Abstract
Toxoplasma gondii modulates pro- and anti-inflammatory responses to regulate parasite multiplication and host survival. Pressure from the immune response causes the conversion of tachyzoites into slowly dividing bradyzoites. The regulatory mechanisms involved in this switch are poorly understood. The aim of this study was to investigate the immunomodulatory role of T. gondii cyclophilin 18 (TgCyp18) in macrophages and the consequences of the cellular responses on the conversion machinery. Recombinant TgCyp18 induced the production of nitric oxide (NO), interleukin-12 (IL-12), and tumor necrosis factor alpha through its binding with cysteine-cysteine chemokine receptor 5 (CCR5) and the production of gamma interferon and IL-6 in a CCR5-independent manner. Interestingly, the treatment of macrophages with TgCyp18 resulted in the inhibition of parasite growth and an enhancement of the conversion into bradyzoites via NO in a CCR5-dependent manner. In conclusion, T. gondii possesses sophisticated mechanisms to manipulate host cell responses in a TgCyp18-mediated process.
Toxoplasma gondii is a ubiquitous protozoan parasite that is able to infect a broad range of warm-blooded animals, including humans (17, 38). Fortunately, T. gondii is a well-adapted parasite which generally causes very little disease unless the host's immune system is compromised in situations such as AIDS (41). Toxoplasma gondii affects pro- and anti-inflammatory host cell signaling in such a way as to maximize parasite multiplication and spread while maintaining host survival (14). One aspect of this manipulation is the upregulation of the interleukin-12 (IL-12)-dependent production of gamma interferon (IFN-γ), which is critical to host survival of acute toxoplasmosis (7, 8, 26, 28). This effect appears to occur by a pathway unique to T. gondii and involves the triggering of cysteine-cysteine chemokine receptor 5 (CCR5) in dendritic cells (DC) and macrophages by secreted T. gondii cyclophilin 18 (TgCyp18) (2). High et al. previously isolated genes encoding two Toxoplasma gondii cyclophilins, TgCyp18 and TgCyp20 (27). In T. gondii, the isolation of cyclosporine-binding proteins on affinity columns yielded only the cyclophilins TgCyp18 and TgCyp20 (27). Both cyclophilins were highly similar to human cyclophilin (hCyp18) in the central core region, but TgCyp20 differed in a 7-amino-acid “insertion” in the same region as that in Plasmodium falciparum cyclophilins (27).
TgCyp18, but not hCyp18 or P. falciparum cyclophilin 19A (PfCyp19A), appears to induce IL-12 production by interacting directly with CCR5, an effect that was blocked by the addition of cyclosporine (2, 4, 63). These observations implied that structural determinants of TgCyp18, related to cyclosporine binding, were responsible for the induction of IL-12 synthesis (4, 63). This idea was confirmed by modeling of the TgCyp18 structure on that of PfCyp19A and site-directed mutagenesis of putatively surface-exposed residues that were absent in PfCyp19A (63). Two of the TgCyp18 mutants, namely, 17GEH19 to 17AAA19 and 149RP150 to 149YV150, located in the N and C termini of the protein, respectively, had reduced interactions with CCR5 and reduced IL-12 induction (63). Moreover, TgCyp18 peptidyl-prolyl cis-trans isomerase (PPIase) activity was not required for its interaction with CCR5, but IL-12 induction by TgCyp18 required both CCR5 binding and PPIase enzymatic activities (63). TgCyp18 appears to act as a structural mimic of CCR5-binding ligands, albeit one with no sequence similarity to the known host ligands, macrophage inflammatory protein 1α/chemokine (C-C motif) ligand 3 (CCL3), macrophage inflammatory protein 1β/CCL4, regulated on activation normal T-cell expressed and secreted (RANTES)/CCL5, or monocyte chemotactic protein 2/CCL8, for this receptor (4, 63). There is also evidence that the closely related protozoan Neospora caninum cyclophilin plays a role in stimulating IFN-γ production by bovine peripheral blood mononuclear cells and N. caninum-specific CD4+ T cells (59). This effect is also blocked by cyclosporine (59). IFN-γ production induced by N. caninum tachyzoites is thought to be critical in controlling the acute phase of neosporosis (59).
Pressure from the immune response causes tachyzoites to differentiate into slowly multiplying bradyzoites, which form cysts within muscle and brain cells (19). The cysts are protected from the host immune response and establish a life-long chronic infection (18). If the tissue cysts are ingested, for example, through the consumption of undercooked meat, bradyzoites are released into the gut, invade epithelial cells, and differentiate into tachyzoites, initiating a new asexual cycle (10). In vitro models of tachyzoite-to-bradyzoite differentiation have been established by using a variety of stress conditions that mimic the stresses of the host immune response. These conditions include treatment with IFN-γ (5), mitochondrial inhibitors (6), alkaline pH (pH 8.1) (53), and high temperature (54). The stress response is controlled in part by eukaryotic initiation factor 2 kinase in the parasite, which is well characterized as a stress response in eukaryotic cells (55), and differentiation also involves parasite-derived cyclic nucleotide kinases (20). Large-scale sequencing of stage-specific cDNA (36, 39), microarray studies (10), and serial analysis of gene expression tags (44) revealed that stage conversion involves changes in the levels of expression of a large number of genes, although the regulatory mechanism(s) involved in the conversion is poorly understood.
Although previous reports mentioned the ability of TgCyp18 to induce the production of the IL-12 (1, 2, 14, 63), the productions of other cytokines were not elucidated. Here we show that TgCyp18 induces the production of other cytokines and nitric oxide (NO) and enhances the bradyzoite conversion of T. gondii.
MATERIALS AND METHODS
Parasite and cell cultures.
T. gondii (PLK strain) tachyzoites were maintained on monkey kidney adherent fibroblasts (Vero cells) cultured in Eagle's minimum essential medium (Sigma, St. Louis, MO) supplemented with 8% heat-inactivated fetal bovine serum. For the purification of tachyzoites, parasites and host cell debris were washed in cold phosphate-buffered saline (PBS), and the final pellet was resuspended in cold PBS and passed through a 27-gauge needle and a 5.0-μm-pore-size filter (Millipore, Bedford, MA).
Animals.
C57BL/6J female mice (B6 mice), 6 to 8 weeks of age, and a female Japanese white rabbit were obtained from Clea Japan (Tokyo, Japan). CCR5 knockout (CCR5−/−) mice (B6.129P2-Ccr5tm1Kuz/J, stock no. 005427) were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed under specific-pathogen-free conditions in the animal facility of the National Research Center for Protozoan Diseases at the Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan. Animals used in this study were treated and used according to the Guiding Principles for the Care and Use of Research Animals of the Obihiro University of Agriculture and Veterinary Medicine.
Reagents.
Lipopolysaccharide (LPS) from Escherichia coli (10-μg/ml stock solution in RPMI 1640 medium), cyclosporine from Tolypocladium inflatum (1 mM stock solution in 0.01% ethanol-RPMI 1640 medium), and NG-monomethyl-l-arginine acetate (l-NMMA) (100 mM stock solution in Dulbecco's modified Eagle's medium) were obtained from Sigma.
