Abstract
Human monocytic ehrlichiosis (HME) is a tick-borne disease caused by Ehrlichia chaffeensis. Patients exhibit diagnostically important hematological changes, including anemia and thrombocytopenia, although the basis of the abnormalities is unknown. To begin to understand these changes, we used a mouse model of ehrlichiosis to determine whether the observed hematological changes induced by infection are associated with altered hematopoietic activity. Infection with Ehrlichia muris, a pathogen closely related to E. chaffeensis, resulted in anemia, thrombocytopenia, and a marked reduction in bone marrow cellularity. CFU assays, conducted on days 10 and 15 postinfection, revealed a striking decrease in multipotential myeloid and erythroid progenitors. These changes were accompanied by an increase in the frequency of immature granulocytes in the bone marrow and a decrease in the frequency of B lymphocytes. Equally striking changes were observed in spleen cellularity and architecture, and infected mice exhibited extensive extramedullary hematopoiesis. Splenomegaly, a characteristic feature of E. muris infection, was associated with an expanded and disorganized marginal zone and a nearly 66-fold increase in the level of Ter119+ erythroid cells, indicative of splenic erythropoiesis. We hypothesize that inflammation associated with ehrlichia infection suppresses bone marrow function, induces the emigration of B cells, and establishes hematopoietic activity in the spleen. We propose that these changes, which may be essential for providing the innate and acquired immune cells to fight infection, are also responsible in part for blood cytopenias and other clinical features of HME.
Human monocytic ehrlichiosis (HME) is a tick-borne disease caused by the intracellular bacterium Ehrlichia chaffeensis (23). HME can vary in severity, but it often presents with nonspecific symptoms such as fever, lethargy, and headache (6, 27). In some cases, however, especially in immunocompromised patients, this disease can be severe or even fatal (23). Clinically important diagnostic observations include hematological abnormalities such as anemia and thrombocytopenia, as well as elevated levels of liver transaminases and, occasionally, hepatosplenomegaly (22, 23).
The ehrlichiae are obligate intracellular bacteria with tropism for monocytes and macrophages in mammalian hosts and for hemocytes in the tick vector (9). E. chaffeensis causes a largely subclinical infection in immunocompetent mice (37); however, infection of C57BL/6 mice with a closely related pathogen, E. muris, results in cytopenias similar to those observed in HME patients. Importantly, the underlying etiology of the hematological changes during HME and during E. muris infection of the mouse is not known.
Hematopoiesis is an essential homeostatic process by which the cells of the blood system are continuously replenished. Stress, such as the stress induced by infection, injury, or inflammation, has been shown to have profound effects on hematopoiesis (26, 29, 31, 32, 36). Infection-induced suppression of hematopoiesis has been suggested to occur via the induction of inflammatory cytokines (18, 24, 35, 41). Endotoxin-mediated alterations in bone marrow function associated with gram-negative bacterial infections involve the release of colony-stimulating factors, tumor necrosis factor alpha, and interleukin-1β. These factors have been shown to promote the production of granulocytes and to inhibit lymphopoiesis, and they may enhance innate immunity (33, 34, 41). Although such changes have been shown to be mediated via Toll-like receptors (TLRs) (20), the ehrlichiae are unique among gram-negative bacteria in that they do not have genes that code for lipopolysaccharide (LPS) biosynthesis and lack most of the genes that code for peptidoglycan synthesis (15). LPS and peptidoglycan are well-known TLR ligands responsible for the production of inflammatory mediators. The mechanisms by which the ehrlichiae trigger host innate responses in the absence of such TLR ligands are not known.
A number of acute and chronic infections, including ehrlichiosis, are associated with anemia and thrombocytopenia. Anemia, a decrease in the number of red blood cells (RBCs), can lead to a decrease in the amount of oxygen supplied to tissues and organs, which in some circumstances can be life threatening (19). A variety of different mechanisms can contribute to anemia, including cytokine-mediated suppression of erythropoiesis, loss of erythroid progenitors, unresponsiveness to erythropoietin, reduced release of RBCs from the bone marrow, and sequestration of RBCs (5, 19, 31, 36). Thrombocytopenia, a reduction in the number of circulating platelets, has been noted during acute infections with viruses and LPS-containing bacteria and during ehrlichiosis and anaplasmosis (3, 8, 23, 39, 40). The cause of thrombocytopenia is typically a decrease in the number of megakaryocytes, but it may also involve decreased platelet production, increased platelet sequestration, and increased platelet destruction (1).
Given that bone marrow hypocellularity has been reported during ehrlichia infections (8), we addressed here whether alterations in hematopoiesis are associated with, and contribute to, cytopenias. Our data reveal that ehrlichia infection causes major changes in bone marrow progenitor cell activity, as well as splenic extramedullary hematopoiesis. Thus, cytopenias, which are temporally correlated with bone marrow failure, may result from both inadequate progenitor function and sequestration of hematopoietic progenitor cells by the spleen. Depletion of splenic B cells during infection may result, in a similar fashion, from the loss of B-cell progenitor cells in the bone marrow. We propose that such alterations are essential for providing innate and acquired immune cells to fight infection.
MATERIALS AND METHODS
Mice.
All mice used in these studies were obtained from The Jackson Laboratory (Bar Harbor, ME) or were bred in the Animal Care Facility at the Wadsworth Center under microisolater conditions. Gender- and age-matched C57BL/6 mice were used for all experiments. All animal studies were performed in accordance with Institutional Animal Care and Use Committee guidelines. In all experiments, we analyzed at least two mock-infected and three E. muris-infected mice per time point, and the data presented below are representative of at least two experiments.
Bacteria and infections.
Mice were infected, via intraperitoneal injection, at 6 to 12 weeks of age with approximately 50,000 copies of E. muris. The inoculum was generated from spleen mononuclear cells harvested from infected mice that had been stored at −80°C, as previously described (2).
Quantification of bacteria.
