Abstract
Infection with Anaplasma phagocytophilum, a gram-negative, lipopolysaccharide (LPS)-negative, obligate intracellular bacterium, results in multiple peripheral blood cytopenias. We hypothesized that infection with this organism would result in decreased bone marrow (BM) function and shifts in hematopoietic progenitor cells (HPCs) and lineage-committed cells in a well-established murine model of infection. HPCs and lineage-committed progenitors were enumerated in the BM and spleen during acute infection. BM cytokine production and BM CXCL12 expression were determined. Infection resulted in peripheral blood bicytopenia, marked decreases in the number of lineage-committed HPCs in the BM along with concurrent increases in the number of lineage-committed HPCs in the spleen, and a mixed, predominantly myelosuppressive BM cytokine environment. There was significant downregulation of CXCL12 in BM cells that may have been partially responsible for changes in HPC trafficking observed. Changes occurred in the absence of direct pathogen infection of BM cells. Hematopoietic lineage assessment demonstrated that there was loss of erythrocytes and B lymphocytes from the BM along with increased granulopoiesis. These changes were accompanied by splenomegaly due to lymphoid hyperplasia and increased hematopoiesis, most notably erythropoiesis. These changes largely mimic well-described inflammation and endotoxin-mediated effects on the BM and spleen; however, the numbers of peripheral blood neutrophils appear to be independently modulated as granulocytic hyperplasia does not result in neutrophilia. Our findings highlight a well-conserved series of events that we demonstrate can be instigated by an LPS-negative pathogen in the absence of an endotoxin-mediated acute proinflammatory response.
Hematologic complications result from a wide variety of infectious diseases and may significantly complicate the prognosis and outcome of these diseases. Infection and inflammation elicit alterations in all hematopoietic subsets. For example, endotoxin mediates a proinflammatory cascade of events including the release of systemic, proinflammatory cytokines, granulocyte efflux from the bone marrow (BM), the release of colony-stimulating factors, and a compensatory granulocytic hyperplasia (49, 54, 61, 65). The expansion of the granulocytic compartment fuels a reactive neutrophilia that is balanced by reciprocal depletion of the BM lymphoid compartment by either mobilization or apoptosis of lymphocytes (58, 59). Inflammation, infection, and injury also profoundly alter erythropoiesis and generally result in anemia. The pathogenesis behind alterations in erythropoiesis involves multiple factors, including cytokine-mediated erythroid apoptosis, a blunted BM response to erythropoietin, reduced iron availability, suppression of erythroid progenitor cell proliferation, and BM egress of erythroid progenitor cells (34, 40, 50, 51). Infection and inflammation also modulate the numbers of platelets. Acute endotoxemia and acute viral infections are most frequently associated with thrombocytopenia. There are multiple mechanisms of thrombocytopenia, and they include increased platelet sequestration and destruction and inhibition of megakaryopoiesis (37). Platelet kinetics can also be modulated by inflammation-induced production of interleukin-6 (IL-6), which promotes a reactive thrombocytosis (37).
Anaplasma phagocytophilum, the agent of granulocytic anaplasmosis (GA) (formerly granulocytic ehrlichiosis), is a gram-negative, lipopolysaccharide (LPS)-negative (38), obligate intracellular bacterium that resides primarily within circulating granulocytes (20, 25). GA is an emerging, tick-borne infectious disease (26). Unlike endotoxin-mediated events, infection with A. phagocytophilum typically results in multiple cytopenias in natural disease in both humans (5) and animals (7, 43, 48), as well as in animal models of infection (10, 12, 33). The cytopenias typically include mild nonregenerative anemia, mild to moderate leukopenia, and moderate to marked thrombocytopenia. The mechanism(s) underlying the cytopenias are not fully understood. Immune-mediated destruction or splenic sequestration of cells is unlikely as cytopenias occur within the first 2 to 5 days of infection, before a significant acquired immune response is mounted, SCID mice (lacking functional B and T cells) become cytopenic (10, 12, 33), and splenectomized mice become thrombocytopenic (12).
Multiple cytopenias are frequently associated with alterations in BM production of cells. Primary BM progenitor cells are susceptible to A. phagocytophilum infection (36), and A. phagocytophilum DNA is present and may persist in BM during natural and experimental infections (8, 32). Nonetheless, pathological changes associated with infection are largely thought to be independent of direct pathogen infection (17, 53). Although routine histopathologic evaluation of BM from GA patients can reveal normo- to hypercellular marrow (39), pathogens can have profound effects on hematopoiesis with limited morphological alterations of BM. Such effects include nonvisible effects on stromal cells, increased apoptosis of proliferating cells, and a lack of differentiation and proliferation of immature hematopoietic precursors (ineffective hematopoiesis) (22, 24, 44). Hematopoietic cell trafficking and mobilization can be initiated by disruption of the CXCL12/CXCR4 axis in BM (15, 41, 59). The CXCL12/CXCR4 axis is a key regulator of stem cell and lineage-committed progenitor cell trafficking.
The objective of this study was to characterize the hematopoietic response to infection with A. phagocytophilum, a pathogen that elicits multiple peripheral blood cytopenias. We hypothesized that infection would result in altered BM function. We found that infection with A. phagocytophilum resulted in rapid and profound multilineage deficits in hematopoietic progenitor proliferation or differentiation. This quantitative defect in hematopoiesis was accompanied by induction of myelosuppressive chemokines within the BM, shifts in BM hematopoietic subsets, including B-lymphocyte depletion, erythroid depletion, and granulocytic hyperplasia, and significant downregulation of CXCL12 in BM cells. Changes were independent of the pathogen burden or the route of pathogen inoculation. Our data, combined with data for characteristic multiple-lineage cytopenias, suggest that kinetic alterations in hematopoietic cell subsets may contribute to infection-induced cytopenias.
MATERIALS AND METHODS
Mice and in vivo experimental infection.
Mouse experiments were performed as previously described (11). Female, 5- to 8-week-old C3H/HeN (C3H) and C3H/Smn.ClcrHsd-scid (SCID) mice were purchased from National Cancer Institute Animal Production Program, Frederick Cancer Research Center (Frederick, MD) and Harlan Sprague-Dawley (Indianapolis, IN), respectively. Mice were maintained according to approved institutional animal use and care protocols. Infection of C3H mice was performed either by infestation with infected nymphal ticks (11) or by intraperitoneal (i.p.) injection of infected SCID mouse blood (10, 33), exactly as previously described. Both routes of inoculation result in successful infection with similar kinetics (32); however, tick infestation is more complicated and time-consuming. Thus, tick infestation was used to document that cytopenias and BM alterations occur in the mouse model using a physiologically relevant route of infection. For tick infestation, C3H mice were infested with A. phagocytophilum-infected or uninfected nymphal Ixodes scapularis ticks exactly as previously described (11). Nymphal I. scapularis ticks were kindly provided by Durland Fish (Yale School of Public Health, New Haven, CT). The nymphal ticks included uninfected nymphs and nymphs infected with either the Spooner or Bradford isolate of A. phagocytophilum (both the Spooner and Bradford isolates infect mice and induce cytopenias; significant differences were not noted between A. phagocytophilum isolates). In brief, mice were anesthetized with ketamine/xylazine i.p. (Vetamine [Schering-Plough Animal Health Corp., Union, NJ] and TranquiVed [Vedco Inc., St. Joseph, MO]). One half of the mice were infested with four A. phagocytophilum-infected nymphal ticks each, and the other half were infested with uninfected ticks. Mice were killed with CO2 on various days postinfestation depending on the experimental design (pathogen transfer from ticks delays infection by 48 h compared to i.p. inoculation) (32, 47). Tick infestation experiments were repeated once. For i.p. administration, C3H mice were inoculated with 100 μl of pooled infected (A. phagocytophilum strain NCH-1) or pooled uninfected SCID mouse blood exactly as previously described (33). Infections in SCID mice were maintained through serial i.p. passage of infected blood (100 μl of blood per injection). Experiments utilizing i.p. inoculation were repeated at least once. Serial hematology data were collected during all experiments to verify typical infection-induced hematologic alterations.
