Abstract
A set of enzymes dedicated to recycling of the amino sugar components of peptidoglycan has previously been identified in Escherichia coli. The complete pathway includes the nagA-encoded enzyme, N-acetylglucosamine-6-phosphate (GlcNAc6P) deacetylase, of the catabolic pathway for use of N-acetylglucosamine (GlcNAc). Mutations in nagA result in accumulation of millimolar concentrations of GlcNAc6P, presumably by preventing peptidoglycan recycling. Mutations in the genes encoding the key enzymes upstream of nagA in the dedicated recycling pathway (ampG, nagZ, nagK, murQ, and anmK), which were expected to interrupt the recycling process, reduced but did not eliminate accumulation of GlcNAc6P. A mutation in the nagE gene of the GlcNAc phosphotransferase system (PTS) was found to reduce by 50% the amount of GlcNAc6P which accumulated in a nagA strain and, together with mutations in the dedicated recycling pathway, eliminated all the GlcNAc6P accumulation. This shows that the nagE-encoded PTS transporter makes an important contribution to the recycling of peptidoglycan. The manXYZ-encoded PTS transporter makes a minor contribution to the formation of cytoplasmic GlcNAc6P but appears to have a more important role in secretion of GlcNAc and/or GlcNAc6P from the cytoplasm.
Peptidoglycan (PG) or murein, the rigid shape-forming layer of the bacterial cell envelope, undergoes extensive degradation and resynthesis during normal bacterial growth. It is estimated that 40 to 50% of the PG is broken down and reused each generation (for a review, see reference 22). PG is a matrix of chains of alternating N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) sugars cross-linked by peptide bridges. Over the last 20 years the pathways for recycling both the peptide and amino sugar portions of the PG have been elucidated, and a number of genes involved in this process have been identified. Most of the genes involved encode dedicated enzymes whose only function seems to be to recover the material produced during PG turnover and to reuse it to synthesize more PG or as a source of energy. However, some of the enzymes shown to be involved have apparently been recruited from another metabolic pathway (e.g., murQ- and nagA-encoded enzymes [see below]), while other specialized PG-recycling enzymes have a subsidiary function (e.g., ampG- and ampD-encoded enzymes in β-lactamase induction [20]).
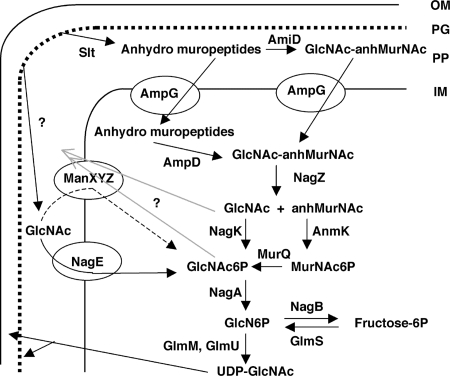
The pathway for recycling the amino sugar part of PG in Escherichia coli is shown in Fig. 1 (for a review, see reference 22). Periplasmic hydrolases (lytic transglycosylases, Slt) and endopeptidases break the PG backbone, liberating anhydro-muropeptides (principally GlcNAc-anhydro-MurNAc [anhMurNAc]-tetrapeptide), which are transported into the cytoplasm by the ampG-encoded transporter (10). The peptide portion is cleaved off either by the membrane-associated amiD-encoded amidase (28) or by the ampD-encoded cytoplasmic amidase (11), liberating the disaccharide. The tetrapeptide is converted to a tripeptide and free d-Ala, both of which are reused to produce UDP-MurNAc-pentapeptide (11). The GlcNAc-anhMurNAc disaccharide is cleaved by the nagZ-encoded β-N-acetylglucosaminidase (2, 32), and then both sugars are converted to their 6-phosphate forms by the specific kinases NagK (29) and AnmK (31). The latter produces MurNAc-6-phosphate (MurNAc6P), which is converted to GlcNAc6P by the murQ-encoded etherase (12, 30). MurNAc6P is also the product of transport of MurNAc by the MurNAc-specific phosphotransferase system (PTS) transporter MurP. The murP and murQ genes form an operon for use of MurNAc as a carbon source (4). Thus, the MurQ protein has both catabolic and recycling functions (12, 30). Similarly, further use of the GlcNAc6P involves an enzyme normally involved in the catabolism of GlcNAc, the nagA-encoded GlcNAc6P deacetylase of the GlcNAc degradation pathway (21). The deacetylase converts GlcNAc6P to glucosamine-6-phosphate (GlcN6P), which can be converted to UDP-GlcNAc, the first dedicated compound for the synthesis of the cell wall components, by the glmM- and glmU-encoded enzymes (16, 17).
FIG. 1.
Scheme for recycling of PG in E. coli. The enzymes and substrates are described in the text. Slt is the major soluble lytic transglycosylase. OM, outer membrane; PP, periplasm; IM, inner membrane. The enzymes involved in converting UDP-GlcNAc into the components of the PG and outer membrane are not shown. Arrows with a question mark indicate the pathways postulated to exist based on the results described in this work.
