Abstract
Purified superdormant spores of Bacillus cereus, B. megaterium, and B. subtilis isolated after optimal heat activation of dormant spores and subsequent germination with inosine, d-glucose, or l-valine, respectively, germinate very poorly with the original germinants used to remove dormant spores from spore populations, thus allowing isolation of the superdormant spores, and even with alternate germinants. However, these superdormant spores exhibited significant germination with the original or alternate germinants if the spores were heat activated at temperatures 8 to 15°C higher than the optimal temperatures for the original dormant spores, although the levels of superdormant spore germination were not as great as those of dormant spores. Use of mixtures of original and alternate germinants lowered the heat activation temperature optima for both dormant and superdormant spores. The superdormant spores had higher wet-heat resistance and lower core water content than the original dormant spore populations, and the environment of dipicolinic acid in the core of superdormant spores as determined by Raman spectroscopy of individual spores differed from that in dormant spores. These results provide new information about the germination, heat activation optima, and wet-heat resistance of superdormant spores and the heterogeneity in these properties between individual members of dormant spore populations.
Spores of Bacillus species are formed in sporulation and are metabolically dormant and extremely resistant to a variety of stress factors (31, 32). While spores can remain dormant for long periods, if given the proper stimulus, they can rapidly “return to life” in the process of spore germination followed by outgrowth (30). Since spores are generally present in significant amounts on many foodstuffs and growing cells of a number of Bacillus species are significant agents of food spoilage and food-borne disease (32), there is continued applied interest in spore resistance and germination. While dormant spores can be killed by a treatment such as wet heat, this requires high temperatures that are costly and detrimental to food quality. Consequently, there has long been interest in triggering spore germination in foodstuffs, since germinated spores have lost the extreme resistance of dormant spores and are relatively easy to kill. However, this strategy has been difficult to apply because of the significant heterogeneity in germination rates between individual spores in populations. One reflection of this heterogeneity is the extremely variable lag times following addition of germinants but prior to initiation of germination events; while these lag times can vary from 10 to 30 min for most spores in populations, some spores have lag times of many hours or even many days (2, 12, 13, 15, 25). The spores that are extremely slow to germinate have been termed superdormant spores, and populations of superdormant spores have recently been isolated from three Bacillus species, and their germination properties characterized (9, 10). These superdormant spores germinate extremely poorly with the original germinants used to remove dormant spores from spore populations, thus allowing superdormant spore isolation, and also poorly with a number of other germinants, in particular, germinants that target nutrient germinant receptors different than those activated to isolate the superdormant spores. However, the superdormant spores germinate reasonably well with mixtures of nutrient germinants that target multiple germinant receptors. All reasons for spore superdormancy are not known, but one contributing factor is the number of nutrient germinant receptors in the spore's inner membrane that trigger spore germination by binding to nutrient germinants (9). The levels of these receptors are most likely in the tens of molecules per spore (24), and thus stochastic variation in receptor numbers might result in some spores with such low receptor numbers that these spores germinate very poorly (23). Indeed, 20- to 200-fold elevated levels of at least one nutrient germinant receptor greatly decreases yields of superdormant spores of Bacillus subtilis (9).
Spores of Bacillus species generally exhibit a requirement for an activation step in order to exhibit maximum germination (17). Usually this activation is a sublethal heat treatment that for a spore population exhibits an optimum of 60 to 100°C depending on the species. Spores are also extremely resistant to wet heat, generally requiring temperatures of 80 to 110°C to achieve rapid spore killing, with the major factor influencing the wet-heat resistance of spores of mesophilic strains being the spore core's water content, which can be as low as 30% of wet weight as water in a fully hydrated spore (8, 19, 27, 28, 31). Invariably, increases in core water content are associated with a decrease in spore wet-heat resistance (8, 19, 22, 25). While spore populations most often exhibit log-linear kinetics of wet-heat killing, the observation of tailing in such killing curves at high levels of killing is not uncommon, suggesting there is significant heterogeneity in the wet-heat resistances of individual spores in populations (27, 28). While there has been no comparable work suggesting that there is also heterogeneity in the temperature optima for heat activation of individual spores in populations, this certainly seems possible and indeed was suggested as one cause of spore superdormancy, as yields of superdormant spores from spore populations that are not heat activated are much higher (9, 10). Consequently, the current work was initiated to test the hypothesis that superdormant spores require heat activation temperatures that are higher than those of the original dormant spores. Once this was found to be the case, the wet-heat resistance and core water content of the superdormant and original dormant spores were compared, and the environment of the spore core's major small molecule, pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) was assessed by Raman spectroscopy of individual spores.
MATERIALS AND METHODS
Bacillus strains used and spore preparation.