Tissue collection and processing.
Blood, spleens, and BM were collected at necropsy. Blood was collected into EDTA by cardiocentesis. Blood was used for quantitative PCR (qPCR) to detect A. phagocytophilum p44 DNA and to obtain complete blood cell counts and differential leukocyte counts. Complete blood cell counts were determined using an automated analyzer (Advia 120; Bayer Corporation, Norwood, MA) within 4 h after blood was collected.
The spleen was removed and weighed, and tissue imprints were made for cytological evaluation. A section of the spleen was placed in 10% formalin for routine histology analysis. A single-cell suspension was made from the rest of the spleen for flow cytometic analysis. In brief, the spleen was finely minced with sterile scissors, resuspended in medium, and transferred to a 70-μm nylon cell strainer (BD Biosciences, San Jose, CA). The parenchyma was pressed through the strainer, medium was added, and cells were allowed to settle for ∼15 min.
The sternum was removed and placed in 10% formalin for routine histology analysis. Tissue imprints were made for cytological evaluation. BM was sterilely harvested from both femurs and tibias. In brief, intact bones with their musculature removed were placed in 70% ethanol for 2 min for disinfection and then washed with sterile phosphate-buffered saline. BM was flushed from the right tibia with Iscove's modified Dulbecco's medium (IMDM) containing 2% fetal bovine serum, and a single-cell suspension was prepared. One aliquot of BM was counted (AcT hematology analyzer; Beckman/Coulter, Holbrook, NY) and frozen for qPCR. The remaining BM was processed for hematopoietic progenitor assays, flow cytometric analysis, and culture for cytokine analysis.
DNA extraction and qPCR.
DNA was extracted from 50 μl of whole blood and 2.5 × 105 unfractionated BM cells from each mouse using a Qiagen DNeasy tissue kit (Qiagen, Valencia, CA) according to manufacturer's instructions and exactly as previously described (10, 11). DNA amplification to determine the A. phagocytophilum p44 copy number, data acquisition, and data analysis were performed with an ABI Prism 7700 sequence detector (Perkin-Elmer, Applied Biosystems, Foster City, CA) exactly as previously described (10, 32).
Hematopoietic progenitor cell (HPC) assays.
Aliquots of unfractionated BM were plated in duplicate according to the manufacturer's instructions in 35-mm tissue culture dishes and cultured in methylcellulose medium with recombinant cytokines for colony-forming (CFU) assays of murine cells (MethoCult GF M3434; StemCell Technologies, Vancouver, British Columbia, Canada). Aliquots of unfractionated splenic cells were plated in duplicate using identical methods. Colonies derived from mixed granulocyte-macrophage, granulocyte or macrophage (CFU-GM colonies), and erythroid (BFU-E colonies) progenitors were scored after 7 to 10 days of incubation at 37°C with 5% CO2. Megakaryopoiesis cannot be evaluated using this system as the serum-based medium contains transforming growth factor β (TGF-β), which inhibits megakaryocyte production.
Flow cytometry.
Single-cell suspensions of BM and spleen cells were labeled with antibodies to detect granulocytes (Gr-1), erythrocytes (TER-119, which labels proerythroblasts to mature erythrocytes), or B lymphocytes (B220) by flow cytometry. Briefly, cells were isolated from tissue, and the concentration was adjusted to 1 × 106 cells/ml in staining buffer (phosphate-buffered saline with 3% fetal bovine serum). Then 2.5 × 105 cells were labeled with Gr-1-phycoerythrin (0.025 μg; BD Pharmingen, San Jose, CA), TER119-phycoerythrin (0.05 μg; BD Pharmingen), or B220-fluorescein isothiocyanate (0.125 μg; R&D Systems, Minneapolis, MN). Fluorescein isothiocyanate- and phycoerythrin-conjugated isotype antibodies were used to prepare negative controls. Cells were incubated with antibody at room temperature for 20 min, washed, fixed for 30 min at room temperature with CytoPerm/CytoFix (BD Biosciences), and washed again in staining buffer. Samples were stored at 4°C overnight before they were analyzed with a Cytomics FC500 flow cytometer using CXP software (Beckman Coulter, Miami, FL); 10,000 events were acquired per sample.
Spontaneous BM cytokine production.
BM cells from infected and uninfected mice (days 2 and 6 postinfection) were washed twice with IMDM (1,500 rpm, 8 min), placed on top of 10 ml of Lymphoprep (Greiner Bio-One, Monroe, NC), and centrifuged at 1,700 rpm for 20 min. The mononuclear cells were removed, washed, and resuspended in IMDM. Cells were diluted by adding 100 μl of cells to 900 μl of StemSpan medium (StemCell Technologies) and plated at a final concentration of 7.5 × 105 cells/ml/well in a 24-well culture plate (Corning, Cambridge, MA). BM supernatants were collected after 24, 48, and 72 h and frozen at −80°C until they were analyzed. All BM supernatants were submitted to the Cytokine Reference Laboratory (University of Minnesota). BM cytokine concentrations were determined for undiluted samples by performing a multiplex bead-based assay using the Luminex system (Luminex Systems, Austin, TX), Bioplex software (Bio-Rad, Hercules, CA), and mouse-specific bead sets (R&D Systems) according to the manufacturers' instructions. Concentrations were interpolated from standard curves for recombinant mouse proteins (R&D Systems). The cytokines measured included granulocyte-monocyte colony-stimulating factor, tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein 1 (JE), keratinocyte-derived chemokine (KC), macrophage inflammatory protein 2 (MIP-2), IL-1β, IL-6, and gamma interferon (IFN-γ). TGF-β1 was assayed separately using a standard enzyme-linked immunosorbent assay format (Cytokine Reference Laboratory, University of Minnesota).
RNA isolation and real-time qPCR.
Total BM RNA was obtained from banked BM cells stored at −80°C. RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA). RNA was isolated using the manufacturer's protocol. cDNA was prepared using a QuantiTect reverse transcription kit (Qiagen, Valencia, CA). Expression of CXCL12 was assessed using an inventoried gene expression assay (Applied Biosystems, Foster City, CA). A murine glyceraldehyde-3-phosphate dehydrogenase assay was performed as an endogenous control, using a gene expression assay (catalog no. 4352932E; Applied Biosystems, Foster City, CA).
Statistical analyses.
Statistical analyses were performed using Student's t test (Microsoft Office Excel 2003; Microsoft Corporation, Bellevue, WA) or simple regression analysis (InStat 3 for Windows; GraphPad Software, Inc., San Diego, CA). A P value of <0.05 was considered significant.
RESULTS
Mice infected with A. phagocytophilum via tick bites become bicytopenic.
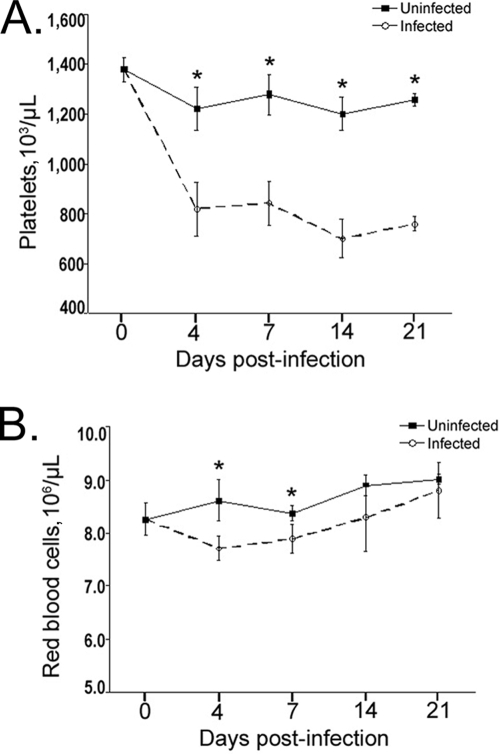
Cytopenias characterize A. phagocytophilum infection of humans, other animals (5, 43, 48), and mice following i.p. inoculation (10, 12, 33). However, the occurrence of cytopenias in mice when they are infected via tick bites, the physiologically relevant route of infection, has not been established. Thrombocytopenia occurred in all infected mice. It occurred rapidly, by day 4 postinfection, and persisted throughout the duration of the study (3 weeks) (Fig. 1A) (P < 0.01 for all days sampled). The platelet counts for infected mice were 33% to 52% lower than the platelet counts for uninfected mice on all sampling dates. The mean platelet volumes for infected and uninfected mice did not differ on any day postinfection (P > 0.05 on all days sampled) (data not shown). Infected mice also became anemic, as shown by a significant decrease in the number of red blood cells on days 4 and 7 postinfection (P < 0.03 for both days) (Fig. 1B). The anemia was mild and lasted only a short time, consistent with the anemia observed in naturally infected animals, humans, and the i.p. infection murine model (5, 33, 43). Leukocyte abnormalities, including leukopenia and lymphopenia, were not observed in this study; however, they have been described in previous studies (4, 7, 10).
