Abstract
A species-specific RNA colony blot hybridization protocol was developed for enumeration of culturable Vibrio cholerae and Vibrio mimicus bacteria in environmental water samples. Bacterial colonies on selective or nonselective plates were lysed by sodium dodecyl sulfate, and the lysates were immobilized on nylon membranes. A fluorescently labeled oligonucleotide probe targeting a phylogenetic signature sequence of 16S rRNA of V. cholerae and V. mimicus was hybridized to rRNA molecules immobilized on the nylon colony lift blots. The protocol produced strong positive signals for all colonies of the 15 diverse V. cholerae-V. mimicus strains tested, indicating 100% sensitivity of the probe for the targeted species. For visible colonies of 10 nontarget species, the specificity of the probe was calculated to be 90% because of a weak positive signal produced by Grimontia (Vibrio) hollisae, a marine bacterium. When both the sensitivity and specificity of the assay were evaluated using lake water samples amended with a bioluminescent V. cholerae strain, no false-negative or false-positive results were found, indicating 100% sensitivity and specificity for culturable bacterial populations in freshwater samples when G. hollisae was not present. When the protocol was applied to laboratory microcosms containing V. cholerae attached to live copepods, copepods were found to carry approximately 10,000 to 50,000 CFU of V. cholerae per copepod. The protocol was also used to analyze pond water samples collected in an area of cholera endemicity in Bangladesh over a 9-month period. Water samples collected from six ponds demonstrated a peak in abundance of total culturable V. cholerae bacteria 1 to 2 months prior to observed increases in pathogenic V. cholerae and in clinical cases recorded by the area health clinic. The method provides a highly specific and sensitive tool for monitoring the dynamics of V. cholerae in the environment. The RNA blot hybridization protocol can also be applied to detection of other gram-negative bacteria for taxon-specific enumeration.
Vibrio cholerae is autochthonous to the aquatic environment, but some strains produce enterotoxins and are capable of causing epidemics of the human disease cholera. Strains of V. cholerae are classified by their O antigen, with over 210 serogroups recognized to date. Seven cholera pandemics have occurred since 1832: while microbiologic data on the earlier pandemics are not available, the last two are known to have been caused by strains within serogroup O1, with the major pathogenic factor being production of cholera toxin. The genes encoding cholera toxin and other pathogenic factors have been shown to reside in a mobile genetic element of phage origin, designated CTXΦ (20).
Standard microbiologic methods for isolation of V. cholerae present in natural waters rely primarily on a method originally developed for clinical diagnosis, namely, enrichment in alkaline peptone water, followed by subculture on selective media and confirmation using selected biochemical and immunological tests (7). The alkaline nature of the enrichment broth allows differential multiplication of Vibrio species but renders this method inappropriate for enumeration. PCR methods and oligonucleotide hybridization have been used to detect and enumerate toxigenic V. cholerae bacteria (3, 11, 12, 14, 15, 21). These methods typically rely on amplification of or hybridization to pathogenic markers, such as O1/O139 wbe, tcpA, and ctxA DNA sequences.
However, occasional localized outbreaks of cholera have been caused by non-O1, non-O139 V. cholerae, which may be toxigenic or nontoxigenic. Conversely, many environmental V. cholerae O1 strains isolated from areas of endemicity do not harbor ctx genes (9). It has also been shown that CTXΦ is capable of lysogenic conversion of strains that are CTXΦ negative (20). Additionally, the cholera toxin (CTX) prophage has also been detected in clinical strains of V. mimicus, and V. mimicus has been proposed as a natural reservoir for CTXΦ (2). Furthermore, ecological studies of V. cholerae are often hampered by the fact that toxigenic strains represent only a small percentage of the total V. cholerae population in the environment, especially in areas where cholera is not endemic. These facts underline the need for a method of detection of the total number of V. cholerae bacteria present in environmental samples.
