Abstract
Proliferation of the human-pathogenic bacterium Streptococcus pneumoniae is fundamentally linked to the bacterial proteins that function in cell division. Here, we show that LytR, a pneumococcal protein from the LytR-CpsA-Psr family, is essential to this process.
Recently, we and others have described a mechanism for active acquisition of homologous DNA that increases the efficiency of gene exchange between pneumococci and their close relatives (2, 5, 6, 8, 9, 19, 20). This mechanism, termed “fratricide,” enables competent Streptococcus pneumoniae cells to attack and lyse noncompetent bacteria from closely related species, resulting in the release of chromosomal DNA from the target cells. In liquid cultures, competent pneumococci trigger lysis of the target cells by production and secretion of the murein hydrolase CbpD (10). CbpD represents a double-edged sword, since this enzyme also has an intrinsic ability to attack the cell wall of the producers. In order to protect their own cell wall, competent pneumococci therefore produce a competence-induced immunity protein, ComM (13). The comM operon also includes lytR (Fig. 1A), which clearly encodes a protein that belongs to the LytR-CpsA-Psr family (1, 7, 11, 17). Although LytR-CpsA-Psr family members are omnipresent in gram-positive bacteria, their cellular function remains unknown. Interestingly, such proteins are generally not present in gram-negative bacteria. The family consists of proteins that carry a LytR-CpsA-Psr domain with a hitherto unidentified function. Typically, these proteins are composed of an N-terminal part comprising one or several transmembrane helices and an extracellular C-terminal part that comprises the LytR-CpsA-Psr domain (7). Transcriptional analyses have previously demonstrated that transcription of pneumococcal lytR increases upon binding of ComE to the comM promoter during competence induction, indicating that LytR perhaps could serve a specific function in fratricide immunity (3, 14). However, it has also been speculated that a putative extended −10 promoter element (5′-TNTGNTATAAT-3′) situated immediately upstream of spr1760 could drive expression of lytR in noncompetent cells (6) (Fig. 1A). To investigate the basal expression of lytR in noncompetent cells, we inserted a luciferase reporter gene downstream of lytR in strain R704, giving rise to strain RH424 (Table 1; and see the supplemental material for a full description of methods). As shown in Fig. 1B, RH424 displayed high luciferase activity during the early logarithmic growth phase. In comparison, a strain carrying the luciferase reporter gene fused to the coding region of comM (RH21) displayed little or no luciferase activity. These results demonstrate that the genes downstream of comM are regulated separately from comM during normal growth. To further confirm the results from the luciferase reporter study, we next exchanged the wild-type lytR gene with a mutant lytR gene encoding a C-terminal polyhistidine tag. In a previous study aimed at identifying fratricide immunity factors in S. pneumoniae, it was demonstrated that lytR could not be deleted by mariner mutagenesis, suggesting that lytR is an essential gene in S. pneumoniae (6). To facilitate the exchange of wild-type lytR with a His-tagged version of the gene, we therefore used the counterselectable Janus system to insert an additional copy of lytR into the genome of RH426, resulting in strain RH428. The ectopic lytR copy (pEXT1::lytR), which upon integration substitutes for a Janus cassette introduced into the truncated IS1167 element located downstream of the ami operon, is expressed from a strong synthetic version of the dpnM extended −10 promoter previously described by Sabelnikov et al. (16). Next, a Janus cassette (lytR::Janus) was inserted into the wild-type lytR locus. The resulting strain, RH429, did not display any altered growth characteristics compared to the parent strain, demonstrating that pEXT1::lytR fully compensates for deletion of the wild-type lytR locus (data not shown). Next, the lytR::Janus cassette in RH429 was exchanged with a His-tagged copy of lytR (RH439). Subsequent deletion of pEXT1::lytR resulted in strain RH440. Samples of strain RH440 were subsequently harvested at different stages during growth and analyzed by Western blotting (Fig. 1C). The immunoblotting results confirmed that LytR is constitutively synthesized during exponential growth. Taken together with the fact that a previous study suggested that pneumococcal lytR is essential in wild-type cells, the above data strongly support a housekeeping function of LytR in dividing cells. In our experience, when kanamycin is used as a selection marker for gene deletion, mutant cells normally emerge on agar plates after 12 to 16 h of incubation at 