The number of copies of a bacterial gene in bone marrow was determined by real-time quantitative probe-based PCR, using primers and probes for the E. muris dsb gene, as described previously by Stevenson et al. (30). To obtain DNA for analysis, cells were flushed from the femurs of mock-infected and infected mice, the erythrocytes were lysed, and DNA was isolated using DNAzol (Molecular Research Center, Cincinnati, OH) and quantitated spectroscopically. The PCR products were analyzed using an Applied Biosystems 7500 fast real-time PCR platform (Applied Biosystems, Foster City, CA), and the quantity of the 6-carboxytetramethylrhodamine probe was determined by measuring the optical density. The number of copies of the E. muris dsb gene in 10 ng of DNA was determined using known quantities of dsb amplicons as standards.
Complete blood counts.
Mice were euthanized with carbon dioxide, and peripheral blood was collected in EDTA by cardiocentesis. Complete blood counts were determined using an automated hematology analyzer (Advia 120; Bayer Corporation, Norwood, MA).
Hematopoietic progenitor cell assays.
Bone marrow cells were plated at a concentration of 2.5 × 104 cells per 35-mm tissue culture dish in duplicate and cultured in methylcellulose medium (MethoCult GF M3434; StemCell Technologies, Vancouver, British Columbia, Canada) along with recombinant cytokines for colony assays of murine cells by following the manufacturer's instructions. Colonies derived from granulocyte/macrophage, erythroid, and multipotential progenitor cells were scored after 7 to 10 days of incubation at 37°C in 5% CO2.
RNA isolation and quantitative real time-PCR.
RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). Total bone marrow RNA from two fractions of bone marrow cells, the cells within the medullary cavity, and the cells lining the medullary cavity was pooled. RNA isolation was carried out using the manufacturer's protocol. cDNAs were prepared from DNase I-treated mRNAs using an RT2 first-strand kit (SABiosciences, Frederick, MD). Expression of CXCL12 was determined using the Superarray platform (PAMM-011; SABiosciences).
Histology and cytology of spleen and bone marrow.
For immunohistology, sections of spleen tissue were fixed in formalin for 24 h and then embedded in paraffin. Sections (7 μm) were cut and stained with hemotoxylin and eosin (H&E). Cytological specimens were generated using tissues from the same animals. To visualize bone marrow cells, the end of the humerus was removed, and a fine paintbrush was used to transfer bone marrow cells onto glass microscope slides. The cells were then fixed, stained with Wright-Giemsa stain, and imaged.
Immunohistochemistry.
Sections of spleen were embedded in Neg-50 medium (Richard-Allan Scientific), frozen on dry ice, and stored at −80°C. Cryostat sections (7 μm) were fixed in ice-cold acetone for 15 min and rehydrated in phosphate-buffered saline. Tissue sections were blocked for 30 min at 37°C in Fc blocking solution, as described previously (25), and were stained successively for expression of the mucosal endothelial adhesion molecule with immunoglobulin and mucin-like domains (MAdCAM-1), B cells, and T cells. B cells were detected using biotinylated rat anti-mouse B220, and T cells were detected using rat anti-mouse Thy1.2 (both obtained from BD Biosciences). The stained sections were mounted in an antifading reagent (Slow Fade Gold; Invitrogen). Images were acquired with an epifluorescence microscope (Azioskop2; Carl Zeiss SMT, Peabody, MA) equipped with a Hamamatsu camera and were processed with OPENLAB software (Zeiss).
Flow cytometry and antibodies.
Mononuclear cells were harvested from spleens by homogenization of tissue between frosted glass slides in Hanks' balanced salt solution. Homogenates were passed through a 70-μm filter, and the cells were collected by centrifugation. RBCs were lysed in a hypotonic buffer containing 0.84% ammonium chloride. The cells were enumerated and washed with a buffer containing 5% fetal calf serum and 0.01% sodium azide in phosphate-buffered saline. Bone marrow mononuclear cells were harvested by flushing the marrow from femurs after removal of the ends of the bones. The cells were flushed from the bones using a 26.5-gauge needle and 2 ml Hanks' balanced salt solution. The RBCs were lysed, and cells were enumerated and washed, as described above. Prior to staining, the cells were incubated in blocking buffer for 15 min at 4°C, as previously described (25).
The following antibodies were purchased from eBiosciences (San Diego, CA): CD11b-fluorescein isothiocynanate (M1/70), Ter119-fluorescein isothiocynanate (Ly76), and B220-peridinin-chlorophyll-protein complex-Cy5.5 (RA3-6B2). The Ly-6G-phycoerythrin (RB6-8C5) antibody was purchased from BD Biosciences. Data were acquired using a FACSCalibur flow cytometer (BD Biosciences), and data analysis was performed using FloJo software (TreeStar, Ashland, OR).
Statistical analyses.
Statistical analyses were performed using a Student t test (Prism; GraphPad Software, La Jolla, CA); a P value of <0.05 was considered significant.
RESULTS
E. muris infection induces cytopenias, splenomegaly, and bone marrow hypocellularity.
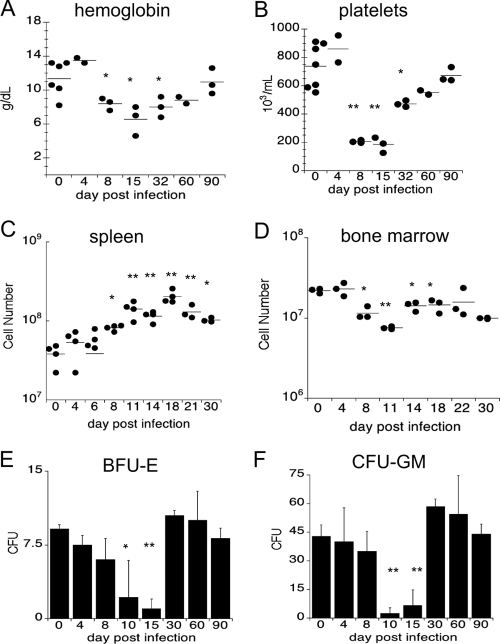
Cytopenias are a diagnostic feature of ehrlichiosis in humans and animals, including laboratory mice (2, 8, 37). To begin to address the mechanisms underlying cytopenias, we examined the development of anemia and thrombocytopenia, using a mouse model of ehrlichiosis. C57BL/6 mice were inoculated via intraperitoneal injection with 5 × 104 copies of E. muris, and on various days postinfection blood was harvested via cardiac puncture and hematology analyses were performed. During the first week of infection, only mild changes in hemoglobin were observed; however, by day 8 postinfection infected mice developed anemia (Fig. 1A). The anemia resolved over the next month, and the hemoglobin levels reflected those in mock-infected animals by day 90 postinfection. Thrombocytopenia, another characteristic of human ehrlichiosis, was observed as early as 8 days postinfection, and the numbers of platelets did not return to normal levels until day 90 postinfection (Fig. 1B). The hematological changes were accompanied by large increases in spleen cellularity (Fig. 1C) and wet weight, as has been reported previously (25). Splenomegaly was most pronounced on day 18 postinfection (Fig. 1C). Major changes in bone marrow cellularity were also observed during E. muris infection (Fig. 1D). On day 11 postinfection, the number of bone marrow cells was threefold lower in the infected mice than in the mock-infected mice; thus, the total cellularity was reduced by 65% (Fig. 1D). These studies demonstrate that the cytopenias observed during ehrlichia infection are associated with marked changes in the numbers of cells in the spleen and bone marrow.
