Abstract
Staphylococcus aureus is a highly versatile and evolving bacterium of great clinical importance. S. aureus can evolve by acquiring single nucleotide polymorphisms and mobile genetic elements and by recombination events. Identification and location of novel genomic elements in a bacterial genome are not straightforward, unless the whole genome is sequenced. Optical mapping is a new tool that creates a high-resolution, in situ ordered restriction map of a bacterial genome. These maps can be used to determine genomic organization and perform comparative genomics to identify genomic rearrangements, such as insertions, deletions, duplications, and inversions, compared to an in silico (virtual) restriction map of a known genome sequence. Using this technology, we report here the identification, approximate location, and characterization of a genetic inversion of ∼500 kb of a DNA element between the NRS387 (USA800) and FPR3757 (USA300) strains. The presence of the inversion and location of its junction sites were confirmed by site-specific PCR and sequencing. At both the left and right junction sites in NRS387, an IS1181 element and a 73-bp sequence were identified as inverted repeats, which could explain the possible mechanism of the inversion event.
Staphylococcus aureus is a gram-positive bacterium of immense clinical importance. This opportunistic pathogen is capable of causing a wide range of diseases from skin and soft-tissue infections to life-threatening bacteremia, endocarditis, and osteomyelitis (14). Approximately 75% of the S. aureus genome is composed of a core genome that is common in all strains, and 25% of the genome is composed of variable regions which can differ between different strains (4, 16, 24-26). S. aureus evolves primarily by introducing single nucleotide polymorphisms in its core genome and by acquiring mobile genetic elements (MGEs) through horizontal gene transfer. These MGEs include pathogenicity/genomic islands, plasmids, transposons, and bacteriophages that become integrated in the chromosome (4, 11, 16, 31, 32). Despite being a heterogeneous organism, genetic recombination in S. aureus is proposed to be rather rare (20, 24, 29, 35). Its clones are more likely to evolve by point mutations than by recombination events (12). The MGEs contribute to the phenotypic and genotypic diversity seen with the S. aureus population. Acquisition of the staphylococcal cassette chromosome (SCCmec) elements through site-specific recombinases has led to the epidemic of methicillin-resistant S. aureus (MRSA) strains in hospitals and communities all over the world (6, 10, 15). In recent years, the integration of arginine catabolite mobile element in the USA300 lineage of MRSA has been proposed to give the pathogen its epidemiological advantage, including traits for surviving in low-pH conditions and oxygen tension environments (11). In addition, chromosomal replacements have been observed within lineages of sequence type 34 (ST34) and ST42 (34) and ST8 and ST30 (13).
Genomic rearrangements, such as inversions, have been observed with genomes of enteric bacteria, such as Salmonella enterica, Shigella flexneri, Yersinia pestis KIM, Escherichia coli (K12 and O157:H7), and group A Streptococcus pyogenes (8, 9, 18, 27, 28, 30, 37). No genomic inversions in S. aureus have been reported to date. With the use of optical mapping, large genomic rearrangements, such as inversions, that would otherwise be missed with other comparative genotyping approaches, including microarray analysis, can be identified. Optical mapping uses high-resolution restriction maps (optical maps) of a bacterial genome to determine its genomic organization (5, 21, 23, 33, 36). These optical maps can be compared to an in silico (virtual) restriction map of a known genome sequence and can be used to identify gene rearrangements and their locations. Using optical mapping in conjunction with subsequent site-specific PCR and sequencing, we report the identification, approximate location, and partial characterization of an ∼500-kb DNA element in NRS387, a USA800 strain that was found to be inverted relative to USA300FPR3757. Identification of IS1181 elements and a novel 73-bp element at both ends of the ∼500-kb element in NRS387 could suggest the possibility of an inversion event in an ancestral strain of NRS387.
MATERIALS AND METHODS
Bacterial strains.
The S. aureus strains used were MW2, USA300FPR3757 (NRS384), and USA800 (NRS387). These strains were grown overnight, and an aliquot was embedded into low-melting agar for cell lysis, similar to the preparation for pulsed-field gel electrophoresis.
Preparation of the optical maps.