The Bacillus strains used were B. megaterium QM B1551 (ATCC 12872), B. cereus T (originally obtained from H. O. Halvorson), and B. subtilis PS533, a derivative of a prototrophic laboratory 168 strain that carries plasmid pUB110 encoding resistance to kanamycin (10 μg/ml) (29). Spores of these strains were prepared as follows: B. subtilis at 37°C on 2× SG medium plates without antibiotics (21, 22), B. megaterium at 30°C in liquid-supplemented nutrient broth medium (11), and B. cereus at 30°C in liquid-defined sporulation medium (5), all essentially as described previously. Spores were harvested by centrifugation, washed extensively with 4°C water over a period of ∼7 days and stored at 4°C in water protected from light (21). All spores used in this work were free (>98%) from growing or sporulating cells, germinated spores, and cell debris, as observed by phase-contrast microscopy.
Isolation of superdormant spores and spore germination.
Superdormant spores of various Bacillus species were isolated as described previously (9, 10) by germination of spores with a high concentration of a good germinant for 2 h, removal of germinated spores by buoyant density centrifugation, and repetition of the germination and removal of germinated spores. This procedure routinely yielded 1 to 3% of starting dormant spores as superdormant spores, and these spores germinated extremely poorly with the germinants used to isolate them, as found previously (9, 10). Prior to germination to isolate superdormant spores, the spores were heat activated and then cooled on ice for ≥15 min. Heat activation conditions routinely used were optimal for germination of the spore populations (see Results) and were as follows: B. cereus, 20 min, 65°C; B. megaterium, 15 min, 60°C; and B. subtilis, 30 min, 75°C. The specific germination conditions for superdormant spore isolation were as follows, all with spores at an optical density at 600 nm (OD600) of 1.0: B. cereus, 37°C in 25 mM KPO4 buffer (pH 7.4) and 5 mM inosine; B. megaterium, 30°C in 25 mM KPO4 buffer (pH 7.4) and 10 mM d-glucose; and B. subtilis, 37°C in 25 mM Tris-HCl buffer (pH 7.4) and 10 mM l-valine. These germinants are defined as original germinants, and their concentrations are saturating (9, 10; data not shown). Spores were also germinated in alternate germinants for each species, and these were as follows: B. cereus, 37°C in 25 mM KPO4 buffer (pH 7.4) and 50 mM l-alanine; B. megaterium, 30°C in 25 mM KPO4 buffer (pH 7.4) and 5 mM l-proline; and B. subtilis, 37°C in a mixture of 12 mM l-asparagine, 13 mM D-glucose, 13 mM d-fructose, and 25 mM KPO4 buffer (pH 7.4) (AGFK) (9, 10). The progress of spore germination was tracked by monitoring the OD600s of germinating cultures, which fall 50 to 60% upon complete spore germination (3), and after 2 h, the extent of spore germination was determined by examination of several hundred spores by phase-contrast microscopy.
Raman spectroscopy of individual spores.
Raman spectra of individual spores were obtained at 23°C by laser-tweezers Raman spectroscopy (LTRS) essentially as described previously (4, 16). The LTRS system was in a confocal configuration. A laser beam from a wavelength-stabilized diode laser at 785 nm was introduced in an inverted differential interference contrast microscope (Nikon TE2000) equipped with an objective (100×; numerical aperture, 1.30), to form a single-beam optical trap. A bacterial spore in distilled water is trapped ∼10 μm above the bottom surface of a sample holder in the focus of the laser beam so that it remains in the laser focus during the acquisition time for Raman spectroscopy. The backward Raman scattering light is collected and focused on the entrance slit of a spectrograph and detected by a liquid nitrogen-cooled, charge-coupled detector (Symphony CCD; Jobin Yvon, Edison, NJ). The Raman spectra were recorded in the “fingerprint” range from 500 to 2,000 cm−1, with a spectral resolution of ∼6 cm−1. A background spectrum was taken under the same acquisition conditions without a spore in the trap and subtracted from spectra of individual spores. The spectra of 50 individual spores of each sample were analyzed with a laser power of 30 mW and an acquisition time of 20 s, and the spectra were averaged.
Other methods.
While heat activation was routinely for the times and at the temperatures noted above, in one experiment various heat activation temperatures were used, but again for 30 min (B. subtilis), 20 min (B. cereus), or 15 min (B. megaterium), and spores were cooled on ice for at least 15 min prior to germination.
Spore viability was determined by spotting 10 μl of dilutions in water of heat-treated dormant or superdormant spores on Luria broth (LB) (23) plates with or without a mixture of the original and alternate germinants for spores of that species. The plates were incubated for 24 to 48 h at 30°C (B. megaterium) or 37°C (B. cereus and B. subtilis), and colonies were counted.
The wet-heat resistance of dormant and superdormant spores was determined by incubating spores at an OD600 of 1 in water at 80°C (B. cereus and B. megaterium) or 93°C (B. subtilis). Samples were removed at various times and diluted in room temperature water, aliquots of dilutions were spotted on LB medium plates plus the mixture of the alternate and original germinants, plates were incubated, and colonies counted as described above.