The many copies of 16S rRNA molecules in each V. cholerae cell offer appropriate targets for species-specific enumeration. In this study, the probe Vchomim1276, previously described by Heidelberg et al. (4-6), was employed in an RNA colony blot hybridization protocol. The specificity and sensitivity of the probe were tested using type strains and environmental and clinical isolates. The method was evaluated using laboratory microcosms to which cells of V. cholerae were added, and the protocol was used to enumerate V. cholerae bacteria in samples collected from ponds in a region of cholera endemicity in Bangladesh.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table 1. These included strains from the American Type Culture Collection (Manassas, VA) and clinical and environmental isolates from the United States, Mexico, Bangladesh, England, and Ecuador. Unless specified, all strains were grown on LB-20 agar (Luria-Bertani agar amended with NaCl to a final concentration of 2%). The bacterial cultures were maintained at −80°C in LB broth to which 25% glycerol had been added and on LB-20 agar slants at room temperature.
TABLE 1.
Bacterial strains used to evaluate RNA colony blot hybridization method employing probe Vchomim1276
| Strain | Hybridization result |
|---|---|
| V. cholerae O1 classical ATCC 14035 | + |
| V. cholerae O1 classical ATCC 11623 | + |
| V. cholerae O139 AI1877 | + |
| V. cholerae O139 EM-0208 | + |
| V. cholerae O1 El Tor N16961 | + |
| V. cholerae O1 El Tor EB-0184 | + |
| V. cholerae non-O1, non-O139 CB98-203 | + |
| V. cholerae non-O1, non-O139 CB99-18 | + |
| V. cholerae non-O1, non-O139 EC1 | + |
| V. cholerae non-O1, non-O139 UM4089 | + |
| V. cholerae non-O1, non-O139 TMA21 | + |
| V. cholerae non-O1, non-O139 EB-0172 | + |
| V. cholerae non-O1, non-O139 EM-0232 | + |
| V. mimicus ATCC 33563 | + |
| V. mimicus UM4198 | + |
| Aeromonas caviae ATCC 15468 | − |
| Escherichia coli K-12 | − |
| V. salmonicida ATCC 43839 | − |
| V. vulnificus ATCC 27562 | − |
| V. orientalis ATCC 33934 | − |
| V. splendidus ATCC 33125 | − |
| V. furnissii ATCC 35016 | − |
| V. anguillarum ATCC 19264 | − |
| Grimontia (Vibrio) hollisae ATCC 33564 | −a |
| V. fluvialis CB99-14 | − |
Weak cross-reactivity. V. hollisae was reclassified as Grimontia hollisae by Thompson et al. (19), based on its 16S rRNA-based genetic distance from representative Vibrio species.
V. cholerae-specific probe.
We examined 207 16S rRNA gene sequences of Vibrio or Photobacterium origin deposited in GenBank to determine V. cholerae-specific sequence motifs in 16S rRNA genes (22). However, a stretch of parsimony-informative sites between 16S rRNA gene sequences of V. cholerae and V. mimicus was not found. A previously described 16S rRNA gene probe, Vchomim1276, was selected as an oligonucleotide probe specific for V. cholerae and V. mimicus (5, 6). The probe (ACT TTG TGA GAT TCG CTC CAC CTC G; melting temperature [Tm] = 72°C), was 5′ end labeled with either fluorescein or Cy3 at the time of oligomer manufacturing by Sigma-Genosys (St. Louis, MO).
Colony blot lifting.
Strains were inoculated onto LB-20 agar and grown overnight at room temperature to a colony size no larger than 5 mm in diameter. A previously reported RNA colony blot lift method (8) was employed to liberate, denature, and immobilize nucleic acids on nylon or nitrocellulose membranes (GE Osmonics). Briefly, colony-containing agar plates were overlaid with membranes, and transfer of colony materials was allowed to proceed for at least 15 min. Membranes were transferred colony side up to filter papers wetted with 10% sodium dodecyl sulfate (SDS) and incubated for 5 to 10 min. Membranes were then transferred to filter paper wetted with 3× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), prewarmed to 65°C, and incubated at 65°C for 10 to 15 min. Membranes were dried at 37°C for 10 min and baked at 70°C for 15 min. Methylene blue was used for qualitative examination of RNA and/or DNA immobilized on membranes.
