Abstract
Comparative genomic analysis of a wild-type strain of the ovine pathogen Chlamydia abortus and its nitrosoguanidine-induced, temperature-sensitive, virulence-attenuated live vaccine derivative identified 22 single nucleotide polymorphisms unique to the mutant, including nine nonsynonymous mutations, one leading to a truncation of pmpG, which encodes a polymorphic membrane protein, and two intergenic mutations potentially affecting promoter sequences. Other nonsynonymous mutations mapped to a pmpG pseudogene and to predicted coding sequences encoding a putative lipoprotein, a sigma-54-dependent response regulator, a PhoH-like protein, a putative export protein, two tRNA synthetases, and a putative serine hydroxymethyltransferase. One of the intergenic mutations putatively affects transcription of two divergent genes encoding pyruvate kinase and a putative SOS response nuclease, respectively. These observations suggest that the temperature-sensitive phenotype and associated virulence attenuation of the vaccine strain result from disrupted metabolic activity due to altered pyruvate kinase expression and/or alteration in the function of one or more membrane proteins, most notably PmpG and a putative lipoprotein.
The Chlamydiaceae represent a group of obligate intracellular bacteria responsible for a wide range of diseases in many different hosts with significant and broad economic and public health impacts. Despite the global impact of chlamydial disease, vaccines are available for only two chlamydial species, Chlamydia abortus and Chlamydia felis, which infect ovine and feline species, respectively. A formalin-inactivated vaccine against C. abortus infection, which in the United Kingdom costs the agricultural industry an estimated £20 million each year (2), was developed in the 1960s but did not offer complete protection and required revaccination every 3 years. This vaccine subsequently became demonstrably ineffective when C. abortus infections occurred in vaccinated flocks in the late 1970s (1, 14). Although inactivated vaccines are still used, more effective C. felis and C. abortus vaccines derived from live attenuated strains are also available. However, C. felis reinfection is not completely controlled with the live attenuated C. felis vaccine, as vaccinated animals still shed chlamydiae (32). In contrast, the live attenuated C. abortus vaccine blocks reinfection and shedding (24, 25).
The C. abortus vaccine is based on a live attenuated strain obtained by nitrosoguanidine (NTG) mutagenesis of the virulent strain AB7, isolated from an aborted lamb (8). NTG causes mutations by methylation of nucleotides, especially of guanine, which can then pair with thymine or cytosine (16), giving rise to a GC-to-AT conversion. Other nucleotide substitutions are seen less frequently with deletions resulting from lesion repair. Treatment of AB7 with NTG produced two temperature-sensitive mutants, 1B and 1H, which were attenuated in the mouse model (21). Unlike 1H, which was unstable in temperature-sensitive growth studies, the 1B mutant was stable after multiple passages in vitro or in vivo (23). The 1B mutant is characterized by a reduced ability to induce death in utero of baby mice or lambs (21, 24) and was able to protect against C. abortus infection in goats and sheep (6, 22, 24-26). Additionally, this vaccine elicited protective immunity when administered in conjunction with live Brucella and Salmonella vaccines or with a Toxoplasma live vaccine (6, 19, 28). Although the 1B mutant strain is currently used commercially in the United Kingdom and other parts of the world for vaccination of sheep and goats, the genetic basis of the attenuation remains unknown, owing to the random nature of NTG mutagenesis.
Comparative genome resequencing (CGR) methodologies allow comparative genomic analysis of closely related genomes by using two microarray-based steps, a mapping phase and a resequencing phase, with approximately 95% of single nucleotide polymorphisms (SNPs) correctly identified. In this study, CGR was used to identify differences between the reference strain S26/3 (30) and the wild-type strain AB7 and to identify the mutations specific to the NTG-generated mutants 1B and 1H. In addition to potentially identifying pathogenic mechanisms, this approach enables the “tolerance” for genomic variation between two virulent isolates of C. abortus to be examined. Finally, as the first whole-genome analysis of an NTG-induced mutant microorganism, our study provides a model for the distribution and specificity of NTG mutagenesis in bacteria.
MATERIALS AND METHODS
Bacterial strains.