Construction and expression of recombinant TgCyp18 and recombinant MTgCyp18.
A cDNA was synthesized from RNA isolated with Tri reagent (Sigma) using a SuperScript first-strand synthesis system for reverse transcription-PCR (Invitrogen, Carlsbad, CA). The cDNA was used as a template to amplify the coding region of wild-type TgCyp18. The DNA and amino acid sequences of TgCyp18 are stored in the GenBank database under accession number U04633.1. To clone wild-type recombinant TgCyp18 without the signal peptide (amino acids 1 to 17), consisting of 163 amino acids, one set of oligonucleotide primers that included a BamHI restriction enzyme site (boldface type) and a methionine start codon in the forward primer (5′-CTG GAT CCA TGG AAA ATG CCG GAG TCA GAA AG-3′) and an EcoRI site (boldface type) in the reverse primer (5′-GCG AAT TCT TAC TCC AAC AAA CCA ATG TC-3′) was designed. The PCR products were digested with BamHI and EcoRI and then ligated into the glutathione S-transferase (GST) fusion protein in Escherichia coli expression vector pGEX-4T1 (GE Healthcare, Buckinghamshire, England), which had been digested with the same set of restriction enzymes (pGEX-TgCyp18). To generate the indicated mutant TgCyp18 (MTgCyp18), the following primers were used: the forward primer contained an EcoRV restriction site (boldface type) (5′-CAT GGA TAT CGA CAT CGA CGC AGC AGC TGC CGG GCG CAT TAT CTT-3′) (17GEH19 to 17AAA19), and the reverse primer contained a NruI restriction site (boldface type) (5′-TCC GTG ATT TTC GCG ACC TTA GAC ACG TAG CCG CCG CTG CCG CCG-3′) (149RP150 to 149YV150). The PCR products were digested with EcoRV and NruI and then ligated into EcoRV- and NruI-treated pGEX-TgCyp18 (pGEX-MTgCyp18). The nucleotide sequences of the plasmid were analyzed with a model ABI 3100 DNA sequencer (Applied Biosystems, Foster City, CA). Wild-type and MTgCyp18s were expressed as GST fusion proteins in E. coli DH5α cells (Takara, Bio, Inc., Japan). The GST tags of the recombinant proteins were removed with thrombin protease (GE Healthcare) according to the manufacturer's instructions. Proteins were dialyzed in PBS and purified with Detoxi-Gel endotoxin-removing gel (Pierce Biotechnology Inc., Rockford, IL) to remove endotoxins. For cell culture use, the proteins were filtered using a 0.45-μm low-protein-binding Supor membrane (Pall Life Sciences, Ann Arbor, MI). The purity and quantity of the proteins were detected as a single band by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) followed by Coomassie brilliant blue R250 staining (MP Biomedicals Inc., France). The concentration was measured using a BCA protein assay kit (Pierce Biotechnology Inc.).
Production of anti-TgCyp18 serum and purification of IgG.
Three hundred micrograms of recombinant TgCyp18 in Freund's complete adjuvant (Sigma) was intradermally injected into a female Japanese white rabbit. The same antigen in Freund's incomplete adjuvant (Sigma) was intradermally injected into the rabbit on days 14, 28, and 42. Serum from the immunized rabbit was collected 7 days after the last immunization. Immunoglobulin G (IgG) was purified from 2 ml of rabbit sera through protein A chromatography columns according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA). The fractions containing IgG were pooled and resolved by SDS-PAGE to test purity and quantity.
Monolayer cultures of peritoneal macrophages.
Mouse peritoneal macrophages were collected from B6 and CCR5−/− mice 4 days after intraperitoneal injection of 1 ml of 4.05% Brewer modified BBL thioglycolate medium (Becton Dickinson, Sparks, MD) by peritoneal washing with 5 ml of cold PBS. After harvesting, the cells were centrifuged at 800 × g for 10 min and suspended in RPMI 1640 medium containing 10% fetal bovine serum. The macrophage suspension was then added to 24-well tissue culture microplates at 1 × 106 cells/well. The suspensions were incubated at 37°C for 3 h, washed thoroughly to remove nonadherent cells, and further incubated at 37°C. The purities of the peritoneal macrophage cultures were quantified by flow cytometry using anti-CD11b and anti-CD3 monoclonal antibodies. The percentages of CD11b+ cells were 99.4% ± 0.17% at 0 h and 98.6% ± 1.01% at 24 h (n = 3). Moreover, the percentages of CD3+ cells were 0.02% ± 0% at 0 h and 0.04% ± 0.06% at 24 h. These results indicate the high purity of the peritoneal macrophages used in this study.
Cytokine enzyme-linked immunosorbent assay.
Macrophage culture supernatants were collected for measurements of IL-12p40, IL-6, tumor necrosis factor alpha (TNF-α), and IFN-γ levels by an enzyme-linked immunosorbent assay (Pierce Biotechnology Inc.) according to the manufacturer's recommendations. Cytokine concentrations were calculated using standard cytokine curves run on the same plates.
Measurement of NO.
Supernatants from peritoneal macrophages cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum were collected for analysis of NO levels. Levels of nitrate and nitrite production in the culture medium were measured using a nitrite/nitrate assay kit (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturer's recommendations. Nitrite and nitrate levels were calculated with a standard absorbance curve run on the same plate.
Examination of parasite growth.
Measurement of [3H]uracil uptake in the tachyzoites was performed because [3H]uracil serves as a parasite-specific metabolic label for measuring viability, as previously described (42). In order to study the role of TgCyp18 during primary and secondary infections, pre- and post-TgCyp18 treatment experiments were designed. In the posttreatment experiment mimicking a primary infection, peritoneal macrophages were infected with 2 × 105 parasites for 12 h before the culture was incubated for 24 h with TgCyp18, MTgCyp18, GST as a negative control, or LPS as a positive control. In the pretreatment experiment mimicking a secondary infection, peritoneal macrophages were pretreated with TgCyp18, MTgCyp18, GST, or LPS for 12 h, and the culture was then infected with 2 × 105 parasites. After incubation for 24 h at 37°C, [5,6-3H] uracil (Moravek Biochemicals, Brea, CA) was added to the plate at 1 μCi/well, and the cell mixtures were further incubated for 2 h at 37°C. After fixation with 10% trichloroacetic acid, the cell mixtures were incubated with 0.2 N NaOH for 30 min at 37°C. The radioactivity incorporated into the parasites was measured using a beta counter.
Effects of l-NMMA on parasite growth in vitro.
Peritoneal macrophages (1 × 106 macrophages) in a 24-well culture plate were incubated with recombinant proteins or LPS for 12 h and then cultured with T. gondii tachyzoites for 24 h at 37°C with or without l-NMMA at the indicated concentrations as described previously (43). After incubation, cells were processed for the parasite growth assay as described above.