37°C. During the current work, we discovered that small colony-sized kanamycin-resistant mutants could be rescued at a low frequency when pEXT1::lytR in strain RH430 was deleted by replacement with a Janus kan-rpsL+ cassette (strain RH433). However, mutant cells would only emerge on the agar plates when the incubation time at 37°C was extended to 36 h. As compared to the parent strains RH426 and RH430, RH433 grew at a much slower rate and displayed premature autolysis (Fig. 2). Next, pEXT1::lytR was reintroduced into this strain, thus replacing the Janus kan-rpsL+ fragment. This backcross resulted in cells (RH436) with significantly higher growth rate than the RH433 parent strain (Fig. 2). However, reintroduction of lytR could not fully restore the growth rate back to the level displayed by strain RH426 or strain RH430. The fact that wild-type growth could not be fully restored hinted at the presence of a suppressor mutation in strain RH433. Induction of competence in S. pneumoniae results in production of the murein hydrolase CbpD. Competent cells also produce the ComM immunity protein, which by an unknown mechanism protects the competent cells from the activity of CbpD. Interestingly, competence induction in the lytR-deficient strain RH433 resulted in a drastic drop in optical density (data not shown), indicating that rapid autolysis was taking place. Autolysis was not observed in competent RH435 cells (ΔlytR ΔcbpD). Reversion of the RH433 lytR deletion mutant back to a lytR+ phenotype could not fully restore immunity toward CbpD, as a CbpD-dependent drop in optical density was observed when cultures of RH436 were induced to competence (data not shown). This observation supports that a suppressor mutation is formed upon lytR deletion. DNA sequencing demonstrated that this mutation does not locate to the region of the comM operon, and the genomic location of the suppressor hence remains unknown. In S. pneumoniae, stationary-phase autolysis is known to be mediated by the murein hydrolase LytA. Although the cells appear to constitutively synthesize LytA during growth, the enzymatic activity of LytA normally manifests itself some hours after the onset of stationary phase (12, 21). The observation that lytR deletion mutants undergo rapid premature autolysis led us to investigate whether this lysis was dependent upon LytA. Indeed, deletion of lytR in a ΔlytA background resulted in cells (RH434) that displayed a growth rate similar to that of RH433 but did not autolyse (Fig. 2). Hence, cells deleted for lytR become highly sensitized toward both LytA and CbpD. It has previously been demonstrated that LytA-dependent autolysis can be triggered by treatment with a number of different compounds, such as detergents and penicillin, as well as other cell wall inhibitors (4, 13, 15). Common for these compounds is that they damage the cell envelope. We therefore hypothesized that deletion of lytR generates alterations in the structure of the pneumococcal cell wall that trigger LytA activity. To investigate whether these putative alterations resulted in a visible change in cell morphology, we analyzed lytR deletion mutants by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM analysis revealed that strains RH426 and RH430 grew as well-defined diplococci of relatively uniform size. In contrast, lytR mutant cells displayed high variability in both size and shape. Some of the mutant cells were two to three times larger than wild-type cells, whereas others grew as irregularly shaped minicells. In contrast, lytR deletion mutants that were reverted to a lytR+ phenotype by reinsertion of the pEXT1::lytR copy (RH436) displayed a morphological phenotype similar to that of strains RH426 and RH430 (data not shown). The basis for the altered morphology of lytR mutants was identified in the TEM analysis. As expected, thin sections of strain RH426 displayed cells with symmetrical septum formation that divided into evenly shaped progeny of the expected size (Fig. 3A). In contrast, a significant number of the RH433 cells displayed nonsymmetrical septum formation in which several septa often were observed to be distributed at multiple sites within the same cell. In contrast to the perpendicular septa found in strain RH426, the septa in RH433 were frequently found to form at odd angles with respect to the long axis of the cell (Fig. 3B and C). Thus, the abnormal morphology of RH433 cells results from lack of nonsymmetrical cell division and frequent formation of multiple septa. As shown in Fig. 3D, symmetrical septum formation was restored upon reversion of the RH433 lytR deletion mutant back to a lytR+ phenotype by insertion of pEXT1::lytR (RH436). This result confirmed that the observed defect in control of septum placement in RH433 is due to the deletion of lytR.