For determination of spore core water content, dormant and superdormant spores of various species at an OD600 of 10 to 25 were first decoated by incubation in 1 ml of 0.1 M NaOH, 0.1 M NaCl, 1% SDS, and 0.1 M dithiothreitol for 2 h at 70°C (B. subtilis), 68°C (B. cereus), or 63°C (B. megaterium); washed 10 times with 1 ml water; and suspended in 0.1 ml water (1). The core wet densities of these decoated spores were determined in duplicate by buoyant density gradient centrifugation on Nycodenz gradients, and spore core water contents were calculated with the formula y = −0.00254x + 1.460, where y is the core wet density in g/ml and x is the percentage of core wet weight as water (18). All gradients to measure spore core wet densities also contained small amounts of B. subtilis CU1065 (33) spores that were labeled with the dye Procion MX-5B (14) (Sigma Aldrich, St. Louis, MO) to serve as an internal standard.
RESULTS
Heat activation temperature requirement for germination of dormant and superdormant spores.
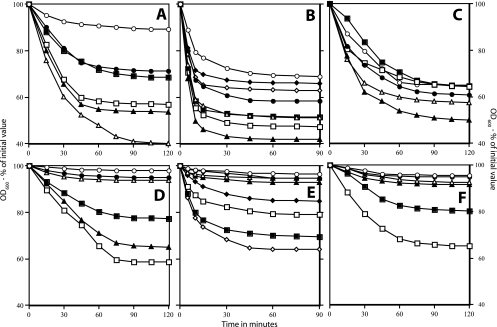
Previous work showed that superdormant spores of B. cereus, B. megaterium, and B. subtilis isolated using heat activation at the temperature optima for the dormant spores germinated extremely poorly with the germinant used to remove dormant spores from spore populations, thus allowing isolation of superdormant spores (9, 10) (Fig. 1). One explanation for this phenomenon is that the superdormant spores require a higher temperature for heat activation than the great majority of individual spores in the dormant spore population. To test this possibility, dormant spores of B. cereus, B. megaterium, and B. subtilis were germinated with the original germinants without heat activation or following activation at various temperatures (Fig. 1A to C). Without heat activation, the extent of dormant spore germination was only 20 to 55%, with B. subtilis spores exhibiting the most germination without heat activation (Fig. 1 A to C; Fig. 2). The reason that many dormant spores germinated even without heat activation is not clear, but it is likely that there is slow spore activation even during sporulation, as activation is both temperature and time dependent (17). Dormant spores also activate slowly during storage at low temperatures (17), although our experiments used spores within several weeks of their preparation.
FIG. 1.
Germination of dormant and superdormant spores of Bacillus species following heat activation at different temperatures. Spores of Bacillus species were obtained, and superdormant spores were isolated following germination with inosine (B. cereus), glucose (B. megaterium) or l-valine (B. subtilis) as described in Materials and Methods. The dormant and superdormant spores were heat activated at various temperatures, cooled on ice, and germinated with the original germinants, and the OD600 of cultures was measured. The spores analyzed were B. cereus (A and D), B. megaterium (B and E), B. subtilis (C and F), original dormant (A, B, and C), and superdormant (D, E, and F). The symbols for the heat activation temperatures used in the various panels follow. Panels A and D: ○, no activation; •, 60°C; ▵, 65°C; ▴, 70°C; □, 75°C; ▪, 82.5°C. Panels B and E: ○, no activation; •, 50°C; ▵, 55°C; ▴, 60°C; □, 65°C; ▪, 70°C; ⋄, 75°C; ♦, 80°C. Panels C and F: ○, no activation; •, 65°C; ▵, 70°C; ▴, 75°C; □, 82.5°C; ▪, 87.5°C.
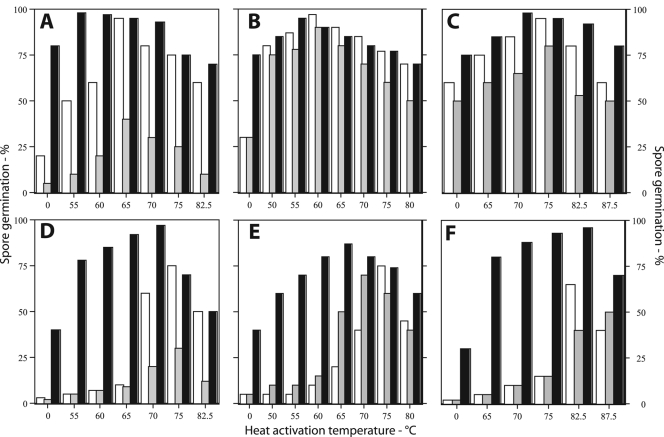
FIG. 2.