FIG. 1.
Kinetics of hematological abnormalities, splenomegaly, and bone marrow cellularity. Peripheral blood from C57BL/6 mice was analyzed after intraperitoneal infection with E. muris. The hemoglobin concentration (A) and number of platelets (B) were analyzed using an automated hematology analyzer (Advia). Total numbers of spleen cells (C) and bone marrow cells (D) were determined after tissue homogenization; bone marrow cellularity represents the total number of cells harvested from two femurs obtained from each mouse. Bone marrow cells were plated in semisolid methylcellulose media, and CFU assays were performed to determine the frequency of progenitor cells after E. muris infection. (E) Erythroid progenitors (BFU-E) were nearly absent by day 15 in infected mice compared to mock-infected animals. (F) Numbers of granulocyte/monocyte progenitors (CFU-GM) were significantly reduced by day 10 postinfection. A Student t test was used to compare mean values for experimentally infected mice to mean values for mock-infected mice (*, P < 0.05; **, P < 0.001).
Loss of committed progenitor cells in the bone marrow during E. muris infection.
Due to the loss of bone marrow cells during infection, we next addressed whether bone marrow function was affected by E. muris infection, using in vitro colony-forming assays. Bone marrow harvested from the femurs of uninfected mice consistently contained colonies derived from erythroid and granulocyte/monocyte progenitor cells (Fig. 1E and F). However, by day 10 postinfection, the number of erythroid colonies was significantly reduced (by more than 50%), and by day 15 postinfection few, if any, colonies were detected (Fig. 1E). The reduction in the number of erythroid colonies was first observed on day 10 postinfection and coincided with anemia in the E. muris-infected mice (Fig. 1A). The numbers of colonies derived from granulocyte/monocyte progenitor cells were also reduced during acute ehrlichia infection. Compared to the number of colonies for uninfected mice, the number of colonies for infected mice was reduced on days 10 and 15 postinfection (Fig. 1F). By day 30, the frequencies of erythroid and granulocyte/monocyte precursors had returned to normal levels. Together, these data demonstrate that during acute E. muris infection, the hematopoietic activity of the bone marrow is greatly impaired, and the diminished bone marrow cellularity and the reduced frequency of progenitor cells in the infected mice are temporally correlated with the observed blood cytopenias.
To determine whether infection of the bone marrow played an important role in the hematopoietic defects in our model, we quantitated bacterial infection within the bone marrow at days 4, 8, 10, 14, and 33 postinfection. The levels of bacteria were barely above the limit of detection on days 10 and 14 postinfection, and bacteria were undetectable by day 33 postinfection (data not shown).
Infection is associated with reciprocal changes in the numbers of granulocytes and B lymphocytes in the bone marrow.
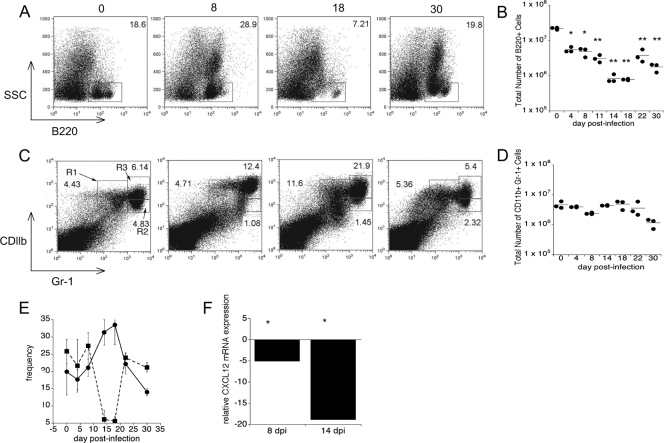
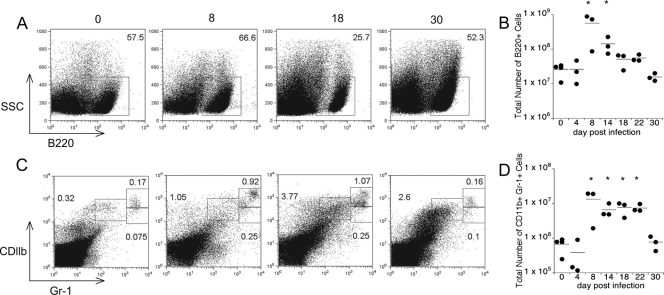
To address whether changes in progenitor cell frequency were accompanied by changes in lineages of more mature white blood cells, we next analyzed the composition of the bone marrow, using both cytology and flow cytometry. Cytological examination of bone marrow harvested from tibiae on day 11 postinfection revealed higher numbers of granulocytes, but lower numbers of lymphocytes, than the numbers that were present in controls (Fig. 2). Flow cytometric analysis revealed that following a transient increase in the frequency of bone marrow B220+ B cells on day 8 postinfection, a marked decrease in the frequency of B cells occurred (Fig. 3A and B). Between days 14 and 18 postinfection, the percentage of B220+ cells declined nearly 2.5-fold, from 18.6 to 7.21% (Fig. 3A and E). This decline in the percentage amounted to a >10-fold decrease in the number of B220+ cells (Fig. 3B). By day 22 postinfection, the frequency of B220+ cells returned to a value near the normal value. Concomitant with the loss of B cells, we observed an increase in the frequency of granulocytes in the bone marrow (Fig. 3C and E). Because the total number of granulocytes in the bone marrow remained fairly stable during the course of infection (Fig. 3D), we attribute the loss in cellularity of the bone marrow primarily to a loss of lymphocytes within this tissue.