It has been known for many years that mutations in nagA lead to very high levels of GlcNAc6P (33). Strains carrying nagA mutations are NagSensitive (i.e., they do not grow in medium containing GlcNAc and another carbon source). The toxicity of the accumulated sugar phosphates means that secondary mutations that alleviate this toxicity arise spontaneously in vivo (33). GlcNAc6P is the inducing signal for the NagC repressor of the nag regulon, and the accumulation of GlcNAc6P in the nagA strain results in derepression (endogenous induction) of the nag regulon (25). One class of suppressor mutations result in noninducible versions of NagC that are not sensitive to GlcNAc6P, so that the nag genes stay repressed (23), implying that overexpression of the nag regulon genes is one cause of the toxicity. Amino sugars are essential constituents of the bacterial PG and lipopolysaccharide (LPS) in gram-negative bacteria. In the absence of an exogenous supply of amino sugars, glmS, encoding GlcN6P synthase, is an essential gene (for a review, see reference 7). As GlcNAc6P accumulates in nagA cells growing in medium devoid of amino sugars, it must ultimately be derived from the de novo synthesis of GlcN6P by GlmS, which is destined for synthesis of PG and the LPSs of the outer membrane. As no acetyltransferase for GlcN6P has been characterized, the most likely origin of the GlcNAc6P in nagA strains is recycling of the PG. The LPS of the outer membrane of gram-negative bacteria also contains GlcN, but it is not known to undergo any turnover and the work of Park (21) showed that radioactive GlcN was stably incorporated into the LPS fraction, whereas radioactivity was slowly lost from the PG of isolated sacculi.
In this work the effect of mutations in the recycling pathway on the accumulation of GlcNAc6P in vivo was investigated. The results show that mutations in one or more genes of the recycling pathway reduce but do not eliminate GlcNAc6P accumulation in nagA strains. However, when these mutations are present in the same strain with a mutation in the nagE gene encoding the GlcNAc6P-specific transporter of the GlcNAc PTS, GlcNAc6P levels decrease to the background level. This shows that the GlcNAc PTS is another pathway that is involved in recycling the GlcNAc component of PG. The manXYZ-encoded PTS transporter is also capable of GlcNAc uptake, and its effect on the recycling process was also examined.
MATERIALS AND METHODS
Bacterial methods.
The starting strains carrying antibiotic resistance replacement-deletion mutations in the PG-recycling genes and PTS genes used in this work are listed in Table 1. They were introduced sequentially by P1 transduction into MC-B1. MC-B1 is MC4100 carrying a single copy of the nagB-lacZ fusion on a λ lysogen. Expression of the nagB-lacZ fusion depends on the level of GlcNAc6P, which is the inducing signal for the NagC repressor. The ΔnagA::FRTkan and ΔnagA::FRTcm mutations remove only the 5′ half of nagA, leaving the nagC promoters intact (24). Antibiotic cassettes which are flanked by FRT sites were cured by transformation with the pCP20 plasmid expressing the Flp recombinase (5). Curing strains that carried nagE::FRTkan and nagA::FRTcm simultaneously resulted in loss of the intervening nagB gene. The loss of nagB did not affect the accumulation of GlcNAc6P in strains when the cured and uncured versions were both tested. The nagA::tc534 insertion mutation (25) had the same effect on GlcNAc6P accumulation as nagA::FRTcm or kan replacement, but it affected nagC expression and the nagB-lacZ fusion was derepressed. The final genotypes of the strains tested are shown in Table 1, in which genes are listed in the order in which they were introduced. Strains bearing nagA mutations are not very stable, especially on LB medium, and were kept on minimal glucose plates containing Casamino Acids. Several independent constructs carrying different alleles of the same gene and/or with the mutations introduced in a different order when possible were tested. Mutants with similar genotypes are organized in eight mutant groups, which are shown in Fig. 2B. The groups with the suffix “m” all carry a manXYZ mutation. The presence of the mutations, either cured or with the antibiotic cassette, was verified by PCR analysis of the bacteria using pairs of oligonucleotides located upstream and downstream of the mutated gene. The nagZ::cm mutation cotransduces about 50% with nagK, and the presence of the correct nagK allele was verified systematically by PCR.