RNA colony blot hybridization.
All solutions used for RNA colony blot lift and hybridization were treated with diethyl pyrocarbonate (DEPC) to eliminate RNase contamination. Colony blots were washed in 3× SSC-0.1% SDS three times at room temperature for 10 min each and once at 65°C for 2 h to prevent cellular debris from interfering with hybridization. Membranes were prehybridized at 60°C for 30 min in hybridization solution base (10 ml per 100-cm2 membrane; 0.9 M NaCl, 50 mM sodium phosphate, pH 7.0, 5 mM EDTA, 0.5% SDS in DEPC-treated water). Hybridizations were performed overnight (approximately 16 h) at 60°C, using hybridization solution base (10 ml per 100-cm2 membrane) containing fluorescently labeled Vchomim1276 probe (80 ng/ml). After hybridization, membranes were washed in washing solution (1% SDS, 1× SSC in DEPC-treated water) at the hybridization temperature for 30 min. Membranes were wrapped in plastic wrap or placed in a hybridization bag and then visualized using a Typhoon 9410 variable-mode imager (GE Healthcare, Piscataway, NJ) in fluorescence mode. Alternatively, colony blots hybridized with Cy3- or fluorescein-labeled Vchomim1276 were visualized with a Dark Reader hand lamp (Clare Chemical, Dolores, CO).
Estimation of sensitivity and specificity of RNA colony blot hybridization.
The sensitivity and specificity of the Vchomim1276 probe, under conditions employed for RNA colony blot hybridization, were estimated using colony blots containing pure cultures of V. cholerae and sister species. According to statistical definition (1, 18), sensitivity was determined using the following equation: sensitivity = number of true-positive results/(number of true-positive results + number of false-negative results). Specificity was determined using the following equation: specificity = number of true-negative results/(number of true-negative results + number of false-positive results). Extensive testing was also previously performed on the specificity of the Vchomim1276 probe (4).
Spiking experiments.
For evaluation of the sensitivity and specificity of the overall hybridization assay, a freshwater sample with a known abundance of V. cholerae was employed. Aliquots from freshwater (Lake Artemesia, MD) samples were amended with luminescent V. cholerae non-O1, non-O139 strain CB99-18, isolated from Chesapeake Bay, MD (13). Appropriate dilutions were made to obtain approximately 150 total colonies, including approximately 40 V. cholerae colonies, per spread plate, using heterotrophic plate count agar or LB-20 agar. Colony lift and RNA colony blot hybridization were performed as described above, and the prevalence of Vchomim1276-positive colonies was compared with the expected prevalence of the amended culture, based on luminescence of the V. cholerae CB99-18 strain. Thus, we were able to confirm that the lake water did not contain luminescent bacteria.
Copepod microcosm.
Microcosms containing copepods collected from Baltimore Harbor, MD, during the winter months were incubated with cells of V. cholerae CB99-18 at room temperature for 18 h. The copepods in the microcosms were collected using a nylon mesh (64 μm) plankton net, washed to remove unattached bacteria, homogenized, and plated for enumeration of attached V. cholerae bacteria. Plates were incubated at room temperature for 1 to 2 days, after which RNA colony blots were prepared using Cy3-labeled Vchomim1276.
Field trial.