FIG. 1.
Hematologic changes induced by tick-borne A. phagocytophilum infection. Mice were infected via tick bites and bled at days 0, 4, 7, 14, and 21 postinfestation. (A) Mice became moderately thrombocytopenic on day 4 after infestation. The thrombocytopenia was sustained for the duration of the study (through day 21) with an approximately 50% reduction in the number of circulating platelets. (B) Mice were anemic (decreased number of red blood cells) on days 4 and 7 postinfection. For all experiments, there were four mice per group, the bars indicate the standard errors of the means, and an asterisk indicates a significant difference from the results for uninfected control mice sampled on the same day; the experiment was repeated once. Hematologic data for mice infected via i.p. injection have been published previously (10, 12, 33).
A. phagocytophilum infection results in profound multilineage alterations in committed HPC populations in BM and in the spleen.
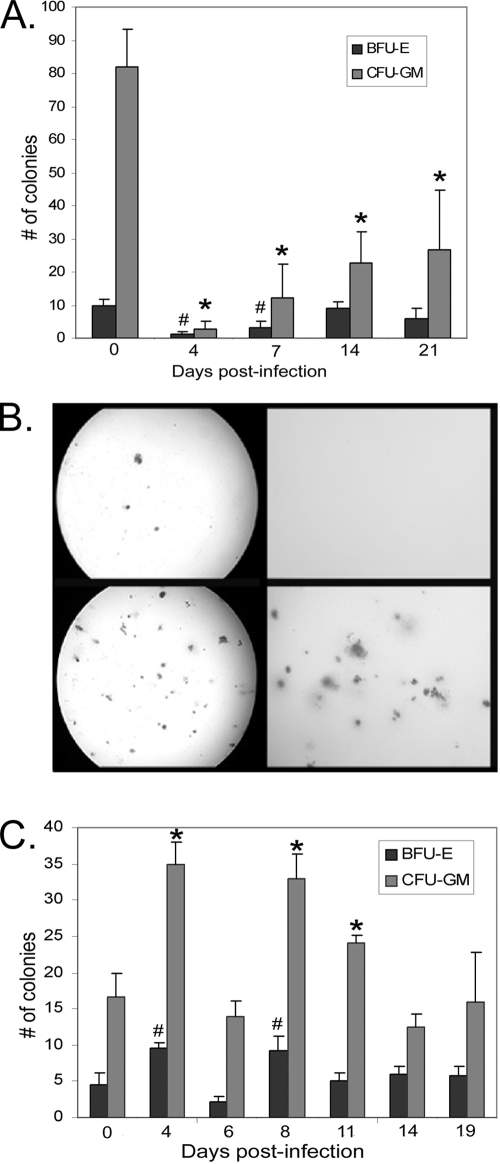
Lineage-committed myeloid cells arise from multipotent progenitor cells in the BM and, potentially, the spleen. These restricted progenitor cells can be enumerated using CFU assays. A. phagocytophilum-infected mice showed a marked decrease in total BM colony formation from day 4 to day 21 postinfection. Significant decreases were noted in both granulocytic-macrophage (CFU-GM) and erythroid (BFU-E) lineages (Fig. 2A and 2B [the data shown are data for tick infestation]) (P < 0.05 for all days sampled). Marked decreases in the number of BM CFU also occurred after i.p. infection with A. phagocytophilum (data not shown). Our findings support the conclusion that there were infection-induced global decreases in the number of lineage-restricted colony-forming cells in murine BM. Conversely, A. phagocytophilum infection resulted in a marked increase in the number of splenic CFU (Fig. 2C). This increase included a significant increase in the number of CFU-GM colonies on days 4, 8, and 11 postinfection (Fig. 2C) (P < 0.05) and a significant increase in the number of BFU-E colonies on days 4 and 8 postinfection (Fig. 2C) (P < 0.05). These findings support the conclusion that there was infection-induced augmentation of HPCs in the spleen as a result of mobilization of progenitor cells from BM or expansion of preexisting splenic progenitor cells.
FIG. 2.
A. phagocytophilum infection results in a global reduction in the number of BM CFU and a global increase in the number of splenic CFU. Unfractionated BM or splenic cells were plated in semisolid methocellulose medium, and CFU assays were performed to determine the frequency of progenitor cells after A. phagocytophilum infection. (A) The number of BM granulocyte-monocyte (CFU-GM) progenitors (light gray bars) was significantly reduced on days 4 to 21 after tick-borne infection compared to the results for control mice infested with uninfected nymphal ticks (*, P < 0.05). The number of BM erythroid (BFU-E) progenitors (dark gray bars) was significantly de creased on days 4 and 7 after tick-borne infection compared to the results for mice infested with uninfected nymphal ticks (#, P < 0.05). The bars indicate the average number of colonies from at least four mice per group, and the error bars indicate the standard errors of the means. (B) Photomicrographs from representative BM colony assays after tick-borne infection. (Upper panels) Low-power (left panel) and high-power (right panel) images for CFU assays for an infected mouse at day 7 postinfection. The few colonies present are almost exclusively granulocyte, macrophage, or mixed granulocyte-macrophage colonies. No BFU-E colonies are evident in these images. (Lower panels) Low-power (left panel) and high-power (right panel) images for CFU assays for an uninfected mouse at day 7 postinfection. The visible colonies are almost exclusively granulocyte, macrophage, or mixed granulocyte-macrophage colonies. Individual BFU-E colonies are not evident at the magnifications used. (C) The number of splenic CFU-GM progenitors was significantly increased (light gray bars) on days 4, 8, and 11 postinfection compared to the results for uninfected control mice (*, P < 0.05). The numbers of splenic erythroid (BFU-E) progenitors (dark gray bars) were significantly increased on days 4 and 8 postinfection compared to the results for uninfected mice (#, P < 0.05). The bars indicate the average numbers of colonies from at least four mice per group, and the error bars indicate the standard errors of the means; the experiment was repeated once. Increased numbers of splenic CFU may represent infection-induced mobilization of BM progenitor cells to the spleen or expansion of preexisting splenic progenitor cells.
BM alterations are associated with a predominantly myelosuppressive cytokine profile.