TABLE 1.
Bacterial strainsa
| Strain | Relevant genotype | Reference(s) | Mutant group |
|---|---|---|---|
| Strains with mutations in PTS and PG-recycling genes | |||
| DY nagA::FRTcm | nagA::FRTcm | ||
| DY nagA::FRTkan | nagA::FRTkan | ||
| IBPC534 | nagA::tc534 | 25 | |
| HfrH01 | ampG::kan | 10 | |
| TP77 | nagZ::cm | 2 | |
| TB71BK | nagK::FRTcm | 29 | |
| TP71B anmK | anmK::FRTcm | 31 | |
| TJ2 | murQ::FRTkan | 12 | |
| MG1655 murP | murP::FRTkan | 4 | |
| JW0662 | nagE::FRTkan | 1 | |
| DY manXYZ::FRTcm | manXYZ::FRTcm | ||
| IBPC707 | manXYZ::Tn9 | 26 | |
| IBPC590 | ΔnagEBACD::tc | 23, 24 | |
| Strains analyzed | |||
| MC-B1 | MC4100 λRS/nagE-B | B | |
| MC-B11 | nagA::tc534 | 1 | |
| MC-B31 | nagA::FRTkan | 1 | |
| MC-B48 | nagKmurQ anmK ΔnagEBACD::tc | 11 | |
| MC-B51 | nagKmurQ anmKnagA::FRTkan | 3 | |
| MC-B52 | ΔnagEBACD::tc | 10 | |
| MC-B61 | nagA::FRTcm | 1 | |
| MC-B62 | nagKmurQ anmK ampG::kan nagZ::cm | B | |
| MC-B71 | manXYZ::FRTcmnagE::FRTkan | B | |
| MC-B90 | nagE::FRTkannagA::FRTcm | 5 | |
| MC-B120 | nagKmurQ anmK ampG::kan nagZ::cm ΔnagEBACD::tc | 12 | |
| MC-B123 | nagKmurQ anmK nagZ::cm nagA ampG::kan | 4 | |
| MC-B124 | nagKmurQ anmK ampG::kan nagA nagZ::cm | 4 | |
| MC-B125 | manXYZnagE::FRTkan nagA::FRTcm | 5m | |
| MC-B128 | nagE::FRT kannagA::FRTcm | 5 | |
| MC-B134-1P | nagKmurQ anmK manXYZ nagE nagB nagA ampG::kannagZ::cm | 8m | |
| MC-B134-2R | murQanmK manXYZnagEnagB nagA ampG::kan nagZ::cm | 9m | |
| MC-B137 | nagKmurQ anmK manXYZ nagE ampG::kan nagZ::cm | B | |
| MC-B139 | nagKmurQ anmK manXYZ::FRTcmnagA::FRTkan | 3m | |
| MC-B141 | manXYZnagE nagB nagA ampG::kan nagZ::cm | 6m | |
| MC-B144 | nagKmurQ anmK nagE::FRTkannagA::FRTcm | 7 | |
| MC-B157 | ampG::kannagZ::cm nagA::tc534 | 2 | |
| MC-B158 | ampG::kannagA nagZ::cm | 2 | |
| MC-B159 | nagKmurQ anmK nagA::FRTcm | 3 | |
| MC-B160 | nagKmurQ anmK nagE::FRTkannagA::FRTcm | 7 | |
| MC-B162 | nagKmurQ anmK manXYZnagE::FRTkannagA::FRTcm | 7m | |
| MC-B163 | nagKmurQ anmK nagA::tc534 | 3 | |
| MC-B166 | nagKmurQ anmK manXYZ nagE::FRTkannagA::tc534 | 7m | |
| MC-B170 | nagKmurQ anmK nagE nagB nagA manXYZ::FRTcm | 7m | |
| MC-B171 | nagE::FRTkannagA::FRTcm | 5 | |
| MC-B174 | nagA::FRTcm | 1 | |
| MC-B174C | nagA | 1 | |
| MC-B175 | nagE::FRTkannagA::FRTcm | 5 | |
| MC-B175C | nagEnagB nagA | 5 | |
| MC-B177 | nagA::FRTcm | 1m | |
| MC-B178 | nagE::FRTkannagA::FRTcm | 5m | |
| MC-B179 | nagE::FRTkannagA::FRTcm | 5m | |
| MC-B180 | nagAmanXZ::FRTcm | 1m | |
| MC-B181 | nagEnagB nagA manXZ::FRTcm | 5m | |
| MC-B182 | nagAmanXYZ::Tn9 | 1m | |
| MC-B204 | nagEnagB nagA ampG::kan nagZ::cm | 6 | |
| MC-B205 | nagKmurQ anmK nagE nagB nagA nagZ::cm ampG::kan | 8 | |
| MC-B206 | nagKmurQ anmK manXYZ nagE nagB nagA nagZ::cm ampG::kan | 8m | |
| MC-B210 | manXYZnagA ampG::kan nagZ::cm | 2m | |
| MC-B212 | nagAampG::kan nagZ::cm | 2 | |
| MC-B221 | nagKmurQ anmK nagA ampG::kan nagZ::cm | 4 | |
| MC-B222 | nagKmurQ anmK manXYZ nagA ampG::kan nagZ::cm | 4m | |
| MC-B223 | nagEnagB nagA ampG::kan nagZ::cm | 6 | |
| MC-B224 | manXZnagE nagB nagA ampG::kan nagZ::cm | 6m | |
| MC-B225 | nagKmurQ anmKnagA::FRTkanmanXYZ::FRTcm | 3m | |
| MC-B265 | murPnagA::FRTcm | ||
| MC-B270 | murPmanXYZ nagA::FRTcm | ||
| MC-B275 | murPnagE::FRTkannagA::FRTcm | ||
| MC-B280 | murPmanXYZ nagE::FRTkannagA::FRTcm |
All strains whose designation begins with MC-B are derivatives of MC-B1 (MC4100 carrying a nagB-lacZ fusion on a λ lysogen). Strains are divided into mutant groups based on the set of mutations carried (Fig. 2B). The manXYZ derivatives of the mutant groups are indicated by the suffix m. Mutant group B contains the nagA+ strains tested in the experiments whose results are shown in Table 2. The strains in a group can carry the same or different alleles introduced in a different order and/or cured for the antibiotic cassette. Mutations are listed in the order in which they were introduced. FRT indicates that the antibiotic resistance cassette is surrounded by FRT sites. The suffix C in a strain designation indicates that the antibiotic cassette was cured by transformation with pCP20 (5).
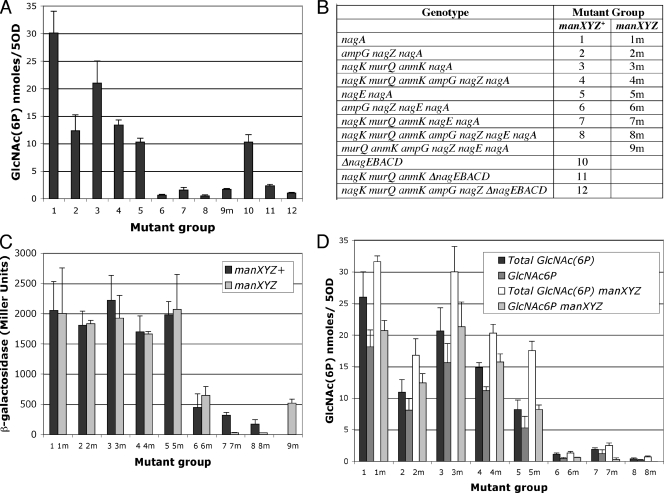
FIG. 2.