The Vchomim1276 probe and the RNA colony blot hybridization protocol were employed to enumerate V. cholerae bacteria in water samples collected from an area of cholera endemicity, Mathbaria, in Bangladesh. This area is located adjacent to the Bay of Bengal and approximately 400 km southwest of Dhaka, the capital city of Bangladesh. In this study, samples from Mathbaria were collected biweekly from July 2006 to March 2007 from six man-made ponds that are heavily used as a source of drinking water and for other domestic purposes. A major river, Baleshwar, flows along the western boundary of Mathbaria, and on its other side is the tropical mangrove forest of the Sundarbans. RNA colony blot lifts from L agar spread plates of unconcentrated water samples were prepared and hybridized using the Vchomim1276 probe. A total of 108 samples were tested. In parallel, colony blots from the same water samples, using the same medium, were assayed for culturable toxigenic V. cholerae bacteria using an alkaline phosphatase-labeled DNA probe as described previously (21). Total bacteria were determined for each sample, using the fluorescent dye 4′,6-diamidino-2-phenylindole (DAPI) and epifluorescence microscopy (16).
RESULTS
Development of RNA colony blot hybridization method.
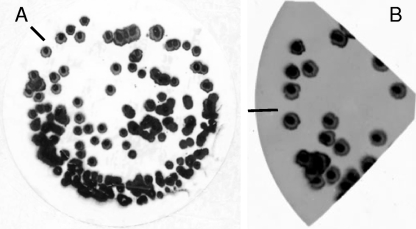
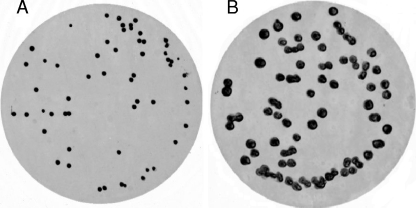
In developing an RNA colony blot hybridization method specific for V. cholerae, four methods of colony blot lifting, two targeting DNA (GE Osmonics [membrane manufacturer]) (21) and two targeting RNA (8, 10), were evaluated. To assess the amount of nucleic acid immobilized on membranes by each method, the blots were stained with methylene blue (Fig. 1A). All four methods were effective in liberating, denaturing, and immobilizing nucleic acid on the nylon membrane. The nylon membranes were then cut into quarters and hybridized with Cy3-labeled Vchomim1276 probe. The method of Ivanov and Gigova (8), targeting RNAs on the blot, proved far superior to the other three methods in terms of hybridization signals (Fig. 1B). A modified method was developed which included prehybridization washing and the use of DEPC-treated solutions, and the full method is presented in Materials and Methods.
FIG. 1.
(A) Methylene blue staining of RNA colony blot on nylon membrane prepared using spread plate of V. cholerae CB99-18 on LB-20 agar. (B) Digital fluorescence image of colony blot from panel A, cut into quarters (for optimization experiments) and hybridized with the Cy3-labeled Vchomim1276 probe. The inserted line is for orientation purposes only.
Evaluation of RNA colony blot hybridization.
The Vchomim1276 probe applied for RNA colony blot hybridization was evaluated for accuracy in detecting colonies of the targeted species. For qualitative variables such as species identification, the accuracy of the probe can be evaluated in terms of sensitivity (proportion of positive samples correctly identified by the test) and specificity (proportion of negative samples correctly identified by the test). According to the results for application of the probe in RNA colony lift hybridization (Table 1), the probe had 100% sensitivity. However, the specificity was 90% because of a weak positive signal produced by Grimontia hollisae (19) under the conditions used for RNA colony blot hybridization (Fig. 2). Sequence comparison at the probe target site revealed that the cross-reactivity was due to 16S rRNA sequence similarity at the target site rather than to nonspecific binding of the probe to other cellular components. The cross-reaction was easily distinguished from that of V. cholerae or V. mimicus (Fig. 2) by comparing the intensities of the signals. Signal intensity statistics can be used to develop separation of the weak false reactivity displayed by G. hollisae, i.e., >25% of the signal strength of a positive control signal, to resolve false-positive results.
FIG. 2.