Following cell lysis, single DNA molecules were captured using a microfluidic device comprised of a polydimethylsiloxane overlay, into which 100-μ-wide grooves had been etched, that was attached to a positively charged glass cover slip (Fig. 1). DNA applied to the device was drawn by capillary action into the channels formed between the overlay and the glass. Contact between single DNA molecules and the positively charged surface resulted in the DNA becoming attached by electrostatic interaction in an elongated and slightly stretched configuration, leaving the molecules accessible by restriction endonucleases. The linearized DNAs were subjected to in situ digestion with XbaI. Following digestion, the DNA molecules were stained with a fluorescent intercalating agent, JoJo-1 (Invitrogen, Carlsbad, CA). The digested and stained DNA fragments were imaged on an automated fluorescent microscope for image capture and single-molecule markup, resulting in high-resolution single-molecule restriction maps. A collection of the single-molecule restriction maps, consisting of 600 to 700 restriction fragments, were assembled to produce whole-genome-ordered restriction maps using the GENTIG program (2, 3). Restriction map alignments between different strains were generated using OpGen's Mapviewer program. This dynamic programming algorithm finds the optimal alignment of two restriction maps according to a scoring model that incorporates fragment sizing errors, false and missing cuts, and missing small fragments.
FIG. 1.
Creation of optical maps. (A) Presence of linearized DNA molecules on a glass chip. (B) In situ-digested DNA molecules seen as gaps in the linear DNA molecules. (C) Visualization of a single digested and JoJo-1 stained DNA molecule. (D) Representation of a linear ordered restriction map of a bacterial genome in a bar coded form. (E) Circular representation of an optical map.
Creation of MW2 in silico and in situ map.
A virtual XbaI restriction digestion of the MW2 genome sequence (GenBank accession no. BA000033) was generated to create an in silico map of the reference strain. In situ optical maps were generated for the NRS384 and NRS387 strains.
Accuracy and reliability of optical maps.
In order to ensure that all optical maps that were generated were reliable and accurate, the XbaI in silico map of MW2 was compared to its XbaI in situ map using the MapViewer program.
Comparison between the FPR3757 and NRS387 strains.
The comparison of the restriction profiles of these two strains showed the presence of a large genomic region that existed in opposite orientation relative to each other. We exploited the genome sequence data of FPR3757 to design PCR primers at the two ends of the inverted region to confirm the inversion relative to the other strain. Putative inversion junction regions were identified based on alignment of the restriction pattern. Six PCR primer sets, three pairs flanking each of the putative inversion junction regions, were designed using the nucleotide positions relative to the sequenced reference strain, USA300FPR3757 (primer sets 1a to 6f; Table 1). Each primer pair covered ∼3 to 4 kb of the region. Ten additional primer sets (primer sets 1a1 to 6f4 and IS1181; Table 1) were designed by primer walking to further refine the junction sites. These primers were designed from the sequences obtained from primer sets 1a and 6f. Primer sets 1a1 to 1a5 covered the 1a primer region, whereas 6f1 to 6f4 covered the 6f primer region of the FPR3757 strain (Table 1). Primer set IS1181 was designed after one of the obtained sequences had homology with IS1181. Specificity of all the primers to FPR3757 was confirmed by doing a BLAST analysis against the published genome sequence of the reference strain, FPR3757. A contiguous region spanning ∼4.8 kb at the left junction and ∼4.7 kb at the right junction of NRS387 was sequenced from respective amplicons.
TABLE 1.
Oligonucleotide primers used to determine the left and right junctions of the inversion and sequence of the regions
| Strain or primer | Forward or reversea | Sequence (5′-3′) | PCR product size (bp) | Location of primers |
|---|---|---|---|---|
| FPR3757 | ||||
| 1a | F | CGAAGGATACGGTCCAAGAG | 3,402 | 1153285-1153304 |
| R | ACCCGGTATATGGCAATCAA | 1156667-1156686 | ||
| 2b | F | TGAAGTCCAGTGCAATTGGT | 3,875 | 1156463-1156482 |
| R | TCAAAGGAGAAATTCGAATGAA | 1160316-1160337 | ||
| 3c | F | GCCATAACGTACTGTTAGAGAAGG | 3,490 | 1160116-1160139 |
| R | TTCAGGGGGTGCACATAAAT | 1163586-1163605 | ||
| 4d | F | AGCACGTTCGAAAGGAAGAT | 3,176 | 1683350-1683369 |
| R | GTCTTGGGCCGAGAATTTTA | 1686506-1686525 | ||
