Abstract
Florfenicol resistance was analyzed in 230 enteric pig isolates collected between 1998 and 2006. PCR, plasmid profiling, Southern blot hybridization, and a mixed-broth conjugation assay suggested the intra- and interspecies plasmid-mediated transfer of florfenicol resistance among the isolates that exhibited MICs for florfenicol between 4 to 128 mg/liter.
Florfenicol, a fluorinated chloramphenicol derivative, is a broad-spectrum antimicrobial agent active against a wide range of both gram-positive and gram-negative bacteria. In South Korea, it was initially approved for the treatment of bovine and porcine respiratory disease in 1999 (10, 16). Recent reports have shown increasing use of florfenicol in the treatment of target respiratory pathogens as well as in Escherichia coli infection in bovine, porcine, and poultry production (4, 6, 11, 18). This may have lead to the emergence and spread of florfenicol resistance in a wide range of gram-negative and gram-positive bacteria (5, 8, 15, 17, 18). Florfenicol resistance in enteric microbes, such as E. coli, Klebsiella pneumoniae, and Salmonella spp., is of special concern because they share a common gut environment and are liable to transferring the resistance genes through mobile genetic structures and plasmids bearing antibiotic-resistant determinants (1, 2, 17). Florfenicol resistance is mediated by the floR gene in gram-negative bacteria, and chromosomal location of this gene, especially in the pentadrug-resistant gene cluster of Salmonella enterica serovar Typhimurium phage type (PT) DT104, has attracted wide interest in light of its contribution to multidrug resistance and the development of DT104 detection methods using PCR (1, 7, 9).
Though recent reports have shown increased consumption of florfenicol in farms, no reports are available on the prevalence of florfenicol resistance among microbes of enteric origin from South Korean farms (10, 12, 19). Past work on florfenicol resistance has reported a wide range of MICs for florfenicol for E. coli animal isolates, with and without the floR gene (12, 14, 17, 18). The floR gene, however, has been identified in most E. coli isolates with MICs for florfenicol of >8 mg/liter (6, 15, 18). However, some strains with MICs between 8 mg/liter and 16 mg/liter have been reported to have other mechanisms of reduced susceptibility to florfenicol (15, 17). Currently, CLSI breakpoints are approved to indicate florfenicol resistance only for bovine and porcine respiratory disease pathogens (Pasteurella multocida, Mannheimia haemolytica, and Histophilus somni); no approved CLSI breakpoint is currently available for enteric bacteria except for S. enterica serovar Cholerasuis.
In light of this, our study focused on an analysis of phenotypic and genotypic florfenicol resistance in enterobacteria isolated from the samples of clinically sick animals (pigs) between the years 1998 and 2006. We further tested whether or not this resistance was plasmid mediated. Conjugation experiments were performed to evaluate the ease with which resistance-associated plasmids would move across species or genera of enterobacteria.
E. coli (n = 121), S. enterica serovar Typhimurium (n = 71), S. enterica serovar Enteritidis (n = 12), and K. pneumoniae (n = 26) strains were obtained from the feces, intestines, lungs, and lymph nodes of pigs with mixed clinical signs of digestive and respiratory disorders after necropsy. Identification of the suspected colonies, isolated from selective media incubated overnight with necropsied samples, was made by Vitek (Vitek system; bioMérieux, Marcy l'Etoile, France). All Salmonella spp. were serotyped by slide agglutination and tube agglutination with Salmonella O and H group antisera, respectively (Difco Co., Franklin Lakes, NJ), at the National Veterinary Research and Quarantine Service (NVRQS; Anyang, South Korea). Phage typing was performed for the isolates showing ACSSuTF resistance at NVRQS in accordance with the guidelines provided by the Public Health Laboratory Service (PHLS), London, United Kingdom.