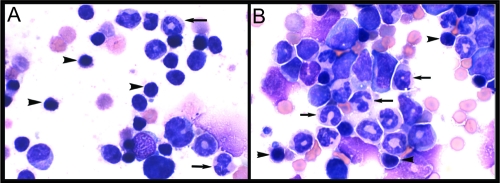
FIG. 2.
Reduced bone marrow lymphopoiesis and enhanced granulopoiesis during ehrlichiosis: representative images of bone marrow cell populations during E. muris infection. (A) Bone marrow from uninfected normal C57BL/6 murine bone marrow consists of approximately 30% small lymphocytes (arrowheads) and approximately 40% granulocyte precursors (arrows). (B) On day 11 postinfection, there was a marked alteration in bone marrow cell populations, as shown by lymphocyte depletion (lymphocytes accounted for approximately 5% of all cells [arrowheads indicate remaining lymphocytes]) and by granulocytic hyperplasia (granulocytes accounted for approximately 75% of all cells [the granulocytes are indicated by arrows]). Magnification, ×50.
FIG. 3.
Reciprocal changes in the granulocyte/lymphocyte ratio in the bone marrow. Bone marrow cells were harvested from the femurs of infected mice and analyzed for B220-positive B cells and CD11b- and Gr1 (Ly6-G)-positive granulocytes. (A) Representative flow cytometry dot plots for analyses of B lymphocytes performed on the days indicated above the plots. (B) Numbers of bone marrow B220+ cells detected during infection. (C) Representative flow cytometry dot plots for analyses of granulocytes performed on the days indicated. (D) The total number of granulocytes in R1, R2, and R3 remained constant during infection. (E) Inverse relationship between the frequency of B220+ cells (▪) and the frequency of granulocytes (•) between days 14 and 18. (F) CXCL12 expression in bone marrow obtained from infected animals (on days 8 and 14 postinfection) relative to the CXCL12 expression in bone marrow obtained from mock-infected animals. A Student t test was used to compare mean values for experimentally infected mice to mean values for mock-infected mice (*, P < 0.05; **, P < 0.001). dpi, days postinfection.
To address the maturation status of the bone marrow granulocytes, we next examined the cell surface expression of CD11b and Gr-1. Immature granulocytes express intermediate levels of both of these cell surface receptors, whereas cells at an intermediate stage of development are Gr-1hi CDllblo and mature neutrophils are characterized by high levels of expression of both CDllb and Gr-1 (33). The frequency of the mature neutrophil population (Fig. 3C, region R3) on day 8 postinfection was twice that of the same population in uninfected mice, and the frequency on day 18 postinfection was nearly 3.5-fold higher than that in control mice. The size of the immature population (Gr-1int CDllbint) (Fig. 3C, region R1) doubled by day 18 postinfection; in contrast, the size of the developmentally intermediate population (region R2) decreased during infection. These data are consistent with the interpretation that infection enhances granulocyte maturation in the bone marrow. The analyses of B cells and granulocytes revealed that granulopoiesis is heavily favored in the bone marrow during infection and is accompanied by a reciprocal loss of B lymphocytes.
B-cell loss is accompanied by diminished expression of CXCL12 in bone marrow.
The inflammatory factors responsible for the major changes in bone marrow cellularity and composition that we have observed during E. muris infection are not known. However, using a model of immunization-induced inflammation, it has been demonstrated that decreased CXCL12 expression in the bone marrow favors granulopoiesis by causing B-lymphocyte egress (33). One explanation proposed for the previously described finding was that granulocytes and B lymphocytes share a physical niche in the bone marrow, and, therefore, the loss or removal of one subset allows expansion of the other. To address this possibility using our infection model, we tested whether E. muris-induced inflammation altered the expression of CXCL12 within the bone marrow. The expression of CXCL12 mRNA in total bone marrow cells on day 8 postinfection was 5-fold lower than that in total bone marrow cells of uninfected mice, and it was 20-fold lower by day 14 postinfection (Fig. 3F). The decrease in the expression of CXCL12 on day 14 postinfection was closely correlated with the inversion of the ratio of B lymphocytes to granulocytes that was observed (Fig. 3E), suggesting that loss of CXCL12 may drive B-cell egress from the bone marrow during E. muris infection, as has been reported in studies of immunization-induced inflammation described above (33, 34).
E. muris-induced splenomegaly is characterized by changes in splenic architecture and cellular composition.
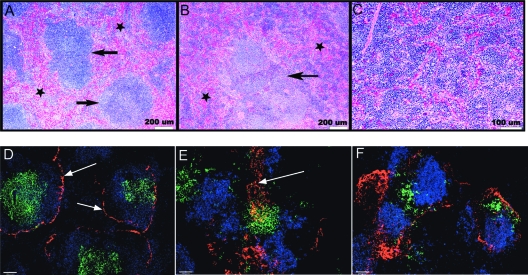
During infection the spleen is a site where the bacterial burden is high, and there is a massive increase in size and cellularity (22, 25). Based on the observed decrease in the number of progenitor cells in the bone marrow, we considered the possibility that the loss of bone marrow function was compensated for in part by changes in the spleen, including extramedullary hematopoiesis. To determine which cells contributed to splenomegaly, we performed histological, immunohistological, and flow cytometric analyses. H&E staining revealed major changes in splenic architecture throughout infection, as shown by a loss of follicular structure and expansion of the red pulp (Fig. 4A to C). The marginal zone was poorly defined, and the red pulp appeared to be denser and contained more nucleated cells, primarily due to extramedullary erythropoiesis. We also examined the distribution of T cells and B cells relative to the marginal sinus by performing an immunofluorescence assay. Normal spleen tissue contained clearly delineated lymphocyte follicles surrounded by the marginal sinus (Fig. 4D). In contrast, on day 13 postinfection the spleen marginal sinus was enlarged, and the T- and B-cell zones were disorganized (Fig. 4E and F). These data reveal that the enhanced hematopoietic activity in the spleen is accompanied by pathological changes in splenic architecture.