The reference strain, C. abortus S26/3, was isolated from a vaccinated ewe in 1979 (30). C. abortus AB7 is a wild-type strain isolated from an aborted lamb (8), while C. abortus 1B and 1H are NTG-derived mutants of the wild-type AB7 strain (21, 24). C. abortus strains were cultured essentially as previously described in McCoy cells in minimal essential medium with 10% fetal bovine serum (21). Three additional low-passage-number wild-type isolates, EBA-Colorado (Cola), isolated from placenta from a bovine abortion; EBA-LX-578-12 (LX), isolated from an aborted bovine fetus; and LW-508 (LW508), isolated from a calf with enteritis, were obtained from Bernhard Kaltenboeck (Auburn University) and cultured in Iscove's modified Dulbecco medium with 2 μg/μl amphotericin B (Fungizone), 25 μg/μl gentamicin, and 10% fetal bovine serum, as previously described (13).
DNA isolation.
Chlamydiae were purified from culture lysates as previously described (4). DNA was extracted from purified chlamydiae by using phenol-chloroform-isoamyl alcohol extraction as previously described (3). DNA concentration and quality were determined by optical density at 260 nm/optical density at 280 nm and agarose gel electrophoresis.
Resequencing and analysis.
DNA isolated from the four strains was processed for mapping and resequencing by Nimblegen, Inc. Mapping arrays were designed based on the genome sequence of the C. abortus S26/3 strain (30), referred to here as the reference strain, with 28-bp probes staggered every 8 bp along the genome, and hybridized to DNA of the three test strains, AB7, 1B, and 1H. Following mapping analysis, a second set of microarrays was designed for resequencing. These arrays had probes designed for each possible SNP within a region of interest, as defined by a significant signal ratio identified upon analysis of the mapping data. The resequencing arrays were run twice for each of the test strains. The mapping and resequencing data were analyzed to identify SNPs and regions of interest without called SNPs, by using SignalMap (Nimblegen, Inc.) and Microsoft Office Excel.
PCR sequencing.
Probes were designed to regions approximately 350 bp upstream and downstream of a called SNP or other sites of interest by using DNAStar PrimerSelect (see Table S1 in the supplemental material). The amplicon was then generated by PCR using Taq HiFi polymerase (Invitrogen), according to the manufacturer's protocol. The amplicon sequence was aligned against the reference genome sequence using BLAST and DNAStar's Megalign.
Analysis of mutations.
Nonsynonymous SNPs were further analyzed, using a BLOSUM80 matrix, to determine if the amino acid substitution resulted in a conservative or a nonconservative change.
RESULTS
Mapping analysis.
Three test strains (AB7, 1B, and 1H) were compared to the S26/3 reference strain to determine the feasibility of resequencing and to gain a preliminary assessment of strain relatedness (data not shown). This analysis confirmed that the two mutants were derived from AB7, as all regions of AB7 with significant signal ratios relative to that of S26/3 were also found in 1B and 1H. In addition, all regions of 1H with significant signal ratios were also identified in 1B, suggesting that 1B was derived from 1H. Results of the mapping analysis showed that the strains were sufficiently closely related for resequencing.
Verification of resequencing results.
A total of 677 potential SNPs present in at least one strain were identified by the resequencing arrays. For each strain, results of two resequencing microarrays were compared for reproducibility. Any putative SNP found only once in all six microarrays (n = 55) was deemed a false positive. Selected SNPs among these and corresponding apparent missed calls were amplified and sequenced for confirmation. The remaining 622 SNPs were categorized according to the probability of representing a true mutation and based on the perceived level of importance of the corresponding mutation. With respect to SNP call accuracy, three categories included SNPs called consistently in all genomes, SNPs called in only one or two genomes, and SNPs near other SNPs. Among these, SNPs generating putative virulence-associated mutations and mutant-associated SNPs leading to intergenic or nonsynonymous mutations received particular attention. SNPs called in only one or two genomes were often characterized by a significantly altered signal ratio in the second and/or third genome and were presumed to result from an apparent missed SNP call at the same location in the other genome(s). Several of these SNPs were amplified and sequenced to verify their presence in all genomes. A second questionable SNP category was composed of SNPs occurring within 20 bp of each other, likely representing errors arising from the overlapping microarray probes. The majority of these neighboring SNP segments were sequenced, and most were confirmed with one valid SNP. All SNPs that had the potential to encode specific alterations in proteins of 1H or 1B were confirmed by PCR sequencing. Additionally, several SNPs mapping to intergenic regions or resulting in silent mutations in the mutants were confirmed by sequencing. However, most SNPs identified in the mutants (n = 24) were also present in AB7, while another four were found to be false positives. Finally, a set of regions with significantly altered ratios that lacked any associated SNPs were sequenced based on the presumption that these might contain nucleotide insertions and deletions that the resequencing microarrays could not resolve.
