-terminal region. The N-terminal residues at positions 7 to 9 (Leu-Ser-Cys) and 15 to 17 (Ser-Val-Asp) are predicted (4) to form β strands, followed by two α helices at positions 18 to 25 (Trp-Gly-Lys-Ala-Ile-Gly-Ile-Ile) and 29 to 32 (Ser-Ala-Ala-Asn).
FIG. 2.
Amino acid alignment of mundticin L (Mun L), enterocin CRL35 (Ent CRL35) (22), and mundticin KS (Mun KS) (18). The leader peptides are indicated by gray typeface. The residues that are different are shaded.
Besides the single amino acid difference from mundticin KS and enterocin CRL35 in the mature peptide, the leader peptide of mundticin L has alanine and serine residues at positions −6 and −10, respectively, which are identical to the residues of enterocin CRL35 but different from the residues of mundticin KS, which are serine and alanine, respectively. Although the mature peptides of mundticin KS and enterocin CRL35 are the same, the sequences of the leader peptides are different in these two bacteriocins (Fig. 2). Therefore, based on the amino acid sequences of the bacteriocin precursors, there are three different bacteriocins in the mundticin group of class IIa bacteriocins: mundticin L, mundticin KS (ATO6), and enterocin CRL35.
The ABC transporter for mundticin L has 674 amino acids with 98% identity to those for enterocin CRL35 and mundticin KS, while the lengths of all three sequences are the same. It is homologous to ABC transporters for other class IIa bacteriocins, but the levels of identity are less than 50% in addition to the differences in length.
The deduced immunity protein of mundticin L was determined to be comprised of 98 amino acids (Fig. 3). Compared to the immunity proteins of mundticin KS and enterocin CRL35, the mundticin L immunity protein is the same length, but it contains different amino acids at positions 30 (Ile), 31 (Glu), 46 (Lys), and 89 (Ile); the enterocin CRL35 immunity protein contains Ile, Asp, Glu, and Ile, the mundticin KS immunity protein contains Met, Asp, Lys, and Met, and Mun-im (15) contains Ile, Asp, Lys, and Met at the same positions.
FIG. 3.
Amino acid alignment of the immunity proteins for mundticin L (Mun L im), enterocin CRL35 (Ent CRL35 im) (22), mundticin KS (Mun KS im) (18), and mundticin (Mun-im) (15). Different amino acid residues are shaded.
Determination of the genetic locus.
The chromosomal and plasmid DNA from the bacteriocin producer were used to identify the genetic locus of the gene cluster responsible for bacteriocin production. Isolated DNA was subjected to electrophoresis in a 0.6% agarose gel. The DNA was blotted onto a nylon membrane and hybridized with the 16S rRNA gene, which was a PCR product obtained using the primers and conditions described above. The PCR-amplified structural gene for mundticin L was used as a hybridization probe to differentiate between chromosomal and plasmid DNA and to determine the fragment containing the genetic locus. The 16S rRNA gene of E. mundtii CUGF08 was used as the control. It was found that the mundticin L structural gene hybridized only with the plasmid DNA. Therefore, the genetic determinants of mundticin L are plasmid associated.
Electroblotting and N-terminal sequencing of the bacteriocin.
The bacteriocin was extracted by pH-dependent adsorption and desorption of the bacteriocin on the producer cells (32). The producer strain was grown in 1 liter of MRS medium at 30°C for 18 h, when the maximum bacteriocin concentration in the medium was observed. After the bacteriocin was released, the cells were removed by centrifugation, and the supernatant was sterilized by using a 0.2-μm-pore-size cellulose acetate membrane and dialyzed three times in dialysis tubing (molecular weight cutoff, 2,000; Spectrum) in 2 liters of Milli-Q water for 24 h at 4°C with stirring. The bacteriocin extract was then lyophilized overnight and reconstituted in 3 ml of 50 mM phosphate buffer (pH 7.0). The resulting extract was subjected to Tricine-SDS-PAGE as described above. The bacteriocin in the polyacrylamide gel was electroblotted onto a polyvinylidene fluoride membrane (Micron Separations Inc., Westborough, MA) in transfer buffer containing 10 mM NaHCO3, 3 mM Na2CO3, and 20% methanol (9) at 100 V for 1 h at 4°C. The blotted bacteriocin band was excised from the membrane and loaded onto a Perkin-Elmer sequencer (Waltham, MA) for Edman degradation (Synthesis and Sequencing Facility, Johns Hopkins University School of Medicine). The N terminus of mundticin L was determined to be Lys-Tyr-Tyr-Gly-Asn-Gly-Leu-Ser-Xaa-Asn, which corroborates the amino acid sequence deduced from the structural gene.