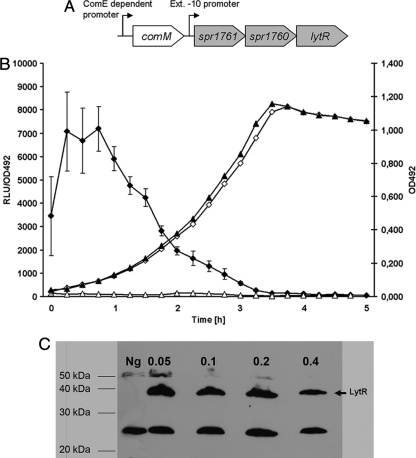
FIG. 1.
LytR is constitutively expressed during growth. (A) Genetic organization of lytR. Both the ComE-dependent promoter located upstream of comM and the constitutive extended −10 promoter located upstream of spr1761 are indicated. (B) Luciferase expression from RH424 (lytR::luc) and RH21 (comM::luc). Luciferase activity is presented as relative luminescence units (RLU)/optical density (OD). Black diamonds, RH424; white triangles, RH21. Growth was measured as OD at 492 nm (OD492). White diamonds, RH424; black triangles, RH21. RLU and OD492 were determined at 15-min intervals. (C) Detection of His-tagged LytR by Western blotting. Cells were harvested at the indicated points of growth, and whole-cell extracts were loaded onto a 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. After transfer to polyvinylidene difluoride membranes, His-tagged LytR was detected using the SuperSignal West Pico HisProbe kit (Pierce). A sample of RH425 harvested at an OD492 of 0.1 was used as the negative control (Ng). The specific bands observed upon immunodetection correspond to the calculated molecular mass of His-tagged LytR (38 kDa). Note that LytR still could be detected at an OD of 0.8 (data not shown).
TABLE 1.
Characteristics of the strains used in this study
| Strain or plasmid | Genotype/relevant feature(s)a | Source or reference |
|---|---|---|
| Strains | ||
| R704 | R6 derivative; comA::ermAM Eryr | 6 |
| CP 1200 | Rx, but malM511 str-1 Smr | 18 |
| RH21 | R704, but comM::luc (pRcomM) comM Eryr Cmr | 8 |
| RH424 | R704, but lytR::luc (pRlytR) lytR+ Eryr Cmr | This study |
| RH425 | R704, but Smr by transformation with CP1200 chromosomal DNA, Eryr Smr | This study |
| RH426 | RH425, but Δ IS1167::Janus Eryr Kanr | This study |
| RH428 | RH426, but substitution of Janus cassette with pEXT1::lytR, Eryr Smr | This study |
| RH429 | RH428, but deletion of wild-type lytR locus by insertion of a lytR::Janus cassette, Eryr Kanr | This study |
| RH430 | RH429, but ΔlytR by transformation with a PCR fragment substituting for the lytR::Janus insertion, Eryr Smr | This study |
| RH431 | RH430, but ΔlytA::spc Eryr Smr Spcr | This study |
| RH432 | RH430, but ΔcbpD::spc Eryr Smr Spcr | This study |
| RH433 | RH430, but ΔpEXT1::lytR by insertion of ΔIS1167::Janus PCR fragment, Eryr Kanr | This study |
| RH434 | RH431, but ΔpEXT1::lytR by insertion of ΔIS1167::Janus PCR fragment, Eryr Kanr Spcr | This study |
| RH435 | RH432, but ΔpEXT1::lytR by insertion of ΔIS1167::Janus PCR fragment, Eryr Kanr Spcr | This study |
| RH436 | RH433, but substitution of Janus cassette with pEXT1::lytR, Eryr Smr | This study |
| RH437 | RH436, but ΔcbpD::Janus Eryr Kanr | This study |
| RH438 | RH436, but ΔcomM::Janus Eryr Kanr | This study |
| RH439 | RH429, but lytR::his by transformation with a PCR fragment substituting for the lytR::Janus insertion, Eryr Smr | This study |
| RH440 | RH439, but deletion of pEXT1::lytR by transformation with a ΔIS1167::Janus PCR fragment, Eryr Kanr | This study |
| Plasmid | ||
| pRlytR | pR424 derivative, Cmr, carries a lytR targeting fragment and luc gene, insertion duplication in S. pneumoniae generates a lytR::luc (lytR+) fusion | This study |
Cm, chloramphenicol; Ery, erythromycin; Kan, kanamycin; Spc, spectinomycin; Sm, streptomycin. “Janus” indicates the presence of a kan::rpsL cassette.
