Abstract
The filamentous fungus Graphium sp. (ATCC 58400) grows on gaseous n-alkanes and diethyl ether. n-Alkane-grown mycelia of this strain also cometabolically oxidize the gasoline oxygenate methyl tert-butyl ether (MTBE). In this study, we characterized the ability of this fungus to metabolize and cometabolize a range of cyclic ethers, including tetrahydrofuran (THF) and 1,4-dioxane (14D). This strain grew on THF and other cyclic ethers, including tetrahydropyran and hexamethylene oxide. However, more vigorous growth was consistently observed on the lactones and terminal diols potentially derived from these ethers. Unlike the case in all previous studies of microbial THF oxidation, a metabolite, γ-butyrolactone, was observed during growth of this fungus on THF. Growth on THF was inhibited by the same n-alkenes and n-alkynes that inhibit growth of this fungus on n-alkanes, while growth on γ-butyrolactone or succinate was unaffected by these inhibitors. Propane and THF also behaved as mutually competitive substrates, and propane-grown mycelia immediately oxidized THF, without a lag phase. Mycelia grown on propane or THF exhibited comparable high levels of hemiacetal-oxidizing activity that generated methyl formate from mixtures of formaldehyde and methanol. Collectively, these observations suggest that THF and n-alkanes may initially be oxidized by the same monooxygenase and that further transformation of THF-derived metabolites involves the activity of one or more alcohol dehydrogenases. Both propane- and THF-grown mycelia also slowly cometabolically oxidized 14D, although unlike THF oxidation, this reaction was not sustainable. Specific rates of THF, 14D, and MTBE degradation were very similar in THF- and propane-grown mycelia.
The cyclic ether tetrahydrofuran (THF) is a colorless, volatile, highly flammable liquid that is widely used as a solvent in the manufacture and processing of polymers such as polyvinyl chloride. Annually, over 1 million pounds of THF is generated in or imported into the United States (43, 44, 49). Many actinomycetes have been identified that can utilize THF as a sole source of carbon and energy under aerobic conditions. Among these organisms are multiple Rhodococcus isolates, including Rhodococcus ruber strains 219 (2, 3) and M2 (12), a number of closely related Pseudonocardia strains, including Pseudonocardia dioxanivorans (25, 35), Pseudonocardia benzenivorans (17), Pseudonocardia sulfidooxydans (17, 27), Pseudonocardia hydrocarbonoxydans (17), and Pseudonocardia tetrahydrofuranoxydans strains K1 (21), M1 (12), and ENV478 (45), and the Nocardia-like propanotroph strain ENV 425 (45). Fungal growth on THF by Aureobasidium pullulans has been reported for a patent (36), and growth of Cordyceps sinensis on THF, tetrahydropyran, and other cyclic ethers, including 1,3-dioxane (13D), 1,4-dioxane (14D), and several methyl dioxanes, was also recently described (33). The Cordyceps strain generates ethylene glycol during 14D catabolism, and a similar pathway appears to operate in a recently described 14D-metabolizing bacterium, Mycobacterium sp. strain PH06 (20).
Despite the variety of aerobic microorganisms that are known to metabolize THF, the mechanism and pathway of THF oxidation have not been established firmly. Bernhardt and Diekmann (2) examined the growth of R. ruber strain 219 on 1,4-butanediol, THF, and several potential THF metabolites, including 3-hydroxytetrahydrofuran, γ-butyrolactone, and succinate. They suggested that the initial oxidation of THF involves a monooxygenase-catalyzed reaction that generates 2-hydroxytetrahydrofuran as an immediate oxidation product. Further analysis of the degradation pathway was prevented by the lack of availability of pure forms of this metabolite. In aqueous solution, this cyclic hemiacetal (a lactol) forms an equilibrium mixture with its tautomer, 4-hydroxybutyraldehyde (14). 4-Hydroxybutyraldehyde is also the putative immediate product of 1,4-butanediol catabolism. An annotated summary of the biological and abiotic transformations potentially involved in THF and 1,4-butanediol oxidation (2) is shown in Fig. 1.
FIG. 1.
Potential biotic and abiotic reactions involved in the degradation of THF and 1,4-butanediol. The figure shows the potential pathways of THF and 1,4-butanediol metabolism discussed in this study. The figure is based on the pathway originally suggested by Bernhardt and Diekmann (2) but has been annotated to include potential enzymes involved in each transformation as well as abiotic processes that can account for the interconversion of some metabolites. The compounds in the inset include compounds that are unrelated to THF except that their metabolism has been reported to generate 4-hydroxybutyrate.
Among bacteria, the most extensively characterized THF-utilizing organism is P. tetrahydrofuranoxydans strain K1. Molecular evidence supporting a monooxygenase-catalyzed oxidation of THF was obtained from cloning and transcriptional studies using this strain (42). A plasmid-borne gene cluster was identified and found to encode a four-component binuclear-iron-containing monooxygenase, THF monooxygenase, and to be transcribed in the presence of THF. Another gene, encoding a putative succinate semialdehyde dehydrogenase (sad), is also transcribed during growth of strain K1 on THF, 4-hydroxybutyrate, and 1,4-butanediol, whereas a separate aldehyde dehydrogenase gene (alhH) is transcribed only in cells grown on 1,4-butanediol, not in cells grown on THF. These observations support the conclusion that the predominant pathway of THF oxidation in strain K1 does not involve 4-hydroxybutyraldehyde but has γ-butyrolactone as an obligate intermediate generated from the enzymatic oxidation of 2-hydroxytetrahydrofuran. However, like all other previous studies with THF-degrading microorganisms, this pathway remains speculative, as no THF-derived intermediates were detected or reported. In addition, no supporting evidence for a hemiacetal-oxidizing activity required for the oxidation of 2-hydroxytetrahydrofuran has been presented for this or any other THF-metabolizing microorganism.
Biodegradation of THF has emerged as an area of interest because of the close association between THF and other cyclic ethers, such as 13D and 14D. A number of cyclic ethers, and particularly 14D, have been used as stabilizing compounds in commercial solvent mixtures containing 1,1,1-trichloroethane (29). Because this chlorinated hydrocarbon is one of the most frequently encountered groundwater pollutants in the United States, there is concern over the environmental fate of cyclic ethers that are likely cocontaminants at sites contaminated with 1,1,1-trichloroethane (27, 40, 44). Currently, 14D is not known to be used widely as a growth substrate by microorganisms, but a number of studies have demonstrated that 14D can be oxidized cometabolically by aerobic organisms that grow on THF or other substrates, such as toluene or n-alkanes (4, 28, 45, 49). Notably, this group of organisms includes Mycobacterium vaccae JOB5 (4) and Pseudonocardia sp. strain ENV478. These strains also oxidize another important ether pollutant, methyl tert-butyl ether (MTBE) (41, 45).
In the present study, we characterized the biodegradation of THF and other cyclic ethers by a filamentous fungus, a Graphium sp. This unusual organism was first isolated from enrichment cultures grown on natural gas (11, 48). In addition to growth on nonmethane n-alkanes, we have shown that this Graphium sp. also grows on diethyl ether (DEE) (13). Mycelia grown on n-alkanes such as propane (39), n-butane (13), and DEE (13) all cometabolically degrade MTBE. Our results show that Graphium sp. grows on THF as well as a variety of related lactones and terminal diols. Our results also demonstrate that n-alkane- and THF-grown mycelia oxidize 14D, although this compound is not used as a sole carbon and energy source. Our physiological and inhibitor studies suggest that THF degradation is initiated by the same cytochrome P450 responsible for oxidizing n-alkanes and MTBE. We also provide evidence for a hemiacetal-oxidizing activity in both THF- and propane-grown mycelia. Our findings are discussed in terms of their contributions to our understanding of the diversity in cyclic ether-degrading organisms and the metabolism and cometabolism of cyclic ethers.
MATERIALS AND METHODS
Materials.