Nucleotide and amino acid sequence analyses.
The cloned nucleotide sequence was analyzed by using Vector NTI Advance 10 (Invitrogen). The promoters for operons were determined by using a prokaryotic promoter prediction program obtained from Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen, Groningen, The Netherlands (http://bioinformatics.biol.rug.nl/websoftware/ppp/ppp_start.php). The primary and secondary structures of the bacteriocin were analyzed by using the ExPASy Proteomics System (http://ca.expasy.org/).
A unique feature of mundticin L is the single Val-to-Leu amino acid mutation in the YGNGV motif compared to the previously identified molecules mundticin ATO6, mundticin KS, and enterocin CRL35. Bacteriocin 31 (28), plantaricin C19 (2), and sakacin 5x (30) belonging to class IIa have been reported to have the YGNGL motif but have additional mutations outside the YGNGX region. The single conservative mutation in the YGNGX motif may provide insight into the role, if any, that residue 5 of the YGNGX motif plays in the activity or spectrum of activity compared to the activities of mundticin ATO6 (KS) and enterocin CRL35. Although leucine and valine have similar hydrophobicities, leucine does not have the β-methyl side chain of valine, which provides a force restraining the freedom of the φ and ψ torsion angles and may have an effect on the stereostructure of the bacteriocin. Whether this type of conformational change phenotypically affects the thermodynamic properties of the bacteriocin (in other words, whether the residue mutation is redundant in structure and function) is not clear.
In this study, comparisons of the activity and the antimicrobial spectrum were performed for mundticins L and ATO6, but no significant differences were observed for either the level of activity or the spectrum of indicator bacteria tested (data not shown). This suggests that the fifth residue in the YGNGX complex is not essential for the activity of mundticin bacteriocins or that the difference in activity is not sufficient to be detected using traditional activity assays. Among class I bacteriocins, nisin Z differs from nisin A by one amino acid (Asn27His). The antimicrobial activities of these molecules were determined to be identical, while the diffusion rate of nisin Z is higher than that of nisin A (7), which should not be the case for mundticin L compared to other mundticins due to the similarity of the hydrophobicities of Leu and Val. The three-dimensional structures determined so far for leucocin A (11), carnobacteriocin B2 (31), sakacin P (29), and curvacin A (14) indicate that Val is located in a β-strand-like region of the N terminus. The positive electrostatic potential is polarized toward the N-terminal region, but the role of Val as a hydrophobic residue may be different. Although studies (16, 23) have indicated that the C-terminal α-helix region may be responsible for the inhibitory specificity of various bacteriocins and the N terminus may be responsible for activity, the function of the YGNGX motif has yet to be clarified.
The crystal structure of the immunity protein (Mun-im) for a recently discovered mundticin produced by E. mundtii 15-1A was resolved (15). Mun-im has only two different residues, at positions 31 (Asp) and 89 (Met), compared to the immunity protein (Fig. 3) for mundticin L (Glu and Ile). The Mun-im protein is a bundle of four antiparallel α helices forming a hydrophobic core, while the cationic and anionic patches are on the surface.