Effect of recycling and nagE mutations on levels of GlcNAc plus GlcNAc6P and nagB-lacZ expression. (A) Soluble extracts of strains belonging to the different mutant groups (see panel B) were tested to determine the levels of GlcNAc plus GlcNAc6P by the modified Morgan-Elson method. Values for different strains belonging to the same mutant group are combined, and the results are the means and standard deviations for between 2 to 10 independent cultures. (B) Genotypes of strains in the different mutant groups. (C) β-Galactosidase activities of the nagB-lacZ fusion in strains belonging to mutant groups 1 to 8 and the isogenic manXYZ groups 1m to 9m. The data are the means and standard deviations for 2 to 10 independent cultures. (D) Total GlcNAc reacting material was measured by the Morgan-Elson method in soluble extracts of mutant group 1 to 8 strains and the isogenic manXYZ strains (mutant groups 1m to 8m) before and after treatment of the extracts with NagA (GlcNAc6P deacetylase). The first bar in each set of four bars indicates the total amount of GlcNAc plus GlcNAc6P in the manXYZ+ strain; the second bar indicates the amount of GlcNAc6P in the manXYZ+ strain; the third bar indicates the total amount of GlcNAc plus GlcNAc6P in the manXYZ strain; and the fourth bar indicates the amount of GlcNAc6P in the manXYZ strain. A subset of the extracts tested to obtain the data in panel A were reanalyzed in this test. The data are the means and standard deviations for two to six independent cultures. 5OD, 5 OD650 units.
Bacteria used for β-galactosidase assays and GlcNAc6P measurements were grown aerobically in morpholinepropanesulfonic acid (MOPS) medium with 0.2% glucose and 0.5% Casamino Acids at 37°C. For β-galactosidase assays aliquots were removed throughout the exponential phase, and values are reported for cultures whose absorbance at 650 nm ranged from 0.5 to 1.0.
GlcNAc(6P) measurement.
Bacteria were grown to an optical density at 650 nm (OD650) of about 1.0 and then rapidly chilled in ice. The OD650 was then measured precisely, and the equivalent of 25 OD650 units was harvested by centrifugation, resuspended in 1.5 ml H2O, transferred to an Eppendorf tube, and recentrifuged. The pellet was resuspended in 1.0 ml H2O and placed in a boiling water bath for 5 min to obtain a hot water extract (10, 21). Denatured proteins and debris were removed by centrifugation, and the supernatant was lyophilized. In other experiments soluble metabolites were extracted by the cold methanol procedure (15). The lyophilized sample was dissolved in water. The GlcNAc level was estimated by a modification of the Morgan-Elson procedure basically as described previously (8). Fifty microliters of extract (equivalent to 5 OD650 units of bacteria) was mixed with 75 μl 2 M potassium borate (12.36 g boric acid/100 ml, adjusted to pH 9.2 with KOH), boiled for 3 min, and then cooled to room temperature in water. Then 625 μl of Ehrlich's reagent [1 g 4-(dimethylamino)benzaldehyde acidified with 1.25 ml HCl and dissolved in 100 ml glacial acetic acid] was added and incubated at 37°C for 25 min. The tubes were centrifuged at 4°C to cool them and remove any precipitated material, and the optical density of the purple color was measured at 585 nm. Standard curves were constructed using GlcNAc. Similar values were obtained for GlcNAc6P accumulation by the hot water and cold methanol methods. The background values were lower with the cold methanol method. The Morgan-Elson procedure does not distinguish GlcNAc from GlcNAc6P, and this is indicated in the text by GlcNAc(6P).
To determine the amount of GlcNAc6P in the extracts, aliquots were treated with 3 μg/ml GlcNAc6P deacetylase in 50 mM Tris (pH 8.0), 25 μM ZnCl2 for 30 min at 37°C before the Morgan-Elson procedure was carried out. This amount of deacetylase was sufficient to remove at least an extra 50 nmol of GlcNAc6P added to the extract-containing reaction mixture. In addition to GlcNAc and GlcNAc6P, some other compounds, like MurNAc6P (but not anhMurNAc, GlcNAc-anhMurNAc disaccharide, or UDP-GlcNAc, in which carbon 1 of the amino sugar is not available for chromogen formation [3]), should react with Ehrlich's reagent. High concentrations of some other compounds are also reported to interfere but were not likely to be important in the extracts used in this study since for the wild-type strain grown on Glc the background values are ≤1 nmol/5 OD650 units of bacteria. The material referred to as nonphosphorylated GlcNAc included any (potential) cross-reacting compounds.
RESULTS
Mutations in the PG-recycling pathway reduce but do not eliminate GlcNAc6P accumulation in strains carrying nagA mutations.
The GlcNAc levels in the soluble extracts of glucose-grown strains carrying a nagA mutation were measured and were found to be about 30 nmol per 5 OD650 units of bacteria (Fig. 2A, group 1). Assuming that 1 OD650 unit of E. coli is about 109 cells (18) and that the volume of one E. coli bacterium is about 10−12 ml (9, 13), this means that the intracellular concentration of accumulated GlcNAc6P was about 5 mM. White (33) estimated that there was a similar concentration in his nagA mutant. The Morgan-Elson reaction does not distinguish between the free sugar and the 6-phosphorylated form. As shown below, most of GlcNAc measured in the extracts was in the 6-phosphorylated form. Where free GlcNAc is not distinguished from the phosphorylated form, the term GlcNAc(6P) is used. All the strains used carry a nagB-lacZ fusion on a λ lysogen, which is repressed by the GlcNAc6P-sensitive NagC repressor. The nagA mutation causes the nagB-lacZ fusion to be fully derepressed (Fig. 2C, group 1) (25).
The nagA mutation was introduced into strains carrying mutations in genes involved in PG recycling, including ampG (GlcNAc-anhMurNAc peptide permease), nagZ (β-N-acetylglucosaminidase), anmK (anhMurNAc kinase), murQ (MurNAc6P etherase), and nagK (GlcNAc kinase) (Table 1 and Fig. 2B). For convenience, these mutations are considered in the following two classes: mutations in ampG and nagZ, which are responsible for production of nonphosphorylated GlcNAc and anhMurNAc; and mutations in anmK, murQ, and nagK, whose products convert anhMurNAc and GlcNAc to GlcNAc6P. The mutant strains are divided into groups according to the sets of mutations that they carry, which are listed in Fig. 2B. For example, group 3 strains carry mutations in anmK, murQ, and nagK, as well as in nagA. Several strains carrying similar sets of mutations were tested. The exact genotypes of the different strains are shown in Table 1.