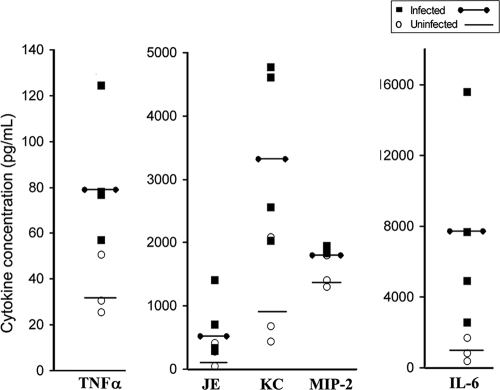
Hematopoiesis is regulated by a variety of chemokines, cytokines, and growth factors. Infection-induced alterations in these soluble BM factors may contribute to myelosuppression. We hypothesized that infection would induce myelosuppressive cytokines preferentially over proinflammatory or stimulatory cytokines, including growth factors. A. phagocytophilum infection did not modulate the BM concentrations of granulocyte-monocyte colony-stimulating factor, IL-1β, IFN-γ, or TGF-β1 (data not shown). There were no differences in the cytokines produced by BM cells taken from infected and uninfected mice on day 2 postinfection. Conversely, BM cells taken from mice on day 6 postinfection produced significantly more KC, MIP-2, JE, TNF-α, and IL-6 than BM cells taken from uninfected mice produced after 24, 48, and 72 h of ex vivo culture (Fig. 3 [the data shown are cytokine concentrations after ex vivo culture for 72 h]). The CXC chemokines KC and MIP-2 and the CC chemokine JE are well-documented myelosuppressive chemokines (13, 14, 16, 52), whereas IL-6 and TNF-α are classic proinflammatory cytokines. The level of TNF-α was elevated twofold compared to the level for uninfected mice (Fig. 3) and was ∼80 pg/ml, a concentration far lower than the blood concentration of TNF-α induced by Escherichia coli (64). Our findings are compatible with the findings obtained in a previous study performed with human BM cells (35) and support the hypothesis that there is a mixed BM cytokine response skewed toward myelosuppressive chemokine production.
FIG. 3.
A. phagocytophilum infection modulates BM cytokine production. BM cells from infected and uninfected mice were isolated and plated in medium for 72 h. BM supernatant was harvested, and the cytokine protein concentrations were measured using multiplex Luminex technology. BM cells isolated from mice at day 6 after i.p. infection produced significantly elevated levels of TNF-α, JE, KC, MIP-2, and IL-6 compared to BM cells isolated from uninfected mice (P < 0.05 for all comparisons). Each filled square represents an infected mouse, each open circle represents an uninfected mouse, four mice were used for each time point, the lines indicate the average concentrations for infected or uninfected mice, and the experiment was repeated twice.
A. phagocytophilum-induced myelosuppression and thrombocytopenia are independent of the pathogen burden in BM and blood, respectively.
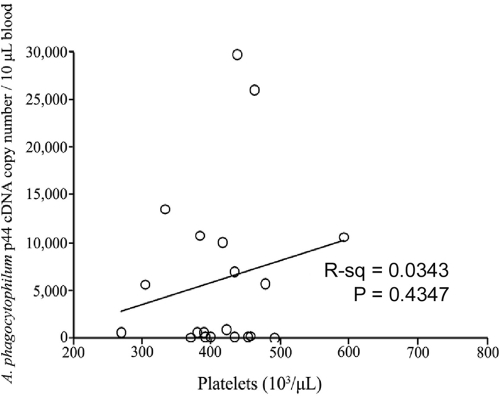
A. phagocytophilum infection is an acute infection in C3H mice, and the peak pathogen DNA level is found in peripheral blood from day 7 to day 12 postinfection (1, 33). C3H mice generally fully clear A. phagocytophilum by 3 to 4 weeks postinfection (32, 33). In our studies, all control mice were PCR negative for A. phagocytophilum DNA at all time points. The peripheral blood of all infected mice was PCR positive. Unfractionated BM was PCR positive for 87.5% of the experimental mice. The quantity of pathogen DNA (per 2.5 × 105 cells) was greatest in peripheral blood (Table 1). In blood, infection peaked at day 7 and for the most part was cleared by day 21 postinfection. The pathogen burden in BM was <1% of that in whole blood from the same animal (Table 1). Thrombocytopenia is the most consistent hematologic alteration associated with GA in all species (4, 12, 43, 47). Thrombocytopenia was present even when the pathogen burden in blood was negligible (Fig. 1 and 4). The pathogen burden in blood did not correlate with platelet number (Fig. 4) (R2 = 0.03, P = 0.43). These findings suggest that alterations in hematopoietic proliferation or differentiation are not directly related to the presence of significant pathogen DNA in BM. However, even a small pathogen presence could play a large role in hemic cell trafficking or cytokine production in BM. Similar to other reports (17, 53), we found that significant infection-induced pathology, including myelosuppression and thrombocytopenia, is likely not related to the presence of the pathogen in affected tissues.
TABLE 1.
Pathogen burden is greatly increased in the blood compared with BM after tick-borne infectiona
| Day postinfection | Avg A. phagocytophilum p44 DNA copy no./2.5 × 105 cells
|
|
|---|---|---|
| Blood | BM | |
| 4 | 27,015 | 87 |
| 7 | 34,481 | 30 |
| 14 | 8,192 | 64 |
| 21 | 431 | 4 |
The average A. phagocytophilum DNA copy number in mouse blood and BM was determined on days 4 to 21 after tick-borne infection. The level of pathogen DNA was significantly higher in blood than in BM.
FIG. 4.
Pathogen burden in blood is not related to thrombocytopenia. There was no correlation (R2 = 0.03, P = 0.43) between the pathogen burden in blood and the platelet count on any day postinfection, and the experiment was repeated twice. The mice with the lowest platelet counts often had very low pathogen burdens, as assessed by qPCR for A. phagocytophilum p44 DNA.
A. phagocytophilum infection results in a constellation of changes in hematopoietic cell compartments.
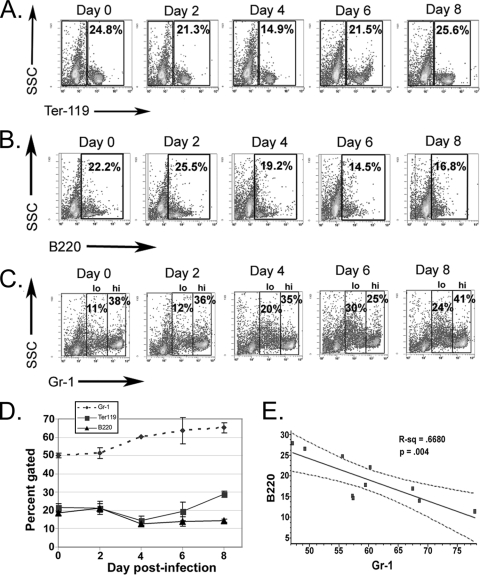
Given the profound loss of committed progenitor cells in the BM, we assessed specific kinetic alterations in the erythroid, granulocytic, and lymphoid BM lineages. Infection with the LPS-negative pathogen A. phagocytophilum resulted in significant depletion of Ter-119+ BM erythroid cells on day 4 postinfection (P = 0.02) (Fig. 5A), concurrent with anemia. Depletion of BM B lymphocytes began at day 6 postinfection (P = 0.08) (Fig. 5B), and there was a significantly lower percentage of B lymphocytes in BM by day 8 postinfection (P = 0.007) (Fig. 5B). Reciprocal hyperplasia was noted in the granulocytic lineage along with significant increases in the total number of Gr-1+ cells on days 4 to 8 postinfection (P ≤ 0.01 for all days) (Fig. 5C). Gr-1lo/int cells represent mitotic granulocyte progenitor cells (promyelocytes and myelocytes) (56, 58). A wave of mitotic activity in the granulocyte lineage (Gr-1lo/int cells) began at day 4 postinfection (20% of all Gr-1+ cells, compared to 11 to 12% of the cells in control mice), and there was a significantly increased granulocyte mitotic pool on days 6 and 8 postinfection (P < 0.001 for both days) (Fig. 5C), concurrent with B-lymphocyte and erythroid depletion (Fig. 5D). Infection with A. phagocytophilum elicits an inflammation-like profile of changes in BM (58) with a strong reciprocal relationship between granulocytic hyperplasia and B-lymphocyte depletion (R2 = 0.67) (Fig. 5E) (37). However, unlike the effects of other inflammatory stimuli, such as endotoxin and TNF-α, A. phagocytophilum infection-induced alterations in the BM are accompanied by thrombocytopenia and anemia but not by a reactive neutrophilia.
FIG. 5.