Graphium sp. (ATCC 58400) was obtained from the American Type Culture Collection (Manassas, VA). Although the conidial morphology of the asexual stage of this fungus identifies it as a Graphium species, ribosomal DNA analyses suggest that its teleomorph is most closely related to Pseudallescheria boydii (1). 1,4-Butanediol (99+% purity), γ-butyrolactone (99+% purity), calcium carbide (∼80% technical-grade purity; for acetylene production), ɛ-caprolactone (≥99% purity), 13D (99.8% purity), 14D (99.8% purity), formaldehyde (37% solution), 3-hydroxytetrahydrofuran (99% purity), methyl formate (99% purity), morpholine (99+% purity), MTBE (99.8% purity), 1,5-pentanediol (96% purity), piperazine (99% purity), piperidine (99% purity), 1,3-propanediol (98% purity), β-propiolactone (90%), pyrrolidine (99% purity), sodium 3-hydroxybutyrate (97% purity), sodium succinate (99% purity), THF (99% purity; inhibitor free), tetrahydropyran (99% purity), and δ-valerolactone (≤25% polymer content) were obtained from Aldrich Chemical Company (Milwaukee, WI). Hexamethylene oxide (98% purity) was obtained from Alfa Aesar (Pelham, NH). Methanol was obtained from Fisher Scientific Inc. (Fair Lawn, NJ). Propane (instrument grade) was obtained from Matheson Gas Products, Inc. (Montgomeryville, PA). Other gases (H2, N2, and air) were obtained from local vendors. To minimize the potential for effects arising from chemical decomposition of ethers to peroxides, the ethers used in this study were all purchased and used within 3 months.
Culture conditions.
Mycelia of Graphium sp. were grown on potato dextrose agar (Difco, Franklin Lakes, NJ) plates at 25°C. After 14 days, the plates were wrapped with Parafilm (American National Can, Menasha, WI) and stored at 4°C until the conidia were collected. Conidia were harvested from potato dextrose agar plates, counted with a hemocytometer, and used to inoculate liquid cultures as previously described (10). All manipulations of cultures were conducted under sterile conditions to ensure that axenic cultures were maintained throughout all experiments. Cultures were also grown attached to glass filters, as previously described (13).
In all experiments investigating the growth of Graphium sp. on novel substrates, the fungus was grown in glass bottles (700 ml) sealed with screw caps fitted with butyl-rubber septa (Wheaton Scientific, Millville, NJ). The culture bottles contained 50 ml of mineral salts medium (MSM) (13) inoculated with conidia (2.5 × 106). Liquid potential growth substrates were added to the cultures as undiluted compounds. Gases such as acetylene and ethylene were added to the sealed vials as an overpressure (2% [vol/vol] gas phase). The gases were added using a plastic syringe fitted with a sterile filter (0.25-μm pore size). The culture bottles were placed on an orbital shaker (100 rpm) at 27 ± 3°C and incubated for 10 to 14 days. The dry weight of the mycelia was calculated as previously described (13), except that the filter paper was dried for 24 h at 55°C.
Short-term degradation reactions.
To obtain sufficient homogeneous biomass for multiple short-term (<5 h) degradation assays, cultures of Graphium sp. were first grown in flasks (500 ml) containing 50 ml of potato dextrose broth (PDB) (Difco, Franklin Lakes, NJ). The medium was inoculated with conidia (2.5 × 106), and the flasks were placed on an orbital shaker (100 rpm) at 27 ± 3°C for 3 days. With work performed in a laminar flow hood, the resulting mycelial mass was collected by gentle vacuum filtration onto sterile filter paper held in an autoclaved ceramic Büchner funnel. The mycelial mass was washed twice with sterile MSM (100 ml) to remove residual PDB. The washed mycelia were then transferred to a sterile glass bottle (700 ml) containing sterile MSM (50 ml). The bottles were sealed with screw caps, and either propane (10% [vol/vol] gas phase) or THF (10 mM) was added to the sealed bottles to induce the expression of enzymes involved in n-alkane or THF metabolism, respectively. Propane was added using a plastic syringe fitted with a sterile filter (0.25-μm pore size). The culture bottles were incubated on an orbital shaker (as above) for 2 days. The mycelia were harvested again by vacuum filtration, washed twice with sterile MSM (100 ml), and finally resuspended in a sterile 700-ml glass bottle containing sterile MSM (50 ml), as described above. The bottle was sealed with a screw cap fitted with a butyl-rubber septum. The degradation assays were then initiated by the addition of pure THF, MTBE, or 14D, and the flasks were placed on an orbital shaker (100 rpm) at 27 ± 3°C. At the indicated times, aqueous-phase samples were removed from these reaction mixtures and immediately analyzed for substrate depletion by gas chromatography (GC) (see below). Previously reported Henry's law coefficients were used to calculate the total mass of the remaining substrate (26).
Methyl formate synthase activity was assayed in cultures grown under similar conditions to those described above. PDB-grown mycelia were harvested, washed with MSM, transferred to 700-ml glass bottles containing sterile MSM (50 ml), and exposed to either propane (10% [vol/vol] gas phase), THF (20 mM), or dextrose (20 mM). These cultures were incubated on an orbital shaker for 2 days. The cultures were again collected, washed, and resuspended in a 700-ml glass bottle containing sterile MSM (50 ml). The reactions were initiated by the addition of methanol and formaldehyde to the sealed culture bottles to initial concentrations of 0.5 M and 50 mM, respectively (20). At the indicated times, headspace samples (25 μl) were removed from the bottle and immediately analyzed by GC (see below) to quantify methyl formate production.
Analytical methods.
Concentrations of THF, 1,4-dioxane, γ-butyrolactone, and MTBE were determined using a Shimadzu GC-8A GC (Kyoto, Japan) fitted with a flame ionization detector. In most experiments, aqueous samples (2 μl) were injected directly into the GC and analytes were separated using a stainless steel column (0.3 by 183 cm) packed with Porapak Q (80/20 mesh) (Waters Associates, Framingham, MA). The GC was operated with a constant column temperature of 140°C and with injector and detector temperatures of 200°C and 220°C, respectively. Nitrogen was used as the carrier gas, at a flow rate of 15 ml min−1. Methyl formate was detected using a Shimadzu GC-14A instrument fitted with a flame ionization detector. Gas-phase samples (25 μl) were injected directly into the GC, and analytes were separated using a stainless steel column (0.3 by 50 cm) packed with Porapak Q (80/20 mesh) (Waters Associates, Framingham, MA). The GC was operated with a constant column temperature of 110°C and with injector and detector temperatures of 200°C and 220°C, respectively. Nitrogen was used as the carrier gas, at a flow rate of 15 ml min−1. THF, γ-butyrolactone, 14D, methyl formate, and MTBE concentrations were determined from eight-point calibration plots obtained using the authentic compounds. To account for possible sorption effects, calibration plots were developed with 700-ml glass bottles containing heat-killed (30 min, 121°C) mycelia suspended in MSM (50 ml). In the experiments examining the effects of propane on THF degradation, headspace samples (25 μl) were analyzed with a Hewlett Packard 6890 (Palo Alto, CA) GC fitted with a flame ionization detector. A 30-m by 0.45-mm-internal-diameter by 2.55-μm DB-MTBE capillary column was used (J&W Scientific, Folsom, CA) at an oven temperature of 50°C. Helium was used as the carrier gas (15 ml min−1).
GC-MS.