Mutations either in nagZ and ampG or in nagK, anmK, and murQ were found to reduce the level of GlcNAc(6P) by 40 to 60% (Fig. 2A, mutant groups 2 and 3). Moreover, there was no additive effect when the five mutations were combined in the same strain (group 4), which is consistent with the hypothesis that they affect the same pathway. Thus, despite the loss of the five genes of the dedicated recycling pathway for amino sugars, high levels of GlcNAc(6P) still accumulated in a strain lacking the nagA-encoded deacetylase.
Mutations in nagE reduce GlcNAc(6P) levels in strains carrying nagA mutations.
Another enzyme known to produce GlcNAc6P is the nagE-encoded PTS transporter for GlcNAc (19). Introduction of the nagE mutation together with the nagA mutation (mutant group 5) resulted in an approximately 60% decrease in GlcNAc(6P) levels (Fig. 2A, compare group 5 with group 1). Similarly, a strain carrying a deletion of the entire nag operon (ΔnagEBACD::tc), including nagE and nagA, also accumulated much less GlcNAc(6P) than a strains with the simple nagA mutation (Fig. 2A, group 10).
Mutations in the recycling pathway together with a nagE mutation reduce the GlcNAc6P levels to basal levels.
In strains carrying ampG and nagZ mutations and/or anmK, murQ, and nagK mutations of the recycling pathway together with nagE and nagA mutations, the levels of GlcNAc(6P) detectable in the soluble extracts were low (Fig. 2A, mutant groups 6, 7, and 8). The values obtained were similar to the values obtained for the wild-type strain growing on glucose (about 1 nmol/5 OD650 units of bacteria), showing that together the two pathways encoded by ampG, nagZ, anmK, murQ, nagK, and nagE were responsible for the accumulation of all the GlcNAc(6P) in nagA strains. Similarly, introducing the anmK, murQ, and nagK mutations or the five recycling mutations into the ΔnagEBACD strain also resulted in levels of GlcNAc(6P) near the background level (Fig. 2A, mutant groups 11 and 12).
ManXYZ contributes to the GlcNAc6P pool.
Determining the level of expression of the nagB-lacZ fusion is an alternative method for monitoring the levels of GlcNAc6P in the cell since nagB is repressed by the GlcNAc6P-sensitive repressor NagC. In all of the strains in which only the recycling pathway or the nagE PTS pathway was eliminated the nagB-lacZ fusion was still fully induced (Fig. 2C, mutant groups 1 to 5), even though the GlcNAc(6P) level dropped about 50%. However, for the strains carrying mutations in both pathways lower levels of nagB-lacZ activity were measured (Fig. 2C, mutant groups 6 to 8). In the presence of the five recycling mutations and the nagE mutation the β-galactosidase activity was about 150 U (Fig. 2C, mutant group 8), but this value is still higher than the value obtained for the wild-type nagA+ strain (MC-B1) growing on glucose (38 U) (Table 2). The manXYZ operon encodes another PTS capable of transporting and phosphorylating several hexoses, including GlcNAc. Introduction of a manXYZ deletion reduced the β-galactosidase activity to about 30 U in strains carrying mutations in nagK, murQ, anmK, and nagE, showing that ManXYZ is also involved in the conversion of amino sugars derived from the PG to GlcNAc6P (Fig. 2C, mutant groups 7m and 8m). Interestingly, the presence of a nagK+ allele (introduced by cotransduction with the nagZ mutation [see Materials and Methods]) increased the GlcNAc(6P) level slightly (Fig. 2A, group 9m) and increased nagB-lacZ β-galactosidase activities from 30 U to about 500 U (Fig. 2C, group 9m). As this strain has the ampG nagZ genotype (as well as nagE and manXYZ), this observation implies that there is some mechanism that generates intracellular GlcNAc, the substrate of NagK, other than the AmpG-NagZ pair.
TABLE 2.
Effect of PG recycling on the basal level of nagB expressiona
| Strain | Genotype | β-Galactosidase activity (Miller units) (mean ± SD) |
|---|---|---|
| MC-B1 | Wild type | 38 ± 3 |
| MC-B62 | ampGnagZ anmK murQ nagK | 33 ± 2 |
| MC-B71 | nagEmanXYZ | 34 ± 4 |
| MC-B137 | anmKmurQ nagK ampG nagZ nagE manXYZ | 33 ± 2 |
MC-B1 carrying mutations in either the dedicated recycling pathway, the PTS pathway, or both pathways were grown in minimal glucose medium at 37°C, and the β-galactosidase activities were determined.
Further roles of ManXYZ.
A manXYZ mutation was introduced into strains belonging to each of the other groups of PG-recycling and nagE mutants, resulting in mutant groups 1m to 8m (Fig. 2B). GlcNAc(6P) accumulation and nagB-lacZ activity were measured. As described above, in the presence of the other mutations preventing GlcNAc phosphorylation (nagK, murQ, anmK, and nagE; mutant groups 7 and 8), the manXYZ mutation reduced nagB-lacZ activity to background levels (Fig. 2C, groups 7m and 8m), while in other strains it had no significant effect on nagB-lacZ expression (Fig. 2C, groups 1m to 6m).