FIG. 4.
Splenomegaly is characterized by disorganized follicular architecture and extramedullary hematopoiesis. Histologic and immunofluorescent analyses of the spleen during E. muris infection were performed. (A) H&E staining of spleen tissue obtained from a normal mouse revealed multiple, discrete, uniform lymphoid nodules (arrows). The red pulp contained erythrocytes, blood vessels, and few nucleated cells (stars). (B) Spleen section from a mouse on day 11 postinfection. The lymphoid nodules are pale and poorly defined. The nodule has an enlarged germinal center and an expanded marginal zone (arrow). The red pulp (dark blue) exhibited increased cellularity due to the extramedullary hematopoiesis, primarily erythropoiesis (stars). (C) Higher magnification of the spleen shown in panel B, showing the marked increase in red pulp cellularity, primarily due to hematopoiesis. (D to F) Immunofluorescence analysis of spleen sections stained with MAdCAM-1 (red), anti-B220 (blue), and anti-Thy1.2 (green). (D) In an uninfected mouse the marginal zone of the spleen is clearly delineated by expression of MAdCAM-1 (arrows), and the B-cell and T-cell zones are organized within the white pulp; T and B cells were largely absent from the red pulp. (E) Immunofluorescence analysis of spleen tissue on day 13 postinfection, demonstrating a breakdown in follicular organization. The marginal zone was no longer clearly defined (arrow). (F) By day 16 postinfection, MAdCAM-1 staining was diffuse and T cells were no longer found in organized structures.
In contrast to the results for the bone marrow, flow cytometry analyses of the spleen revealed that the numbers of both B lymphocytes and granulocytes increased during infection (Fig. 5B and D). However, due to the large increase in spleen cellularity, the frequency of B220+ cells was actually twofold lower than that in control mice on day 18 postinfection (Fig. 5A). This moderate increase in B220+ cells in the spleen likely reflects the expansion of a CD11c-expressing plasmablast population that has been described previously (25). Thus, we propose that the loss of B220+ B lymphocytes within the bone marrow is directly or indirectly related to the accumulation of plasmablasts within the spleen.
FIG. 5.
B-cell loss and granulocytic hyperplasia in the spleen. Spleen cells were harvested from infected mice, and expression of B220, CD11b, and Gr-1 was examined to determine the frequencies and numbers of B cells and granulocytes. (A) Representative flow cytometry dot plots of splenocytes obtained on the days postinfection indicated above the plots. The numbers indicate the frequencies of B220+ B cells. SSC, side scatter. (B and D) Numbers of B cells (B) and granulocytes (D) in the spleen. (C) Representative flow cytometry dot plots of spleen granulocytes. Three gates are indicated on the upper right in each dot plot. The gate on the left defines immature granulocytes; the bottom gate on the right includes granulocytes at an intermediate stage of development, and the upper gate on the right indicates mature granulocytes. The error bars indicate standard deviations; a Student t test was used to compare mean values for experimentally infected mice to mean values for mock-infected mice (*, P < 0.05).
The moderate increase in the level of B220+ cells likely includes a plasmablast population, identified by expression of CD11c and responsible for producing immunoglobulin M, recently identified in our laboratory (25). Like the frequency in the bone marrow, the frequency of total granulocytes in the spleen increased during the course of infection and was reflected by a nearly 10-fold increase in the number of cells (Fig. 5C and D).
Extramedullary erythropoiesis during infection.
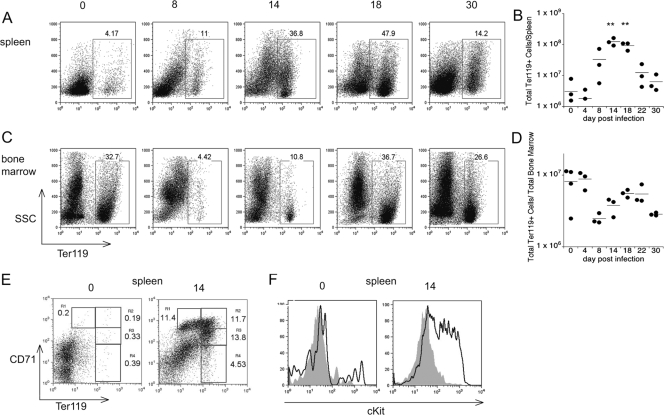
Because spleen pathology was associated with extramedullary hematopoiesis, we next sought to identify cell populations that underwent expansion in the spleen during infection. Using lineage-specific markers for B cells (B220), T cells (CD3, CD4, and CD8), macrophages (CDllb), and erythroid cells (Ter119), we observed that the frequencies of most lineage-specific cells increased moderately (data not shown). One notable exception was the frequency of Ter119+ cells, which increased by >10-fold on day 18 postinfection, corresponding to a 66-fold increase in the number of Ter119 cells (Fig. 6A and B). The kinetics of Ter119+ cell population expansion and splenomegaly were closely linked, as both Ter119+ cell population expansion and splenomegaly were maximal on day 18 postinfection. By comparison, in normal spleen tissue, the percentage of Ter119+ cells is very low, between 3 and 5%. Thus, Ter119+ cells accounted for nearly one-half of the total cells in the spleen and were a major factor contributing to splenomegaly.
FIG. 6.
The spleen is a major site of extramedullary erythropoiesis. Bone marrow and spleen cells were monitored for surface expression of Ter119. (A and C) Representative flow cytometry analyses of the spleen and bone marrow. (B and D) Numbers of Ter119+ cells in the spleen and bone marrow. (E) Analysis of the expression of CD71, a marker of reticulocyte maturation, in the spleen on day 15 postinfection. Reticulocyte maturation involves the upregulation of Ter119 and the downregulation of CD71; thus, as cells mature, they progress sequentially from R1 to R4. (F) Expression of c-Kit on cells within the R1 (solid line) and R4 (shaded area) gates shown in panel E. The data are representative of at least two experiments; a Student t test was used to compare mean values for experimentally infected mice to mean values for mock-infected mice (**, P < 0.001).