The production of γ-butyrolactone and other possible metabolites during growth of Graphium sp. on THF was also investigated using GC-mass spectrometry (GC-MS). Samples (1 μl) of the culture medium were injected into a Shimadzu GC-17A, GCMS-QP5000 instrument (Kyoto, Japan). The GC-MS injection port was operated at 150°C in split mode, with a split ratio of 1:5. The GC-MS instrument was fitted with a DB-5ms capillary column (30 m × 0.25 mm × 0.25-μm film thickness; Agilent Technologies, Palo Alto, CA) and operated at 80°C, and eluting compounds were ionized by electron impact (70 eV) after passing through a GC-MS interface held at 250°C. Ions ranging from 20 to 150 atomic mass units were scanned at 0.3-s intervals. He (120 kPa) was used as the carrier gas. Data were collected and further analyzed using Shimadzu GCMS Solutions software. Compounds were identified by coelution with authentic standards and by computer comparison of fragmentation patterns, using the National Institutes of Standards and Technology 107 electron ionization mass spectral library. The relative intensities of each mass fragment for THF for the library, authentic standard, and material detected in our incubations were m/z 42 (100%:100%:100%), m/z 41 (41%:55%:52%), m/z 72 (29%:28%:27%), m/z 71 (26%:33%:32%), m/z 43 (16%:22%:23%), m/z 27 (16%:18%:19%), and m/z 39 (12%:20%:20%). The relative intensities of each mass fragment for γ-butyrolactone for the library, authentic standard, and material detected in our incubations were m/z 42 (100%:69%:68%) and m/z 28 (68%:100%:100%), m/z 41 (61%:35%:34%), m/z 29 (50%:55%:57%), m/z 27 (42%:34%:35%), m/z 86 (25%:19%:17%), and m/z 56 (23%:20%:18%). A GC-MS analysis of THF used in our experiments in this study also did not reveal the presence of detectable amounts of γ-butyrolactone.
RESULTS
We initially examined the ability of a Graphium sp. to grow on a range of cyclic ethers and some of their potential metabolites. The fungus grew well on THF, γ-butyrolactone, and 4-hydroxybutyrate (Fig. 2). Little or no growth was observed with 3-hydroxytetrahydrofuran, while the yield of mycelia grown on 1,4-butanediol was substantially higher than that for THF, γ-butyrolactone, or 4-hydroxybutyrate. A similar pattern was also observed with other cyclic ethers and their corresponding potential metabolites and precursors. For example, mycelia grown on 1,3-propanediol generated more biomass than mycelia grown on β-propiolactone. Slight to moderate growth was also observed with tetrahydropyran and hexamethylene oxide, and in both cases higher biomass yields were obtained with their corresponding lactones, δ-valerolactone and ɛ-caprolactone. Unlike the cyclic ethers containing a single oxygen atom, no growth was observed with either of the cyclic ethers with two oxygen atoms that we tested (13D and 14D). Likewise, no growth was observed for any of the nitrogen heterocycles tested as potential sole sources of carbon and energy (morpholine, pyrrolidine, piperazine, and piperadine). The potential for toxic effects of the compounds shown in Fig. 2 was not evaluated, and the results were not normalized to account for potential differences in substrate consumption.
FIG. 2.
Growth of Graphium sp. on cyclic ethers, lactones, acids, terminal diols, or nitrogen heterocycles. Liquid cultures of Graphium sp. were grown in glass bottles (700 ml) as described in Materials and Methods. Each culture contained MSM (50 ml), conidia (2.5 × 106), and a potential growth substrate (20 mM initial concentration). The figure shows the dry weight of mycelia generated after 14 days, after subtraction of the dry weight of mycelia (0.33 to 6.9 mg) generated in control cultures incubated in MSM alone. The data presented are the means and standard errors for three replicates.
A typical time course for THF consumption and the concurrent increase in mycelial biomass in a batch culture is shown in Fig. 3. Over 12 days, metabolism of THF (1 mmol) generated ∼22 mg dry weight of mycelia (∼0.3 mg dry weight/mg THF). A single metabolite, γ-butyrolactone, identified by both GC and GC-MS, was transiently detected in the medium during this experiment. The maximum amount of γ-butyrolactone (0.16 mmol) was detected after 7 days, at which point this metabolite accounted for ∼20% of the total THF degraded. After this initial maximum, the concentration of γ-butyrolactone decreased (Fig. 3). No other THF-derived metabolites were detected over the culture time course under the GC conditions described in Materials and Methods. Likewise, no additional metabolites other than γ-butyrolactone were detected by GC-MS when mycelia were grown in the presence of an initial concentration of either 20 or 40 mM THF and the growth medium was analyzed at regular intervals throughout the culture time course (data not shown). In previous studies, acetylene was shown to be a potent inhibitor of n-alkane- and MTBE-oxidizing activity in this Graphium sp. (10, 13). In cultures containing both THF (1 mmol) and acetylene (2% [vol/vol] gas phase), no THF consumption, γ-butyrolactone production (not shown), or increase in biomass was observed over the 12-day culture period (Fig. 3).
FIG. 3.
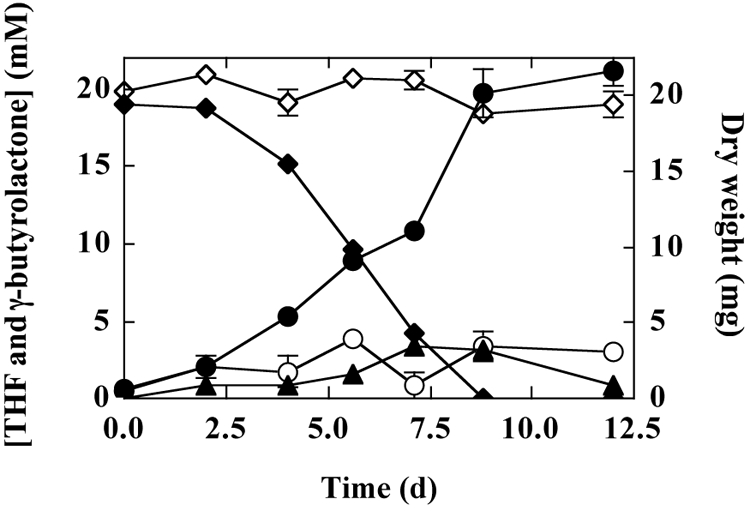
Time course of growth of Graphium sp. on THF. Liquid cultures of Graphium sp. were grown in glass bottles (700 ml) as described in Materials and Methods. Each bottle contained MSM (50 ml), conidia (2.5 × 106), and THF (20 mM initial concentration). Acetylene (1% [vol/vol]) was added to the gas phase of the sealed bottles when required. At the indicated times, six culture bottles (three with and three without acetylene) were randomly selected, and the liquid-phase concentrations of THF and γ-butyrolactone were determined by GC as described in Materials and Methods. The mycelia in these bottles were then harvested to quantify biomass production. The solid symbols show the amounts of THF (⧫), γ-butyrolactone (▴), and fungal biomass (•) (measured as dry weight) at each time point. The open symbols show the amounts of THF (⋄) and fungal biomass (○) in cultures containing acetylene. γ-Butyrolactone was not detected in any cultures containing acetylene. Data presented are the means and standard errors for three replicate cultures analyzed at each time point.
Our previous studies suggested that an n-alkane-inducible cytochrome P450 monooxygenase is responsible for initiating both n-alkane and MTBE oxidation in this Graphium sp. (10, 13). To determine if this enzyme was also involved in THF catabolism, we examined whether propane-grown mycelia were simultaneously adapted to oxidize THF. Mycelia grown on either PDB or propane were exposed to THF (1 mM). Propane-grown mycelia consumed THF without a lag phase (Fig. 4) and consumed ∼75% of the added THF after 5 h. In contrast, PDB-grown mycelia consumed only 8% of the THF over the same period (Fig. 4). After this time, THF-oxidizing activity progressively increased, and over the next 5 h, the rate of THF consumption increased to a level comparable to the maximal rate observed with propane-grown mycelia. γ-Butyrolactone was not observed at any point during the reaction time course for either PDB- or propane-grown mycelia. Mycelia grown on PDB were also incubated with THF in the presence of the protein synthesis inhibitor cycloheximide (100 μg ml−1). The cycloheximide-treated mycelia exhibited no THF-oxidizing activity after 10 h. Likewise, no THF-oxidizing activity was detected after 10 h when propane-grown mycelia were incubated with THF (1 mM) and acetylene (2% [vol/vol] gas phase) (Fig. 4).
FIG. 4.