Western blot analysis.
Peritoneal macrophages were incubated with recombinant proteins for 12 h before the cells were infected with 2 × 104 T. gondii tachyzoites. After 72 h, the cells containing the parasites were washed, scraped, suspended in 100 μl of PBS, sonicated, and mixed with 100 μl of 2× SDS gel-loading buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 140 mM 2-mercaptoethanol, 10% [wt/vol] glycerol, and 0.02% [wt/vol] bromophenol blue) under reducing conditions. The samples were heated at 95°C for 5 min and separated on a 12% polyacrylamide gel. After SDS-PAGE, the protein bands in the gel were electrically transferred onto a membrane (Immobilon-P; Millipore). The membrane was blocked with 1× PBS containing 3% skim milk (PBS-SM) and then incubated with BAG1 or GRA1 polyclonal antibody raised in mice (13) or with a TgSAG1 monoclonal antibody (Advanced Immunochemical Inc., Long Beach, CA) at a 1:100 dilution in PBS-SM at 37°C for 60 min. The membrane was washed three times with PBS for 5 min and then incubated with a 1:1,000 dilution of horseradish peroxidase-conjugated goat anti-mouse IgG (Amersham Pharmacia Biotech, Piscataway, NJ) in PBS-SM at 37°C for 60 min. The membrane was washed three times with PBS for 5 min, incubated with ECL Western blotting detection reagents (Amersham, GE Healthcare) for 1 min, and exposed to a film.
Indirect fluorescent antibody test (IFAT).
Confirmation of the conversion of the parasite was explored using coverslips of confluent peritoneal macrophages pretreated with TgCyp18 and infected with T. gondii parasites. The coverslips were collected 72 h after parasite inoculation, washed twice with PBS containing 1 mM CaCl2 and MgCl2 (PBS++), and then fixed with 3% paraformaldehyde in PBS++. After washing twice with PBS++, the cells were permeabilized with 0.3% Triton X-100 in PBS++ for 5 min at room temperature. After washing, the coverslips were incubated with 3% bovine serum albumin (BSA) in PBS++ at room temperature for 30 min. The coverslips were incubated with BAG1 mouse antiserum and anti-Cyp18 rabbit IgG diluted 1:100 in 3% BSA in PBS++ for 1 h at room temperature. After washing three times with PBS++, the coverslips were incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 594-conjugated goat anti-rabbit IgG (Sigma) diluted 1:1,000 in 3% BSA in PBS++ for 1 h at room temperature and then washed again. The coverslips were placed onto a glass slide coated with Mowiol (Calbiochem, San Diego, CA). The slides were examined using a fluorescence microscope (Nikon, Tokyo, Japan). Three hundred infected cells were counted, and the average numbers as a percentage of BAG1-positive cells, indicating bradyzoites, and Cyp18-positive cells, as total parasites, were determined.
Statistical analysis.
Data are expressed as means ± standard deviations. Various assay conditions were evaluated by using an analysis of variance (ANOVA) test followed by post hoc analysis of group differences that was performed by use of the least significant difference (LSD) test; P values of <0.001 were considered to be statistically significant.
RESULTS
Cytokine production in response to TgCyp18.
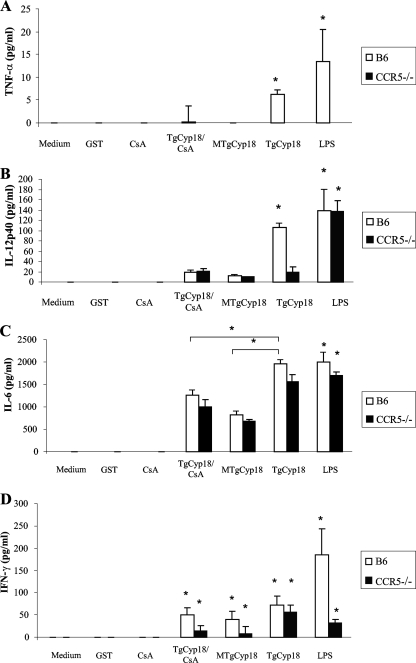
TgCyp18 has been shown to stimulate DC to produce IL-12 in a CCR5-dependent manner (2). However, the effect of TgCyp18 stimulation on the production of other cytokines has not yet been elucidated. We investigated the production of TNF-α, IL-6, IL-12p40, and IFN-γ by peritoneal macrophages treated with TgCyp18, demonstrating the role of CCR5 and the inhibitory effect of cyclosporine (Fig. 1). Interestingly, TgCyp18 induced low-level production of TNF-α and IL-12p40 in a CCR5-dependent manner. MTgCyp18, TgCyp18 plus cyclosporine in combination, and CCR5−/− macrophage treatment with TgCyp18 produced levels of TNF-α and IL-12p40 comparable to those of the negative controls (complete medium, GST, and cyclosporine alone) (Fig. 1A and B). On the other hand, TgCyp18 succeed in upregulating the production of IL-6 and IFN-γ in a CCR5-independent way. The level of IL-6 production in wild-type B6 macrophages was significantly different among the TgCyp18, MTgCyp18, and TgCyp18-plus-cyclosporine treatments (P < 0.001). CCR5−/− macrophages treated with TgCyp18, MTgCyp18, and TgCyp18 plus cyclosporine had comparable differences from each other with no significant differences observed compared to wild-type B6 macrophages (Fig. 1C). It was previously reported that the expression of IFN-γ mRNA was detected upon LPS stimulation of murine macrophages (22). Moreover, a small population of IFN-γ-positive cells with a macrophage phenotype was identified, particularly in chronically Trypanosoma cruzi-infected mice, reinforcing the notion that macrophages can be an alternative source of IFN-γ (3). Human macrophages derived from monocytes in vitro and naturally activated alveolar macrophages immediately secreted IFN-γ upon treatment with IL-12 and IL-18 (12), suggesting that macrophages have the ability to produce IFN-γ in response to certain stimuli. The treatment of wild-type B6 macrophages with TgCyp18, MTgCyp18, and the combination of TgCyp18 plus cyclosporine led to significantly increased levels of IFN-γ compared to those of the negative controls (P < 0.001), although there were no differences among the treatment groups. Similar results were seen with CCR5−/− macrophages, which also significantly increased IFN-γ production levels of over those of the negative controls (P < 0.001) (Fig. 1D). The level of production of TNF-α and IFN-γ by LPS-treated CCR5−/− macrophages was significantly less than that in LPS-treated wild-type B6 macrophages, which might help to clarify the direct or the indirect role of CCR5 in controlling both TNF-α and IFN-γ induction by LPS. Altogether, TgCyp18 induced the production of TNF-α and IL-12p40 in a CCR5-dependent manner and succeeded in upregulating the production of IL-6 and IFN-γ in a CCR5-independent way.
FIG. 1.