MICs of antimicrobial agents were determined by the microbroth dilution method, using microtiter plates that contained florfenicol concentrations of 0.5 to 256 mg/liter in serial twofold dilutions (16). Evaluation of the MIC was performed according to the recommendations of the CLSI (13). The MIC was considered to correspond to the first dilution at which no growth was detectable. E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Salmonella serovar Typhimurium DT104 ATCC 2501 were used for quality control.
PCR was performed using genomic DNA (Wizard genomic DNA purification kit; Promega, Madison, WI) of all the isolates with MICs for florfenicol of ≥4 mg/liter (see Table 2) as a template and a set of floR-specific oligonucleotide primers, Flo-F (5′-CTGATCGCTCCTTTCGACAT-3′) and Flo-R (5′-CCGTGGCGTAACAAATCAC-3′) (GenBank accession no. DQ647028.2). Amplified PCR product with the expected size of 1,083 bp from one of the isolates was cloned in the pQE-UA 30 vector system (Qiagen) using the manufacturer's protocol. The identity of floR gene was confirmed by sequencing. For all other PCR-positive isolates, identity of the floR gene was confirmed by the size and Southern blot hybridization result of the PCR products with the biotin-labeled floR probe as described below.
TABLE 2.
Susceptibilities of antimicrobial agents for the donor and the transconjugant strains
| Strain | MIC (mg/liter)a
|
|||||
|---|---|---|---|---|---|---|
| KAN | TE | SuM | STR | FCC | RIF | |
| E. coli 03/16 | >256 | >256 | >256 | 128 | 16 | 2 |
| Transconjugant | 128 | 128 | 128 | 128 | 16 | >256 |
| E. coli 04/18 | >256 | >256 | >256 | >256 | 32 | 4 |
| Transconjugant | >256 | >256 | >256 | 64 | 16 | >256 |
| Salmonella serovar Enteritidis 03/22 | 128 | 128 | >256 | >256 | 32 | <0.5 |
| Transconjugant | 128 | 64 | >256 | >256 | 16 | 128 |
| K. pneumoniae 04/22 | >256 | 64 | >256 | >256 | 32 | <0.5 |
| Transconjugant | 128 | 32 | 128 | >256 | 16 | 128 |
KAN, kanamycin; TE, tetracycline; SuM, sulfamethoxazole; STR, streptomycin; FCC, florfenicol; RIF, rifampin.
Mixed-broth culture mating was performed to observe the transferability of the florfenicol resistance gene, as described by Kang et al. (7). All isolates with the floR gene (see Table 2) were included as putative donors in a conjugation experiment. E. coli RG488 Rifr, kindly provided by Je Chul Lee (Kyungpook National University), was used as the recipient to detect the transfer of resistance gene. The transconjugants were selected on MacConkey agar supplemented with florfenicol (2 mg/liter) and rifampin (rifampicin) (100 mg/liter). Transfer frequency was calculated as the number of transconjugants per recipient.
Transfers of resistance to florfenicol, tetracycline, streptomycin, kanamycin, and sulfamethoxazole in transconjugants were confirmed by MIC, following the procedures described above. Presence of the florfenicol resistance gene, floR, in the transconjugants was confirmed by PCR and DNA hybridizations.
Single colonies of the transconjugants (E. coli RG488 Rifr) of E. coli 03/16, E. coli 04/18, Salmonella serovar Enteritidis 03/22, and K. pneumoniae 04/22 and single colonies of isolates that failed to transfer the florfenicol resistance (E. coli 04/1, Salmonella serovar Typhimurium 04/13, and Salmonella serovar Enteritidis 04/8) in broth conjugation assay were picked from the MacConkey agar plates with and without antibiotics. Plasmid DNA was extracted using the midi extraction kit (Qiagen, Valencia, CA) following the manufacturer's protocol and was digested by the EcoRI restriction enzyme. The digested plasmids and PCR-amplified products for all the floR-positive strains (see Table 2) were electrophoresed through agarose gels and transferred to a positively charged nylon membrane (GE Healthcare, Little Chalfont, England). Hybridization experiment was performed by using a psoralen-biotin (BrightStar psoralen-biotin nonisotopic labeling kit; Ambion Inc, Austin, TX)-labeled PCR product of the floR gene. Detection was performed using a BrightStar BioDetect nonisotopic detection kit (Ambion Inc., Austin, TX) following the manufacturer's protocol.