| 5e | F | ATTTTCAGCCCATCAAGTGC | 3,330 | 1680210-1680229 |
| R | TGGTATGCGTTTACGTCGTG | 1683520-1683539 | ||
| 6f | F | ATCTGGCGAAGCGATTAAGA | 3,197 | 1677418-1677437 |
| R | AAGACGATGCTGAGCTTTCTG | 1680594-1680614 | ||
| NRS387 | ||||
| 1a1 | F | CGAAGGATACGGTCCAAGAG | 771 | |
| R | TTTAAGCTTCCTGCAGCTAATG | |||
| 1a2 | F | TCATCAACTGGGATAGCAACT | 1,052 | |
| R | TTGTTTGAAAAGTGTCATCATGC | |||
| 1a3 | F | TTTCCAGTTCTAGTGCTATATTGGT | 1,087 | |
| R | AGAAGTATAAGGTGGTGAACAAAAA | |||
| 1a4 | F | GGATAATCGACGTAAGAAGAATCA | 1,128 | |
| R | ACCAATTGCACTGGACTTCA | |||
| 1a5 | F | CAACTTCGTACAATATCTAGATGAACA | 379 | |
| R | TTTAGCCGATAACTTCAGAAATG | |||
| 6f1 | F | ATCTGGCGAAGCGATTAAGA | 965 | |
| R | AGTGGCGAGGTATGGGAAC | |||
| 6f2 | F | TCAATGGAACAAGTTCGTATGC | 288 | |
| R | TCGTGAATATGCAGGTATGTTTT | |||
| 6f3 | F | CAGAGCGGCATAACTGCTTA | 1,053 | |
| R | ACAAGAGATGACGCGCAAAT | |||
| 6f4 | F | GCGACTTTCATCATATAGTCACCT | 1,082 | |
| R | GATCATATGACTGAAGCGGATG | |||
| IS1181 | F | TGCAATGGCACTTTTACTGC | 593 | |
| R | TCCATCAATAGGACTTTCCAGTT |
F, forward; R, reverse.
DNA extraction and PCR.
All strains were grown on blood agar plates for 24 to 36 h at 37°C and used to extract genomic DNA using the QIAmp DNA minikit (Qiagen, Surrey, United Kingdom). Standard PCRs were set up in the GeneAmp 9700 thermocycler (Applied Biosystems, Foster City, CA) using the Expand Long Range PCR kit (Roche Diagnostics, Indianapolis, IN). The expected amplicon sizes ranged between 3 and 4 kb. All PCR products were resolved in a 0.8% NuSieve agarose gel in 1× Tris-borate-EDTA buffer.
Sequencing of inversion junction sites.
Column-purified PCR products were sequenced with PCR primers and by primer walking for the amplicons generated from NRS387 using the BigDye Terminator cycle sequencing kit (Applied Biosystems). A contiguous sequence was created for the left and right junction region amplicons with a DNAstar software package (Lasergene version 7.2.1; Madison, WI). The consensus sequences from the NRS387 amplicons were compared against the USA300FPR3757 genome to determine the identity and location of the left and right junction boundaries.
RESULTS
Reliability of the optical maps.
In order to ensure that optical maps were accurate and reproducible, an in silico XbaI restriction map of the MW2 strain was compared to an XbaI in situ optical map using the MapViewer program (see Fig. S1 in the supplemental material). The restriction pattern-based alignment of the two maps showed that they were nearly identical in their genome size and linear restriction profile, suggesting that optical maps could be used reliably for comparing major genomic changes.
Comparison of the FPR3757 strain with the NRS387 strain.
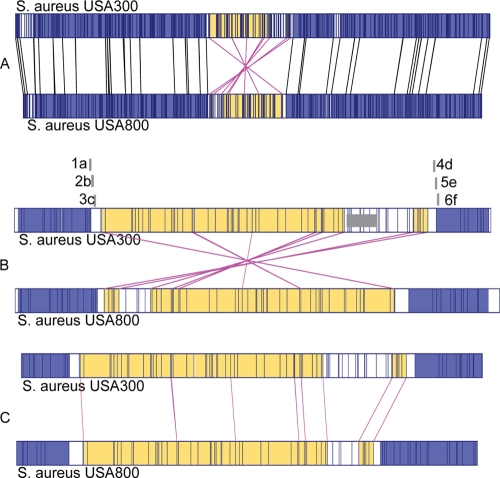
Pairwise comparison of the optical maps of FPR3757 and NRS387 revealed the presence of a putative genomic inversion of an ∼500-kb region in the FPR3757 strain relative to the NRS387 optical map (Fig. 2A). This inversion was identified by comparing XbaI restriction patterns of the two strains by the MapViewer program. This region appeared to be smaller in size in NRS387 by ∼55 kb compared to FPR3757, due to the apparent lack of lukSF-PV-harboring ΦSLT (staphylococcal leukocytolytic toxin) in that region in NRS387. Not surprisingly, NRS387 was PCR negative for lukSF-PV. Using the MapViewer software, a horizontally flipped image of the inverted region of the NRS387 strain showed that the XbaI restriction profiles for the inverted region were very similar to those for FPR3757, suggesting that, indeed, an inversion event had occurred (Fig. 2C). In addition to the inversion, other notable differences were the absence of three MGEs from NRS387, including arginine catabolite mobile element, ΦSLT (containing lukSF-PV), and υSAβ (containing lukD and lukE). In addition, the gain/loss of 22 XbaI restriction sites, four insertions in the sizes of 2 to 5 kb, and three deletions in the sizes of 5 to 6.2 kb was observed with the NRS387 genome relative to FPR3757. Size differences in the SCCmec cassettes between these two strains were also noted. The similar restriction profiles of the inverted region excluding ΦSLT in FPR3757 implies very similar gene contents. There were likely to be ∼380 genes, including 116 genes with hypothetical functions, present in the inverted region of NRS387 based upon the FPR3757 genome sequence (GenBank accession no. NC_007793). Overall, this region harbored genes that are involved in all cellular functions, including cell division, energy production, DNA metabolism, etc. There appeared to be no preference for any cellular function in this inverted region.