Although no listeriosis outbreaks associated with consumption of fresh alfalfa sprouts have been reported, food recalls have been made due to contamination of alfalfa sprouts with L. monocytogenes (13; http://www.fda.gov/consumer/updates/sprouts040909.html). The potential risk of listeriosis from contaminated alfalfa sprouts cannot be underestimated. E. mundtii may have potential as a probiotic in select food systems and as a food grade protective culture to improve safety and retard spoilage bacteria. E. mundtii CUGF08 was isolated from alfalfa sprouts, and its ability to grow during sprouting combined with its production of acid and mundticin L may prove to be useful for protecting seed sprouts by inhibition of the growth of food-borne pathogens that are associated with sprouts. Use of indigenous protective cultures isolated from sprouts to control food-borne pathogens may be more acceptable for sprout producers and their customers than preservatives or physical treatment of the finished sprouts.
Nucleotide sequence accession number.
The cloned nucleotide sequence that contains the genetic determinants for mundticin L has been deposited in the GenBank database under accession number FJ899708.
Acknowledgments
We acknowledge Charles M. Franz of the Federal Research Center for Nutrition and Food, Germany, for providing E. mundtii ATO6 and M. B. Herman, J. Gu, and H. W. Lange of Christine D. Smart's group in the Department of Plant Pathology and Plant-Microbe Biology and D. C. Manns of Food Science and Technology, Cornell University, for their technical assistance.
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.Anderson, D. G., and L. L. McKay. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl. Environ. Microbiol. 46:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atrih, A., N. Rekhif, A. J. G. Moir, A. Lebrihi, and G. Lefebvre. 2001. Mode of action, purification and amino acid sequence of plantaricin C19, an anti-Listeria bacteriocin produced by Lactobacillus plantarum C19. Int. J. Food Microbiol. 68:93-104. [DOI] [PubMed] [Google Scholar]
- 3.Bennik, M. H. J., B. Vanloo, R. Brasseur, L. G. M. Gorris, and E. J. Smid. 1998. A novel bacteriocin with a YGNGV motif from vegetable-associated Enterococcus mundtii: full characterization and interaction with target organisms. Biochim. Biophys. Acta 1373:47-58. [DOI] [PubMed] [Google Scholar]
- 4.Cole, C., J. D. Barber, and G. J. Barton. 2008. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36:W197-W201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, M. D., J. A. E. Farrow, and D. Jones. 1986. Enterococcus mundtii sp. nov. Int. J. Syst. Bacteriol. 36:8-12. [Google Scholar]
- 6.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 7.De Vos, W. M., J. W. Mulders, R. J. Siezen, J. Hugenholtz, and O. P. Kuipers. 1993. Properties of nisin Z and distribution of its gene, nisZ, in Lactococcus lactis. Appl. Environ. Microbiol. 59:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drider, D., G. Fimland, Y. Héchard, L. M. McMullen, and H. Prévost. 2006. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn, S. D. 1986. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on western blots by monoclonal antibodies. Anal. Biochem. 157:144-153. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, U., T. Rogall, H. Blöcker, M. Emde, and E. C. Böttger. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fregeau Gallagher, N. L., M. Sailer, W. P. Niemczura, T. T. Nakashima, M. E. Stiles, and J. C. Vederas. 1997. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062-15072. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez, C. F., and B. S. Kunka. 1987. Plasmid-associated bacteriocin production and sucrose fermentation in Pediococcus acidilactici. Appl. Environ. Microbiol. 53:2534-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorski, L., D. Flaherty, and J. M. Duhé. 2008. Comparison of the stress response of Listeria monocytogenes strains with sprout colonization. J. Food Prot. 71:1556-1562. [DOI] [PubMed] [Google Scholar]
- 14.Haugen, H. S., G. Fimland, J. Nissen-Meyer, and P. E. Kristiansen. 2005. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide curvacin A. Biochemistry 44:16149-16157. [DOI] [PubMed] [Google Scholar]