A. phagocytophilum infection results in dynamic and substantial alterations in BM lineage-committed cells. BM was harvested from infected and control mice on days 0, 2, 4, 6, and 8 after i.p. infection. Cells were labeled with lineage-specific markers that recognize erythrocytes (Ter-119), B lymphocytes (B220), and granulocytes (Gr-1). The plots are representative dot plots (the experiment was repeated twice). (A) There was a significant depletion of erythroid precursors on day 4 postinfection (P = 0.02). (B) The number of B lymphocytes began to decrease at day 6 postinfection and was significantly lower in infected mice by day 8 postinfection (P = 0.007). (C) Infected mice had significantly increased numbers of granulocytes in their BM on days 4 to 8 postinfection (P < 0.01 for all comparisons), and there was a specific increase in the mitotic granulocytic compartment (Gr-1lo/int) on days 6 and 8 postinfection (P < 0.005 for both comparisons). (D) Alterations in BM lineage-committed cells over the course of infection. BM from infected mice undergoes B-lymphocyte and erythroid depletion and concurrent granulocytic hyperplasia that peaks at day 6 postinfection. The error bars indicate the standard errors of the means. (E) There was a strong and significant reciprocal correlation (R2 = 0.668, P = 0.004) between the percentage of B lymphocytes and the percentage of granulocytes in the BM of infected mice.
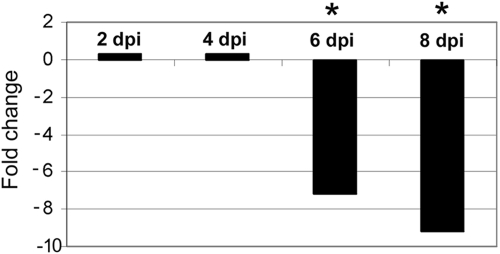
BM lineage shifts are accompanied by decreased expression of CXCL12.
The mechanisms responsible for A. phagocytophilum-induced hematopoietic alterations in progenitor and lineage-committed cells are not known. However, in one model of immunization-induced inflammation, decreased CXCL12 expression in BM cells was associated with increased granulopoiesis and B-lymphocyte depletion from the BM (59). We hypothesized that A. phagocytophilum infection may similarly result in downregulation of BM CXCL12. The expression of CXCL12 mRNA was significantly decreased (seven- to ninefold) in total BM cells on days 6 and 8 after i.p. infection compared to the expression in uninfected mice (Fig. 6). This significant decrease in CXCL12 expression was closely correlated with the B-lymphoid depletion and increased granulopoiesis that were observed (Fig. 5D and 6). Our findings suggest that CXCL12 may favor B-lymphocyte retention within the BM during A. phagocytophilum infection.
FIG. 6.
Relative CXCL12 expression in BM obtained from infected and uninfected mice. There was significant downregulation of CXCL12 in BM from infected mice on days 6 (sevenfold) and 8 (ninefold) postinfection compared to the results for uninfected mice (*, P < 0.05). dpi, day postinfection.
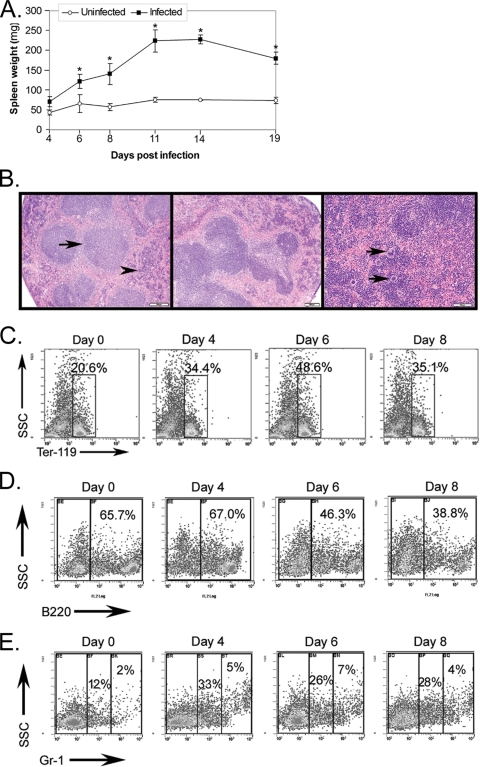
Infection with A. phagocytophilum results in marked splenomegaly characterized by lymphoid hyperplasia and extramedullary hematopoiesis, predominantly erythropoiesis.
Splenomegaly is a well-characterized pathology associated with A. phagocytophilum infection (33). In addition, inflammation and endotoxin can induce BM granulocytic hyperplasia and shunting of erythropoiesis to the spleen (27, 50). Given our comparable BM findings, we investigated whether splenomegaly may be due in part to increased numbers of lineage-committed progenitor cells. Spleen weight was significantly increased by day 6 postinfection and remained significantly increased through day 19 postinfection (P < 0.05 for all days) (Fig. 7A). This splenomegaly was associated with marked hypercellularity (data not shown). The histopathologic evaluation results concurred with previous observations indicating that splenomegaly was due to expansion of lymphoid follicles (lymphoid hyperplasia) and marked extramedullary hematopoiesis in the red pulp (Fig. 7B) (33). Hematopoietic alterations were characterized by a significant expansion of Ter-119+ erythroid precursors at days 6 to 8 postinfection (P < 0.001) (Fig. 7C), significant increases in the number of B lymphocytes at days 6 to 8 postinfection (P < 0.001) (Fig. 7D), and significant marked increases in the number of Gr-1+ granulocytic cells at days 4 to 8 postinfection (P < 0.001) (Fig. 7E). Splenomegaly associated with lymphoid hyperplasia and extramedullary hematopoiesis accompanies many inflammatory conditions, and a shift of erythropoiesis from the BM to the spleen is a well-characterized response to endotoxin and inflammation in the mouse (27, 50, 57).
FIG. 7.
A. phagocytophilum infection results in splenomegaly characterized by lymphoid hyperplasia and extramedullary hematopoiesis, regardless of the route of inoculation. (A) Significant splenomegaly occurred by day 6 postinfection and was sustained through day 19 postinfection, the last day sampled. The error bars indicate the standard errors of the means, and asterisks indicate significant differences between infected and uninfected mice on the same day. (B) Representative histologic sections of spleens stained with routine hematoxylin and eosin. Photomicrographs were taken using a BX40 microscope, a DP25 camera, and DP2-BSW v. 1.4 software (all obtained from Olympus America Inc., Center Valley, PA). (Left panel) Normal murine spleen at day 0 with uniform, small lymphoid follicles (arrow) and mildly cellular red pulp (arrowhead) (magnification, ×4). (Middle panel) Spleen from tick-infected mouse at day 6 with expansion and bridging of lymphoid follicles (magnification, ×4). (Right panel) Spleen from tick-infected mouse at day 8 with a marked increase in red pulp cellularity, including an increase in megakaryocytes (arrows) and darkly stained erythroid precursors (extramedullary hematopoiesis) (magnification, ×10). (C to E) Splenic cells were harvested from infected and control mice on days 0, 2, 4, 6, and 8 after i.p. infection. Cells were labeled with lineage-specific markers that recognize erythrocytes (Ter-119), B lymphocytes (B220), and granulocytes (Gr-1). The plots are representative dot plots. (C) There was a significant increase in the absolute numbers of erythroid precursors at days 6 to 8 postinfection (P < 0.001 for both comparisons). (D) The relative percentage of B lymphocytes was decreased at days 6 to 8 postinfection. However, due to splenomegaly, the absolute numbers of B lymphocytes were significantly increased at days 6 to 8 postinfection (P < 0.001 for both comparisons). (E) Infected mice had significantly increased numbers of granulocytic cells in their spleens on days 4 to 8 postinfection (P < 0.001 for all comparisons). The experiment was performed twice.