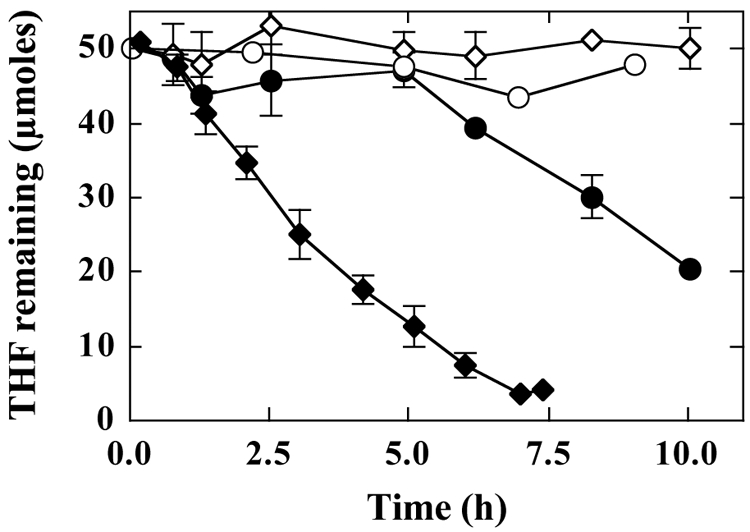
Induction of THF-oxidizing activity in cultures of Graphium sp. Cultures of Graphium sp. were grown on either propane or PDB, as described in Materials and Methods. The washed biomass from these cultures was transferred to glass bottles (700 ml) containing MSM (50 ml). Cycloheximide (100 μg/ml) or acetylene (2% [vol/vol] gas phase) was added to the sealed bottles as required. The reactions were initiated by the addition of THF (∼1 mM initial concentration). At the indicated times, the concentration of THF was determined by GC as described in Materials and Methods. The solid symbols show the changes in THF concentration for incubations containing Graphium sp. mycelia grown on propane (⧫) or PDB (•). The open symbols show the changes in THF concentration over time for propane-grown cultures exposed to THF and acetylene (○) and for PDB-grown cultures exposed to THF and cycloheximide (⋄). Data presented are the means and standard errors for three replicate incubations.
Our previous studies with this Graphium sp. demonstrated that only the initial step of the n-alkane oxidation pathway is sensitive to inhibition by gaseous alkenes (ethylene and propylene) and alkynes (acetylene and propyne) (10). In contrast, growth on later metabolites, such as primary alcohols or acids, is unaffected by these gases. Similar studies were therefore conducted with mycelia grown on THF, γ-butyrolactone, and succinate. Growth of Graphium sp. on THF was fully inhibited by acetylene, ethylene, propylene, or propyne (2% [vol/vol] gas phase) (Fig. 5), while growth on either γ-butyrolactone or succinate was unaffected by the presence of these gases at this concentration.
FIG. 5.
Effects of alkenes and alkynes on growth of Graphium sp. on THF and its metabolites. Liquid cultures of Graphium sp. were grown in sealed glass bottles (700 ml) as described in Materials and Methods. The cultures contained either THF, γ-butyrolactone, or succinate (20 mM initial concentration) as a growth substrate. When required, acetylene, ethylene, propylene, or propyne was added individually to the gas phase (2% [vol/vol] gas phase). The cultures were grown for 14 days, and the final culture biomass was then determined as described in Materials and Methods. The figure shows the effects of acetylene (gray bars), ethylene (checkered bars), propylene (white bars), and propyne (stripped bars) on the final culture dry weight compared to cultures grown in the absence of these gaseous inhibitors (black bars). Data presented are the means and standard errors for three replicate reactions.
Although our inhibitor studies (Fig. 5) suggested that the initial oxidation of THF and n-alkanes could be catalyzed by the same enzyme, these experiments did not eliminate the possibility that n-alkanes and THF are oxidized by two different enzymes with overlapping inhibitor profiles. We therefore examined whether propane and THF are mutually competitive substrates. Filter-attached cultures of propane-grown mycelia were exposed to THF (∼50 μmol) in the presence and absence of propane (200 μmol). In the absence of propane, most (≥90%) of the THF was consumed in 5 h (Fig. 6A). However, in the presence of propane, only 37% of the THF was consumed over the same period (Fig. 6A). When we examined a range of propane concentrations, the initial rates of THF degradation in the presence of propane (100 and 200 μmol) were significantly different (P < 0.0001) from the rates measured in the absence of propane (Fig. 6B). However, smaller amounts of propane (50 and 32 μmol) did not significantly alter the rate of THF degradation (Fig. 6B). Conversely, cultures of Graphium sp. exposed to propane (32 μmol) and THF (50 μmol) oxidized propane at 50% of the oxidation rate in the absence of THF (data not shown). Collectively, these results suggest that propane and THF each exert an inhibitory effect on the oxidation of the other.
FIG. 6.
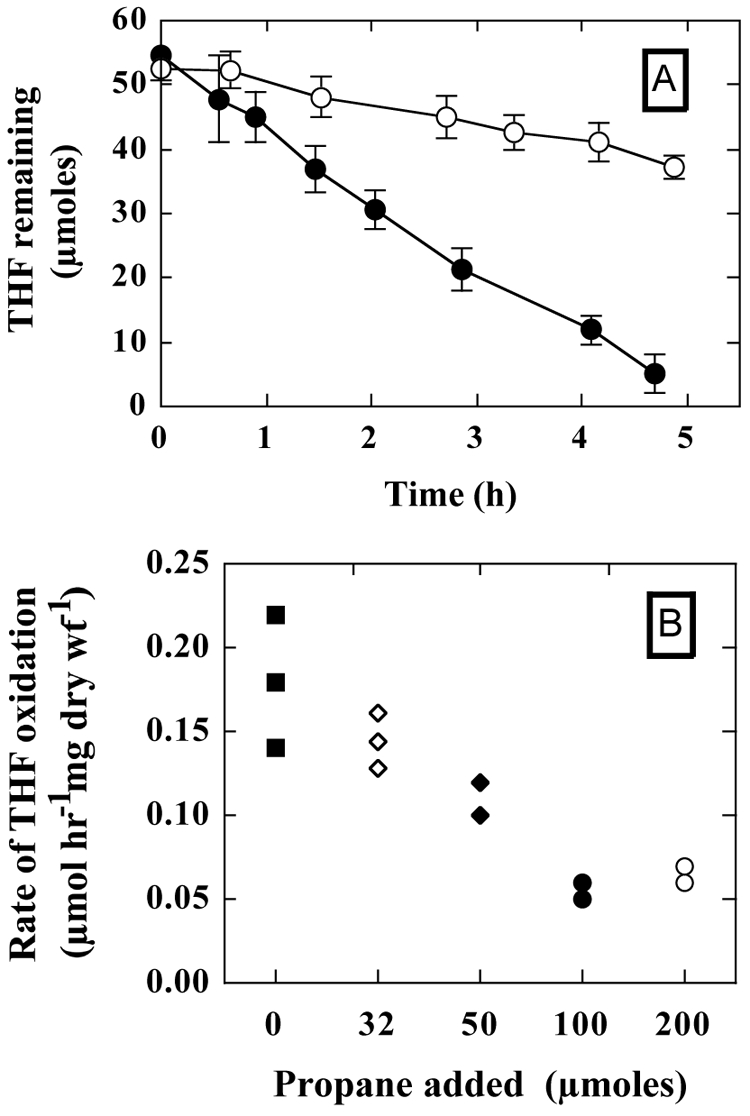
Propane inhibition of THF degradation by filter-attached cultures of Graphium sp. Filter-attached cultures of Graphium sp. were grown on propane as described in Materials and Methods. (A) Three filter-attached cultures were transferred to serum vials (160 ml). The vials were then sealed, and THF (50 μmol) was added as needed to initiate the reaction. Propane (200 μmol) was also added to the reaction vials as needed. At the indicated times, the amount of THF remaining in the reaction vial was determined by GC as described in Materials and Methods. The figure shows the amounts of THF remaining in incubations conducted in the absence (•) and presence (○) of propane. Data presented are the means and standard errors for three replicate reactions. (B) A series of incubations were conducted as described for panel A. The figure shows the specific rates of THF consumption for reactions containing 0 (▪), 32 (⋄), 50 (⧫), 100 (•), or 200 (○) μmol propane.