Reports on the uses of antimicrobial agents have shown that the use of florfenicol gradually increased from 387 kg/year in 2001 to 17,159 kg in 2005 (10). More than half of this increase was used in the pig industry alone, followed by poultry and bovine farms (10). This figure could increase for subsequent years because the in vitro antimicrobial activity of florfenicol against respiratory pathogens is very effective, and no report of resistance in target pathogens is available from South Korea (16). In this study, 7.43% (9/121) of E. coli, 8.45% (6/71) of Salmonella serovar Typhimurium, 16.6% (2/12) of Salmonella serovar Enteritidis, and 7.69% (2/26) of K. pneumoniae strains exhibited MICs for florfenicol that ranged from 4 to 128 mg/liter. The floR gene was amplified by 14 out of 19 isolates that exhibited MIC for florfenicol of ≥4 mg/liter (Fig. 1). Two E. coli (MIC, 4 and 8 mg/liter) and three Salmonella serovar Typhimurium (MIC, 16 mg/liter each) isolates (Table 1) did not amplify the floR gene. The reduced susceptibility of these isolates to florfenicol might be due to the involvement of other mechanisms or resistance genes (15, 17).
FIG. 1.
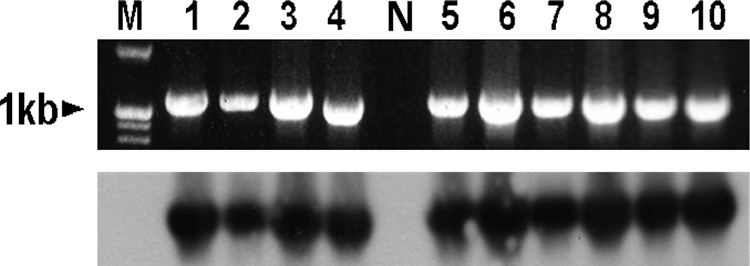
PCR amplification of the floR gene (upper panel). Lane M, 100-bp DNA marker (iNtRON Biotechnology, South Korea); lanes 1 to 4, E. coli (03/16; MIC, 16 mg/liter), E. coli (04/18; MIC, 32 mg/liter), Salmonella serovar Enteritidis (03/22; MIC, 32 mg/liter), and K. pneumoniae (04/22; MIC, 32 mg/liter); lane N, negative control; lanes 5 to 10, E. coli (04/1; MIC, 16 mg/liter), Salmonella serovar Typhimurium (04/13; MIC, 16 mg/liter), Salmonella serovar Enteritidis (04/8; MIC, 32 mg/liter), E. coli (03/17; MIC, 16 mg/liter), E. coli (04/19; MIC, 32 mg/liter), and K. pneumoniae (04/21; MIC, 64 mg/liter). Identity confirmation of floR gene in these isolates was performed based on the size and Southern blot hybridization of PCR products (lower panel).
TABLE 1.