Extents of germination of superdormant spores of Bacillus species with different germinants after heat activation at various temperatures. Dormant and superdormant spores of Bacillus species were isolated and heat activated at various temperatures as described in the legend for Fig. 1. After cooling on ice, the spores were germinated for 2 h in either the original germinants (white bars), the alternative germinants (gray bars), or a mixture of both germinants (black bars), and the extent of spore germination was determined by phase-contrast microscopy as described in Materials and Methods. The spores analyzed were B. cereus (A and D), B. megaterium (B and E), B. subtilis (C and F), dormant (A, B, and C), and superdormant (D, E, and F).
The heat activation temperature had a significant effect on the germination of both dormant and superdormant spores, with most of this effect on the extent of spore germination and less of it on the rate of spore germination, as determined by the fall in the OD600s of cultures, after correcting for the percentage of spores that actually germinated (Fig. 1A to F and data not shown). In addition, there were clear temperature optima for heat activation, and above these temperature optima, the extent of spore germination fell off significantly (Fig. 1A to F; Fig. 2). Several additional results of this experiment were that superdormant spores of all three species (i) exhibited almost no germination with the original germinants when there was no heat activation or with activation temperatures up to the optima for dormant spores and (ii) had heat activation temperature optima with the original germinants that were 8 to 15°C higher than those for dormant spores (Fig. 2; Table 1). However, even with optimal heat activation, the extent of germination of superdormant spores with original germinants was only 60 to 70%, while optimally heat-activated dormant spore populations routinely exhibited 90 to 95% germination under the same conditions (Fig. 1 and 2; Table 1).
TABLE 1.
Heat activation temperature optima for germination of dormant and superdormant spores of Bacillus species with various germinantsa
| Species | Heat activation temp optimum (°C)b
|
|||||
|---|---|---|---|---|---|---|
| Original germinant
|
Alternate germinant
|
Mixture
|
||||
| D | SD | D | SD | D | SD | |
| B. cereus | 65 (95) | 75 (75) | 65 (40) | 75 (30) | 55 (98) | 70 (97) |
| B. megaterium | 60 (97) | 75 (75) | 60 (91) | 70 (70) | 55 (95) | 65 (87) |
| B. subtilis | 75 (95) | 82.5 (65) | 75 (80) | 87.5 (50) | 70 (97) | 82.5 (94) |
Dormant (D) and superdormant (SD) spores of Bacillus species were heat activated at various temperatures; cooled on ice; and germinated with the original germinants, alternate germinants, or a mixture of the original and alternate germinants, and the heat activation temperatures giving maximum extents of germination after 2 h were determined as described in Materials and Methods.
Values in parentheses are the extent (%) of spore germination after 2 h as determined by phase-contrast microscopy as described in Materials and Methods.
Effect of alternate germinants and germinant mixtures on the heat activation requirements of dormant and superdormant spores.
The results noted above indicated that superdormant spores had higher heat activation temperature optima for germination with original germinants than did dormant spores. Previous work has shown that superdormant spores that were isolated following germination with germinants that target one germinant receptor also germinate poorly with germinants that target a different germinant receptor or a different cooperating pair of germinant receptors (9, 10). Thus, it was of interest to examine the heat activation requirements of dormant and superdormant spores for germination with alternate germinants. The results of this analysis (Fig. 2; Table 1) showed that (i) the dormant spores of all three species exhibited the same heat activation optima for maximum germination with either the original or alternate germinants, (ii) the superdormant spores exhibited significantly higher heat activation temperature optima for germination with alternate germinants than the initial dormant spores, and (iii) the extent of germination of the superdormant spores with alternate germinants following optimum heat activation was again lower than that for dormant spores (Fig. 2; Table 1).
Previous work has shown that while superdormant spores germinate poorly with either original or alternate germinants following heat activation optimal for dormant spores, a mixture of original and alternate germinants promotes rapid superdormant spore germination (9, 10). The mixture had an effect largely on the extent of spore germination, as the viability of superdormant spores following heat activation optimal for dormant spores on LB medium plates, a medium in which superdormant spores generally germinate poorly (9, 10), was 10- to 100-fold lower than that of the original dormant spores (Table 2). However, when the LB medium plates were supplemented with a mixture of the original and alternate germinants, the dormant and superdormant spores had essentially the same viabilities, even when the spores had not been heat activated prior to their application to plates (Table 2). This suggested that germination of both dormant and superdormant spores in a mixture of the original and alternate germinants might modify these spores' requirement for heat activation. This was indeed the case, as with these germinant mixtures both dormant and superdormant spores (i) exhibited more germination without heat activation than with either the original or alternate germinants alone; (ii) exhibited higher levels of germination over a much wider range of heat activation temperatures than with either the original or alternate germinants alone (although levels for superdormant spores were lower than those for dormant spores); and (iii) had heat activation temperature optima that were ∼5°C lower than heat activation temperature optima for the original or alternate germinants alone (Fig. 1 and 2; Table 1).