(A) Schematic representation. (B) Digital fluorescence image of RNA colony blot hybridization with Cy3-labeled Vchomim1276 to determine specificity of probe. A, clinical V. cholerae O1; B, environmental V. cholerae O1; C, clinical V. mimicus; D, environmental V. mimicus; E, V. anguillarum; F, V. fluvialis; G, G. hollisae; H, V. vulnificus; I, Aeromonas caviae; J, Escherichia coli.
To evaluate the accuracy of the overall RNA colony blot hybridization protocol, experiments were performed whereby natural water samples were amended with laboratory cultures of V. cholerae. The RNA colony blot method was able to identify and enumerate V. cholerae bacteria in two different types of environmental samples. Figure 3A is a digital fluorescence image of a colony blot in which luminescent V. cholerae CB99-18 was added to Lake Artemesia, MD, pond water and spread plated, using 10-fold serial dilutions. A total of 58 V. cholerae colonies were enumerated by the probe on this membrane. A highly luminescent strain of V. cholerae, CB99-18, was used as the surrogate to validate blot results. Plate counts done prior to RNA colony blot lift and hybridization yielded a total of 187 colonies, 58 of which were luminescent, indicating that the probe hybridization method detected V. cholerae CB99-18 cells without false-positive or false-negative results, yielding 100% sensitivity and 100% specificity for aquatic bacteria in the lake water when G. hollisae was absent.
FIG. 3.
Digital fluorescence images of RNA colony blots to which V. cholerae CB99-18 had been added and hybridized with Cy3-labeled Vchomim1276 probe. (A) Pond water from Lake Artemisia, MD. (B) Copepod microcosm.
Additionally, the RNA colony blot hybridization protocol was used to identify V. cholerae isolates in a complex community of surface-attached bacteria on copepods (Fig. 3B). These experiments indicated that individual copepods can carry 1 × 104 to 5 × 104 cells of V. cholerae, with an attachment rate of 0.3 to 0.05%, after overnight cocultivation. As expected, the negative control, copepods without V. cholerae added to the microcosm, did not produce culturable V. cholerae signals by the RNA colony blot hybridization protocol, since the copepods were collected during a time when the species was most likely in a viable but nonculturable state.
Application of method to population dynamics of V. cholerae.
To assess the practicality of the RNA colony blot lift and hybridization method, water samples collected from an area of cholera endemicity were tested. Figure 4 shows results for two of the positive blots prepared using water collected from Mathbaria Province, Bangladesh (site 1 and site 2). A survey of V. cholerae in freshwater ponds in Bangladesh over a 9-month period demonstrated an abundance of planktonic cells, as high as 104 CFU ml−1, 1 to 2 months prior to an environmental peak in pathogenic V. cholerae isolation and typical seasonal epidemics (October-November and April-May) (Fig. 5). In fact, the trend was a reduction in the number of V. cholerae cells in the water immediately prior to the onset of an outbreak, suggesting that cases may have a longer-than-expected incubation period. In contrast to this trend, the total bacterial abundance, given by total direct DAPI counts, remained relatively constant, at approximately 106 cells/ml. Interestingly, at the time points during which typical seasonal epidemics occur, the number of ctxA-positive colonies was larger than the number of species-positive colonies, suggesting that either free CTX phage can be detected or the phage has infected other species. At all other times, the number of ctxA-positive colonies was smaller than the number of V. cholerae colonies, as expected.
FIG. 4.
Representative RNA colony blots hybridized with Vchomim1276 probe. Blots were prepared from spread plates of 100 μl of water and/or dilution. The blots shown were prepared using 100-μl water samples from pond 3 (July 2006, round 60) (A) and pond 1 (January 2007, round 73) (B) in Mathbaria, Bangladesh.
FIG. 5.
Seasonality and abundance of pathogenic V. cholerae bacteria, determined by conventional culture (solid line) and molecular methods (targeting ctxA), and of total V. cholerae bacteria, determined by RNA colony blot hybridization, compared to total bacterial counts (DAPI) (dashed line) in coastal areas of Mathbaria, Bangladesh.