FIG. 2.
Observation and confirmation of a genetic inversion between FPR3757 and NRS387 by MapViewer. (A) Comparison of optical maps from reference strains FPR3757 and NRS387. The inverted region relative to strain FPR3757 is highlighted in yellow. (B) Enlarged view of the inverted region along with positions of primer sets 1a, 2b, and 3c on the left junction and primer sets 4d, 5e, and 6f on the right junction of the putative inversion. (C) An artificial horizontal flipping of the inverted region in NRS387, demonstrating similarity between the XbaI restriction profiles of FPR3757 and NRS387.
Confirmation of the genetic inversion between FPR3757 and NRS387.
The inversion was confirmed by analyzing the results of a number of serial PCRs performed at the suspected inversion junction sites on both strains. We hypothesized that two primer pairs (designed from the FPR3757 sequence) that overlap the left and right inversion junction sites in FPR3757 would yield an amplicon in this strain only, but not in NRS387 due to the disruption of this region by the inversion. When the two primer sets are used in combinations of two forward (F/F) and two reverse (R/R) primers, an amplicon would then be generated not in FPR3757, but in NRS387, confirming the inversion event (see Fig. S2 in the supplemental material).
The production or lack of amplicons from primer sets 1a, 2b, and 3c identified the left junction region in the NRS387 strain. With the FPR3757 strain, these primers yielded products of ∼3,400, 3,900, and 3,500 bp, respectively, as expected (data not shown). However, only primer sets 2b and 3c yielded products of the expected sizes from the NRS387 strain, as they primed upstream to the right junction region due to the “flipping” status in this strain (see Fig. S2 in the supplemental material). Primer set 1a did not yield any product in the NRS387 strain, suggesting that it probably overlapped with the left junction of the inversion (Fig. 3A). Similarly, amplicons or the lack thereof from primer sets 4d, 5e, and 6f identified the right inversion boundaries in the NRS387 strain. As expected, these primers yielded products of ∼3,200, ∼3,400, and ∼3,200 bp, respectively, with the FPR3757 strain (data not shown). However, only primer sets 4d and 5e yielded products of expected size in the NRS387 strain, as they primed downstream of the left junction region. Primer set 6f did not yield any product in the NRS387 strain, suggesting that it probably overlapped with the right junction of the inversion (Fig. 3B). As mentioned in the hypothesis, when the F/F (i.e., 1a-F/6f-F) and R/R (i.e., 1a-R/6f-R) primer combinations of primer sets 1a and 6f were used, both yielded amplicons within the expected range in the NRS387 strain only (Fig. 3C). The sizes of the amplicons were ∼4,800 and ∼4,700 bp, respectively. The sizes of these amplicons suggested the presence of an additional ∼1,500 bp sequences in them compared to the corresponding regions in the FPR3757 strain. While PCR products from the F/F and R/R primer combinations confirmed an inversion event, the presence of additional sequences in NRS387 suggested the presence of additional genetic elements that could have facilitated the inversion of this large DNA region in NRS387. As expected, the F/F and R/R combinations of primer sets did not yield any products in the FPR3757 strain, as it harbored the inversion relative to NRS387.
FIG. 3.