Susceptibilities, amplification, and hybridization patterns of floR in E. coli, Salmonella serovar Typhimurium, Salmonella serovar Enteritidis, and K. pneumoniae isolates
| Strainsc | Yr/no. | FFC MIC (mg/liter) | PCR resulta | TF | FCC/RIF MIC (TCs) (mg/liter) | Southern blot hybridizationb |
|---|---|---|---|---|---|---|
| E. coli | 04/1 | 16 | + | − | ||
| 04/3 | 32 | + | − | |||
| 05/9 | 32 | + | − | |||
| 05/13 | 4 | − | − | |||
| 03/14 | 8 | − | − | |||
| 03/16 | 16 | + | 1.3 × 10−5 | 16/>256 | + | |
| 03/17 | 16 | + | − | |||
| 04/18 | 32 | + | 1.4 × 10−5 | 16/>256 | + | |
| 04/19 | 32 | + | − | |||
| ST (PT193) | 04/13 | 16 | − | − | ||
| 04/26 | 16 | − | − | |||
| 04/15 | 16 | − | − | |||
| ST (U302) | 05/24 | 128 | + | − | ||
| ST (PT120) | 05/21 | 128 | + | − | ||
| ST (DT104) | 03/41 | 32 | + | − | ||
| SE | 04/8 | 32 | + | − | ||
| 03/22 | 32 | + | 1.3 × 10−4 | 16/128 | + | |
| KP | 04/21 | 64 | + | − | ||
| 04/22 | 32 | + | 1.2 × 10−4 | 16/128 | + | |
| E. coli (RG488 Rifr) | <0.5 | − | − |
PCR amplification of floR gene, using whole-cell genomic DNA.
Hybridization signal was in the plasmid DNA of the transconjugants (E. coli RG4888 Rif) of E. coli 03/16 (MIC of 16 mg/liter), E. coli 04/18 (MIC of 16 mg/liter), Salmonella serovar Enteritidis 03/22 (MIC of 16 mg/liter), and K. pneumoniae 04/22 (MIC of 16 mg/liter).
ST, Salmonella serovar Typhimurium; SE, Salmonella serovar Enteritidis; KP, Klebsiella pneumoniae; FCC, florfenicol; RIF, rifampin; TF, transfer frequency; TCs, transconjugant.
Past work on florfenicol resistance in E. coli animal isolates has reported various ranges of MICs. North American and European E. coli strains carrying the floR gene from animal origin have been reported to have MICs for florfenicol of 16 to ≥256 mg/liter and of ≥128 mg/liter, respectively (15). However, MIC values for florfenicol of isolates from South Korea (this study) and E. coli isolates from China were substantially less than those described earlier (6, 11, 15). This indicates that the MIC for florfenicol could vary geographically, so a comparative study of the florfenicol resistance gene from different geographical regions might help elucidate its mechanism.
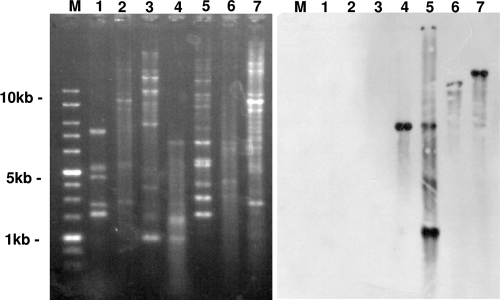
Conjugation assay showed the transfer of reduced florfenicol susceptibility by 4 out of 14 strains. These transconjugants that harbored the plasmid of 23 kb were also resistant to tetracycline, streptomycin, kanamycin, and sulfamethoxazole. Results of the MIC determination of these antibiotics for donor strains (E. coli 03/16, E. coli 04/18, Salmonella serovar Enteritidis 03/22, and K. pneumoniae 04/22) and their transconjugants are listed in Table 2. Out of those 14 strains, 10 isolates amplified the floR gene in PCR and failed to transfer floR gene in conjugation experiments. From those 10 isolates, plasmid extraction was carried out for three randomly selected E. coli (04/1; MIC, 16 mg/liter), Salmonella serovar Typhimurium (04/13; MIC, 16 mg/liter), and Salmonella serovar Enteritidis (04/8; MIC, 32 mg/liter) strains. None of these three strains showed floR-specific probe hybridization in the plasmid profile and Southern blot (Fig. 2, lanes 1 to 3) indicating the chromosomal location of the floR gene in these isolates. The floR-specific probe hybridization of plasmid DNA of the transconjugants (E. coli RG488 Rifr) of E. coli 03/16, E. coli 04/18, Salmonella serovar Enteritidis 03/22, and K. pneumoniae 04/22 indicated the presence of both single (Fig. 2, left panel, lanes 4, 6, and 7) and multiple copies (Fig. 2, left panel, lane 5) of this gene. Likewise, the hybridization signal in the different digested fragments of plasmid DNA indicated the different orientations of the floR gene in the conjugative plasmid of these strains. The floR gene has also been extensively described in Salmonella serovar Typhimurium epidemic strain DT104. However, only one such PT was identified among the Salmonella serovar Typhimurium (MIC, 32 mg/liter) isolates in our study. Two other floR-positive Salmonella serovar Typhimurium isolates were identified as PT 302 and 120. Three Salmonella serovar Typhimurium isolates, identified as PT 193 (MIC, 16 mg/liter), did not amplify the floR gene (Table 1). None of these Salmonella serovar Typhimurium PTs transferred florfenicol resistance in the broth conjugation experiments.