Cytokine production induced by TgCyp18. Peritoneal macrophages (1 × 106 macrophages) from B6 mice and CCR5−/− mice were incubated for 24 h with 50 μg/ml GST, 50 μg/ml TgCyp18, 50 μg/ml MTgCyp18, 1 μM cyclosporine (CsA), or 10 ng/ml LPS, and the supernatants were collected for measurements of the production of TNF-α (A), IL-12p40 (B), IL-6 (C), and IFN-γ (D). Each value represents the mean ± the standard deviation of data from triplicate samples. The results are representative of two repeated experiments with similar results. Statistical significance was calculated between groups compared to the control (medium only or GST) and also for IL-6 among the TgCyp18-, MTgCyp18-, and TgCyp18-plus-cyclosporine-treated macrophage groups, calculated using ANOVA and follow-up test (LSD). *, P < 0.001.
CCR5 controls production of NO in response to TgCyp18.
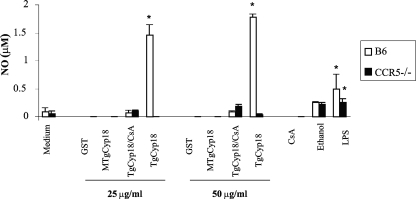
Because of the critical role of NO in the toxoplasma-static effect in activated macrophages, we measured the effect of TgCyp18 on NO production. TgCyp18 at doses of 25 and 50 μg/ml enhanced the production of NO. Pretreatment of B6 macrophages with MTgCyp18 or CCR5−/− macrophages with TgCyp18 failed to produce NO above negative control levels (complete medium and GST). Moreover, the combination of TgCyp18 plus cyclosporine failed to produce NO at levels above those of ethanol, the negative control for this group (Fig. 2). Altogether, TgCyp18 enhanced the production of NO in a CCR5-dependent manner.
FIG. 2.
NO production by TgCyp18. Peritoneal macrophages (1 × 106 macrophages) from B6 mice and CCR5−/− mice were incubated for 24 h with 25 or 50 μg/ml of GST, TgCyp18, or MTgCyp18; 1 μM cyclosporine (CsA); or 10 ng/ml LPS, and supernatants were collected for analysis of NO production. Each value represents the mean ± the standard deviation of data from triplicate samples. The results are representative of two repeated experiments with similar results. Statistical significance was calculated compared to the control groups (medium only or GST) using ANOVA and a follow-up test (LSD). *, P < 0.001.
Effects of TgCyp18 treatment on parasite growth.
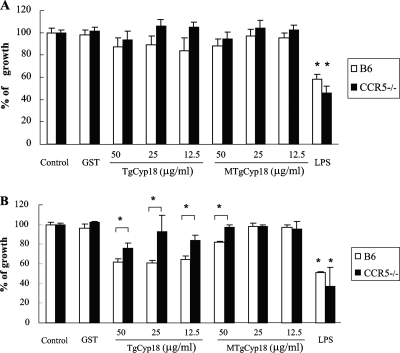
In order to study the role of TgCyp18 in response to uninfected and infected macrophages, the cells were pre- and posttreated with TgCyp18, and parasite growth was measured (Fig. 3). In the posttreatment experiment, the effects of TgCyp18 on infected cells were examined. As shown in Fig. 3A, there were no significant differences in parasite growth in wild-type B6 or CCR5−/− macrophages compared to each other and to the negative controls (complete media and GST). LPS-treated B6 and CCR5−/− macrophages showed levels of downregulated parasite growth similar to those of untreated control macrophages. Next, we investigated the effects of TgCyp18 on uninfected cells through the pretreatment experiment. In this experiment, we mimicked the cell status after parasite egress by adding larger amounts of TgCyp18 (Fig. 3B). All doses (12.5, 25, and 50 μg/ml) of TgCyp18 inhibited parasite growth in wild-type macrophages compared to the control groups and the CCR5−/− macrophages. Only 50 μg/ml of TgCyp18 downregulated parasite growth in CCR5−/− macrophages compared to control groups. Moreover, 50 μg/ml of MTgCyp18 inhibited parasite growth in B6 wild-type macrophages compared to CCR5−/− macrophages and to control groups, suggesting that TgCyp18 might act with other receptors to regulate parasite growth. LPS-treated macrophages again showed a downregulation of parasite growth in both wild-type B6 and CCR5−/− macrophages. These results suggest that the treatment of macrophages with TgCyp18 results in the inhibition of parasite growth under conditions that mimic secondary infection in a CCR5-dependent manner.
FIG. 3.
Inhibition of T. gondii growth by treatment of macrophages with TgCyp18. (A) Posttreatment experiment. Peritoneal macrophages (1 × 106 macrophages) from B6 mice and CCR5−/− mice were infected with T. gondii (2 × 105 parasites) for 12 h, and the culture was then incubated with TgCyp18, MTgCyp18, 50 μg/ml GST, or 10 ng/ml LPS. After 24 h, [3H]uracil uptake in tachyzoites was measured. (B) Pretreatment experiment. Peritoneal macrophages (1 × 106 macrophages) from B6 mice and CCR5−/− mice were incubated with TgCyp18, MTgCyp18, 50 μg/ml GST, and 10 ng/ml LPS for 12 h before the cells were infected with T. gondii (2 × 105 parasites). After incubation for 24 h, the [3H]uracil uptake in tachyzoites was measured. The percentage of growth was calculated by dividing each value (control or tested) by the average means of the control samples (parasite growth in nontreated macrophages) multiplied by 100. Each value represents the mean ± the standard deviation of data from triplicate samples. Statistical significance was calculated between the TgCyp18- or MTgCyp18-pretreated B6 wild-type macrophages and the pretreated CCR5−/− macrophages or the negative control groups. Moreover, significant differences were calculated between the positive controls for both macrophages and the negative control using ANOVA and a follow-up test (LSD). *, P < 0.001.
Inhibition of parasite growth by TgCyp18-induced NO.
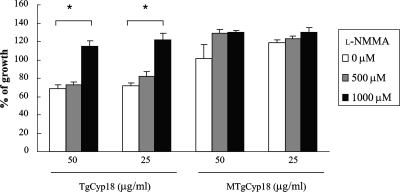
As shown in Fig. 2, the amounts of NO produced by wild-type peritoneal macrophages treated with TgCyp18 were significantly higher than those of MTgCyp18-treated B6 macrophages or CCR5−/− macrophages treated with TgCyp18. To confirm the role of NO induced by TgCyp18, the effect of the inducible NO synthase (iNOS) inhibitor l-NMMA on parasite growth was investigated (Fig. 4). The addition of l-NMMA restored the growth of the parasite in wild-type macrophages pretreated with TgCyp18, while the inhibitor did not have any significant effects on wild-type macrophages pretreated with MTgCyp18. Our data demonstrated that anti-Toxoplasma activity was induced in an NO-dependent manner in wild-type macrophages pretreated with TgCyp18.
FIG. 4.