However, introduction of the manXYZ mutation had a paradoxical effect: it increased the GlcNAc(6P) levels measured chemically (Fig. 2D, compare the first and third bars in each section of the histogram). It should be recalled that the chemical assay for GlcNAc does not distinguish between GlcNAc and GlcNAc6P. To try to understand the effect of the manXYZ mutation on increasing GlcNAc(6P) reacting material, I investigated whether the GlcNAc reacting material in the extracts was GlcNAc6P. The GlcNAc levels in the extracts were measured after treatment with GlcNAc6P deacetylase (the NagA gene product) to convert GlcNAc6P to GlcN6P. The amounts of GlcNAc6P were estimated by determining the difference between the GlcNAc reacting material before treatment with NagA and the GlcNAc reacting material after treatment with NagA (Fig. 2D, compare the second and fourth bars in each section of the histogram). The amount of GlcNAc6P varied in the different strains, but it was mostly in the range from 60 to 75% of the total GlcNAc reacting material. The manXYZ mutation caused the level of GlcNAc6P to increase, but not enough to account for all of the increase in the GlcNAc(6P) level detected chemically, implying that the manXYZ mutation increased the levels of both phosphorylated and nonphosphorylated GlcNAc.
Mutations in the recycling and PTS pathways and the basal level of expression of nagB.
The fact that the combination of PTS and recycling mutations prevented accumulation of GlcNAc6P in nagA strains raised the question of whether these mutations also affected the GlcNAc6P levels in wild-type (nagA+) strains and hence the level of repression of NagC-controlled genes in the absence of exogenous GlcNAc. Combinations of recycling pathway and PTS mutations were tested to determine if there was any effect on nagB-lacZ expression in the nagA+ background (Table 2). The presence of recycling and PTS mutations resulted in only a very small (maximum, 15%) decrease in nagB-lacZ β-galactosidase activity, suggesting that the pool of GlcNAc6P from PG recycling does not strongly affect NagC binding to its operator sites.
DISCUSSION
Role of the PTS in PG recycling.
According to the most recent model for PG recycling (22), lytic transglycosylases produce anhydro-muropeptides in the periplasm. Anhydro-muropeptides and anhydro-disaccharides are transported into the cell by AmpG. As mutations in the ampG and nagZ genes reduced the amount of GlcNAc(6P) that accumulated in a nagA strain only by about one-half, GlcNAc must be able to enter the cytoplasm by an alternative route. The results presented here show that the NagE PTS transporter is also responsible for at least one-half of the GlcNAc(6P) that accumulates in a nagA strain. As mutations in nagE, together with mutations in the dedicated recycling pathway, reduced GlcNAc(6P) levels to values near the background value, this shows that the two pathways are jointly responsible for the majority of the recycling of PG. The generic hexose PTS transporter encoded by manXYZ contributes slightly to the level of GlcNAc6P since a mutation in this operon was necessary to really reduce GlcNAc6P levels to the background level, as measured by the nagB-lacZ reporter. The nagB-lacZ fusion is thus a sensitive method for measuring low levels of GlcNAc6P, which are not distinguishable by the chemical assay.
The only known sugar substrate for NagE is GlcNAc. This implies that some other enzyme is capable of cleaving the anhydro-disaccharide formed in the periplasm by the lytic transglycosylases to produce GlcNAc, the substrate of the NagE PTS, and anhMurNAc. However no periplasmic glucosaminidase has been described for E. coli. It is interesting that the Bacillus subtilis homologue of nagZ (ybbD) has a signal sequence and is predicted to be anchored to the periplasmic side of the membrane (22).
The two pathways, the dedicated recycling pathway encoded by ampG, nagZ, nagK, murQ, and anmK and the PTS encoded by nagE and manXYZ, function completely independently, since mutations that eliminate one pathway reduce GlcNAc(6P) accumulation by about 50%. This seems to eliminate the possibility that the NagE-dependent recycling pathway involves NagE phosphorylating free GlcNAc produced by AmpG and NagZ in the cytoplasm. Phosphorylation of cytoplasmic sugars has been described for some PTS transporters (27), and NagE, in reconstituted vesicles, is capable of nonvectorial phosphorylation (19).
Treating the soluble extracts with GlcNAc6P deacetylase removed 60 to 75% of the chemically detectable GlcNAc, showing that not all the accumulated material in the nagA strains is GlcNAc6P and that the remaining 25 to 40% is probably free GlcNAc. In the nagA single mutant (group 1) the nonphosphorylated GlcNAc accounts for about 30% (7.8 nmol) of the total GlcNAc reacting material (Fig. 2D, group 1). In the nagA strains carrying nagK, murQ, and anmK mutations, which should prevent the phosphorylation of intracellular GlcNAc and anhMurNAc (group 3), the amount of nonphosphorylated GlcNAc (5.4 nmol) was not greater than in group 1 (Fig. 2D), implying that that there is some other route for converting intracellular GlcNAc into GlcNAc6P or that the free GlcNAc is exported to the medium. NagE is a possible alternative route for phosphorylating GlcNAc since it was shown to produce nonvectorial phosphorylation of GlcNAc (19). The ampG and nagZ mutations (groups 2 and 4), which should prevent the formation of nonphosphorylated GlcNAc in the cytoplasm, reduced the amount of the GlcNAc reacting material measured after digestion with deacetylase (to about 3 nmol) but did not eliminate it (Fig. 2D, groups 2 and 4), implying that there is an alternative route for production of intracellular GlcNAc. Similarly, the increase in nagB-lacZ expression due to the nagK+ allele in group 9m (murQ anmK ampG nagZ nagE nagA strain) (Fig. 2C) also implies that there is a source of phosphorylatable cytoplasmic GlcNAc other than AmpG and NagZ. In theory, the GlcNAc reaction after digestion with GlcNAc6P deacetylase could be due to MurNAc6P (but not anhMurNAc). Uehara et al. (30) obtained evidence that MurP transports anhMurNAc into the cytoplasm in a nonphosphorylated form because the 1,6-anhydro ring prevents phosphorylation. This would allow anhMurNAc produced by the postulated periplasmic glucosaminidase to be transported into the cytoplasm by MurP. In a anmK+ strain (group 2), anhMurNAc should be converted to MurNAc6P, but this cannot account for the GlcNAc reacting material in group 4 anmK murQ mutants. Any major role for the murP-encoded transporter in PG recycling seems to be ruled out by the observation that strains carrying a murP mutation, with or without nagE and manXYZ mutations (strains MC-B265, MC-B270, MC-B275, and MC-B280 [Table 1]), behaved just like the equivalent murP+ strains (data not shown).