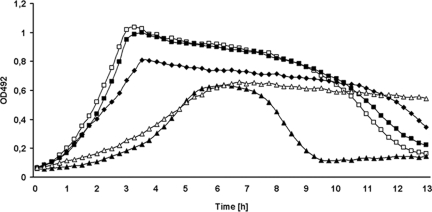
FIG. 2.
Deletion of lytR results in reduced growth and premature autolysis. Cells were grown at 37°C in C medium, and the optical density at 492 nm (OD492) was measured at 15-min intervals. White squares, RH426 (ΔIS1167::Janus); black squares, RH430 (ΔlytR pEXT1::lytR); black triangles, RH433 (ΔlytR); black diamonds, RH436 (ΔlytR; backcross of pEXT1::lytR); white triangles, RH434 (ΔlytR ΔlytA).
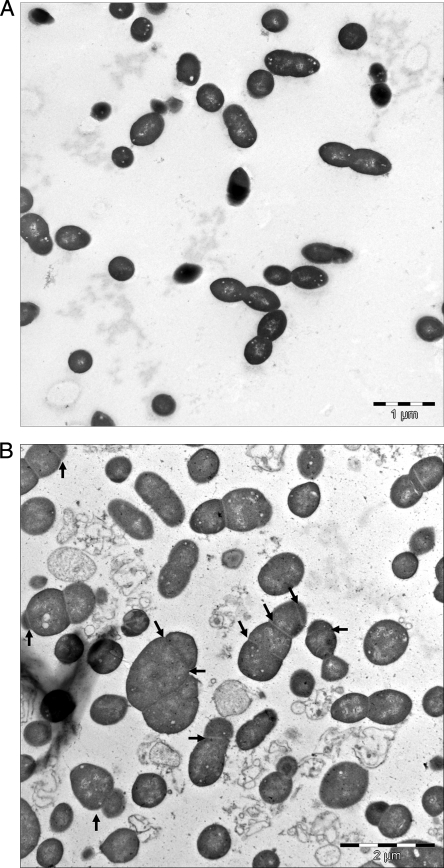
FIG. 3.
TEM of S. pneumoniae cells. Thin sections of early exponential phase cells were prepared as described in Materials and Methods. (A) RH426; (B and C) RH433 (ΔlytR); (D) RH436 (ΔlytR; backcross of pEXT1::lytR). Anomalous division sites in RH433 are indicated by arrows. Note that although normal septum formation was restored in RH436, some of the RH436 cells appeared to inherit a slightly elongated or “stretched” shape compared to the cells of strain RH426.
The present work was originally initiated to investigate whether LytR participates in the CbpD immunity mechanism during competence-induced fratricide in S. pneumoniae. Although lytR mutants appeared to be sensitized toward murein hydrolase activity in general, the data do not support that lytR is expressed to perform a specific role in fratricide immunity. Rather, the data suggest that the main role of LytR is performed during exponential growth, where the protein is essential for proper septum placement. Further work to define the exact role of LytR in pneumococcal cell division is in progress.
Supplementary Material
Acknowledgments
We thank H. R. Kolstad, E. Ørmen, and T. Krekling at the IPM imaging laboratory for technical assistance with the SEM and TEM analyses.
This work was supported by the Research Council of Norway.