Effect of l-NMMA on T. gondii growth in response to TgCyp18. Peritoneal macrophages (1 × 106 macrophages) from B6 mice were pretreated with 0 μM, 500 μM, or 1,000 μM l-NMMA for 15 min. The cells were incubated with TgCyp18, MTgCyp18, 50 μg/ml GST, or 10 ng/ml LPS for 12 h before infection with 2 × 105 parasites. After incubation for 24 h, the [3H]uracil uptake in tachyzoites was measured. The percentage of growth was calculated by dividing each value (control or tested) by the average means of the control samples (parasite growth in nontreated macrophages) multiplied by 100. Each value represents the mean ± standard deviation of data from triplicate samples. Statistical significance was calculated against 0 μM l-NMMA treatment using ANOVA and a follow-up test (LSD). *, P < 0.001.
TgCyp18 enhanced bradyzoite conversion via NO production.
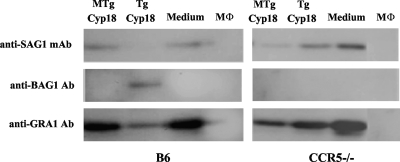
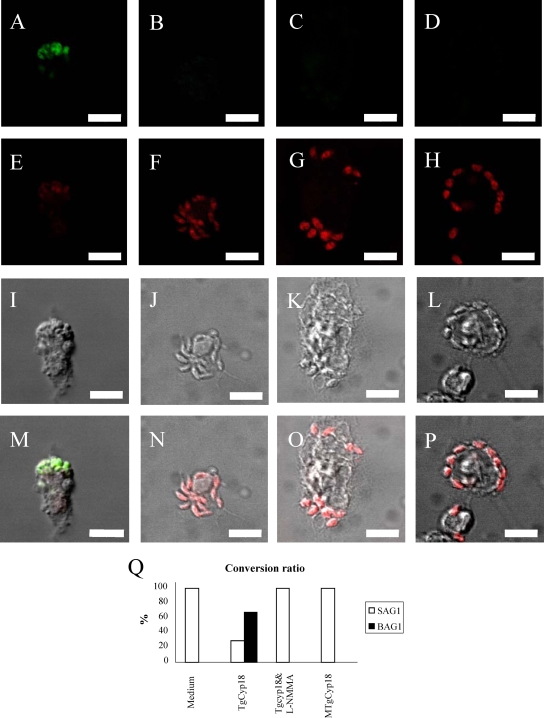
Since TgCyp18 had the ability to induce the production of NO related to the inhibition of parasite growth, we speculated that TgCyp18 would have an influence on bradyzoite conversion. In order to investigate the ability of TgCyp18 to induce the conversion of T. gondii from the tachyzoite stage to the bradyzoite stage, Western blot analysis and IFAT were performed with specific markers for each stage. As shown in Fig. 5, Western blots of lysates from infected wild-type B6 macrophages pretreated with TgCyp18 were strongly positive for the 30-kDa bradyzoite-specific marker BAG1 (13) but showed only weak staining for the 31-kDa tachyzoite-specific marker SAG1. The lysates from infected wild-type B6 macrophages cultured with MTgCyp18 or complete medium were positive for SAG1 only. On the other hand, TgCyp18 did not have any effects on T. gondii conversion in CCR5−/− macrophages because BAG1 expression was not detected in infected CCR5−/− macrophages pretreated with TgCyp18. Anti-GRA1 was used as a control because it is a suitable marker for characterizing both tachyzoites and bradyzoites (21, 60). We then confirmed the induction of bradyzoites following TgCyp18 treatment by IFAT (Fig. 6). The conversion was confirmed using BAG1- and TgCyp18-specific antibodies (for both stages). BAG1 expression was detected on parasites in wild-type B6 macrophages pretreated with TgCyp18 (Fig. 6A), while no expression of BAG1 was detected on parasites in wild-type macrophages pretreated with TgCyp18 plus l-NMMA or MTgCyp18 (Fig. 6B and C). Three hundred infected cells were counted, and percentages of BAG1-positive and TgCyp18-positive parasites were determined (Fig. 6Q). Pretreatment with TgCyp18 resulted in the staining of 67.3% of the parasites with antibodies to BAG1 by day 3 of culture, while other pretreatments failed to express any considerable levels of BAG1 (Fig. 6Q). Taken together, these results suggest that the treatment of macrophages with TgCyp18 resulted in the induction of bradyzoite conversion in an NO- and CCR5-dependent manner.
FIG. 5.
Western blot analysis of T. gondii-infected macrophages pretreated with TgCyp18. Peritoneal macrophages (1 × 106 macrophages) were incubated with TgCyp18 or MTgCyp18 (50 μg/ml) for 12 h before the culture was infected with T. gondii (2 × 104 parasites). After 72 h, the lysates of cells containing the parasite were subjected to Western blot analysis using a SAG1 monoclonal antibody (mAb), BAG1 polyclonal antibody (Ab), or GRA1 antibody. B6 macrophages and CCR5−/− macrophages were preincubated with MTgCyp18, TgCyp18, complete medium, or macrophages (MΦ) without infection.
FIG. 6.
IFAT of T. gondii-infected macrophages pretreated with TgCyp18. Peritoneal macrophages (1 × 106 macrophages) were incubated with TgCyp18 or MTgCyp18 (50 μg/ml) or pretreated with 1,000 μM l-NMMA for 15 min, followed by 50 μg/ml of TgCyp18 for 12 h before the culture was infected with T. gondii (2 × 104 parasites). After 72 h, the cells containing the parasite were subjected to IFAT. Cells treated with TgCyp18 (A, E, I, and M), TgCyp18 plus l-NMMA (B, F, J, and N), MTgCyp18 (C, G, K, and O), or complete medium (D, H, L, and P) were stained with a BAG1 antibody as a bradyzoite-specific marker (A to D) or a TgCyp18 antibody as a tachyzoite and bradyzoite marker (E to H). Light images (I to L) and merged images (M to P) are also shown. For the conversion ratio of T. gondii from the tachyzoite stage to the bradyzoite stage (Q), the data are presented as mean percentages of duplicated samples of BAG1-positive and TgCyp18-positive cells of 300 cells infected with T. gondii. Scale bars, 10 μm.
DISCUSSION
Elucidation of the molecular mechanisms underlying the regulation of the protective immune response has provided important clues for an understanding of the basis of successful long-term interactions between parasites and their vertebrate hosts (15). Toxoplasma so far appears to be unique in that it possesses two mechanisms to trigger IL-12 production from DC (2, 14, 46). One is dependent upon the common adaptor protein myeloid differentiation protein 88 (MyD88) and is likely to involve Toll-like receptors (14, 46, 62). The other is through an 18-kDa cyclophilin, TgCyp18, which is released by extracellular tachyzoites, triggering IL-12 production through binding to CCR5 (2). DC from CCR5−/− mice display defects in IL-12 production and have increased susceptibility to T. gondii (1). The present study aimed to clarify the immunomodulatory role of TgCyp18 in relation to CCR5 in macrophages and the consequences of immunoregulation on the parasite growth and stage conversion of T. gondii parasites.