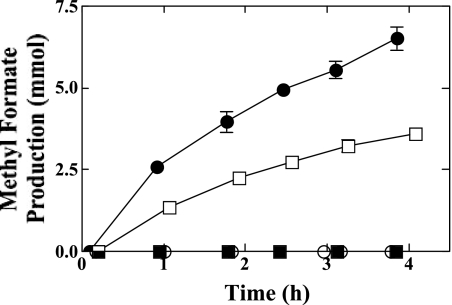
The immediate product of THF oxidation has been suggested to be 2-hydroxytetrahydrofuran (3, 38), and the production of γ-butyrolactone from this cyclic hemiacetal requires oxidation of the hydroxyl group (Fig. 1). To determine if THF-grown mycelia possess a hemiacetal-oxidizing activity, we examined the rate of methyl formate production when mycelia were incubated with high concentrations of methanol and formaldehyde (22, 31). Our results (Fig. 7) indicate that THF-grown mycelia rapidly generated methyl formate, and the rate of production (330 nmol min−1 mg dry weight−1) was ∼60% of the rate observed with propane-grown mycelia (530 nmol min−1 mg dry weight−1). No methyl formate production was observed with mycelia grown on PDB or for incubations containing no fungal biomass. In separate experiments (not shown), the rate of hydrolysis of exogenous methyl formate was also evaluated for THF-, propane-, and PDB-grown mycelia and was ≤5% of the rates of production of methyl formate shown in Fig. 7.
FIG. 7.
Methyl formate production by THF- and propane-grown cultures of Graphium sp. Cultures of Graphium sp. were grown on either propane, THF, or PDB as described in Materials and Methods. The washed biomass from these cultures was transferred to glass bottles (700 ml) containing MSM (50 ml). The reactions were initiated by the addition of methanol and formaldehyde to initial concentrations of 0.5 M and 50 mM, respectively. At the indicated times, the concentration of methyl formate in the headspace was determined by GC as described in Materials and Methods. The figure shows the time course of methyl formate production for incubations containing mycelia of Graphium sp. initially grown on propane (•), THF (□), or PDB (▪) or for abiotic control incubations without fungal mycelia (○). Data presented are the means and standard errors for three replicate incubations.
Although this Graphium sp. was unable to grow on 14D, mycelia grown on either propane or THF cometabolically oxidized this compound. For example, when propane-grown mycelia were exposed to 14D (1 mM initial concentration), ∼50% of the 14D was consumed after 12 h (Fig. 8). However, this reaction was not sustainable, and only ∼65% of the 14D was consumed after 36 h (data not shown). The consumption of 14D with propane-grown mycelia was fully inhibited by acetylene (2% [vol/vol] gas phase) (Fig. 8). Substantially similar results were also observed for 14D degradation by THF-grown mycelia (data not shown). We also compared the abilities of propane-, THF-, and PDB-grown mycelia of Graphium sp. to degrade three ethers (THF, MTBE, and 14D). Propane- and THF-grown mycelia both rapidly oxidized THF (≥100 nmol h−1 mg dry weight−1) and also oxidized 14D and MTBE, albeit at substantially lower rates (≤10% of the rate of THF oxidation). In contrast, no consistent ether-oxidizing activity was observed in PDB-grown mycelia (Table 1).
FIG. 8.
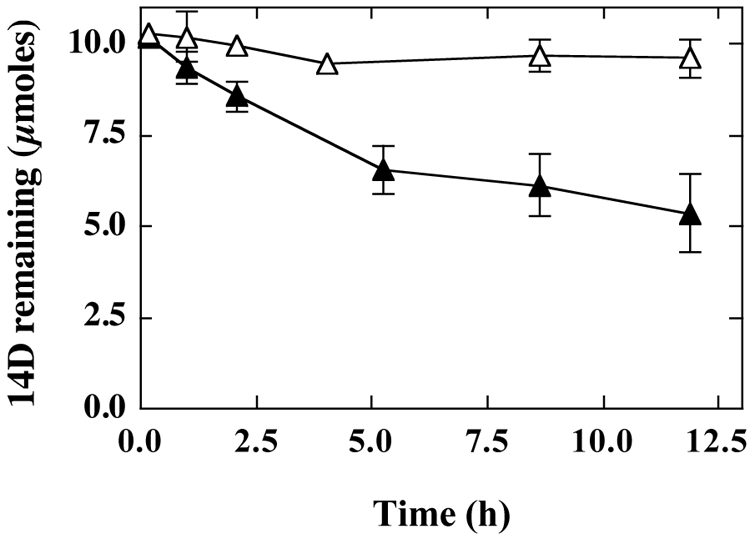
14D degradation by propane-grown cultures of Graphium sp. Cultures of Graphium sp. were initially grown on PDB and then incubated with propane as described in Materials and Methods. The PDB-grown, propane-induced mycelia were then transferred to glass bottles (700 ml) containing MSM (50 ml). The bottles were sealed, and if required, acetylene (2% [vol/vol] gas phase) was added. The reaction was initiated by the addition of 14D (10 μmol), and at the indicated times, the concentration of 14D was determined by GC as described in Materials and Methods. The figure shows the time course for 14D consumption for incubations conducted in the presence (▵) and absence (▴) of acetylene. The data presented show the means and standard errors for three replicate incubations.
TABLE 1.
Initial rates of 14D, THF, and MTBE oxidation by propane-, THF-, and dextrose-grown mycelia of Graphium sp.
| Induction compound | Initial rate of substrate oxidation (nmol substrate consumed min−1 mg dry weight−1)a
|
||
|---|---|---|---|
| 14D | THF | MTBE | |
| Propane | 4 ± 1 | 115 ± 3 | 10 ± 1 |
| THF | 9 ± 5 | 123 ± 22 | 13 ± 2 |
| Dextrose | 0 ± 6 | 20 ± 25 | 2 ± 6 |
Data are presented as means and standard errors for three replicate reactions.
DISCUSSION
Potential enzymes and pathways associated with THF oxidation.
Microbial alkane-oxidizing monooxygenases are often inactivated by alkynes but can also often oxidize alkenes to their corresponding epoxides (10). In contrast, cytochrome P450s are characteristically inactivated by both alkenes and alkynes, through their action as heme-derivatizing, mechanism-based inactivators (10). Our observation that growth of a Graphium sp. on THF, like propane and MTBE oxidation (10, 13), was selectively inhibited by both gaseous alkanes and alkynes (Fig. 5) therefore suggests that THF oxidation by this Graphium sp. is also initiated by a cytochrome P450. Our results also demonstrated that propane-grown mycelia were simultaneously adapted to oxidize THF (Fig. 4) and that THF and propane were mutually competitive substrates (Fig. 6). These observations therefore suggest that THF and propane may also be oxidized by the same cytochrome P450. We recently explored this possibility using RNA-induced silencing of a cytochrome P450 gene (GSPALK1) in this Graphium sp. (40). Our preliminary results indicated that silencing of this gene simultaneously abolishes the ability of this fungus to grown on propane and THF but does not affect growth on their immediate stable metabolites, 1-propanol and γ-butyrolactone (40). However, alkane-utilizing fungi often simultaneously express multiple alkane-oxidizing cytochrome P450s in response to alkanes and other organics, and the possibility of coexpression of multiple cytochrome P450s with overlapping substrate specificities cannot be excluded at this point (15, 34, 46, 51). Further molecular studies with this Graphium sp. will therefore be needed to unequivocally identify the enzymes responsible for initiating both alkane and THF oxidation and to determine the degree to which these enzymes are involved in alkane and THF metabolism.