Perhaps the most surprising observation was the systematic increase in the amount of GlcNAc(6P) reacting material produced by a manXYZ mutation in otherwise isogenic strains. This was true for all the groups of mutations tested (Fig. 2D). The simplest interpretation of this observation is that the ManXYZ transporter is involved in reducing the concentration of intracellular GlcNAc(6P) and could secrete GlcNAc and/or GlcNAc6P. Alternatively, the manXYZ mutation stimulates the turnover of PG. If ManXYZ behaves as an efflux pump, it is not obvious whether it is the phosphorylated or nonphosphorylated form that is secreted. The increase in the amount of GlcNAc(6P) occurs in strains completely missing the dedicated PG-recycling enzymes encoded by ampG, nagZ, nagK, murQ, and anmK (group 4), where the majority of the recycled GlcNAc(6P) is due to NagE and so should be in the phosphorylated form, and in the nagE mutant (group 5), where both GlcNAc and GlcNAc6P can be formed. The effect of the manXYZ mutation is most dramatic in ampG+ nagZ+ strains, which might suggest that GlcNAc is the preferred secreted substrate (Fig. 2D, mutant groups 3 and 5). If we assume that the same amount of GlcNAc and anhMurNAc was transported into the cytoplasm by AmpG and NagZ in group 7 mutants (nagK murQ anmK nagE) as in group 5 mutants (nagE nagK+ murQ+ anmK+), then the absence of phosphorylation by NagK and AnmK significantly reduced the intracellular concentration of GlcNAc(6P) (Fig. 2A and D, compare groups 5 and 7), presumably due to efflux of the nonphosphorylated sugars. Uehara et al. showed that neither GlcNAc nor anhMurNAc was retained in the cytoplasm of nagK or anmK mutants, suggesting that there are efflux pumps for both GlcNAc and anhMurNAc (29, 31). Thus, both GlcNAc and anhMurNAc are candidates for the secreted sugar in a ManXYZ-dependent mechanism.
Interestingly, the effect of the manXYZ mutation was apparent even in strains in which both the dedicated recycling and PTS routes are mutated (groups 6 to 8), although the GlcNAc(6P) values were very low and near the background levels (Fig. 2D, groups 6 to 8). The introduction of a manXYZ mutation into group 7, which has the nagK murQ anmK nagE ampG+ nagZ+ genotype, increased the amount of GlcNAc reacting material slightly from 1.9 nmol to 2.5 nmol/5 OD650 units. More significantly, in the manXYZ strain the GlcNAc is almost all nonphosphorylated GlcNAc, as shown by chemical measurements (Fig. 2D, group 7m) and confirmed by the activity of the nagB-lacZ fusion, which falls from 300 U to 30 U (Fig. 2C, compare groups 7 and 7m). This shows that nagK, murQ, anmK, nagE, and manXYZ code for the only enzymes capable of generating phosphorylated GlcNAc. Mutating manXYZ eliminated the residual GlcNAc6P and simultaneously increased the intracellular GlcNAc level. Thus, although the work described here seems to have revealed the existence of additional pathways implicated in amino sugar transport across the cytoplasmic membrane, the two PTS transporters (NagE and ManXYZ) and the sugar kinases (NagK and indirectly AnmK) are the only enzymes able to generate GlcNAc6P.
Sugar excretion (called inducer expulsion) is a phenomenon described for certain gram-positive bacterial PTS and is thought to involve a sugar phosphatase, but the secretion mechanism has not been identified (6). Some sugar efflux pumps have been described for E. coli (e.g., the Set family [14]), and various phosphatases, including alkaline phosphatase, have been described for the periplasm, which could generate free sugar as a substrate for the NagE PTS transporter. The relationship of these systems to the manXYZ mutant effect on the recycling of PG should be addressed.
Acknowledgments
I am very grateful to Ted Park, Christoph Mayer, and Philippe Bouloc for the gift of strains, to José Souza and Mario Calcagno for the gift of purified GlcNAc6P deacetylase and for advice on use of this enzyme, and to Ted Park for helpful discussions and comments on the manuscript. I thank Carole Pennetier for technical assistance in the early stages of this work.
This work was supported by grants from the CNRS and Université Paris 7-Denis Diderot.
Footnotes
Published ahead of print on 17 July 2009.