Results of left- and right-junction site-specific PCR with NRS387 and FPR3757. Lane 1 is the DNA size marker in all seven panels. The sizes of the molecular mass markers (in thousands) are indicated on the left. (A) Lane 2, lack of amplicon from primer set 1a in the NRS387 strain due to the inversion junction. Lanes 3 and 4, amplicons of 3,402 and 3,490 bp from primer sets 2b and 3c from NRS387. (B) Lanes 2 and 3, amplicons of expected sizes (3,176 and 3,330 bp) from primer sets 4d and 5e from NRS387. Lane 4, lack of amplicon from primer set 6f in NRS387. (C) Lane 2, lack of amplicon from the F/F primer combinations of 1a and 6f in the FPR3757 strain. Lane 3, An ∼4,800-bp amplicon from the F/F primer combinations of 1a and 6f in the NRS387 strain. Lane 4, lack of amplicon from R/R primer combinations of 1a and 6f in the FPR3757 strain. Lane 5, An ∼4,700-bp amplicon from the R/R primer combinations of 1a and 6f in the NRS387 strain. (D and F) PCR results from FPR3757 with primer pairs 1a5 and 6f2, respectively. (E and G) PCR results from NRS387 with the F/F and R/R combination of primer pairs 1a5 and 6f2, respectively. In panels D to G, lane 2 corresponds to FPR3757 and lane 3 corresponds to NRS387. The presence of IS1181 and 73-bp conserved sequences within the left and right junction sites is seen by the increase in size of the PCR products from NRS387 compared to those from FPR3757.
Sequencing of the left and right genetic elements in NRS387 at the junction region.
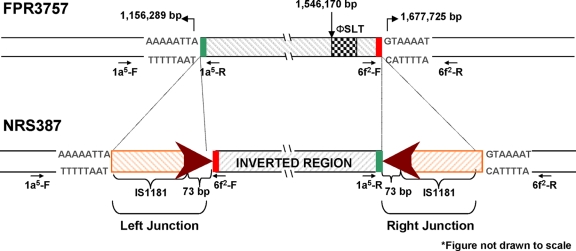
The ∼4.8-kb amplicon (primer set 1a-F/6f-F) encompassing the left junction site was sequenced by the primer walking method using primers sets 1a1-F/R to 1a5-F/R and 6f1-F/R plus 6f2-F/R (Table 1). These overlapping subsets of primers were designed from the sequences obtained from the ∼4.8-kb amplicon from the NRS387 strain to provide a contiguous sequence of the region. The sequencing analysis of the ∼4.8-kb amplicon from the left junction site in the NRS387 strain showed that it consisted of three distinct sequences: (i) 3,031 bp of the expected FPR3757 sequence, (ii) 1,439-bp IS1181 element plus a 73-bp conserved sequence, and (iii) 256 bp of the FPR3757 sequence. The 3.0-kb sequence corresponded to the immediate upstream sequence of the left junction region in NRS387 and the IS element/73-bp sequence at the junction. The 256-bp sequence corresponded to the relative right junction in FPR3757 (Fig. 4).
FIG. 4.
Schematic diagram (not drawn to scale) of the genetic elements at the left and right junction sites in NRS387, in reference to FPR3757. In both junction sites, a genetic element consisting of IS1181 (orange diagonal box) and a 73-bp conserved sequence (red arrowhead) was identified with primer sets 1a5 and 6f2. The genetic element in the right junction site was inverted. The green and red boxes (not drawn to scale) correspond to sequences from FPR3757 found in the inverted region.
Similarly, the ∼4.7-kb amplicon from NRS387 (primer set 1a-R/6f-R) encompassing the right junction site was sequenced by primer sets 6f1-F/R to 6f4-F/R and 1a4-F/R plus 1a5-F/R by the primer walking method. Starting from the 5′ end, this sequence consisted of (i) 372 bp of the FPR3757 sequence, (ii) a 1,439-bp IS1181 element plus a 73-bp conserved sequence, and (iii) 2,850 bp of the FPR3757 sequence. The 372-bp sequence corresponded to the relative left junction in FPR3757 (Fig. 4). The IS1181 element/73-bp sequences were inverted relative to the same sequence at the left junction. As expected, the 2.8-kb sequence corresponded to the immediate downstream sequence of the right junction in NRS387 (Fig. 4). The insertion sequence (IS) elements and 73-bp conserved sequences accounted for the increases in the amplicon sizes from the F/F and R/R primer combinations in the NRS387 strain (Fig. 3D to G). Since these genetic elements were not present at the junction sites in the FPR3757 strain, it suggests that they had a role in the inversion event in NRS387.