The results of the present study demonstrated that TgCyp18 induced the production of NO, TNF-α, and IL-12p40 in a CCR5-dependent manner. MTgCyp18 or TgCyp18 plus cyclosporine-treated wild-type B6 and CCR5−/− macrophages did not produce comparable levels of these cytokines (Fig. 1A and B and 2). In susceptible mouse strains (such as C57BL/6 mice), moderate inflammation characterized by the presence of gene transcripts for IFN-γ, TNF-α, granulocyte-macrophage colony-stimulating factor, IL-6, IL-1, and IL-10 is found in the central nervous system of chronically infected animals (25, 29, 30). Moreover, it was previously reported that TgHSP70-induced NO release was dependent on Toll-like receptor 2, MyD88, and IRAK4 (40), which means that T. gondii has several proteins that work through different pathways to induce NO production. On the other hand, TgCyp18 succeeded in upregulating the production of IFN-γ and IL-6 in a CCR5-independent way, which may provide some clues about the roles of other receptors for TgCyp18 in macrophages. The cytokine IFN-γ is central for resistance to T. gondii at both acute and chronic stages of infection, as demonstrated by cytokine depletion, cytokine repletion, and gene knockout studies (24, 48, 56, 57). Both TNF-α and IFN-γ are required for the induction of optimal levels of iNOS and reactive nitrogen intermediates (31). However, our results showed no significant differences in IFN-γ production among TgCyp18-, MTgCyp18-, and TgCyp18-plus-cyclosporine-treated groups (Fig. 1D), and only TgCyp18 enhanced the production of NO (Fig. 2). Interestingly, LPS-treated macrophages showed comparable differences between wild-type B6 and CCR5−/− macrophages in the production of TNF-α and IFN-γ. A recent report suggested that CCR5-mediated neuron-glia signaling functions to protect neurons by suppressing microglia toxicity through the downregulation of the expression of mRNAs for inflammatory cytokines (IL-1β and TNF-α) and iNOS induced by LPS (23). This might clarify the direct or the indirect role of CCR5 in controlling both TNF-α and IFN-γ induced by LPS. Altogether, these results suggest that CCR5 acts as one of the main receptors controlling NO production in response to T. gondii, playing a direct role in NO production in response to TgCyp18. Moreover, IFN-γ induced by TgCyp18 did not appear to be responsible for the production of NO in our experimental model.
Following infection of intermediate hosts with T. gondii, the parasite initially multiplies in the tachyzoite stage before differentiating into bradyzoites due to pressure from the host immune response (19). Retarded parasite growth seems to be necessary for the induction of bradyzoite-specific antigens (6). Our study demonstrated that the treatment of macrophages with TgCyp18 under conditions that mimic a secondary infection resulted in the inhibition of parasite growth (Fig. 3B and 4) and an enhancement of the conversion into bradyzoites in a CCR5-dependent manner via a NO-dependent pathway (Fig. 5 and 6). In contrast, TgCyp18 under conditions mimicking a primary infection failed to downregulate parasite growth (Fig. 3A). These results emphasized the role of secondary infection in controlling the parasite life cycle.
Two explanations can be imagined to clarify these results. First, the number of parasites during primary infection was not enough to secrete an effective concentration of TgCyp18 capable of enhancing the immune response and NO production. In this scenario, a threshold concentration of TgCyp18 is needed to enhance the production of NO and trigger its consequent effects. We found low levels of secreted endogenous TgCyp18 (216.3 ± 17.5 pg/ml) from extracellular parasites (2 × 107 parasites) after 120 min at 37°C that appeared to be time and temperature dependent (data not shown). Aliberti et al. previously proved that TgCyp18 was actively secreted in a time- and temperature-dependent fashion (2). Therefore, these low levels compared to doses used in our experiments suggest that huge numbers of tachyzoites (proliferated asexually through many cycles) were required to secrete a threshold dose of TgCyp18 capable of mimicking the effects of secondary infection. This concept of a threshold level of TgCyp18 might also explain both the failure of the primary infection experiment to downregulate parasite growth as well as the success of the secondary infection. A second possibility concerns the length of time of macrophage exposure to TgCyp18. In this scenario, macrophages would be required to be exposed to TgCyp18 for a specific duration before NO production proceeds. The critical role of NO for the toxoplasma-static effects in activated macrophages has been well documented by studies that inhibited its formation with the NO synthase inhibitor l-NMMA (35, 37, 50). It is known that endogenous NO is reactive with iron-sulfur centers in proteins and therefore has the potential to inhibit several proteins involved in mitochondrial electron transport (16, 34). Previous in vitro studies indicated that NO, an important effector molecule produced by activated macrophages and an inhibitor of mitochondrial electron transport and ATP formation, induced both parasite stasis and the expression of a subset of bradyzoite-specific antigens (6, 52). NO mediated by TgCyp18 may also be capable of targeting specifically the parasite's mitochondrial electron transfer in a manner similar to the effects of atovaquone. Treatment of parasites with atovaquone resulted in the inhibition of the mitochondrial membrane potential and a subsequent increase of mitochondrial respiration over time, eventually leading to the differentiation of T. gondii from tachyzoites to bradyzoites (58, 61). It is also possible that the differentiation of T. gondii is a combination of these two models requiring both a threshold concentration and prolonged exposure times.
The conversion process of T. gondii is a very complicated process incorporating multiple pathways into this process. Examples of this complexity have been shown when tachyzoite-to-bradyzoite transformation was established in vitro through the inducement of stress including the treatment of host cells with IFN-γ or mitochondrial inhibitors (5, 6) or via the addition of alkaline pH or high temperatures (11, 53, 54). Moreover, while tissue cysts containing T. gondii bradyzoites are found in multiple organs of the host, there appears to be preferential development within neural and muscular tissue (19), suggesting that host cell type plays a role in this process (11). Indeed, changes in host cell transcription can directly influence the molecular environment to enable bradyzoite development (45). Human cell division autoantigen 1, which is involved in cell cycle arrest and the downregulation of cell proliferation (9), played a crucial role in cyst development through its ability to modify the growth state of the host cell (45). Exogenous stress factors appear to influence the developmental differentiation of T. gondii. With this complexity, previous work that found evidence of iNOS independence in cyst formation is not entirely surprising (51); the blockage of iNOS in knockout mice may have led to a compensation of alternate inputs (pathways) leading to T. gondii conversion. Certainly, there is ample evidence of an in vivo role for iNOS in parasite development and disease pathology (32, 47, 49). Moreover, a previous in vivo study reported that CCR5 was required for effective T. gondii control and that CCR5−/− resulted in the death of infected mice due to uncontrolled parasite replication (33). While the present study was carried out in vitro, it does provide enticing indications that TgCyp18 can act as an input for parasite conversion through NO induction and warrants further study in vivo.