REFERENCES
- 1.Baba, T., Y. Ara, M. Hasegawa, Y. Takai, Y. Okurmura, M. Baba, K. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in frame, single-gene knockout mutations: the Keio collection. Mol. Syst. Biol. 22006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng, Q., X. Li, K. Merdek, and J. T. Park. 2000. Molecular characterization of the β-N-acetylglucosaminidase of Escherichia coli and its role in cell wall recycling. J. Bacteriol. 1824836-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cifonelli, J., and A. Dorfman. 1958. A colorimetric method for determination of linkage in hexosamine-containing compounds. J. Biol. Chem. 23111-18. [PubMed] [Google Scholar]
- 4.Dahl, U., T. Jaeger, B. T. Nguyen, J. M. Sattler, and C. Mayer. 2004. Identification of a phosphotransferase system of Escherichia coli required for growth on N-acetylmuramic acid. J. Bacteriol. 1862385-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One step inactivation of chromosomal genes in Escherichia coli K12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutscher, J., C. Francke, and P. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand, P., B. Golinelli-Pimpaneau, S. Mouilleron, B. Badet, and M.-A. Badet-Denisot. 2008. Highlights of glucosamine-6P synthase catalysis. Arch. Biochem. Biophys. 474302-317. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh, S., H. Blumenthal, E. Davidson, and S. Roseman. 1960. Glucosamine metabolism. V. Enzymatic synthesis of glucosamine 6-phosphate. J. Biol. Chem. 2351265-1273. [PubMed] [Google Scholar]
- 9.Hedal, M., S. Norland, and B. Riemann. 1994. Determination of bacterial cell number and cell volume by means of flow cytometry, transmission electron microscopy and epifluorescence microscopy. J. Microbiol. Methods 20255-263. [Google Scholar]
- 10.Jacobs, C., L.-J. Huang, E. Bartowsky, S. Normark, and J. T. Park. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 134684-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs, C., B. Joris, M. Jamin, K. Klarsov, J. Van-Beeumen, D. Mengin-Lecreulx, J. van Heijenoort, J. T. Park, S. Normark, and J.-M. Frère. 1995. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-l-alanine amidase. Mol. Microbiol. 15553-559. [DOI] [PubMed] [Google Scholar]
- 12.Jaeger, T., M. Arsic, and C. Meyer. 2005. Scission of the lactyl ether bond of N-acetylmuramic acid by Escherichia coli etherase. J. Biol. Chem. 28030100-30106. [DOI] [PubMed] [Google Scholar]
- 13.Kubitschek, H., and J. Friske. 1986. Determination of bacterial cell volume with the Coulter counter. J. Bacteriol. 1681466-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, J., P. Miller, J. Willard, and E. Olson. 1999. Functional and biochemical characterization of Escherichia coli sugar efflux transporters. J. Biol. Chem. 27422977-22984. [DOI] [PubMed] [Google Scholar]
- 15.Maharjan, R., and T. Ferenci. 2003. Global metabolite analysis: the influence of extraction methodology on metabolome profiles of Escherichia coli. Anal. Biochem. 313145-154. [DOI] [PubMed] [Google Scholar]
- 16.Mengin-Lecreulx, D., and J. van Heijenoort. 1996. Characterization of the essential gene glmM encoding phosphoglucosamine mutase in Escherichia coli. J. Biol. Chem. 27132-39. [DOI] [PubMed] [Google Scholar]
- 17.Mengin-Lecreulx, D., and J. van Heijenoort. 1994. Copurification of glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase activities of Escherichia coli: characterization of the glmU gene product as a bifunctional enzyme catalyzing two subsequent steps in the pathway for UDP-N-acetylglucosamine synthesis. J. Bacteriol. 1765788-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 19.Mukhija, S., and B. Erni. 1996. Purification by Ni2+ affinity chromatography and functional reconstitution of the transporter for N-acetylglucosamine of Escherichia coli. J. Biol. Chem. 27114819-14824. [DOI] [PubMed] [Google Scholar]
- 20.Park, J. T. 1996. The convergence of murein recycling research with β-lactamase research. Microb. Drug Resist. 2105-112. [DOI] [PubMed] [Google Scholar]
- 21.Park, J. T. 2001. Identification of a dedicated recycling pathway for anhydro-N-acetylmuramic acid and N-acetylglucosamine derived from Escherichia coli cell wall murein. J. Bacteriol. 1833842-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, J. T., and T. Uehara. 2008. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol. Mol. Biol. Rev. 72211-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plumbridge, J. 1992. A dominant mutation in the gene for the Nag repressor of Escherichia coli that renders the nag regulon uninducible. J. Gen. Microbiol. 1381011-1017. [DOI] [PubMed] [Google Scholar]
- 24.Plumbridge, J. 1996. How to achieve constitutive expression of a gene within an inducible operon: the example of the nagC gene of Escherichia coli. J. Bacteriol. 1782629-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plumbridge, J. 1991. Repression and induction of the nag regulon of Escherichia coli K12: the roles of nagC and nagA in maintenance of the uninduced state. Mol. Microbiol. 52053-2062. [DOI] [PubMed] [Google Scholar]
- 26.Plumbridge, J., and E. Vimr. 1999. Convergent pathways for utilization of the amino sugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J. Bacteriol. 18147-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson, J., and B. Chassy. 1985. Intracellular phosphorylation of glucose analogues via the phosphoenolpyruvate:mannose-phosphotransferase system in Streptococcus lactis. J. Bacteriol. 162224-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uehara, T., and J. T. Park. 2007. An anhydro-N-acetylmuramyl-l-alanine amidase with broad specificity tethered to the outer membrane of Escherichia coli. J. Bacteriol. 1895634-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uehara, T., and J. T. Park. 2004. The N-acetyl-d-glucosamine kinase of Escherichia coli and its role in murein recycling. J. Bacteriol. 1867273-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uehara, T., K. Suefuji, T. Jaeger, C. Mayer, and J. T. Park. 2006. MurQ etherase is required by Escherichia coli in order to metabolize anhydro-N-acetylmuramic acid obtained either from the environment or from its own cell wall. J. Bacteriol. 1881660-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uehara, T., K. Suefuji, N. Valbuena, B. Meehan, M. Donegan, and J. T. Park. 2005. Recycling of the anhydro-N-acetylmuramic acid derived from cell wall murein involves a two step conversion to N-acetylglucosamine-phosphate. J. Bacteriol. 1873643-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vötsch, W., and M. Templin. 2000. Characterization of a β-N-acetylglucosamindase of Escherichia coli and elucidation of its role in muropeptide recycling and β-lactamase induction. J. Biol. Chem. 27539032-39038. [DOI] [PubMed] [Google Scholar]
- 33.White, R. J. 1968. Control of aminosugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem. J. 106847-858. [DOI] [PMC free article] [PubMed] [Google Scholar]