FIG. 2.
Southern blot hybridization (left) and EcoRI restriction (right) profiles of plasmids extracted from E. coli, Salmonella serovar Typhimurium, Salmonella serovar Enteritidis, and K. pneumoniae isolates with different MICs. Lanes M, 1-kb DNA marker; lanes 1 to 3, E. coli (04/1; MIC, 16 mg/liter), Salmonella serovar Typhimurium (04/13; MIC, 16 mg/liter), and Salmonella serovar Enteritidis (04/8; MIC, 32 mg/liter) that showed no transfer of florfenicol resistance in broth conjugation experiments; lanes 4 to 7, transconjugants (E. coli RG4888 Rif) of E. coli (03/16; MIC, 16 mg/liter), E. coli (04/18; MIC, 16 mg/liter), Salmonella serovar Enteritidis (03/22; MIC, 16 mg/liter), and K. pneumoniae (04/22; MIC, 16 mg/liter). The hybridization signal in the different digested fragments of plasmid DNA indicated the different orientations of the floR gene in the conjugative plasmid of these strains. Likewise, the hybridization signal in the plasmid DNA of the transconjugant (E. coli RG4888 Rif) of E. coli (04/18; MIC, 16 mg/liter) (lane 4) indicated the multiple copies of this gene.
Our findings are in agreement with those of previous reports on florfenicol resistance in E. coli and support the clinical relevance of an MIC breakpoint of 32 mg/liter in E. coli and other enteric bacteria as proposed by Singer et al. (17). Likewise, plasmid profiling, Southern blotting, phage typing, and conjugation experiment results indicated that emergence and dissemination of floR genes among the Enterobacteriaceae are not due to the prevalence of DT104 or other closely related PTs (4). Indeed, it could be inferred that floR-bearing promiscuous plasmids in these groups of enteric microbes are in circulation due to the local selection pressure imposed by the use of antimicrobial agents on farms (7, 10, 12).
Acknowledgments
This work was supported by Korea Research Foundation grants (KRF-2006-21-E00011 and KRF-2006-005-J502901), a BK-21 grant for Veterinary Science, and a Bio-Green 21 grant (20070401-034-009-007-01-00), RDA, South Korea.
Footnotes
Published ahead of print on 10 July 2009.