DISCUSSION
Prokaryotic genomes have a great degree of plasticity and can adapt and evolve by a variety of mechanisms including single nucleotide polymorphisms, acquisition of MGEs by horizontal gene transfer, and recombination. They could also evolve by both small and large genomic inversions (7, 17). Genomic inversions have frequently been reported for strains of Escherichia coli, Salmonella enterica serovar Typhimurium, Yersinia sp., group A Streptococcus, and Lactococcus lactis (7-9, 18, 27, 28, 30, 37). Multiple types of chromosomal rearrangements have also been observed with food-borne strains of E. coli O157:H7 (19). A common observation with these inversions is the presence of IS elements or prophages flanking the junction sites. An inversion of 556 kb has been identified between the Salmonella serovar groups Typhimurium and Typhi (1). This inversion is thought to be catalyzed by the IS element IS200. Similarly, nearly half of the chromosome was inverted in a L. lactis strain by IS905 (7). In addition, prophages are thought to be involved with inversions seen with group A Streptococcus and E. coli O157:H7. These IS elements or phage proteins allow reciprocal recombination due to alignments of their reverse repeat orientations (22). We present evidence that an approximately 500-kb piece of DNA from a strain belonging to the USA800 linage was present in an inverted orientation in one of the most successful clones of community-associated MRSA. The presence of a large inversion in one of the two strains was rather intriguing and could be explained in the context of these two strains descending from a common ancestor which was probably more like NRS387 (Fig. 5). The ancestral strain harbored this region in an orientation similar to that in FPR3757, along with the IS1181/73-bp elements at the two ends. Following alignment of the IS1181/73-bp elements and reciprocal recombination, this region was inverted. The IS1181/73-bp elements in NRS387 were 100% identical in sequence but in opposite orientation. These elements are 99% identical to IS elements present in FPR3757. This inversion could be a one-time-only event. Since the genotypes of NRS387 and FPR3757 are quite different (FPR3757, ST8, agr type I; NRS387, ST5, agr type II; unrelated spa types), it is likely that the contemporary NRS387 strain diverged from its common ancestor a long time ago.
FIG. 5.
Proposed mechanisms of the genetic inversion in NRS387. (A) Location of the inverted repeat sequences harboring IS1181 in the ancestral NRS387-like strain. (B) Alignment of the inverted repeat sequences followed by reciprocal recombination. This recombination event causes the region to “flip.” (C) The current orientation of the inverted region in NRS387. (D) Divergence of FPR3757 and NRS387 from a common ancestor strain. ACME, arginine catabolite mobile element.
In a rather unlikely scenario, it is possible that a strain like NRS387 could have been the source for this inversion in the FPR3757 strain. It is possible that this region may have flipped first in an intermediate NRS387-like strain due to reciprocal recombination and then been transposed in a strain like FPR3757. Once inserted into the strain like FPR3757, the IS and 73-bp elements could have been lost or jumped to a different location on the chromosome. Indeed, two copies of the IS1181/73-bp elements are present hundreds of kb downstream in the FPR3757 genome. Since ΦSLT was not observed with NRS387, it is possible that it was acquired by the FPR3757 strain after the insertion event.
The inverted region in NRS387 is approximately halfway in the genome from the origin of replication. Based on the location of the inversion and the GC skew data from other sequenced S. aureus genomes, it appears that there will be no significant change in the replicore sizes in these two strains. Not surprisingly, the growth curves of these two strains in Trypticase soy broth were nearly identical. It has been suggested by others that an inversion that does not change the symmetry of the chromosome usually does not have any phenotypic effect or selective disadvantage (7). It is premature to comment on how common the genotype of a strain like NRS387 really is. In summary, this report is the first observation of any genetic inversion within an S. aureus strain and was made possible by comparing the optical maps of two S. aureus strains in the absence of whole-genome sequencing. Using this approach, it would be possible to identify additional novel genetic events in S. aureus genomes. Identification of novel genetic events in successful epidemic strains could possibly explain reasons for their enhanced virulence and epidemicity.
Supplementary Material
Acknowledgments
This work was supported in part by Marshfield Clinic Research Foundation and OpGen Inc. Some of the data used in the manuscript were generated from NIAID grant AI061385 to S.K.S.
We thank anonymous reviewers for making insightful comments to improve the manuscript. We also acknowledge Lindsay Sammons for useful discussion and thank Alice Stargardt in the preparation of the manuscript.