In conclusion, our data suggested that TgCyp18-induced NO production played a critical role not only in the inhibition of parasite replication but also in triggering the induction of bradyzoite development. This led to the hypothesis that TgCyp18 can manipulate the immune response not only to fulfill its life cycle by converting to the slowly replicating bradyzoite but also to protect its host from the highly dividing tachyzoite. T. gondii would possess sophisticated mechanisms to instruct and subvert host cell responses by secreting TgCyp18. This dual potency of the parasite could allow for the establishment of a stable host-parasite interaction.
Acknowledgments
We deeply thank M. Igarashi and A. Ueno (National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine) for supplying the BAG1 and GRA1 antibodies and beneficial help.
H.M.I. has been supported by the Egyptian Ministry of High Education and Scientific Research. This study was supported by grants-in-aid for scientific research on priority areas (grant 19041008) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnage, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat. Immunol. 183-87. [DOI] [PubMed] [Google Scholar]
- 2.Aliberti, J., J. G. Valenzuela, V. B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J. M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4485-490. [DOI] [PubMed] [Google Scholar]
- 3.Bastos, K. R., R. Barboza, L. Sardinha, M. Russo, J. M. Alvarez, and M. R. Lima. 2007. Role of endogenous IFN-gamma in macrophage programming induced by IL-12 and IL-18. J. Interf. Cytok. Res. 27399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, A., P. Monaghan, and A. P. Page. 2006. Peptidyl-prolyl cis-trans isomerases (immunophilins) and their roles in parasite biochemistry, host-parasite interaction and antiparasitic drug action. Int. J. Parasitol. 36261-276. [DOI] [PubMed] [Google Scholar]
- 5.Bohne, W., J. Heesemann, and U. Gross. 1993. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect. Immun. 611141-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohne, W., J. Heesemann, and U. Gross. 1994. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect. Immun. 621761-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai, G., R. Kastelein, and C. A. Hunter. 2000. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect. Immun. 686932-6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai, G., T. Radzanowski, E. N. Villegas, R. Kastelein, and C. A. Hunter. 2000. Identification of STAT4-dependent and independent mechanisms of resistance to Toxoplasma gondii. J. Immunol. 1652619-2627. [DOI] [PubMed] [Google Scholar]
- 9.Chai, Z., B. Sarcevic, A. Mawson, and B. H. Toh. 2001. SET-related cell division autoantigen-1 (CDA1) arrests cell growth. J. Biol. Chem. 27633665-33674. [DOI] [PubMed] [Google Scholar]
- 10.Cleary, M. D., U. Singh, I. J. Blader, J. L. Brewer, and J. C. Boothroyd. 2002. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot. Cell 1329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Fonseca Ferreira da Silva, M., H. S. Barbosa, U. Gross, and C. G. Lüder. 2008. Stress-related and spontaneous stage differentiation of Toxoplasma gondii. Mol. Biosyst. 4824-834. [DOI] [PubMed] [Google Scholar]
- 12.Darwich, L., G. Coma, R. Peña, R. Bellido, E. J. Blanco, J. A. Este, F. E. Borras, B. Clotet, L. Ruiz, A. Rosell, F. Andreo, R. M. Parkhouse, and M. Bofill. 2008. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology 126386-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dautu, G., B. Munyaka, G. Carmen, G. Zhang, Y. Omata, X. Xuenan, and M. Igarashi. 2007. Toxoplasma gondii: DNA vaccination with genes encoding antigens MIC2, M2AP, AMA1 and BAG1 and evaluation of their immunogenic potential. Exp. Parasitol. 116273-282. [DOI] [PubMed] [Google Scholar]
- 14.Denkers, E. Y. 2003. From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii. FEMS Immunol. Med. Microbiol. 39193-203. [DOI] [PubMed] [Google Scholar]
- 15.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drapier, J. C., and J. B. Hibbs, Jr. 1988. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J. Immunol. 1402829-2838. [PubMed] [Google Scholar]
- 17.Dubey, J. P. 1994. Toxoplasmosis. J. Am. Vet. Med. Assoc. 2051593-1598. [PubMed] [Google Scholar]
- 18.Dubey, J. P. 1998. Re-examination of resistance of Toxoplasma gondii tachyzoites and bradyzoites to pepsin and trypsin digestion. Parasitology 11643-50. [DOI] [PubMed] [Google Scholar]
- 19.Dubey, J. P., D. S. Lindsay, and C. A. Speer. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 11267-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton, M. S., L. M. Weiss, and K. Kim. 2006. Cyclic nucleotide kinases and tachyzoite-bradyzoite transition in Toxoplasma gondii. Int. J. Parasitol. 36107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson, D. J. 2004. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int. J. Parasitol. 34347-360. [DOI] [PubMed] [Google Scholar]
- 22.Fultz, M. J., S. A. Barber, C. W. Dieffenbach, and S. N. Vogel. 1993. Induction of IFN-gamma in macrophages by lipopolysaccharide. Int. Immunol. 51383-1392. [DOI] [PubMed] [Google Scholar]
- 23.Gamo, K., S. Kiryu-Seo, H. Konishi, S. Aoki, K. Matsushima, K. Wada, and H. Kiyama. 2008. G-protein-coupled receptor screen reveals a role for chemokine receptor CCR5 in suppressing microglial neurotoxicity. J. Neurosci. 2811980-11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated T. gondii vaccine. J. Immunol. 146286-292. [PubMed] [Google Scholar]
- 25.Gazzinelli, R. T., I. Eltoum, T. A. Wynn, and A. Sher. 1993. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J. Immunol. 1513672-3681. [PubMed] [Google Scholar]
- 26.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 906115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.High, K. P., K. A. Joiner, and R. E. Handschumacher. 1994. Isolation, cDNA sequences, and biochemical characterization of the major cyclosporin-binding proteins of Toxoplasma gondii. J. Biol. Chem. 2699105-9112. [PubMed] [Google Scholar]
- 28.Hunter, C. A., C. S. Subauste, V. H. Van Cleave, and J. S. Remington. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 622818-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter, C. A., C. W. Roberts, M. Murray, and J. Alexander. 1992. Detection of cytokine mRNA in the brains of mice with toxoplasmic encephalitis. Parasite Immunol. 14405-413. [DOI] [PubMed] [Google Scholar]
- 30.Hunter, C. A., C. W. Roberts, and J. Alexander. 1992. Kinetics of cytokine mRNA production in the brains of mice with progressive toxoplasmic encephalitis. Eur. J. Immunol. 222317-2322. [DOI] [PubMed] [Google Scholar]