DISCUSSION
In this study, we developed an RNA colony blot hybridization method using a fluorescently labeled oligonucleotide probe specific for V. cholerae and V. mimicus (4). A simple RNA colony blot method, originally reported in 1986 (8), proved superior to others tested and was modified for use in this study. A commercially available fluorochrome label was preferred over other labels because of laboratory personnel safety, operational regulations, availability, and/or economic reasons. By targeting RNA instead of DNA, the fluorochrome signal was boosted sufficiently for reliable detection. The probe is unable to resolve V. cholerae from V. mimicus, since the 16S rRNA sequences of V. cholerae and V. mimicus differ by only ∼6 of 1,456 nucleotides (17). However, inclusion of V. mimicus in the V. cholerae RNA colony blot hybridization method was deemed acceptable, since V. mimicus has been proposed to be a reservoir for CTXΦ phage (2). Also, these two species can be separated, when necessary, by using thiosulfate-citrate-bile salts-sucrose agar for the spread plates, since V. mimicus typically produces green, non-sucrose-fermenting colonies and V. cholerae produces yellow, sucrose-fermenting colonies.
The results from experiments in which V. cholerae was added to samples and from field trials demonstrate that the RNA colony blot hybridization method can be used to detect and enumerate V. cholerae bacteria in different types of environmental water samples. For samples containing or expected to contain large numbers of V. cholerae bacteria, colony blot lifts from spread plates are satisfactory for enumeration of V. cholerae bacteria. In this case, there is no need for an enrichment step or use of selective media. The method can be adapted based on the expected density of cells of V. cholerae. For samples in which the expected density of V. cholerae is low, such as water samples collected during winter months, filtration can be used to concentrate samples directly onto membranes. In this case, several sample volumes should be filtered to yield well-separated colonies of sufficient size to give a reliable signal (∼2 to 3 mm in diameter). Larger membranes (137 mm) can be used to sample even larger volumes of water (100 to 200 ml) when the number of V. cholerae bacteria is extremely small. When filtration is used, a selective medium such as thiosulfate-citrate-bile salts-sucrose agar should be used to inhibit growth of competitor cells/colonies.
The method can also be adapted for isolation of the organism. If the colony blot is made from a spread plate, the master plate can be stored at 15°C until the hybridization is complete, at which time the positive signals can be correlated to colonies and subsequently subcultured. If the colony blot is made by filtering a water sample directly onto the membrane (and then overlaying it onto an agar plate), subsequent growth on the membrane can be replica plated onto a fresh agar plate prior to colony lysis. Again, positive signals can be correlated with colonies, subcultured, and confirmed. For this purpose, this method is preferred to traditional enrichment/selective medium isolation methods because the necessity for extensive and laborious biochemical tests can be avoided.
Acknowledgments
This research was supported by NIH research grant 1RO1A13912901 under collaborative agreements between the Johns Hopkins Bloomberg School of Public Health and the ICDDR. C. J. Grim was supported by the IC Postdoctoral Fellowship Program (NGA grant HM15820612010). Young-Gun Zo was supported by the second-phase Brain Korea 21 Project in 2008.
Footnotes
Published ahead of print on 26 June 2009.
REFERENCES
- 1.Altman, D. G., and J. M. Bland. 1994. Diagnostic tests. 1. Sensitivity and specificity. BMJ 308:1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd, E. F., K. E. Moyer, L. Shi, and M. K. Waldor. 2000. Infectious CTXΦ and the vibrio pathogenicity island prophage in Vibrio mimicus: evidence for recent horizontal transfer between V. mimicus and V. cholerae. Infect. Immun. 68:1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goel, A. K., S. Ponmariappan, D. V. Kamboj, and L. Singh. 2007. Single multiplex polymerase chain reaction for environmental surveillance of toxigenic-pathogenic O1 and non-O1 Vibrio cholerae. Folia Microbiol. (Prague) 52:81-85. [DOI] [PubMed] [Google Scholar]
- 4.Heidelberg, J. 1997. Seasonal abundance of bacterioplankton populations of bacteria, gamma proteobacteria, Vibrio/Photobacterium, Vibrio vulnificus, Vibrio cholerae/Vibrio mimicus, and Vibrio cincinnatiensis associated with zooplankton in the Choptank River, Maryland. Ph.D. thesis. University of Maryland, College Park.