REFERENCES
- 1.Briggs, C. E., and P. Fratamico. 1999. Molecular characterization of an antibiotic resistance gene cluster of Salmonella typhimurium DT104. Antimicrob. Agents Chemother. 43:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carattoli, A. 2001. Importance of integrons in the diffusion of resistance. Vet. Res. 32:243-259. [DOI] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Cloeckaert, A., S. Baucheron, G. Flaujac, S. Schwarz, C. Kehrenberg, J. L. Martel, and E. Chaslus-Dancla. 2000. Plasmid-mediated florfenicol resistance encoded by the floR gene in Escherichia coli isolated from cattle. Antimicrob. Agents Chemother. 44:2858-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doublet, B., S. Schwarz, C. Kehrenberg, and A. Cloeckaert. 2005. Florfenicol resistance gene floR is a part of a novel transposon. Antimicrob. Agents Chemother. 49:2106-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du, X., C. Xia, J. Shen, B. Wu, and Z. Shen. 2004. Characterization of florfenicol resistance among calf pathogenic Escherichia coli. FEMS Microbiol. Lett. 236:183-189. [DOI] [PubMed] [Google Scholar]
- 7.Kang, H. Y., Y. S. Jeong, J. Y. Oh, S. H. Tae, C. H. Choi, D. C. Moon, W. K. Lee, Y. C. Lee, S. Y. Seol, D. T. Cho, and J. C. Lee. 2005. Characterization of antimicrobial resistance and class 1 integrons found in Escherichia coli isolates from humans and animals in Korea. J. Antimicrob. Chemother. 55:639-644. [DOI] [PubMed] [Google Scholar]
- 8.Kehrenberg, C., and S. Schwarz. 2005. Plasmid-borne florfenicol resistance in Pasteurella multocida. J. Antimicrob. Chemother. 55:773-775. [DOI] [PubMed] [Google Scholar]
- 9.Khan, A. A., M. S. Nawaz, S. A. Khan, and C. E. Cerniglia. 2000. Detection of multidrug-resistance Salmonella typhimurium DT104 by multiplex polymerase chain reaction. FEMS Microbiol. Lett. 182:355-360. [DOI] [PubMed] [Google Scholar]
- 10.Korea Food and Drug Administration (KFDA). 2005. Establishment of control system of antibiotics for livestocks. Korea Food and Drug Administration, Seoul, South Korea.
- 11.Li, X. S., G. Q. Wang, X. D. Du, B. A. Cui, S. M. Zhang, and J. Z. Shen. 2007. Antimicrobial susceptibility and molecular detection of chloramphenicol and florfenicol resistance among Escherichia coli isolates from diseased chickens. J. Vet. Sci. 8:243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim, S. K., H. S. Lee, H. M. Nam, Y. S. Cho, J. M. Kim, S. W. Song, Y. H. Park, and S. C. Jung. 2007. Antimicrobial resistance observed in Escherichia coli strains isolated from fecal samples of cattle and pigs in Korea during 2003-2004. Int. J. Food Microbiol. 116:283-286. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, 2nd ed. Approved standard M31-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 14.Pritchett, L. C., M. E. Konkel, J. M. Gay, and T. E. Besser. 2000. Identification of DT104 and U320 phage type among Salmonella enterica serotype Typhimurium isolates by PCR. J. Clin. Microbiol. 38:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz, S., C. Khenberg, B. Doublet, and A. Cloeckaert. 2004. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Lett. 28:519-542. [DOI] [PubMed] [Google Scholar]
- 16.Shin, S. J., S. G. Kang, N. Rayamajhi, M. L. Kang, and H. S. Yoo. 2005. Evaluation of the antimicrobial activity of florfenicol against bacteria isolated from bovine and porcine respiratory disease. Vet. Microbiol. 106:73-77. [DOI] [PubMed] [Google Scholar]
- 17.Singer, R. S., T. E. Patterson, A. E. Meier, J. K. Gibson, H. L. Lee, and C. W. Maddox. 2004. Relationship between phenotypic and genotypic florfenicol resistance in Escherichia coli. Antimicrob. Agents Chemother. 48:4047-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White, D. G., C. Hudson, J. J. Maurer, S. Ayers, S. Zhao, M. D. Lee, L. Bolton, T. Foley, and J. Sherwood. 2000. Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea. J. Clin. Microbiol. 38:4593-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang, S. J., K. Y. Park, S. H. Kim, K. M. No, B. K. Lee, and Y. H. Park. 2002. Antimicrobial resistance in Salmonella enterica serovars Enteritidis and Typhimurium isolated from animals from Korea: comparison of phenotypic and genotypic resistance characterization. Vet. Microbiol. 86:295-301. [DOI] [PubMed] [Google Scholar]