To determine whether the increase in the level of Ter119+ cells reflected an increase in the number of reticulocytes belonging to a particular subset, we next examined the splenocytes for expression of Ter119, CD71 (the transferrin receptor), and c-Kit (CD117). The level of expression of CD71 on immature reticulocytes is typically high, but it decreases as reticulocytes mature; early-stage reticulocytes also exhibit high surface expression of c-Kit (10, 11, 13). On the basis of the expression of these surface markers, the Ter119+ cells in the E. muris-infected spleens were largely immature erythroid cells as a majority of the Ter119+ cells expressed high levels of CD71 and c-Kit (Fig. 6E and F). These data reveal that the spleen is a major site of extramedullary erythropoiesis during acute E. muris infection. The observation that the spleen was a site of erythropoiesis led us to reexamine Ter119 expression within the bone marrow. Between days 10 and 14 postinfection, we observed a decrease in the number and frequency of Ter119+ cells in the bone marrow (Fig. 6C and D). Whereas the bone marrow in normal mice typically contains between 20 and 30% Ter119+ cells, the percentages of Ter119+ cells in infected mice were 4.42% and 10.8% on days 8 and 14 postinfection, respectively, before they returned to normal levels by day 18 postinfection. This change in the bone marrow was inversely correlated with the increase in the number of Ter119+ cells observed in the spleen (Fig. 6E), although the loss of Ter119+ cells in the bone marrow preceded the increase in the number of spleen Ter119+ cells by about 4 days. These data suggest that the onset of extramedullary erythropoiesis was preceded by migration of erythrocyte precursors from the bone marrow (12). Although the frequency of Ter119+ cells in the bone marrow returned to normal levels by day 18 postinfection, the spleen continued to contain elevated numbers of Ter119+ cells for as long as 30 days postinfection. Despite the increased number of erythroid cells observed in the spleen, the mice were anemic at this time, suggesting that extramedullary hematopoiesis is ineffective.
DISCUSSION
E. muris-induced hematopoietic stress.
Our studies suggest that infection-induced hematopoietic stress is a factor contributing to cytopenias during ehrlichiosis. The loss of hematopoietic progenitor cell function in the bone marrow was temporally correlated with both anemia and thrombocytopenia, suggesting that cytopenias may be a direct consequence of decreased hematopoiesis in the bone marrow. In addition to the effects of infection on progenitor cells, the frequencies and numbers of differentiated, lineage-specific cell populations were also altered in the bone marrow. Whereas the numbers and frequencies of B lymphocytes and Ter119+ erythroid cells were reduced, there was an increase in the percentage of granulocytes in the bone marrow.
These changes were correlated with equally striking changes in the spleen. Splenomegaly was likely due to massive extramedullary hematopoiesis and the robust expansion of the number of Ter119+ cells. We hypothesize that the increase in the number of granulocytes in the spleen was a result of the maturation and migration of granulocytes from the bone marrow in response to infection. We also hypothesize that the increase in the number of B cells in the spleen was a consequence of both extramedullary lymphopoiesis and antigen-specific expansion. We have demonstrated previously that E. muris infection is accompanied by a massive but transient spleen B-cell plasmablast response (25). Thus, E. muris infection induces both alterations in and redistribution of hematopoietic activity, and these effects are likely causally associated with cytopenias. Splenomegaly may also be a consequence of the inability of the tissue to release cells into circulation. This may explain why, paradoxically, massive extramedullary hematopoiesis occurs when the infected mice are anemic.
In HME patients, hypocellular bone marrow has rarely been reported during acute ehrlichiosis (23). This has led to the idea that cytopenias associated with HME result from peripheral events, such as sequestration, consumption, or destruction of infected and noninfected cells (7, 23). We have not observed hemophagocytosis in splenic tissue in our studies (K. C. MacNamara, D. Borjesson, and G. M. Winslow, unpublished data), which suggests that activated macrophage-induced anemia is not a factor that is responsible for cytopenias during E. muris infection. Although our data do not exclude any of the mechanisms described above, we propose that underlying defects in hematopoiesis are also responsible. There are two possible explanations for the apparent discord between the clinical observation that bone marrow is not hypocellular during acute ehrlichiosis and our experimental observations that bone marrow is hypocellular during E. muris infection. First, bone marrow from human patients was likely not available during acute infection, and, second, bone marrow aspirates from human patients are likely not representative of the total cell populations in the bone marrow. Thus, additional studies of human patients are necessary to resolve whether our experimental observations have clinical correlates.
Infection-induced changes in hematopoiesis.
The primary site of hematopoiesis in healthy rodents is the bone marrow. However, infection with several pathogens, including Salmonella enterica, Listeria monocytogenes, and Leishmania donovani, is associated with redistribution of hematopoietic activity from the bone marrow to the spleen (4, 38). Such changes mirror those that we have observed during E. muris infection and similar changes were also observed in a mouse model of anaplasmosis (13a). Viral infection is also known to dramatically alter hematopoiesis in humans and in mouse models of disease. In some cases this is the result of direct infection of progenitor cells (40), while in other instances infection of supporting cells, such as stromal cells of the bone marrow, contributes to bone marrow suppression (28). In general, it is thought that progenitor cells are refractory to infection by bacteria because these primitive cells are not phagocytic (14). In our studies we have found that the number of bacteria is very low in the bone marrow during the first 2 weeks of infection and that bacteria are undetectable by day 33 postinfection. While we cannot rule out the possibility that a very small number of infected cells play a significant role in hematopoietic defects, we favor the hypothesis that the mechanism of bone marrow suppression is indirect. We propose that the loss of hematopoietic progenitor cells is a consequence of inflammation-induced cell death or apoptosis, reduced proliferation of progenitor cells, increased mobilization from the bone marrow to the periphery, or rapid differentiation into committed cells.
Inflammation-induced changes in hematopoiesis.