- 5.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Bacteria of the γ-subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Appl. Environ. Microbiol. 68:5498-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Seasonality of Chesapeake Bay bacterioplankton species. Appl. Environ. Microbiol. 68:5488-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huq, A., C. Grim, R. R. Colwell, and G. B. Nair. 2006. Detection, isolation, and identification of Vibrio cholerae from the environment. Curr. Protoc. Microbiol. 2006:6A.5. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov, I., and L. Gigova. 1986. RNA colony hybridization method. Gene 46:287-290. [DOI] [PubMed] [Google Scholar]
- 9.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann, P., A. Pfefferkorn, M. Teuber, and L. Meile. 1997. Identification and quantification of Bifidobacterium species isolated from food with genus-specific 16S rRNA-targeted probes by colony hybridization and PCR. Appl. Environ. Microbiol. 63:1268-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khuntia, H. K., B. B. Pal, and G. P. Chhotray. 2008. Quadriplex PCR for simultaneous detection of serotype, biotype, toxigenic potential, and central regulating factor of Vibrio cholerae. J. Clin. Microbiol. 46:2399-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch, W. H., W. L. Payne, B. A. Wentz, and T. A. Cebula. 1993. Rapid polymerase chain reaction method for detection of Vibrio cholerae in foods. Appl. Environ. Microbiol. 59:556-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis, V. R., E. Russek-Cohen, N. Choopun, I. N. Rivera, B. Gangle, S. C. Jiang, A. Rubin, J. A. Patz, A. Huq, and R. R. Colwell. 2003. Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 69:2773-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantri, C. K., S. S. Mohapatra, T. Ramamurthy, R. Ghosh, R. R. Colwell, and D. V. Singh. 2006. Septaplex PCR assay for rapid identification of Vibrio cholerae including detection of virulence and int SXT genes. FEMS Microbiol. Lett. 265:208-214. [DOI] [PubMed] [Google Scholar]
- 15.Mendes, C. L., F. G. Abath, and N. C. Leal. 2008. Development of a multiplex single-tube nested PCR (MSTNPCR) assay for Vibrio cholerae O1 detection. J. Microbiol. Methods 72:191-196. [DOI] [PubMed] [Google Scholar]
- 16.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 17.Ruimy, R., V. Breittmayer, P. Elbaze, B. Lafay, O. Boussemart, M. Gauthier, and R. Christen. 1994. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas and Plesiomonas deduced from small subunit rRNA sequencer. Int. J. Syst. Bacteriol. 44:416-426. [DOI] [PubMed] [Google Scholar]
- 18.Sokal, R. R., and F. J. Rohlf. 1995. Biometry: the principles and practices of statistics in biological research, 3rd ed. W. H. Freeman and Co., New York, NY.
- 19.Thompson, F. L., B. Hoste, K. Vandemeulebroecke, and J. Swings. 2003. Reclassification of Vibrio hollisae as Grimontia hollisae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 53:1615-1617. [DOI] [PubMed] [Google Scholar]
- 20.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 21.Wright, A., Y. Guo, J. Johnson, J. Nataro, and J. J. Morris. 1992. Development and testing of a nonradioactive DNA oligonucleotide probe that is specific for Vibrio cholerae cholera toxin. J. Clin. Microbiol. 30:2302-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zo, Y. G., and R. R. Colwell. 2008. A simple binomial test for estimating sequencing errors in public repository 16S rRNA sequences. J. Microbiol. Methods 72:166-179. [DOI] [PubMed] [Google Scholar]