- 15.Jeon, H. J., M. Noda, Y. Matoba, T. Kumagai, and M. Sugiyama. 2009. Crystal structure and mutagenic analysis of a bacteriocin immunity protein, Mun-im. Biochem. Biophys. Res. Commun. 378:574-578. [DOI] [PubMed] [Google Scholar]
- 16.Johnsen, L., G. Fimland, and J. Nissen-Meyer. 2005. The C-terminal domain of pediocin-like antimicrobial peptides (class IIa bacteriocins) is involved in specific recognition of the C-terminal part of cognate immunity proteins and in determining the antimicrobial spectrum. J. Biol. Chem. 280:9243-9250. [DOI] [PubMed] [Google Scholar]
- 17.Katla, T., K. Naterstad, M. Vancanneyt, J. Swings, and L. Axelsson. 2003. Differences in susceptibility of Listeria monocytogenes strains to sakacin P, sakacin A, pediocin PA-1, and nisin. Appl. Environ. Microbiol. 69:4431-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamoto, S., J. Shima, R. Sato, T. Eguchi, S. Ohmomo, J. Shibato, N. Horikoshi, K. Takeshita, and T. Sameshima. 2002. Biochemical and genetic characterization of mundticin KS, an antilisterial peptide produced by Enterococcus mundtii NFRI 7393. Appl. Environ. Microbiol. 68:3830-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-86. [DOI] [PubMed] [Google Scholar]
- 20.Marugg, J. D., C. F. Gonzalez, B. S. Kunka, A. M. Ledeboer, M. J. Pucci, M. Y. Toonen, S. A. Walker, L. C. M. Zoetmulder, and P. A. Vandenbergh. 1992. Cloning, expression and nucleotide sequence of genes involved in production of pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0. Appl. Environ. Microbiol. 58:2360-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mengaud, J., C. Geoffroy, and P. Cossart. 1991. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect. Immun. 59:1043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saavedra, L., C. Minahk, A. P. de Ruize Holgado, and F. Sesma. 2004. Enhancement of the enterocin CRL35 activity by a synthetic peptide derived from the NH2-terminal sequence. Appl. Environ. Microbiol. 48:2778-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvucci, E., L. Saavedra, and F. Sesma. 2007. Short peptides derived from the NH2-terminus of subclass IIa bacteriocin enterocin CRL35 show antimicrobial activity. J. Antimicrob. Chemother. 59:1102-1108. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Schägger, H. 2006. Tricine-SDS-PAGE. Nat. Protoc. 1:16-22. [DOI] [PubMed] [Google Scholar]
- 26.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Chem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 27.Schillinger, U., and F. Lücke. 1989. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 55:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1996. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 178:3585-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uteng, M., H. H. Hauge, P. R. L. Markwick, G. Fimland, D. Mantzilas, J. Nissen-Meyer, and C. Muhle-Goll. 2003. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide sakacin P and a sakacin P variant that is structurally stabilized by an inserted C-terminal disulfide bridge. Biochemistry 42:11417-11426. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan, A., V. G. H. Eijsink, T. F. O'Sullivan, K. O'Hanlon, and D. van Sinderen. 2001. An analysis of bacteriocins produced by lactic acid bacteria isolated from malted barley. J. Appl. Microbiol. 91:131-138. [DOI] [PubMed] [Google Scholar]
- 31.Wang, Y., M. E. Henz, N. L. F. Gallagher, S. Chai, A. C. Gibbs, L. Z. Yan, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 1999. Solution structure of carnobacteriocin B2 and implications for structure-activity relationships among type IIa bacteriocins from lactic acid bacteria. Biochemistry 38:15438-15447. [DOI] [PubMed] [Google Scholar]
- 32.Yang, R., M. C. Johnson, and B. Ray. 1992. Novel method to extract large amount of bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 58:3355-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zendo, T., N. Eungruttanagorn, S. Fujioka, Y. Tashiro, K. Nomura, Y. Sera, G. Kobayashi, J. Nakayama, A. Ishizaki, and K. Sonomoto. 2005. Identification and production of a bacteriocin from Enterococcus mundtii QU 2 isolated from soybean. J. Appl. Microbiol. 99:1181-1190. [DOI] [PubMed] [Google Scholar]