DISCUSSION
A. phagocytophilum is an obligate intracellular, endotoxin-negative pathogen (38) that induces significant alterations in BM and spleen HPCs and peripheral blood cells. Specifically, A. phagocytophilum infection results in anemia and thrombocytopenia in blood, granulocyte hyperplasia and erythroid and B-lymphocyte depletion in the BM, and concomitant increased hematopoiesis in the spleen. These effects mimic those of endotoxin and other inflammatory stimuli, for which early observations revealed splenomegaly due to erythropoiesis and overall BM hypocellularity due to decreased numbers of erythroid cells, along with concurrent increases in granulopoiesis (27, 50, 57, 65). Our findings and other findings support the hypothesis that there is a nonspecific yet highly conserved and orchestrated pattern of hematopoietic alterations resulting from a variety of stimuli, including infection with Anaplasmataceae family pathogens (A. phagocytophilum and Ehrlichia muris [42]) and E. coli (54), administration of endotoxin (27, 57, 65) or turpentine (50), immunization (acting via TNF-α) (58, 59), and shock and injury (40).
Inflammation-mediated shifts in hematopoietic subsets generally result in peripheral blood changes that facilitate infection control, including heightened production and release of neutrophils (neutrophilia and left shift), lymphopenia (altered lymphocyte trafficking and homing to nodes), and sequestration of iron with resultant anemia of inflammatory disease. Infection with many of the Anaplasmataceae family pathogens also results in mild anemia and variable lymphopenia; however, neutrophilia and left shift, reflecting BM hyperplasia, are rarely observed. As A. phagocytophilum resides primarily in host neutrophils, this unique host cell-pathogen interaction may contribute to the absence of neutrophilia in spite of the induction of inflammation-like BM changes. A. phagocytophilum survival strategies include numerous specific host cell functional alterations, such as downregulation of the host cell proinflammatory response (9), reduced oxidative burst (19), and delayed apoptosis (9, 28, 63), many of which may alter neutrophil trafficking. A. phagocytophilum does not appear to rely on Toll-like receptors for stimulation of the innate immune response (60). Pathogen infection also does not elicit a typical acute-phase response characterized by systemic TNF-α and IL-1 cytokine production; however, it does result in production of the chemokine IL-8 (2). Thus, granulocyte hyperplasia in the BM with increased neutrophil release may be mitigated by infection-induced IL-8 production and neutrophil functional alterations directing the increased numbers of neutrophils out of the circulating blood pool. IL-8 production may direct neutrophil trafficking to include splenic or endothelial sequestration. Tissue or endothelial sequestration of neutrophils may promote pathogen transfer.
The marked decrease in lineage-committed progenitor cells in BM may be a result of HPC mobilization, apoptosis, or failure of progenitor differentiation or proliferation. These results are unlikely to be directly attributable to altered stromal cell function or cytokine production as the CFU assays are performed in the absence of stromal cells and with cytokine supplementation. Mobilization is suggested by previous work (27, 40, 54, 58, 59, 64) and by hematopoietic alterations that we noted in the spleen; however, further work is need to fully delineate the mechanism(s) underlying the loss of lineage-restricted progenitor cells from BM. Furthermore, in the absence of cytokine supplementation, BM cells from infected mice exhibited a mixed cytokine-chemokine secretion profile. Notably, production of the murine IL-8 homologues and production of IL-6 are significantly increased. Although it is tempting to speculate that IL-6 production could be partially responsible for the granulocytic hyperplasia noted on days 6 to 8 postinfection, in a separate experiment specific antagonism of IL-6 did not reverse the hyperplasia (data not shown). The IL-8 homologues JE and KC are myelosuppressive (16, 18). Given the role hypothesized for IL-8 in this neutrophil-tropic infection (2), hematopoietic myelosuppression may be a prominent indirect side effect of infection. The absence of IFN-γ production in BM supernatants is interesting given the prominent systemic production of IFN-γ during A. phagocytophilum infection (1). IFN-γ is produced primarily by T lymphocytes (CD3+) and natural killer cells. The concentration of IFN-γ in a particular tissue depends on the number of cells producing the cytokine, as well as the degree of cellular stimulation resulting in activation. We propose that the absence of IFN-γ production in BM supernatants is due to the relative absence of CD3+ cells in BM of C3H mice (∼3.5% of BM cells in C3H mice) and the abundance of these cells in the spleen (∼25% of splenocytes in C3H mice). The spleen also differs from BM in having functional natural killer cells (23). The BM and spleen may also have different levels of cellular stimulation, resulting in variable IFN-γ secretion. Systemic effects of increased IFN-γ levels may influence the BM compartment despite a lack of increased local production by BM cells.
Thrombocytopenia is the hallmark of infection with many Anaplasmataceae family pathogens (particularly Ehrlichia spp., A. phagocytophilum, and Anaplasma platys) (4, 12, 43, 45-47), yet the mechanism of this process is largely unknown. Our work did not specifically investigate BM megakaryopoiesis or mechanisms of peripheral destruction or sequestration. However, marked thrombocytopenia was noted with no evidence of increased mean platelet volume, suggesting that there was a poor BM response to thrombocytopenia in the time frame measured. In addition, routine cytologic and histopathologic evaluation of BM over the course of infection revealed no pattern of megakaryocyte hyperplasia or hypoplasia. This is not surprising as significant infection-induced effects on lineage-committed progenitors in BM are minimally shown by histopathologic evaluation alone. Although there was a weak positive correlation between platelet number and pathogen burden, the findings were not statistically significant and are contrary to what would be expected if thrombocytopenia was a direct result of platelet-pathogen interaction (an increased number of pathogens would result in a decreased number of platelets, a negative or inverse correlation). These findings are consistent with the general conclusion that pathological changes associated with A. phagocytophilum infection are largely independent of direct pathogen infection (17, 53).
Our research demonstrates that A. phagocytophilum infection induces cytopenias, shifts in hematopoietic cell subsets, alterations in BM cytokines, and decreases in the number of lineage-restricted cells in BM (colony assays) regardless of the route of infection. Although these data are consistent with research that examined tissue distribution and copy number of A. phagocytophilum after i.p. and tick-borne inoculation (32), there are subtle differences between the models. In our hands, tick-borne infection almost always elicits more profound changes. For example, the thrombocytopenia lasts longer (>21 days in the current work versus 10 days after i.p. infection) (12), and the decrease in hematopoietic activity is more substantial and prolonged (>21 days in the current work, compared to 12 to 14 days after i.p. infection [data not shown]) after physiologic infection. We postulate that this may be due to the immunomodulatory and infection-promoting effects of tick saliva (6, 29, 62) that do not occur with needle inoculation.
Many infectious agents, especially viral pathogens, cause marked alterations in HPCs either by mobilization, apoptosis, or failure to differentiate or proliferate (24, 55). In addition, several non-LPS pathogens, notably Plasmodium falciparum (3, 31) and Leishmania spp. (21, 30), also alter hematopoiesis. The alterations are variably attributed to pathogen persistence (31), cytokine-mediated BM suppression (21, 30), and hemic cell apoptosis (3). Our findings suggest that BM dysfunction due to infections with Anaplasmataceae family pathogens more likely results from cytokine-mediated effects than from direct BM infection and pathogen persistence. Elucidation of the mechanisms underlying alterations in hematopoiesis may permit more directed interventions for the occasionally severe hematologic consequences of these infections.
Acknowledgments
We thank Emir Hodzic, Rachael Racine, Regina Feferman, and Kim Freet for technical assistance. We also thank the Cytokine Reference Laboratory at the University of Minnesota and the Wadsworth Center Flow Cytometry Facility for assistance with data collection and analysis.
This work was supported in part by grant 5R01AI06467803 to G.M.W. from the NIH.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 29 June 2009.