- 31.James, S. L. 1995. Role of nitric oxide in parasitic infections. Microbiol. Rev. 59533-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan, I. A., J. D. Schwartzman, T. Matsuura, and L. H. Kasper. 1997. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA 9413955-13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan, I. A., S. Y. Thomas, M. M. Moretto, F. S. Lee, S. A. Islam, C. Combe, J. D. Schwartzman, and A. D. Luster. 2006. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolb, H., and V. Kolb-Bachofen. 1992. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol. Today 13157-160. [DOI] [PubMed] [Google Scholar]
- 35.Langermans, J. A. M., M. E. B. van der Hulst, P. H. Nibbering, P. S. Hiemstra, L. Fransen, and R. van Furth. 1992. IFN-γ induced L-arginine dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor alpha. J. Immunol. 148568-574. [PubMed] [Google Scholar]
- 36.Li, L., B. P. Brunk, J. C. Kissinger, D. Pape, K. Tang, R. H. Cole, J. Martin, T. Wylie, M. Dante, S. J. Fogarty, D. K. Howe, P. A. Liberator, C. Diaz, J. Anderson, M. White, M. E. Jerome, E. A. Johnson, J. A. Radke, C. J. Stoeckert, Jr., R. H. Waterston, S. W. Clifton, D. S. Roos, and L. D. Sibley. 2003. Gene discovery in the Apicomplexa as revealed by EST sequencing and assembly of a comparative gene database. Genome Res. 13443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liew, F. Y., and F. E. G. Cox. 1991. Nonspecific defence mechanism: the role of nitric oxide. Immunol. Today 12A17-A21. [DOI] [PubMed] [Google Scholar]
- 38.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15211-222. [DOI] [PubMed] [Google Scholar]
- 39.Manger, I. D., H. Adrian, S. Parmley, L. D. Sibley, M. Marra, L. Hillier, R. Waterston, and J. C. Boothroyd. 1998. Expressed sequence tag analysis of the bradyzoite stage of Toxoplasma gondii: identification of developmentally regulated genes. Infect. Immun. 661632-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mun, H. S., F. Aosai, K. Norose, L. X. Piao, H. Fang, S. Akira, and A. Yano. 2005. Toll-like receptor 4 mediates tolerance in macrophages stimulated with Toxoplasma gondii-derived heat shock protein 70. Infect. Immun. 734634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navia, B. A., C. K. Petito, J. W. Gold, E. S. Cho, B. D. Jordan, and R. W. Price. 1986. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann. Neurol. 19224-238. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa, Y., H. Zhang, H. M. Ibrahim, F. Ui, A. Ogiso, and X. Xuan. 2008. Construction of Toxoplasma gondii bradyzoite expressing the green fluorescent protein. Parasitol. Int. 57219-222. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa, Y., X. Xuenan, L. Makala, O. Vielemeyer, K. A. Joiner, and H. Nagasawa. 2003. Characterisation of Toxoplasma gondii engineered to express mouse interferon-gamma. Int. J. Parasitol. 331525-1535. [DOI] [PubMed] [Google Scholar]
- 44.Radke, J. R., M. S. Behnke, A. J. Mackey, J. B. Radke, D. S. Roos, and M. W. White. 2005. The transcriptome of Toxoplasma gondii. BMC Biol. 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radke, J. R., R. G. Donald, A. Eibs, M. E. Jerome, M. S. Behnke, P. Liberator, and M. W. White. 2006. Changes in the expression of human cell division autoantigen-1 influence Toxoplasma gondii growth and development. PLoS Pathog. 2e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 1685997-6001. [DOI] [PubMed] [Google Scholar]
- 47.Scharton-Kersten, T. M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 1851261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, L. Showe, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-γ mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 1574045-4054. [PubMed] [Google Scholar]
- 49.Schlüter, D., M. Deckert-Schlüter, E. Lorenz, T. Meyer, M. Röllinghoff, and C. Bogdan. 1999. Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii-susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii-resistant BALB/c mice. J. Immunol. 1623512-3518. [PubMed] [Google Scholar]
- 50.Sibley, L. D., L. B. Adams, Y. Fukotomi, and J. L. Krahenbuhl. 1991. Tumor necrosis factor alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J. Immunol. 1472340-2345. [PubMed] [Google Scholar]
- 51.Silva, N. M., W. L. Tafuri, J. I. Alvarez-Leite, J. R. Mineo, and R. T. Gazzinelli. 2002. Toxoplasma gondii: in vivo expression of BAG-5 and cyst formation is independent of TNF p55 receptor and inducible nitric oxide synthase functions. Microbes Infect. 4261-270. [DOI] [PubMed] [Google Scholar]
- 52.Soête, M., and J. F. Dubremetz. 1996. Toxoplasma gondii: kinetics of stage-specific protein expression during tachyzoite-bradyzoite conversion in vitro. Curr. Top. Microbiol. Immunol. 21976-80. [PubMed] [Google Scholar]
- 53.Soête, M., B. Fortier, D. Camus, and J. F. Dubremetz. 1993. Toxoplasma gondii: kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp. Parasitol. 76259-264. [DOI] [PubMed] [Google Scholar]
- 54.Soête, M., D. Camus, and J. F. Dubremetz. 1994. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp. Parasitol. 78361-370. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan, W. J. J., J. Narasimhan, M. M. Bhatti, and R. C. Wek. 2004. Parasite-specific eIF2 (eukaryotic initiation factor-2) kinase required for stress-induced translation control. Biochem. J. 380523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki, Y., F. K. Conley, and J. S. Remington. 1990. Treatment of toxoplasmic encephalitis in mice with recombinant gamma interferon. Infect. Immun. 583050-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 240516-518. [DOI] [PubMed] [Google Scholar]
- 58.Tomavo, S., and J. C. Boothroyd. 1995. Interconnection between organellar functions, development and drug resistance in the protozoan parasite, Toxoplasma gondii. Int. J. Parasitol. 251293-1299. [DOI] [PubMed] [Google Scholar]
- 59.Tuo, W., R. Fetterer, M. Jenkins, and J. P. Dubey. 2005. Identification and characterization of Neospora caninum cyclophilin that elicits gamma interferon production. Infect. Immun. 735093-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueno, A., G. Dautu, B. Munyaka, G. Carmen, Y. Kobayashi, and M. Igarashi. 2009. Toxoplasma gondii: identification and characterization of bradyzoite-specific deoxyribose phosphate aldolase-like gene (TgDPA). Exp. Parasitol. 12155-63. [DOI] [PubMed] [Google Scholar]
- 61.Vercesi, A. E., C. O. Rodrigues, S. A. Uyemura, L. Zhong, and S. N. Moreno. 1998. Respiration and oxidative phosphorylation in the apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 27331040-31047. [DOI] [PubMed] [Google Scholar]
- 62.Yarovinsky, F., D. Zhang, J. F. Andersen, G. L. Bannenberg, C. N. Serhan, M. S. Hayden, S. Hieny, F. S. Sutterwala, R. A. Flavell, S. Ghosh, and A. Sher. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 3081626-1629. [DOI] [PubMed] [Google Scholar]
- 63.Yarovinsky, F., J. F. Andersen, L. R. King, P. Caspar, J. Aliberti, H. Golding, and A. Sher. 2004. Structural determinants of the anti-HIV activity of a CCR5 antagonist derived from Toxoplasma gondii. J. Biol. Chem. 27953635-53642. [DOI] [PubMed] [Google Scholar]