Footnotes
Published ahead of print on 6 July 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Cieslewicz, M. J., D. L. Kasper, Y. Wang, and M. R. Wessels. 2001. Functional analysis in type Ia group B streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J. Biol. Chem. 276139-146. [DOI] [PubMed] [Google Scholar]
- 2.Claverys, J. P., and L. S. Håvarstein. 2007. Cannibalism and fratricide: mechanisms and raison d'être. Nat. Rev. Microbiol. 5219-229. [DOI] [PubMed] [Google Scholar]
- 3.Dagkessamanskaia, A., M. Moscoso, V. Hénard, S. Guiral, K. Overweg, M. Reuter, B. Martin, J. Wells, and J. P. Claverys. 2004. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol. Microbiol. 511071-1086. [DOI] [PubMed] [Google Scholar]
- 4.Dubos, R. J. 1937. Mechanism of the lysis of pneumococci by freezing and thawing, bile, and other agents. J. Exp. Med. 66101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiral, S., T. J. Mitchell, B. Martin, and J. P. Claverys. 2005. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc. Natl. Acad. Sci. USA 1028710-8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Håvarstein, L. S., B. Martin, O. Johnsborg, C. Granadel, and J. P. Claverys. 2006. New insights into the pneumococcal fratricide: relationship to clumping and identification of a novel immunity factor. Mol. Microbiol. 591297-1307. [DOI] [PubMed] [Google Scholar]
- 7.Hübscher, J., L. Lüthy, B. Berger-Bächi, and P. Stutzmann Meier. 2008. Phylogenetic distribution and membrane topology of the LytR-CpsA-Psr protein family. BMC Genomics 9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnsborg, O., V. Eldholm, M. Langedok Bjørnstad, and L. S. Håvarstein. 2008. A predatory mechanism dramatically increases the efficiency of lateral gene transfer in Streptococcus pneumoniae and related commensal species. Mol. Microbiol. 69245-253. [DOI] [PubMed] [Google Scholar]
- 9.Johnsborg, O., and L. S. Håvarstein. 2009. Regulation of natural genetic transformation and acquisition of transforming DNA in Streptococcus pneumoniae. FEMS Microbiol. Rev. 33627-642. [DOI] [PubMed] [Google Scholar]
- 10.Kausmally, L., O. Johnsborg, M. Lunde, E. Knutsen, and L. S. Håvarstein. 2005. Choline-binding protein D (CbpD) in Streptococcus pneumoniae is essential for competence-induced cell lysis. J. Bacteriol. 1874338-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarevic, M. J., P. Margot, B. Soldo, and D. Karamata. 1992. Sequencing and analysis of the Bacillus subtilis lytRABC divergom: a regulatory unit encompassing the structural genes of the N-acetyl-muramoyl-L-alanine amidase and its modifier. J. Gen. Microbiol. 1381949-1961. [DOI] [PubMed] [Google Scholar]
- 12.Lewis, K. 2000. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 64503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mascher, T., M. Heintz, D. Zähner, M. Merai, and R. Hakenbeck. 2006. The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in β-lactam resistance. J. Bacteriol. 1881959-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson, S. N., C. K. Sung, R. Cline, B. V. Desai, E. C. Snesrud, P. Luo, J. Walling, H. Li, M. Mintz, G. Tsegaye, P. C. Burr, Y. Do, S. Ahn, J. Gilbert, R. D. Fleischmann, and D. A. Morrison. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 511051-1070. [DOI] [PubMed] [Google Scholar]
- 15.Regev-Yochay, G., K. Trzcinski, C. M. Thompson, M. Lipsitch, and R. Malley. 2007. SpxB is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J. Bacteriol. 1896532-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabelnikov, A. G., B. Greenberg, and S. A. Lacks. 1995. An extended −10 promoter alone directs transcription of the DpnII operon of Streptococcus pneumoniae. J. Mol. Biol. 250144-155. [DOI] [PubMed] [Google Scholar]
- 17.Sapunaric, F., C. Franssen, P. Stefanic, A. Amoroso, O. Dardenne, and J. Coyette. 2003. Redefining the role of psr in β-lactam resistance and cell autolysis of Enterococcus hirae. J. Bacteriol. 1855925-5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoemaker, N. B., and W. R. Guild. 1974. Destruction of low efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol. Gen. Genet. 128283-290. [DOI] [PubMed] [Google Scholar]
- 19.Steinmoen, H., E. Knutsen, and L. S. Håvarstein. 2002. Induction of natural competence in Streptococcus pneumoniae triggers lysis and DNA release from a subfraction of the cell population. Proc. Natl. Acad. Sci. USA 997681-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinmoen, H., A. Teigen, and L. S. Håvarstein. 2003. Competence-induced cells of Streptococcus pneumoniae lyse competence-deficient cells of the same strain during cocultivation. J. Bacteriol. 1857176-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasz, A., and S. Waks. 1975. Enzyme replacement in a bacterium: phenotypic correction by the experimental introduction of the wild type enzyme into a live enzyme defective mutant pneumococcus. Biochem. Biophys. Res. Commun. 651311-1319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.