The initial monooxygenation of THF by this Graphium sp. can potentially generate two hydroxylated isomers. The stable isomer, 3-hydroxytetrahydrofuran, does not appear to be involved in THF metabolism, as it does not support growth of this Graphium sp. (Fig. 2) and was not observed in any experiment examining THF degradation. The other isomer, 2-hydroxytetrahydrofuran, is a cyclic hemiacetal (a lactol). In aqueous solution, this lactol rapidly equilibrates with its tautomer, 4-hydroxybutyraldehyde, to give an approximately 9:1 lactol-hydroxyaldehyde mixture (14, 18, 23). As originally suggested by Bernhardt and Diekmann (2), one potential downstream pathway of THF catabolism involves alcohol and aldehyde dehydrogenases, with 4-hydroxybutyraldehyde, 4-hydroxybutyrate, succinate semialdehyde, and succinate as sequential intermediates (Fig. 1). Precedents for major elements of this pathway and a role for dehydrogenases have been established in studies of 4-hydroxybutyrate metabolism by Ralstonia eutropha (25) and of acyl-homoserine lactone degradation and γ-butyrolactone metabolism by Agrobacterium tumefaciens (5-7). In A. tumefaciens, the attKLM operon encodes a lactonase (AttM) that hydrolyzes both 3-oxooctanoyl-homoserine lactone and γ-butyrolactone. The other genes in the operon encode putative dehydrogenases that oxidize 4-hydroxybutyrate to succinate semialdehyde (AttL) and succinate semialdehyde to succinate (AttM) (6). Notably, with the inclusion of another dehydrogenase-catalyzed reaction, this pathway (Fig. 1) can also be extended to accommodate 1,4-butanediol, another growth-supporting substrate for this Graphium sp. (Fig. 2).
Mechanism and significance of γ-butyrolactone production.
Our present results are unique among studies of microbial THF biodegradation in that we detected a THF-derived metabolite, γ-butyrolactone (Fig. 3). According to the model presented in Fig. 1, there are two potential routes to account for γ-butyrolactone formation. The first involves lactonization of 4-hydroxybutyrate. In aqueous solution, 4-hydroxybutyrate rapidly lactonizes at low pH but is stable for >200 days in a phosphate-buffered solution under basic conditions (pH 12), as well as under the neutral pH conditions used in the present study (8). Diverse bacterial processes, such as the metabolism of polyhydroxyalkanoates (25) and fungal metabolism of cyclopropanecarboxylic acid (38), all generate 4-hydroxybutyrate (Fig. 1). However, as far as we are aware, γ-butyrolactone has not been identified as a product in any of these processes. In contrast, substantial microbial formation of γ-butyrolactone has been reported during the cometabolic oxidation of bis(4-chloro-n-butyl) ether by DEE-grown cells of Rhodococcus sp. strain DTB (30) and during 1-choroalkane metabolism by Rhodococcus rhodocrous NCIMB 13064 (9). In both cases, γ-butyrolactone is believed to be derived from hydrolytic dehalogenation of 4-chlorobutyrate, which is generated either directly from the scission of bis(4-chloro-n-butyl) ether or through β-oxidation of 1-chloroalkanes.
Based on our observations and the discussion above, the second and most likely route to γ-butyrolactone formation during THF metabolism by this Graphium sp. is an alcohol dehydrogenase-catalyzed oxidation of 2-hydroxytetrahydrofuran (Fig. 1). The ability of alcohol dehydrogenases to oxidize hemiacetals has been recognized for over 50 years (19) and has even been used for preparative-scale production of some lactones (16). More recently, hemiacetal oxidation has been characterized for fungi during investigations of the production of nonlactonized esters, such as the production of methyl formate by methylotrophic yeasts (31, 32, 37, 47). The enzymes responsible for this activity, methyl formate synthases, are zinc-containing alcohol dehydrogenases (32) that catalyze the oxidation of a transient hemiacetal formed in concentrated mixtures of simple alcohols and aldehydes (22). In this study, we used this reaction to show high rates of methyl formate synthase activity in both THF- and propane-grown mycelia and the absence of this activity in PDB-grown mycelia (Fig. 7). Since alcohol dehydrogenases are the only enzymes currently known to catalyze this reaction, our results lend support to the proposed pathway involving 2-hydroxytetrahydrofuran oxidation, as they demonstrate that an appropriate hemiacetal-oxidizing activity is present in both THF- and propane-grown mycelia. Further studies will be needed to determine if these methyl formate synthase activities can be attributed to the same enzyme in both THF- and propane-grown mycelia. If this proves to be the case, then our results would indicate that not only are THF and propane oxidized by the same monooxygenase but the alcohol or hemiacetal products of these reactions are also oxidized by the same dehydrogenases.
The detection of a hemiacetal-oxidizing activity in propane-grown mycelia also impacts our understanding of the cometabolic oxidation of MTBE by this Graphium sp. The consensus pathway for aerobic MTBE oxidation involves an initial monooxygenase-catalyzed hydroxylation of the methoxy carbon to an unstable hemiacetal. In many MTBE-degrading organisms, abiotic decomposition of this hemiacetal generates formaldehyde and tert-butyl alcohol. However, in alkane-grown mycelia of this Graphium sp. (13, 39) and M. vaccae JOB5 (41), the hemiacetal is rapidly oxidized to tert-butyl formate (TBF). Since TBF production during MTBE oxidation has most frequently been observed during MTBE cometabolism by alkane-grown microorganisms (13, 24, 41), the production of TBF, like MTBE oxidation itself, may simply reflect a fortuitous reaction. In the case of TBF production, this is probably facilitated by the high levels of alcohol dehydrogenase activity in n-alkane-grown organisms.
Mechanism and significance of γ-butyrolactone consumption.
For both Graphium sp. and M. vaccae JOB5, the production of TBF from MTBE can account, at least initially, for ∼80% of the MTBE degraded (39, 41). During growth of this Graphium sp. on THF, the maximum amount of γ-butyrolactone detected was a much smaller (∼20%) portion of the THF degraded (Fig. 3). This low-level stoichiometry may indicate that γ-butyrolactone is a minor and fortuitous product of THF metabolism but could also indicate rapid consumption of an obligate intermediate in THF metabolism. Further clarification of the role of γ-butyrolactone will require better estimates of the rates of production and hydrolysis of this compound, but our results at this stage do not exclude the possibility that tautomerism and biological oxidation are two concurrent and competing processes involved in 2-hydroxytetrahydrofuran degradation.
Like 4-hydroxybutyrate, γ-butyrolactone undergoes several biological and chemical transformations. Lactonases are widely distributed in fungi, and hydrolysis of γ-butyrolactone has been used as an assay for some of these enzymes (50). We previously observed the hydrolytic degradation of TBF by this Graphium sp. (13, 39), and biological hydrolysis may therefore also contribute to the growth of this Graphium sp. on γ-butyrolactone and other lactones (Fig. 2). However, although abiotic lactonization of 4-hydroxybutyrate is an unlikely source of γ-butyrolactone in the present study, abiotic hydrolysis of γ-butyrolactone to 4-hydroxybutyrate is potentially a much more significant reaction, especially at neutral pH (8). For example, it has been reported that over a period of 200 days, 97% of a 0.5% (wt/vol) solution of γ-butyrolactone hydrolyzes to 4-hydroxybutyrate at pH 7.0 (8). Although we did not detect abiotic hydrolysis of γ-butyrolactone in control incubations lacking fungal mycelia, the possibility clearly exists that abiotic production of 4-hydroxybutyrate impacted our growth experiments (Fig. 2), and growth on this lactone may have been supported through both the biological and chemical formation of 4-hydroxybutyrate (Fig. 2). The greater stability of larger, less-strained lactones, such as ɛ-caprolactone and δ-valerolactone (Fig. 2), suggests that growth observed with these compounds more likely reflects biological rather than chemical hydrolysis of these compounds to their corresponding hydroxy acids.
Cometabolism of 14D.