REFERENCES
- 1.Akkoyunlu, M., and E. Fikrig. 2000. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect. Immun. 681827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkoyunlu, M., S. E. Malawista, J. Anguita, and E. Fikrig. 2001. Exploitation of interleukin-8-induced neutrophil chemotaxis by the agent of human granulocytic ehrlichiosis. Infect. Immun. 695577-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anantrakulsil, S., Y. Maneerat, P. Wilairatana, S. Krudsood, C. Arunsuriyasak, V. Atichartakarn, R. Kumsiri, K. Pattanapanyasat, S. Looareesuwan, and R. Udomsangpetch. 2005. Hematopoietic features and apoptosis in the bone marrow of severe Plasmodium falciparum-infected patients: preliminary study. Southeast Asian J. Trop. Med. Public Health 36543-551. [PubMed] [Google Scholar]
- 4.Bakken, J. S., M. E. Aguero-Rosenfeld, R. L. Tilden, G. P. Wormser, H. W. Horowitz, J. T. Raffalli, M. Baluch, D. Riddell, J. J. Walls, and J. S. Dumler. 2001. Serial measurements of hematologic counts during the active phase of human granulocytic ehrlichiosis. Clin. Infect. Dis. 32862-870. [DOI] [PubMed] [Google Scholar]
- 5.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275199-205. [PubMed] [Google Scholar]
- 6.Barriga, O. O. 1999. Evidence and mechanisms of immunosuppression in tick infestations. Genet. Anal. 15139-142. [DOI] [PubMed] [Google Scholar]
- 7.Batungbacal, M. R., G. R. Scott, and C. Burrells. 1982. The lymphocytopaenia in tick-borne fever. J. Comp. Pathol. 92403-407. [DOI] [PubMed] [Google Scholar]
- 8.Bayard-Mc Neeley, M., A. Bansal, I. Chowdhury, G. Girao, C. B. Small, K. Seiter, J. Nelson, D. Liveris, I. Schwartz, D. F. Mc Neeley, G. P. Wormser, and M. E. Aguero-Rosenfeld. 2004. In vivo and in vitro studies on Anaplasma phagocytophilum infection of the myeloid cells of a patient with chronic myelogenous leukaemia and human granulocytic ehrlichiosis. J. Clin. Pathol. 57499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borjesson, D. L., S. D. Kobayashi, A. R. Whitney, J. M. Voyich, C. M. Argue, and F. R. Deleo. 2005. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J. Immunol. 1746364-6372. [DOI] [PubMed] [Google Scholar]
- 10.Borjesson, D. L., S. I. Simon, E. Hodzic, C. M. Ballantyne, and S. W. Barthold. 2002. Kinetics of CD11b/CD18 upregulation during infection with the agent of human granulocytic ehrlichiosis in mice. Lab. Investig. 82303-311. [DOI] [PubMed] [Google Scholar]
- 11.Borjesson, D. L., S. I. Simon, E. Hodzic, H. E. DeCock, C. M. Ballantyne, and S. W. Barthold. 2003. Roles of neutrophil beta 2 integrins in kinetics of bacteremia, extravasation, and tick acquisition of Anaplasma phagocytophila in mice. Blood 1013257-3264. [DOI] [PubMed] [Google Scholar]
- 12.Borjesson, D. L., S. I. Simon, F. Tablin, and S. W. Barthold. 2001. Thrombocytopenia in a mouse model of human granulocytic ehrlichiosis. J. Infect. Dis. 1841475-1479. [DOI] [PubMed] [Google Scholar]
- 13.Broxmeyer, H. E., S. Cooper, G. Cacalano, N. L. Hague, E. Bailish, and M. W. Moore. 1996. Involvement of interleukin (IL) 8 receptor in negative regulation of myeloid progenitor cells in vivo: evidence from mice lacking the murine IL-8 receptor homologue. J. Exp. Med. 1841825-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broxmeyer, H. E., and C. H. Kim. 1999. Regulation of hematopoiesis in a sea of chemokine family members with a plethora of redundant activities. Exp. Hematol. 271113-1123. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer, H. E., C. M. Orschell, D. W. Clapp, G. Hangoc, S. Cooper, P. A. Plett, W. C. Liles, X. Li, B. Graham-Evans, T. B. Campbell, G. Calandra, G. Bridger, D. C. Dale, and E. F. Srour. 2005. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2011307-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broxmeyer, H. E., B. Sherry, S. Cooper, L. Lu, R. Maze, M. P. Beckmann, A. Cerami, and P. Ralph. 1993. Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. Interacting effects involving suppression, synergistic suppression, and blocking of suppression. J. Immunol. 1503448-3458. [PubMed] [Google Scholar]
- 17.Bunnell, J. E., E. R. Trigiani, S. R. Srinivas, and J. S. Dumler. 1999. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J. Infect. Dis. 180546-550. [DOI] [PubMed] [Google Scholar]
- 18.Cacalano, G., J. Lee, K. Kikly, A. M. Ryan, S. Pitts-Meek, B. Hultgren, W. I. Wood, and M. W. Moore. 1994. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science 265682-684. [DOI] [PubMed] [Google Scholar]
- 19.Carlyon, J. A., and E. Fikrig. 2006. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr. Opin. Hematol. 1328-33. [DOI] [PubMed] [Google Scholar]
- 20.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotterell, S. E., C. R. Engwerda, and P. M. Kaye. 2000. Leishmania donovani infection of bone marrow stromal macrophages selectively enhances myelopoiesis, by a mechanism involving GM-CSF and TNF-alpha. Blood 951642-1651. [PubMed] [Google Scholar]
- 22.Crawford, T. B., K. J. Wardrop, S. J. Tornquist, E. Reilich, K. M. Meyers, and T. C. McGuire. 1996. A primary production deficit in the thrombocytopenia of equine infectious anemia. J. Virol. 707842-7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das, A., and R. K. Saxena. 2001. Abrogation of tumor induced Ly49 expression on mouse spleen cells by mitomycin C. Immunol. Lett. 7773-77. [DOI] [PubMed] [Google Scholar]
- 24.Donahue, R. E., M. M. Johnson, L. I. Zon, S. C. Clark, and J. E. Groopman. 1987. Suppression of in vitro haematopoiesis following human immunodeficiency virus infection. Nature 326200-203. [DOI] [PubMed] [Google Scholar]
- 25.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 512145-2165. [DOI] [PubMed] [Google Scholar]
- 26.Dumler, J. S., K. S. Choi, J. C. Garcia-Garcia, N. S. Barat, D. G. Scorpio, J. W. Garyu, D. J. Grab, and J. S. Bakken. 2005. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 111828-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fruhman, G. J. 1967. Endotoxin-induced shunting of erythropoiesis in mice. Am. J. Physiol. 2121095-1098. [DOI] [PubMed] [Google Scholar]
- 28.Ge, Y., K. Yoshiie, F. Kuribayashi, M. Lin, and Y. Rikihisa. 2005. Anaplasma phagocytophilum inhibits human neutrophil apoptosis via upregulation of bfl-1, maintenance of mitochondrial membrane potential and prevention of caspase 3 activation. Cell. Microbiol. 729-38. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie, R. D., M. L. Mbow, and R. G. Titus. 2000. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol. 22319-331. [DOI] [PubMed] [Google Scholar]
- 30.Guilpin, V. O., L. Nosbisch, R. G. Titus, and C. J. Swardson-Olver. 2003. Infection with Leishmania major stimulates haematopoiesis in susceptible BALB/c mice and suppresses haematopoiesis in resistant CBA mice. Parasitology 126187-194. [DOI] [PubMed] [Google Scholar]
- 31.Helleberg, M., B. Q. Goka, B. D. Akanmori, G. Obeng-Adjei, O. Rodriques, and J. A. Kurtzhals. 2005. Bone marrow suppression and severe anaemia associated with persistent Plasmodium falciparum infection in African children with microscopically undetectable parasitaemia. Malaria J. 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodzic, E., S. Feng, D. Fish, C. M. Leutenegger, K. J. Freet, and S. W. Barthold. 2001. Infection of mice with the agent of human granulocytic ehrlichiosis after different routes of inoculation. J. Infect. Dis. 1831781-1786. [DOI] [PubMed] [Google Scholar]
- 33.Hodzic, E., J. W. Ijdo, S. Feng, P. Katavolos, W. Sun, C. H. Maretzki, D. Fish, E. Fikrig, S. R. Telford III, and S. W. Barthold. 1998. Granulocytic ehrlichiosis in the laboratory mouse. J. Infect. Dis. 177737-745. [DOI] [PubMed] [Google Scholar]