TABLE 2.
Viability of dormant and superdormant spores on various media with and without heat activationa
| Bacillus species and plating medium | Spore viability (%)b
|
|||
|---|---|---|---|---|
| No heat activation
|
Heat activation
|
|||
| D | SD | D | SD | |
| B. cereus and LB | 40 | 1 | 100 | 3 |
| B. cereus and LB + alanine/inosine | 88 | 110 | 102 | 98 |
| B. megaterium and LB | 87 | 4 | 100 | 5 |
| B. megaterium and LB + glucose/proline | 107 | 100 | 100 | 108 |
| B. subtilis and LB | 87 | 1.5 | 100 | 10 |
| B. subtilis and LB + AGFK/valine | 105 | 95 | 100 | 96 |
Dormant (D) and superdormant (SD) spores at an OD600 of 1 were heat activated at the temperature optima for dormant spores, cooled, and applied to LB medium plates without or with the mixture of the original and alternate germinants; the plates were incubated at 30°C (B. megaterium) or 37°C (B. cereus and B. subtilis) for 24 to 36 h; and colonies were counted.
Spore viability is expressed relative to that of heat-activated dormant spores of the same species on LB medium plates, and this latter value was set at 100%.
Wet-heat resistance of dormant and superdormant spores.
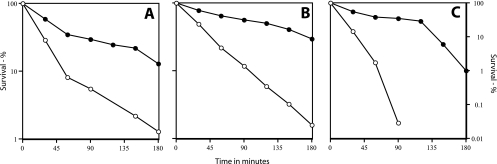
The different heat activation requirements for optimal germination of dormant and superdormant spores likely reflect the heterogeneity in spore populations. There is also heterogeneity in the resistance properties of spores in populations, in particular wet-heat resistance (27, 28), so it was of obvious interest to determine if dormant and superdormant spores exhibited differences in their wet-heat resistance. For this analysis to be successful, it was essential that we obtain similar and essentially complete viabilities for dormant and superdormant spores, since wet-heat treatment could activate spores in the process of killing them and thus lead to an artificial increase in the apparent viability of superdormant spores in particular. As noted above, dormant and superdormant spores of all three species exhibited identical and essentially complete viabilities on LB medium plates plus a mixture of the original and alternate germinants (Table 2). Thus, the extent of spore germination on these plates was even higher than that in the germinant mixtures alone (compare results shown in Fig. 2 and Table 2), presumably because of the additional germinants provided by the LB medium. When these enriched LB medium plates were used to measure the viability of non-heat-activated spores during wet-heat treatment, there were no increases in spore viability, indicating that spores were not being activated and then killed (Fig. 3A). More importantly, the superdormant spores of all three species were significantly more wet-heat resistant than were the dormant spores (Fig. 3A to C).
FIG. 3.
Wet-heat resistance of dormant and superdormant spores of Bacillus species. Dormant and superdormant spores of Bacillus species were isolated, and their wet-heat resistance at various temperatures was determined as described in Materials and Methods. The spores analyzed were B. cereus (A); B. megaterium (B); and B. subtilis (C), and the symbols used are the following: ○, original dormant spores; •, superdormant spores.
A number of factors influence the wet-heat resistance of Bacillus spores, but a particularly important one is the spore core water content, as decreases in core water content are invariably associated with higher spore wet-heat resistance (8, 19, 22, 25). Spore core water content can be determined by buoyant density gradient centrifugation to measure the core wet density, using decoated spores to allow the density gradient medium to penetrate up to the spore's inner membrane (18). When this analysis was done on dormant and superdormant spores of the three Bacillus species, the superdormant spores had a higher core wet density and thus lower core water content than their dormant spore counterparts (Table 3).
TABLE 3.
Core wet density and water content of dormant and superdormant spores of Bacillus speciesa
| Species | Core wet density (g/ml)
|
Core water content (g/100 g wet wt)
|
||
|---|---|---|---|---|
| Dormant | Superdormant | Dormant | Superdormant | |
| B. cereus | 1.340 | 1.350 | 47 | 43 |
| B. megaterium | 1.324 | 1.334 | 54 | 50 |
| B. subtilis | 1.362 | 1.378 | 38 | 32 |
The core wet densities of dormant and superdormant spores were determined as described in Materials and Methods and are the averages of two determinations that varied ≤0.002 g/ml. Core water content was calculated as described in Materials and Methods. Superdormant spores of B. cereus, B. megaterium, and B. subtilis were prepared with inosine, d-glucose, and l-alanine, respectively.
Environment of DPA in dormant and superdormant spores.