Cometabolic degradation of 14D was first reported for propane-grown M. vaccae JOB5 (4) and is initiated by an as yet uncharacterized propane monooxygenase. More recently, 14D cometabolism has also been reported for bacterial strains that express other, better-characterized monooxygenases. These include soluble methane monooxygenase, THF monooxygenase, and toluene-2-, -3-, and -4- monooxygenases (28). As discussed earlier, inactivation of a monooxygenase activity by both alkenes and alkynes is a characteristic of cytochrome P450s. Our observation that both propane- and THF-grown mycelia of this Graphium sp. can oxidize 14D suggests that microbial cytochrome P450s are also likely catalysts for this reaction.
The nonoptimized specific rates of 14D oxidation determined in this study (Table 1) are ≥100-fold lower than the lowest rates recently reported for bacterial 14D oxidation (28), and this difference is comparable to the difference between the rates of MTBE oxidation we reported for this fungus and rates reported for bacterial systems (13, 24, 41). However, specific activity determinations for filamentous fungi based on dry weight are expected to be significant underestimates, as large portions of fungal mycelia contain little metabolically active cytoplasm. Irrespective of the underlying causes of differences in absolute rates of 14D oxidation, a clear similarity between cometabolic 14D degradation by this Graphium sp. and that in bacterial systems is that both processes lack full sustainability (Fig. 8) (28). The lack of sustained 14D oxidation in THF-grown Pseudonocardia ENV478 has been suggested to be due in part to the generation of intermediates such as 2-hydroxyethoxyacetic acid (45). This acid is thought to be generated from 2-hydroxyethoxy-2-acetaldehyde, which is the tautomer of the immediate hydroxylated product of 14D oxidation, 1,4-dioxane-2-ol (45). We have not characterized the products of 14D oxidation by this Graphium sp. or investigated the potential toxicity of 14D metabolites. However, since this Graphium sp. effectively metabolizes THF, 2-hydroxyethoxyacetic acid may also be generated by this organism from 14D, and this may limit the ability of this fungus to sustainably oxidize 14D.
Acknowledgments
We thank Christy Smith (NCSU) and Alan House (NCSU) for the GC-MS analyses of THF biodegradation and γ-butyrolactone production.
This research was supported by a U.S. Environmental Protection Agency Science to Achieve Results (STAR) graduate fellowship awarded to K.S. and in part through a research grant from the U.S. Environmental Protection Agency-sponsored Western Region Hazardous Substance Research Center under agreement R-828772.
This article has not been reviewed by the U.S. Environmental Protection Agency, and no official endorsement should be inferred.
Footnotes
Published ahead of print on 6 July 2009.
REFERENCES
- 1.April, T. M., S. P. Abbott, J. M. Foght, and R. S. Currah. 1998. Degradation of hydrocarbons in crude oil by the ascomycete Pseudoallescheria boydii (Microascaceae). Can. J. Microbiol. 44:270-278. [DOI] [PubMed] [Google Scholar]
- 2.Bernhardt, D., and H. Diekmann. 1991. Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental Rhodococcus strain. Appl. Microbiol. Biotechnol. 36:120-123. [DOI] [PubMed] [Google Scholar]
- 3.Bock, C., R. M. Kroppenstedt, and H. Diekmann. 1996. Degradation and bioconversion of aliphatic and aromatic hydrocarbons by Rhodococcus ruber 219. Appl. Microbiol. Biotechnol. 45:408-410. [Google Scholar]
- 4.Burback, B. L., and J. J. Perry. 1993. Biodegradation and biotransformation of groundwater pollutant mixtures by Mycobacterium vaccae. Appl. Environ. Microbiol. 59:1025-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlier, A., R. Chevrot, Y. Dessaux, and D. Faure. 2004. The assimilation of gamma butyrolactone in Agrobacterium tumefaciens C58 interferes with the accumulation of the N-acyl-homoserine lactone signal. Mol. Plant-Microbe Interact. 17:951-957. [DOI] [PubMed] [Google Scholar]
- 6.Chai, Y., C. S. Tsai, H. Cho, and S. C. Winans. 2007. Reconstitution of the biochemical activities of the AttJ repressor and the AttK, AttL, and AttM catabolic enzymes of Agrobacterium tumefaciens. J. Bacteriol. 189:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherot, R., R. Rosen, E. Haudecoeur, A. Cirou, B. J. Shelp, E. Ron, and D. Faure. 2006. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 103:7460-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciolino, L. A., M. Z. Mesmer, R. D. Satzger, A. C. Machal, B. S. Heather, A. McCauley, and A. S. Mohrhaus. 2001. The chemical interconversion of GHB and GBL: forensic issues and implications. J. Forensic Sci. 46:1315-1323. [PubMed] [Google Scholar]
- 9.Curragh, H., O. Flynn, M. J. Larkin, T. M. Stafford, J. T. Hamilton, and D. B. Harper. 1994. Haloalkane degradation and assimilation by Rhodococcus rhodochrous NCIMB 13064. Microbiology 140:1433-1442. [DOI] [PubMed] [Google Scholar]
- 10.Curry, S., L. M. Ciuffetti, and M. R. Hyman. 1996. Inhibition of growth of Graphium sp. on gaseous n-alkanes by gaseous n-alkynes and n-alkenes. Appl. Environ. Microbiol. 62:2198-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, J. S., J. E. Zajic, and A. Wellman. 1974. Fungal oxidation of gaseous alkanes. Dev. Ind. Microbiol. 15:256-262. [Google Scholar]
- 12.Daye, K. J., J. C. Groff, A. C. Kirpekar, and R. Mazumder. 2003. High efficiency degradation of tetrahydrofuran (THF) using a membrane bioreactor: identification of THF-degrading cultures of Pseudonocardia sp. strain M1 and Rhodococcus ruber isolate M2. J. Ind. Microbiol. Biotechnol. 30:705-714. [DOI] [PubMed] [Google Scholar]
- 13.Hardison, L. K., S. S. Curry, L. M. Ciuffetti, and M. R. Hyman. 1997. Metabolism of diethyl ether and cometabolism of methyl tert-butyl ether by a filamentous fungus, Graphium sp. Appl. Environ. Microbiol. 63:3059-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurd, C. D., and W. H. Saunders. 1952. Ring chain tautomerism of hydroxy aldehydes. J. Am. Chem. Soc. 74:5324-5329. [Google Scholar]
- 15.Iida, T., T. Sumita, A. Ohta, and M. Takagi. 2000. The cytochrome P450ALK multigene family of an n-alkane-assimilating yeast Yarrowia lipolytica: cloning and characterization of genes coding for new CYP52 family members. Yeast 16:1077-1087. [DOI] [PubMed] [Google Scholar]
- 16.Jones, J. B., and K. P. Lok. 1979. Enzymes in organic synthesis. 14. Stereoselective horse liver alcohol dehydrogenase catalyzed oxidations of diols containing a prochiral centre and of related hemiacetals. Can. J. Chem. 57:1025-1032. [Google Scholar]
- 17.Kämpfer, P., U. Kohlweyer, B. Thiemer, and J. R. Andreesen. 2006. Pseudonocardia tetrahydrofuranoxydans sp. nov. Int. J. Syst. Evol. Microbiol. 56:1535-1538. [DOI] [PubMed] [Google Scholar]
- 18.Kang, J. W., and W. L. Hergenrother. December 1978. Preparation of 2,3-dihydrofuran. U.S. patent 4,129,579.
- 19.Kendal, L. P., and A. N. Ramanathan. 1952. Liver alcohol dehydrogenase and ester formation. Biochem. J. 52:430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, Y.-M., J.-R. Jeon, K. Murugesan, E.-J. Kim, and Y.-S. Chang. 2009. Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06. Biodegradation 20:511-519. [DOI] [PubMed] [Google Scholar]
- 21.Kohlweyer, U., B. Thiemer, T. Schrader, and J. R. Andreesen. 2000. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiol. Lett. 186:301-306. [DOI] [PubMed] [Google Scholar]
- 22.Kusano, M., Y. Sakai, N. Kato, H. Yoshimoto, H. Sone, and Y. Tani. 1998. Hemiacetal dehydrogenase activity in alcohol dehydrogenases in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 62:1956-1961. [DOI] [PubMed] [Google Scholar]
- 23.Lawson, A. P., and J. A. Klang. October 1993. Process for preparation of 2-oxytetrahydrofurans. U.S. patent 5,254,702.