- 34.Jongen-Lavrencic, M., H. R. Peeters, H. Rozemuller, W. J. Rombouts, A. C. Martens, G. Vreugdenhil, M. Pillay, P. H. Cox, M. Bijser, G. Brutel, F. C. Breedveld, and A. J. Swaak. 1996. IL-6-induced anaemia in rats: possible pathogenetic implications for anemia observed in chronic inflammations. Clin. Exp. Immunol. 103328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein, M. B., S. Hu, C. C. Chao, and J. L. Goodman. 2000. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 182200-205. [DOI] [PubMed] [Google Scholar]
- 36.Klein, M. B., J. S. Miller, C. M. Nelson, and J. L. Goodman. 1997. Primary bone marrow progenitors of both granulocytic and monocytic lineages are susceptible to infection with the agent of human granulocytic ehrlichiosis. J. Infect. Dis. 1761405-1409. [DOI] [PubMed] [Google Scholar]
- 37.Klinger, M. H., and W. Jelkmann. 2002. Role of blood platelets in infection and inflammation. J. Interferon Cytokine Res. 22913-922. [DOI] [PubMed] [Google Scholar]
- 38.Lee, E. H., and Y. Rikihisa. 1996. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1beta, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect. Immun. 644211-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lepidi, H., J. E. Bunnell, M. E. Martin, J. E. Madigan, S. Stuen, and J. S. Dumler. 2000. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 6229-37. [DOI] [PubMed] [Google Scholar]
- 40.Livingston, D. H., D. Anjaria, J. Wu, C. J. Hauser, V. Chang, E. A. Deitch, and P. Rameshwar. 2003. Bone marrow failure following severe injury in humans. Ann. Surg. 238748-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma, Q., D. Jones, P. R. Borghesani, R. A. Segal, T. Nagasawa, T. Kishimoto, R. T. Bronson, and T. A. Springer. 1998. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA 959448-9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacNamara, K. C., R. Racine, M. Chatterjee, D. Borjesson, and G. M. Winslow. 2009. Diminished hematopoietic activity associated with alterations in innate and adaptive immunity in a mouse model of human monocytic ehrlichiosis. Infect. Immun. 774061-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madigan, J. E., and D. Gribble. 1987. Equine ehrlichiosis in northern California: 49 cases (1968-1981). J. Am. Vet. Med. Assoc. 190445-448. [PubMed] [Google Scholar]
- 44.Mori, T., K. Ando, K. Tanaka, Y. Ikeda, and Y. Koga. 1997. Fas-mediated apoptosis of the hematopoietic progenitor cells in mice infected with murine cytomegalovirus. Blood 893565-3573. [PubMed] [Google Scholar]
- 45.Mylonakis, M. E., A. F. Koutinas, E. B. Breitschwerdt, B. C. Hegarty, C. D. Billinis, L. S. Leontides, and V. S. Kontos. 2004. Chronic canine ehrlichiosis (Ehrlichia canis): a retrospective study of 19 natural cases. J. Am. Anim. Hosp. Assoc. 40174-184. [DOI] [PubMed] [Google Scholar]
- 46.Paparone, P. W., P. Ljubich, G. A. Rosman, and N. T. Nazha. 1995. Ehrlichiosis with pancytopenia and ARDS. N. J. Med. 92381-385. [PubMed] [Google Scholar]
- 47.Pusterla, N., C. M. Leutenegger, J. S. Chae, H. Lutz, R. B. Kimsey, J. S. Dumler, and J. E. Madigan. 1999. Quantitative evaluation of ehrlichial burden in horses after experimental transmission of human granulocytic Ehrlichia agent by intravenous inoculation with infected leukocytes and by infected ticks. J. Clin. Microbiol. 374042-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pusterla, N., J. B. Pusterla, U. Braun, and H. Lutz. 1999. Experimental cross-infections with Ehrlichia phagocytophila and human granulocytic ehrlichia-like agent in cows and horses. Vet. Rec. 145311-314. [DOI] [PubMed] [Google Scholar]
- 49.Quesenberry, P., A. Morley, F. Stohlman, Jr., K. Rickard, D. Howard, and M. Smith. 1972. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N. Engl. J. Med. 286227-232. [DOI] [PubMed] [Google Scholar]
- 50.Reissmann, K. R., and K. B. Udupa. 1978. Effect of inflammation on erythroid precursors (BFU-E and CFU-E) in bone marrow and spleen of mice. J. Lab. Clin. Med. 9222-29. [PubMed] [Google Scholar]
- 51.Robinson, Y., A. Hostmann, A. Matenov, W. Ertel, and A. Oberholzer. 2006. Erythropoiesis in multiply injured patients. J. Trauma 611285-1291. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez, X., K. Suetomi, B. Cousins-Hodges, J. K. Horton, and J. Navarro. 1998. CXC chemokines suppress proliferation of myeloid progenitor cells by activation of the CXC chemokine receptor 2. J. Immunol. 160906-910. [PubMed] [Google Scholar]
- 53.Scorpio, D. G., M. Akkoyunlu, E. Fikrig, and J. S. Dumler. 2004. CXCR2 blockade influences Anaplasma phagocytophilum propagation but not histopathology in the mouse model of human granulocytic anaplasmosis. Clin. Diag. Lab. Immunol. 11963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahbazian, L. M., L. J. Quinton, G. J. Bagby, S. Nelson, G. Wang, and P. Zhang. 2004. Escherichia coli pneumonia enhances granulopoiesis and the mobilization of myeloid progenitor cells into the systemic circulation. Crit. Care Med. 321740-1746. [DOI] [PubMed] [Google Scholar]
- 55.Simmons, P., K. Kaushansky, and B. Torok-Storb. 1990. Mechanisms of cytomegalovirus-mediated myelosuppression: perturbation of stromal cell function versus direct infection of myeloid cells. Proc. Natl. Acad. Sci. USA 871386-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeuchi, K. H., M. P. McGarry, and R. T. Swank. 1987. Elastase and cathepsin G activities are present in immature bone marrow neutrophils and absent in late marrow and circulating neutrophils of beige (Chediak-Higashi) mice. J. Exp. Med. 1661362-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Udupa, K. B., and K. R. Reissmann. 1977. In vivo and in vitro effect of bacterial endotoxin on erythroid precursors (CFU-E and ERC) in the bone marrow of mice. J. Lab. Clin. Med. 89278-284. [PubMed] [Google Scholar]
- 58.Ueda, Y., M. Kondo, and G. Kelsoe. 2005. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 2011771-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ueda, Y., K. Yang, S. J. Foster, M. Kondo, and G. Kelsoe. 2004. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 19947-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Loewenich, F. D., D. G. Scorpio, U. Reischl, J. S. Dumler, and C. Bogdan. 2004. Frontline: control of Anaplasma phagocytophilum, an obligate intracellular pathogen, in the absence of inducible nitric oxide synthase, phagocyte NADPH oxidase, tumor necrosis factor, Toll-like receptor (TLR)2 and TLR4, or the TLR adaptor molecule MyD88. Eur. J. Immunol. 341789-1797. [DOI] [PubMed] [Google Scholar]
- 61.Wengner, A. M., S. C. Pitchford, R. C. Furze, and S. M. Rankin. 2008. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood 11142-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wikel, S. K. 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29851-859. [DOI] [PubMed] [Google Scholar]
- 63.Yoshiie, K., H. Y. Kim, J. Mott, and Y. Rikihisa. 2000. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 681125-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, P., S. Nelson, G. J. Bagby, R. Siggins II, J. E. Shellito, and D. A. Welsh. 2008. The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells 261778-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, P., L. J. Quinton, L. Gamble, G. J. Bagby, W. R. Summer, and S. Nelson. 2005. The granulopoietic cytokine response and enhancement of granulopoiesis in mice during endotoxemia. Shock 23344-352. [DOI] [PubMed] [Google Scholar]