Footnotes
Published ahead of print on 19 June 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alokam, S., S. L. Liu, K. Said, and K. E. Sanderson. 2002. Inversions over the terminus region in Salmonella and Escherichia coli: IS200s as the sites of homologous recombination inverting the chromosome of Salmonella enterica serovar Typhi. J. Bacteriol. 1846190-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anantharaman, T. S, V. Mysore, and B. Mishra. 2005. Fast and cheap genome wide haplotype construction via optical mapping. Pac. Symp. Biocomput. 2005385-396. [DOI] [PubMed] [Google Scholar]
- 3.Anantharaman, T. S., B. Mishra, and D. C. Schwartz. 1999. Genomics via optical mapping III: contiging genomic DNA. In Thomas Lengauer, Reinhard Schneider, Peer Bork, Douglas Brutlad, Janice Glasgow, Hans-Werner Mewes, and Ralf Zimmer (ed.), Proceedings of the Seventh International Conference on Intelligent Systems for Molecular Biology, Heidelberg, Germany. [PubMed]
- 4.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 3591819-1827. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Q., S. J. Savarino, and M. M. Venkatesan. 2006. Subtractive hybridization and optical mapping of the enterotoxigenic Escherichia coli H10407 chromosome: isolation of unique sequences and demonstration of significant similarity to the chromosome of E. coli K-12. Microbiology 1521041-1054. [DOI] [PubMed] [Google Scholar]
- 6.Daum, R. S., T. Ito, K. Hiramatsu, F. Hussain, K. Mongkolrattanothai, M. Jamklang, and S. Boyle-Vavra. 2002. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J. Infect. Dis. 1861344-1347. [DOI] [PubMed] [Google Scholar]
- 7.Daveran-Mingot, M. L., N. Campo, P. Ritzenthaler, and P. Le Bourgeois. 1998. A natural large chromosomal inversion in Lactococcus lactis is mediated by homologous recombination between two insertion sequences. J. Bacteriol. 1804834-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 1844601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng, W., S. R. Liou, G. Plunkett III, G. F. Mayhew, D. J. Rose, V. Burland, V. Kodoyianni, D. C. Schwartz, and F. R. Blattner. 2003. Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J. Bacteriol. 1852330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deurenberg, R. H., C. Vink, S. Kalenic, A. W. Friedrich, C. A. Bruggeman, and E. E. Stobberingh. 2007. The molecular evolution of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 13222-235. [DOI] [PubMed] [Google Scholar]
- 11.Diep, B. A., S. R. Gill, R. F. Chang, T. H. Phan, J. H. Chen, M. G. Davidson, F. Lin, J. Lin, H. A. Carleton, E. F. Mongodin, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367731-739. [DOI] [PubMed] [Google Scholar]
- 12.Feil, E. J., J. E. Cooper, H. Grundmann, D. A. Robinson, M. C. Enright, T. Berendt, S. J. Peacock, J. M. Smith, M. Murphy, B. G. Spratt, C. E. Moore, and N. P. Day. 2003. How clonal is Staphylococcus aureus? J. Bacteriol. 1853307-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feil, E. J., E. K. Nickerson, N. Chantratita, V. Wuthiekanun, P. Srisomang, R. Cousins, W. Pan, G. Zhang, B. Xu, N. P. Day, and S. J. Peacock. 2008. Rapid detection of the pandemic methicillin-resistant Staphylococcus aureus clone ST 239, a dominant strain in Asian hospitals. J. Clin. Microbiol. 461520-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon, R. J., and F. D. Lowy. 2008. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 46(Suppl. 5)S350-S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiramatsu, K., L. Cui, M. Kuroda, and T. Ito. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 9486-493. [DOI] [PubMed] [Google Scholar]
- 16.Holden, M. T., E. J. Feil, J. A. Lindsay, S. J. Peacock, N. P. Day, M. C. Enright, T. J. Foster, C. E. Moore, L. Hurst, R. Atkin, A. Barron, N. Bason, S. D. Bentley, C. Chillingworth, T. Chillingworth, C. Churcher, L. Clark, C. Corton, A. Cronin, J. Doggett, L. Dowd, T. Feltwell, Z. Hance, B. Harris, H. Hauser, S. Holroyd, K. Jagels, K. D. James, N. Lennard, A. Line, R. Mayes, S. Moule, K. Mungall, D. Ormond, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, S. Sharp, M. Simmonds, K. Stevens, S. Whitehead, B. G. Barrell, B. G. Spratt, and J. Parkhill. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 1019786-9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolsto, A. B. 1997. Dynamic bacterial genome organization. Mol. Microbiol. 24241-248. [DOI] [PubMed] [Google Scholar]
- 18.Kotewicz, M. L., S. A. Jackson, J. E. LeClerc, and T. A. Cebula. 2007. Optical maps distinguish individual strains of Escherichia coli O157:H7. Microbiology 1531720-1733. [DOI] [PubMed] [Google Scholar]
- 19.Kotewicz, M. L., M. K. Mammel, J. E. Leclerc, and T. A. Cebula. 2008. Optical mapping and 454 sequencing of Escherichia coli O157:H7 isolates linked to the US 2006 spinach-associated outbreak. Microbiology 1543518-3528. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn, G., P. Francioli, and D. S. Blanc. 2006. Evidence for clonal evolution among highly polymorphic genes in methicillin-resistant Staphylococcus aureus. J. Bacteriol. 188169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latreille, P., S. Norton, B. S. Goldman, J. Henkhaus, N. Miller, B. Barbazuk, H. B. Bode, C. Darby, Z. Du, S. Forst, S. Gaudriault, B. Goodner, H. Goodrich-Blair, and S. Slater. 2007. Optical mapping as a routine tool for bacterial genome sequence finishing. BMC Genomics 8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewin, B. 2007. Genes IX. Jones & Bartlett Publishers, Inc., Sudbury, MA.