- 24.Liu, C. Y., G. E. Speitel, Jr., and G. Georgiou. 2001. Kinetics of methyl t-butyl ether cometabolism at low concentrations by pure cultures of butane-degrading bacteria. Appl. Environ. Microbiol. 67:2197-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lütke-Eversloh, T., and A. Steinbüchel. 1999. Biochemical and molecular characterization of a succinate semialdehyde dehydrogenase involved in the catabolism of 4-hydroxybutyric acid in Ralstonia eutropha. FEMS Microbiol. Lett. 181:63-71. [DOI] [PubMed] [Google Scholar]
- 26.Mackay, D. M., and W. Ying. 1981. Critical review of Henry's law constants for chemicals of environmental interest. J. Phys. Chem. 10:1175-1195. [Google Scholar]
- 27.Mahendra, S., and L. Alvarez-Cohen. 2005. Pseudonocardia dioxanivorans sp. nov., a novel actinomycete that grows on 1,4-dioxane. Int. J. Syst. Evol. Microbiol. 55:593-598. [DOI] [PubMed] [Google Scholar]
- 28.Mahendra, S., and L. Alvarez-Cohen. 2006. Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria. Environ. Sci. Technol. 40:5435-5442. [DOI] [PubMed] [Google Scholar]
- 29.Mohr, T. K. G. 2001. Solvent stabilizers white paper. Santa Clara Valley Water District, San Jose, CA. http://www.valleywater.org/Water/Water_Quality/Protecting_your_water/_Solvents/_PDFs/SolventStabilizers.pdf.
- 30.Moreno-Horn, M., L.-A. Garbe, R. Tressi, and H. Görisch. 2005. Transient accumulation of γ-butyrolactone during degradation of bis(4-chloro-n-butyl) ether by diethylether-grown Rhodococcus sp. strain DTB. Appl. Microbiol. Biotechnol. 69:335-340. [DOI] [PubMed] [Google Scholar]
- 31.Murdanoto, A. P., Y. Sakai, L. Sembiring, Y. Tani, and N. Kato. 1997. Ester synthesis by NAD+-dependent dehydrogenation of hemiacetal: production of methyl formate by cells of methylotrophic yeasts. Biosci. Biotechnol. Biochem. 61:1391-1393. [DOI] [PubMed] [Google Scholar]
- 32.Murdanoto, A. P., Y. Sakai, T. Konishi, F. Tasuda, Y. Tani, and N. Kato. 1997. Purification and properties of methyl formate synthase, a mitochondrial alcohol dehydrogenase, participating in formaldehyde oxidation in methylotrophic yeasts. Appl. Environ. Microbiol. 63:1715-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamiya, K., S. Hashimoto, H. Ito, J. S. Edmonds, and M. Morita. 2005. Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Appl. Environ. Microbiol. 71:1254-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohkuma, M., S.-I. Muraoka, T. Tanimoto, M. Fujii, A. Ohta, and M. Takagi. 1995. CYP52 (cytochrome P450alk) multigene family in Candida maltosa: identification and characterization of eight members. DNA Cell Biol. 14:163-173. [DOI] [PubMed] [Google Scholar]
- 35.Parales, R. E., J. E. Adamus, N. White, and H. D. May. 1994. Degradation of 1,4-dioxane by an actinomycete in pure culture. Appl. Environ. Microbiol. 60:4527-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patt, T. E., and H. M. Abede. March 1993. Microbial degradation of chemical pollutants. U.S. patent 5,399,495.
- 37.Sakai, Y., A. P. Murdanoto, L. Sembiring, Y. Tani, and N. Kato. 1995. A novel formaldehyde oxidation pathway in methylotrophic yeasts: methyl formate as a possible intermediate. FEMS Microbiol. Lett. 127:229-234. [DOI] [PubMed] [Google Scholar]
- 38.Schiller, J. G., and A. E. Chung. 1970. The metabolism of cyclopropanecarboxylic acid. J. Biol. Chem. 245:5857-5864. [PubMed] [Google Scholar]
- 39.Skinner, K. M., A. Martinez-Prado, M. R. Hyman, K. J. Williamson, and L. M. Ciuffetti. 2008. Pathway, inhibition and regulation of methyl tertiary butyl ether oxidation in a filamentous fungus, Graphium sp. Appl. Microbiol. Biotechnol. 77:1359-1365. [DOI] [PubMed] [Google Scholar]
- 40.Skinner, K. M. 2008. Ph.D. dissertation. Oregon State University, Corvallis. http://ir.library.oregonstate.edu/dspace/bitstream/1957/4952/1/KM_Skinner_Dissertation.pdf.
- 41.Smith, C. A., K. T. O'Reilly, and M. R. Hyman. 2003. Characterization of the initial reactions during the cometabolic oxidation of methyl tert-butyl ether by propane-grown Mycobacterium vaccae JOB5. Appl. Environ. Microbiol. 69:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiemer, B., J. R. Andreesen, and T. Schrader. 2003. Cloning and characterization of a gene cluster involved in tetrahydrofuran degradation in Pseudonocardia sp. strain K1. Arch. Microbiol. 179:266-277. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Environmental Protection Agency. January 2006, revision date. 1990 high production volume challenge program chemical list. Office of Prevention, Pesticides, and Toxic Substances, U.S. Environmental Protection Agency, Washington, DC.
- 44.U.S. Environmental Protection Agency. 1995. OPPT-chemical fact sheets. 1,4-Dioxane fact sheet. EPA-749-F-95-0102. Office of Pollution Prevention and Toxic Substances, U.S. Environmental Protection Agency, Washington, DC.
- 45.Vainberg, S., K. McClay, H. Masuda, D. Root, C. Condee, G. J. Zylstra, and R. J. Steffan. 2006. Biodegradation of ether pollutants by Pseudonocardia sp. strain ENV478. Appl. Environ. Microbiol. 72:5218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vatsyayan, P., A. K. Kumar, P. Goswami, and P. Goswami. 2008. Broad substrate cytochrome P450 monooxygenase activity in the cells of Aspergillus terreus MTCC 6324. Bioresour. Technol. 99:68-75. [DOI] [PubMed] [Google Scholar]
- 47.Yurimoto, H., B. Lee, F. Yasuda, Y. Sakai, and N. Kato. 2004. Alcohol dehydrogenases that catalyze methyl formate synthesis participate in formaldehyde detoxification in the methylotrophic yeast Candida boidinii. Yeast 21:341-350. [DOI] [PubMed] [Google Scholar]
- 48.Zajic, J. E., B. Volesky, and A. Wellman. 1969. Growth of Graphium sp. on natural gas. Can. J. Microbiol. 15:1231-1236. [DOI] [PubMed] [Google Scholar]
- 49.Zenker, M. J., R. C. Borden, and M. A. Barlaz. 2003. Occurrence and treatment of 1,4-dioxane in aqueous environments. Environ. Eng. Sci. 20:423-432. [Google Scholar]
- 50.Zhang, X., J. H. Xu, Y. Xu, and J. Pan. 2007. Isolation and properties of a levolactonase from Fusarium proliferatum ECU2002: a robust biocatalyst for production of chiral lactones. Appl. Microbiol. Biotechnol. 75:1087-1094. [DOI] [PubMed] [Google Scholar]
- 51.Zimmer, T., M. Ohkuma, A. Ohta, M. Takagi, and W.-H. Schunck. 1996. The CYP52 multigene family of Candida maltosa encodes functionally diverse n-alkane-inducible cytochromes P450. Biochem. Biophys. Res. Commun. 224:784-789. [DOI] [PubMed] [Google Scholar]