The lower core water content of the superdormant spores of the three Bacillus species noted above could be due to the superdormant spores having lower core water content with the same amount of core dry weight as dormant spores, or a larger amount of core dry weight than dormant spores and a corresponding decrease in core water. One spore small molecule whose levels can greatly influence spore core water content is DPA, which together with its chelated divalent metal ion, predominantly Ca2+ (CaDPA), can comprise ∼25% of core dry weight (8, 22, 25, 31, 32). However, previous work has shown that the DPA contents as the percentages of the dry weights of dormant and superdormant spores are identical, within experimental error (9). Consequently, superdormant spores may have the same amount of core dry weight as dormant spores, but less core water, as appears to be the case for spores prepared at higher temperatures compared to those made at lower temperatures (19).
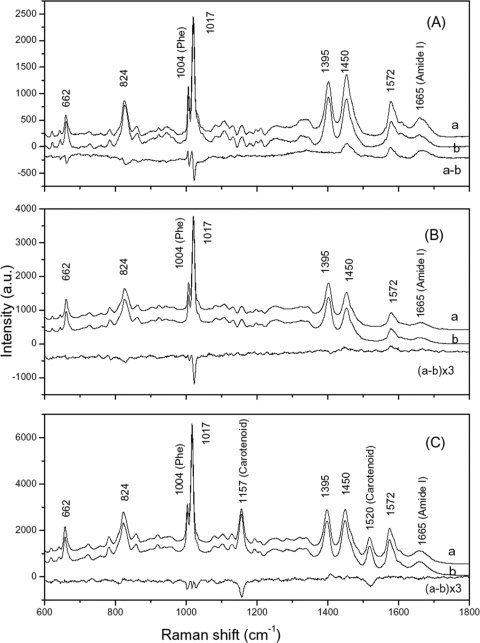
The argument given above suggests that there may be some notable differences in the environment of DPA in dormant and superdormant spores, because the same amount of DPA is associated with different amounts of water in the two types of spores, although we have no real idea of the state of water in dormant spores. To examine this possibility in detail, we carried out LTRS analysis on 50 individual dormant and superdormant spores of each species suspended in water, as a number of peaks due to DPA, in all likelihood as CaDPA, are prominent in an individual dormant spore's Raman spectrum (4, 16). CaDPA-specific bands at 662, 824, 1,017, 1,395, 1,450, and 1,572 cm−1, as well as a phenylalanine band at 1,004 cm−1, and a protein amide I band at 1,665 cm−1 in the average Raman spectra of the dormant and superdormant spores of all three Bacillus species were observed, as expected (Fig. 4A to C) (4, 16). The B. megaterium spores' spectra also had two additional peaks at 1,157 and 1,520 cm−1 that are almost certainly due to carotenoids, as several colored carotenoids were present at significant levels in B. megaterium spores, but not in B. cereus or B. subtilis spores (20, 26). Strikingly, of the Raman spectral bands due to CaDPA in particular, the average difference spectra of the superdormant and dormant spores of all three species were not zero. In B. subtilis superdormant spores the intensities of the 1,017-, 824-, and 662-cm−1 CaDPA bands were reduced, while the intensities of the 1,450- and 1,572-cm−1 CaDPA bands (and the 1,665-cm−1 amide I band) were increased compared to those of dormant spores. In B. cereus superdormant spores, the intensities of 1,017- and 824-cm−1 bands were slightly reduced, while the intensities of the 1,450- and 1,572-cm−1 bands were slightly increased, and in B. megaterium superdormant spores the intensities of the 1,017- and 824-cm−1 bands were slightly reduced, while the intensities of the 1,395-, 1,450-, and 1,572-cm−1 bands were almost unchanged. These results suggest that the environment of CaDPA in superdormant spores of all three species is altered compared to that in dormant spores. In addition, the two carotenoid bands in the B. megaterium spore spectra had significantly lower intensities in the superdormant spores.
FIG. 4.
Average Raman spectra and difference spectra of dormant and superdormant spores of Bacillus species. The average Raman spectra of 50 individual B. subtilis superdormant spores (curve a), dormant spores (curve b) and their difference (curve a-b) (A); B. cereus superdormant spores (curve a), dormant spores (curve b) and their difference (curve a-b), multiplied by a factor of 3 for display (B); and B. megaterium superdormant spores (curve a), dormant spores (curve b), and their difference (curve a-b), multiplied by a factor of 3 for display (C).
DISCUSSION
The work in this communication allows a number of new conclusions about spore heat activation, superdormancy, and wet-heat resistance, and since these conclusions are the same for spores of three different Bacillus species, this suggests they are conclusions for spores of all Bacillus species. These conclusions are as follows. First, the higher heat activation temperature optima for superdormant spores compared to that for dormant spores is consistent with superdormant spores having a higher threshold for a signal to trigger germination compared to dormant spores, as suggested previously (9, 10). The mechanism whereby the threshold level for a signal sufficient for germination is set is not known, but presumably the heat activation temperature is one variable that influences the level of this signal, whatever the signal is. Unfortunately, the mechanism whereby spores are activated by heat or any other activation treatment is not known.