Inflammation-induced changes in hematopoietic activity have been proposed to act in part to generate cell subsets necessary to combat infection. Ueda and colleagues demonstrated that inflammation can alter hematopoiesis so that the production of granulocytes is favored over the production of lymphocytes in the bone marrow (33). As neutrophils are short-lived and contribute to innate immunity against bacterial pathogens, it was proposed that increased granulocyte production is critical for host defense during microbial infections. We propose that granulopoiesis is similarly enhanced in the bone marrow in response to E. muris infection. Likewise, the loss of B lymphocytes in the bone marrow is consistent with the model proposed by Ueda and colleagues. However, during E. muris infection the loss of B lymphocytes in the bone marrow is accompanied by a massive T-cell-independent plasmablast response in the spleen (25). The plasmablast response is first observed in the spleen on day 9 postinfection, a time when the bone marrow has lost a majority of the B lymphocytes and the environment appears to favor granulopoiesis over lymphopoiesis. Thus, we propose that infection favors granulopoiesis in the bone marrow at the expense of B lymphopoiesis, but in response to the changes in the bone marrow, B lymphopoiesis is redirected to the spleen. This scenario allows the host to marshal both granulocytes and B cells for host defense.
Ueda et al. also demonstrated that increased levels of tumor necrosis factor alpha and interleukin-1β and decreased levels of CXCL12 and stem cell factor contributed to the mobilization of B lymphocytes and favored the maturation of granulocytes in the bone marrow (34). Lymphocytes, unlike granulocytes, require chemokine signals to remain in the bone marrow; thus, diminished expression of CXCL12 facilitates the release of lymphocytes from the bone marrow, which is consistent with our observations. In addition, CXCL12 is critical for the retention of myeloid progenitor cells in the bone marrow (16, 21). Together, these data support the hypothesis that the loss of progenitor cell function and lymphocyte egress observed during E. muris infection is due to reduced CXCL12 expression in the bone marrow and may result in enhanced mobilization to the periphery.
The inflammatory factors responsible for the changes that we have observed are unknown. The ehrlichiae do not express LPS or other known TLR ligands (15), which may explain the apparent lack of a requirement for TLRs in our experimental model (K. C. MacNamara and G. M. Winslow, unpublished data). Although it has been demonstrated that E. muris is recognized by CD1d-restricted NK T cells (17), CD1d-deficient mice survive E. muris infection and do not display increased signs of disease (K. C. MacNamara and G. M. Winslow, unpublished data). In fact, CD1d-deficient mice exhibit increased splenomegaly compared to wild-type mice, suggesting that CD1d-dependent recognition of E. muris is not required for inflammation-mediated alterations in hematopoiesis. Additional studies are required to identify the nature of the innate signals that drive the hematological and hematopoietic changes during E. muris infection.
In summary, our findings reveal that E. muris infection is accompanied by major changes in bone marrow function, alterations in granulopoiesis and lymphopoiesis, and extramedullary hematopoiesis. These changes likely contribute to host defense, immunopathology, and immune homeostasis during ehrlichia infections in both animals and humans.
Acknowledgments
We thank Sheila Le, Naomi Walker, and Jennifer Johns for excellent technical assistance, and we thank the Wadsworth Center Immunology Core Facility.
This work was supported by U.S. Public Health Service grant R01AI47963-01 to G.M.W.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 18 May 2009.
REFERENCES
- 1.Alugupalli, K. R., A. D. Michelson, I. Joris, T. G. Schwan, K. Hodivala-Dilke, R. O. Hynes, and J. M. Leong. 2003. Spirochete-platelet attachment and thrombocytopenia in murine relapsing fever borreliosis. Blood 1022843-2850. [DOI] [PubMed] [Google Scholar]
- 2.Bitsaktsis, C., B. Nandi, R. Racine, K. C. MacNamara, and G. Winslow. 2007. T-cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection. Infect. Immun. 754933-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borjesson, D. L., S. I. Simon, F. Tablin, and S. W. Barthold. 2001. Thrombocytopenia in a mouse model of human granulocytic ehrlichiosis. J. Infect. Dis. 1841475-1479. [DOI] [PubMed] [Google Scholar]
- 4.Cotterell, S. E., C. R. Engwerda, and P. M. Kaye. 2000. Enhanced hematopoietic activity accompanies parasite expansion in the spleen and bone marrow of mice infected with Leishmania donovani. Infect. Immun. 681840-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dallalio, G., M. North, and R. T. Means, Jr. 1996. Inhibition of marrow CFU-E colony formation from human immunodeficiency virus-infected patients by beta- and gamma-interferon. Am. J. Hematol. 53118-120. [DOI] [PubMed] [Google Scholar]
- 6.Demma, L. J., R. C. Holman, J. H. McQuiston, J. W. Krebs, and D. L. Swerdlow. 2005. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001-2002. Am. J. Trop. Med. Hyg. 73400-409. [PubMed] [Google Scholar]
- 7.Dumler, J. S., P. Brouqui, J. Aronson, J. P. Taylor, and D. H. Walker. 1991. Identification of ehrlichia in human tissue. N. Engl. J. Med. 3251109-1110. [DOI] [PubMed] [Google Scholar]
- 8.Dumler, J. S., W. L. Sutker, and D. H. Walker. 1993. Persistent infection with Ehrlichia chaffeensis. Clin. Infect. Dis. 17903-905. [DOI] [PubMed] [Google Scholar]
- 9.Dumler, J. S., and D. H. Walker. 2001. Tick-borne ehrlichioses. Lancet Infect. Dis. (Suppl S) 21-28.