- 23.Lim, A., E. T. Dimalanta, K. D. Potamousis, G. Yen, J. Apodoca, C. Tao, J. Lin, R. Qi, J. Skiadas, A. Ramanathan, N. T. Perna, G. Plunkett III, V. Burland, B. Mau, J. Hackett, F. R. Blattner, T. S. Anantharaman, B. Mishra, and D. C. Schwartz. 2001. Shotgun optical maps of the whole Escherichia coli O157:H7 genome. Genome Res. 111584-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay, J. A., and M. T. Holden. 2004. Staphylococcus aureus: superbug, super genome? Trends Microbiol. 12378-385. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay, J. A., and M. T. Holden. 2006. Understanding the rise of the superbug: investigation of the evolution and genomic variation of Staphylococcus aureus. Funct. Integr. Genomics 6186-201. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay, J. A., C. E. Moore, N. P. Day, S. J. Peacock, A. A. Witney, R. A. Stabler, S. E. Husain, P. D. Butcher, and J. Hinds. 2006. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J. Bacteriol. 188669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, S. L., and K. E. Sanderson. 1996. Highly plastic chromosomal organization in Salmonella typhi. Proc. Natl. Acad. Sci. USA 9310303-10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, S. L., and K. E. Sanderson. 1995. Rearrangements in the genome of the bacterium Salmonella typhi. Proc. Natl. Acad. Sci. USA 921018-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore, P. C., and J. A. Lindsay. 2001. Genetic variation among hospital isolates of methicillin-sensitive Staphylococcus aureus: evidence for horizontal transfer of virulence genes. J. Clin. Microbiol. 392760-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa, I., K. Kurokawa, A. Yamashita, M. Nakata, Y. Tomiyasu, N. Okahashi, S. Kawabata, K. Yamazaki, T. Shiba, T. Yasunaga, H. Hayashi, M. Hattori, and S. Hamada. 2003. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 131042-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novick, R. P. 2003. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid 4993-105. [DOI] [PubMed] [Google Scholar]
- 32.Novick, R. P., and A. Subedi. 2007. The SaPIs: mobile pathogenicity islands of Staphylococcus. Chem. Immunol. Allergy 9342-57. [DOI] [PubMed] [Google Scholar]
- 33.Reslewic, S., S. Zhou, M. Place, Y. Zhang, A. Briska, S. Goldstein, C. Churas, R. Runnheim, D. Forrest, A. Lim, A. Lapidus, C. S. Han, G. P. Roberts, and D. C. Schwartz. 2005. Whole-genome shotgun optical mapping of Rhodospirillum rubrum. Appl. Environ. Microbiol. 715511-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson, D. A., and M. C. Enright. 2004. Evolution of Staphylococcus aureus by large chromosomal replacements. J. Bacteriol. 1861060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson, D. A., and M. C. Enright. 2003. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 473926-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valouev, A., D. C. Schwartz, S. Zhou, and M. S. Waterman. 2006. An algorithm for assembly of ordered restriction maps from single DNA molecules. Proc. Natl. Acad. Sci. USA 10315770-15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou, S., A. Kile, M. Bechner, M. Place, E. Kvikstad, W. Deng, J. Wei, J. Severin, R. Runnheim, C. Churas, D. Forrest, E. T. Dimalanta, C. Lamers, V. Burland, F. R. Blattner, and D. C. Schwartz. 2004. Single-molecule approach to bacterial genomic comparisons via optical mapping. J. Bacteriol. 1867773-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.