A second conclusion related to the first one is that with a richer spectrum of germinants, the temperature optima for heat activation are significantly decreased both for dormant and superdormant spores, consistent with heat activation temperature being one factor influencing the level of germination “signal” received by dormant spores and another factor being the “richness” of the nutrient germination signal (9, 10). However, in the absence of knowledge of how heat activation potentiates spore germination, how germination signals are integrated, the mechanism of spore germination, and how threshold levels of signal for germination are set and sensed, it is difficult to interpret the influence of germinant composition on requirements for heat activation in molecular terms.
A third novel conclusion is that there may be a relationship between the optimal heat activation temperature for spores of a given species and the degree of core hydration, and this relationship may even hold true across species, as spores with lower core water required higher temperatures for heat activation. Unfortunately, as noted above, it is not clear how heat activation potentiates spore germination. Consequently, we cannot be sure that the correlation we observed between core water content and heat activation temperature optima is due to a causal connection.
The fourth conclusion concerns the correlation between spore superdormancy, core water content, and wet-heat resistance that we observed. An inverse relationship between core water content and wet-heat resistance has been well established for spores both within a species and across species and for spores of both Clostridium and Bacillus species (8). This correlation is thought to be causal as well, with lower core water contents presumably stabilizing core proteins more efficiently, and core protein damage seems most likely to be the reason for spore killing by wet heat (6, 7). What is novel in our results was that superdormant spores had lower core water content and higher wet-heat resistance than the great majority of the original dormant spore population. This suggests that superdormancy may be related in some way to these spores' lower core water content. While this may also be related to the higher heat activation temperature requirement for germination of superdormant spores as noted above, perhaps a lower core water content also decreases spore germination in some fashion. The fact that the intensity of one CaDPA-specific Raman spectral peak decreased in superdormant spores relative to the intensity in dormant spores, while the intensities of several other peaks increased, strongly suggests that the CaDPA environment differs between dormant and superdormant spores. However, beyond there being more core water in dormant spores, we do not know of any other factors important in determining the CaDPA environment in the core. One potential utility of the differences in the CaDPA-specific Raman spectral peaks in dormant and superdormant spores is that it may allow prediction of the presence of superdormant spores in dormant spore populations by utilizing principal component analysis of an individual spore's Raman spectrum, and detailed examination of this possibility is in progress.
In addition to the differences in the CaDPA Raman spectra of dormant and superdormant spores, there were also significant differences in the intensities of carotenoid-specific Raman spectral bands in dormant and superdormant B. megaterium spores, with notably lower intensity in superdormant spores, suggesting that there is less carotenoid in superdormant spores, although it is possible that the carotenoid environments in dormant and superdormant spores differ slightly, as suggested above for CaDPA. The carotenoid is likely in the spore's inner membrane (20, 26), although this has not been conclusively established. However, it is not clear how a lower level of a carotenoid in the spore's inner membrane would modify spore wet-heat resistance and core water content. Indeed, many significant changes in the phospholipid composition of the B. subtilis spore's inner membrane either have only minimal effects on core water content or cause increases in core water content, and none decrease core water content (14).
A fifth conclusion, albeit not a novel one, from our results is that spore populations are clearly heterogeneous, both in their germination requirements, with a small percentage of spores requiring much greater stimuli to trigger germination than the majority of the population, and in their wet-heat resistance, with a small percentage of spores exhibiting higher wet-heat resistance. In the spore populations we have analyzed, the superdormant spores with slightly elevated wet-heat resistance were 1 to 3% of spore populations, and possibly there are even lower percentages of superdormant spores that have even higher wet-heat resistance. Perhaps it is these latter superdormant spores that contribute most significantly to the tailing often seen in wet-heat killing of dormant spore populations (27, 28).
Perhaps most importantly, our results have clearly identified an additional factor that influences levels of superdormant spores in populations. Previous work indicated that levels of particular nutrient germinant receptors are potentially important in determining levels of superdormant spores in populations, with elevated receptor levels correlated with lower yields of superdormant spores with germinants that target the receptors whose levels are elevated (9). The level of germinant and whether multiple germinants are used also significantly affect yields of superdormant spores, with subsaturating levels of a single germinant giving higher levels of superdormant spores and saturating levels of multiple germinants giving much lower levels (9). In addition, spores not heat activated prior to germination also give lower yields of superdormant spores (9, 10). We now find that heat activation temperature optima for superdormant spores are also different than those for dormant spores. Clearly, the heterogeneity of spore populations is reflected in a number of spore properties, including wet-heat resistance, superdormancy, and heat activation temperature optima. The challenge now is to determine what specific changes promote these differences in properties between individual spores in populations.
Acknowledgments
We are grateful to Keren Griffiths for assistance with preparing dye-labeled spores of B. subtilis.