- 10.Fang, J., M. Menon, W. Kapelle, O. Bogacheva, O. Bogachev, E. Houde, S. Browne, P. Sathyanarayana, and D. M. Wojchowski. 2007. EPO modulation of cell-cycle regulatory genes, and cell division, in primary bone marrow erythroblasts. Blood 1102361-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang, J., M. Menon, D. Zhang, B. Torbett, L. Oxburgh, M. Tschan, E. Houde, and D. M. Wojchowski. 2008. Attenuation of EPO-dependent erythroblast formation by death-associated protein kinase-2. Blood 112886-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruhman, G. J. 1967. Endotoxin-induced shunting of erythropoiesis in mice. Am. J. Physiol. 2121095-1098. [DOI] [PubMed] [Google Scholar]
- 13.Gordon, A. R., S. V. Outram, M. Keramatipour, C. A. Goddard, W. H. Colledge, J. C. Metcalfe, A. L. Hager-Theodorides, T. Crompton, and P. R. Kemp. 2008. Splenomegaly and modified erythropoiesis in KLF13−/− mice. J. Biol. Chem. 28311897-11904. [DOI] [PubMed] [Google Scholar]
- 13a.Johns, J. L., K. C. MacNamara, N. J. Walker, G. M. Winslow, and D. L. Borjesson. 2009. Infection with Anaplasma phagocytophilum induces multilineage alterations in hematopoietic progenitor cells and peripheral blood cells. Infect. Immun. 774070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb-Maurer, A., M. Wilhelm, F. Weissinger, E. B. Brocker, and W. Goebel. 2002. Interaction of human hematopoietic stem cells with bacterial pathogens. Blood 1003703-3709. [DOI] [PubMed] [Google Scholar]
- 15.Lin, M., and Y. Rikihisa. 2003. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 715324-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma, Q., D. Jones, and T. A. Springer. 1999. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity 10463-471. [DOI] [PubMed] [Google Scholar]
- 17.Mattner, J., K. L. Debord, N. Ismail, R. D. Goff, C. Cantu III, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, K. Hoebe, O. Schneewind, D. Walker, B. Beutler, L. Teyton, P. B. Savage, and A. Bendelac. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434525-529. [DOI] [PubMed] [Google Scholar]
- 18.Means, R. T., Jr. 1995. Pathogenesis of the anemia of chronic disease: a cytokine-mediated anemia. Stem Cells 1332-37. [DOI] [PubMed] [Google Scholar]
- 19.Means, R. T., Jr. 2003. Recent developments in the anemia of chronic disease. Curr. Hematol Rep. 2116-121. [PubMed] [Google Scholar]
- 20.Nagai, Y., K. P. Garrett, S. Ohta, U. Bahrun, T. Kouro, S. Akira, K. Takatsu, and P. W. Kincade. 2006. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagasawa, T., S. Hirota, K. Tachibana, N. Takakura, S. Nishikawa, Y. Kitamura, N. Yoshida, H. Kikutani, and T. Kishimoto. 1996. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382635-638. [DOI] [PubMed] [Google Scholar]
- 22.Olano, J. P., G. Wen, H. M. Feng, J. W. McBride, and D. H. Walker. 2004. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am. J. Pathol. 165997-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 1637-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadaki, H. A., H. D. Kritikos, V. Valatas, D. T. Boumpas, and G. D. Eliopoulos. 2002. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood 100474-482. [DOI] [PubMed] [Google Scholar]
- 25.Racine, R., M. Chatterjee, and G. M. Winslow. 2008. CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J. Immunol. 1811375-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson, Y., A. Hostmann, A. Matenov, W. Ertel, and A. Oberholzer. 2006. Erythropoiesis in multiply injured patients. J. Trauma 611285-1291. [DOI] [PubMed] [Google Scholar]
- 27.Schutze, G. E., and R. F. Jacobs. 1997. Human monocytic ehrlichiosis in children. Pediatrics 100E10. [DOI] [PubMed] [Google Scholar]
- 28.Simmons, P., K. Kaushansky, and B. Torok-Storb. 1990. Mechanisms of cytomegalovirus-mediated myelosuppression: perturbation of stromal cell function versus direct infection of myeloid cells. Proc. Natl. Acad. Sci. USA 871386-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Socolovsky, M. 2007. Molecular insights into stress erythropoiesis. Curr. Opin. Hematol. 14215-224. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson, H. L., J. M. Jordan, Z. Peerwani, H. Q. Wang, D. H. Walker, and N. Ismail. 2006. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect. Immun. 744856-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theurl, I., V. Mattle, M. Seifert, M. Mariani, C. Marth, and G. Weiss. 2006. Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood 1074142-4148. [DOI] [PubMed] [Google Scholar]
- 32.Tilg, H., H. Ulmer, A. Kaser, and G. Weiss. 2002. Role of IL-10 for induction of anemia during inflammation. J. Immunol. 1692204-2209. [DOI] [PubMed] [Google Scholar]
- 33.Ueda, Y., M. Kondo, and G. Kelsoe. 2005. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 2011771-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueda, Y., K. Yang, S. J. Foster, M. Kondo, and G. Kelsoe. 2004. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 19947-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitale, M., G. Gobbi, P. Mirandola, C. Ponti, I. Sponzilli, L. Rinaldi, and F. A. Manzoli. 2006. TNF-related apoptosis-inducing ligand (TRAIL) and erythropoiesis: a role for PKC epsilon. Eur. J. Histochem. 5015-18. [PubMed] [Google Scholar]
- 36.Weiss, G., and L. T. Goodnough. 2005. Anemia of chronic disease. N. Engl. J. Med. 3521011-1023. [DOI] [PubMed] [Google Scholar]
- 37.Winslow, G., E. Yager, K. Shilo, D. N. Collins, and F. K. Chu. 1998. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect. Immun. 663892-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young, A. M., and C. Cheers. 1986. Colony-forming cells and colony-stimulating activity during listeriosis in genetically resistant or susceptible mice. Cell. Immunol. 97227-237. [DOI] [PubMed] [Google Scholar]
- 39.Young, D., T. Hussell, and G. Dougan. 2002. Chronic bacterial infections: living with unwanted guests. Nat. Immunol. 31026-1032. [DOI] [PubMed] [Google Scholar]
- 40.Young, N., and P. Mortimer. 1984. Viruses and bone marrow failure. Blood 63729-737. [PubMed] [Google Scholar]
- 41.Zhang, P., L. J. Quinton, L. Gamble, G. J. Bagby, W. R. Summer, and S. Nelson. 2005. The granulopoietic cytokine response and enhancement of granulopoiesis in mice during endotoxemia. Shock 23344-352. [DOI] [PubMed] [Google Scholar]