This work was supported by grants from the National Institutes of Health (GM-19698) and the Army Research Office to P.S. and a grant from the Army Research Office to Y.Q.L. and P.S.
Footnotes
Published ahead of print on 10 July 2009.
REFERENCES
- 1.Bagyan, I., M. Noback, S. Bron, M. Paidhungat, and P. Setlow. 1998. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene 212179-188. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer, R., E. Johnson, and R. Bollinger. 2003. Modeling the optimum duration of antibiotic prophylaxis in an anthrax outbreak. Proc. Natl. Acad. Sci. USA 10010129-10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera-Martinez, R.-M., F. Tovar-Rojo, V. Ramana Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 1852457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, D., S. S. Huang, and Y.-Q. Li. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores in aqueous solution by confocal laser tweezers Raman spectroscopy. Anal. Chem. 786936-6941. [DOI] [PubMed] [Google Scholar]
- 5.Clements, M. O., and A. Moir. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 1801787-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman, W. H., D. Chen, Y.-Q. Li, A. E. Cowan, and P. Setlow. 2007. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 1898458-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman, W. H., and P. Setlow. 2009. Analysis of damage due to moist heat treatment of spores of Bacillus subtilis. J. Appl. Microbiol. 1061600-1607. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt, P., and R. E. Marquis. 1989. Spore thermoresistance mechanisms, p. 43-64. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development: structural and functional analysis of bacterial sporulation and germination. American Society for Microbiology, Washington, DC.
- 9.Ghosh, S., and P. Setlow. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 1911787-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh, S., and P. Setlow. The preparation, germination properties and stability of superdormant spores of Bacillus cereus. J. Appl. Microbiol., in press. [DOI] [PubMed]
- 11.Goldrick, S., and P. Setlow. 1983. Expression of a Bacillus megaterium sporulation-specific gene in Bacillus subtilis. J. Bacteriol. 1551459-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould, G. W. 1969. Spore germination, p. 397-444. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, New York, NY.
- 13.Gould, G. W. 1970. Germination and the problem of dormancy. J. Appl. Bacteriol. 3334-49. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths, K. K., and P. Setlow. 2009. Effects of modification of membrane lipid composition on Bacillus subtilis sporulation and spore properties. J. Appl. Microbiol. 1062064-2078. [DOI] [PubMed] [Google Scholar]
- 15.Heine, H. S., J. Bassett, L. Miller, J. M. Hartings, B. E. Irvine, M. L. Pitt, D. Fritz, S. L. Norris, and W. R. Byrne. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob. Agents Chemother. 511373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, S.-S., D. Chen, P. L. Pelczar, V. R. Vepachedu, P. Setlow, and Y.-Q. Li. 2007. Levels of Ca-DPA in individual Bacillus spores determined using microfluidic Raman tweezers. J. Bacteriol. 1894681-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keynan, A., and Z. Evenchick. 1969. Activation, p. 359-396. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, New York, NY.
- 18.Lindsay, J. A., T. C. Beaman, and P. Gerhardt. 1985. Protoplast water content of bacterial spores determined by buoyant density sedimentation. J. Bacteriol. 163735-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melly, E., P. C. Genest, M. E. Gilmore, S. Little, D. L. Popham, A. Driks, and P. Setlow. 2002. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J. Appl. Microbiol. 921105-1115. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell, C., S. Iyer, J. F. Skomurski, and J. C. Vary. 1980. Red pigment in Bacillus megaterium spores. Appl. Environ. Microbiol. 5264-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, England.
- 22.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 1825505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 1822513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 1833982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paredes-Sabja, D., B. Setlow, P. Setlow, and M. R. Sarker. 2008. Characterization of Clostridium perfringens spores that lack SpoVA proteins and dipicolinic acid. J. Bacteriol. 1904648-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Racine, F. M., and J. C. Vary. 1980. Isolation and properties of membranes from Bacillus megaterium spores. J. Bacteriol. 1431208-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts, T. A., and A. D. Hitchins. 1969. Resistance of spores, p. 611-670. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, New York, NY.
- 28.Russell, A. D. 1982. The destruction of bacterial spores. Academic Press, New York, NY.
- 29.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 1783486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550-556. [DOI] [PubMed] [Google Scholar]
- 31.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101514-525. [DOI] [PubMed] [Google Scholar]
- 32.Setlow, P., and E. A. Johnson. 2007. Spores and their significance, p. 35-67. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 3rd ed. ASM Press, Washington, DC.
- 33.Zahler, S. A., R. Z. Korman, R. Rosenthal, and H. E. Hemphill. 1977. Bacillus subtilis bacteriophage SPβ: localization of the prophage attachment site, and specialized transduction. J. Bacteriol. 129556-558. [DOI] [PMC free article] [PubMed] [Google Scholar]