Comparative genomics of two virulent C. abortus isolates, AB7 and S26/3.
A total of 591 SNPs affecting 363 different predicted annotated genes or coding sequences (CDSs) and 66 predicted intergenic regions were identified in AB7 relative to S26/3 (see Table S2 in the supplemental material). Seven deletions and 11 insertions ranging from single nucleotide insertions (n = 5) or deletions (n = 3) to larger mutations of up to 23 deleted nucleotides and 57 inserted nucleotides were also identified. Mutations were distributed evenly throughout the AB7 genome with no discernible hot spots (Fig. 1). In total, 318 mutations in 255 known or predicted protein-coding CDSs, including 22 pseudogenes, were identified (see Table S3 in the supplemental material).
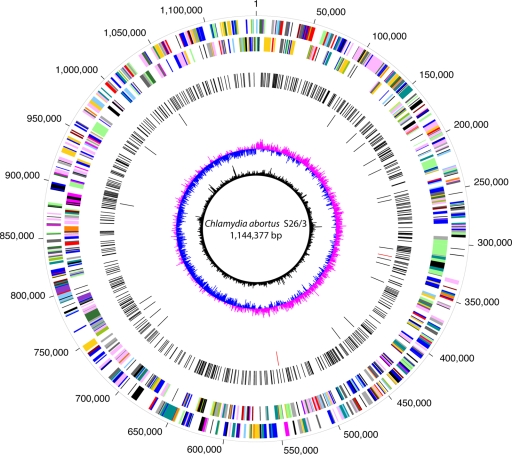
FIG. 1.
Genome comparison of S26/3, AB7, 1B, and 1H. Mutations in AB7 and the two mutant strains, relative to the S26/3 reference genome backbone, are indicated. From the outermost circle inward: circles 1 and 2, CDSs on the plus and minus strands, respectively, color coded as previously described (17) according to role category classification, as determined for the reference S26/3 genome; circle 3, SNPs present in AB7 relative to S26/3; circle 4, SNPs in both 1H and 1B (black) and those unique to 1B (red); circle 5, GC skew of the S26/3 genome; circle 6, G+C content of the S26/3 backbone.
Consistent with their earlier characterization as a highly polymorphic gene family (9, 29), 3 of the 14 genes with more than two protein-altering mutations are pmp genes, one being a predicted pmpG pseudogene. A total of 254 genes/CDSs had at least one mutation leading to an alteration in the predicted protein sequence; however, 16 of these are pseudogenes, suggesting that these SNPs are unlikely to effect a functional change. Most of the remaining CDSs encode hypothetical proteins (n = 64) or proteins with an unknown function (n = 20) (Fig. 2).
FIG. 2.
Role category distribution for genes altered in AB7 relative to S26/3.
The second largest group of SNP-bearing CDSs (n = 31) encode predicted cell envelope proteins (Fig. 2). Consistent with their “housekeeping” roles, genes encoding predicted functions in regulation, protein synthesis, central intermediary metabolism, transcription, fatty acid and lipid metabolism, and synthesis of nucleotides, cofactors, carriers, and prosthetic groups were altered less frequently than were those associated with putative roles in pathogenesis or subjected to innate or adaptive immune pressures (Fig. 2).
Only 10 genes (CAB041, CAB416, CAB502, CAB541, CAB547, CAB557, CAB564, CAB786, CAB852, and CAB853) were found to have intragenic deletions or insertions in AB7 compared to S26/3. CAB541 is a pseudogene conserved in both strains. Of the remaining nine, only CAB786, encoding a 30S ribosomal protein with an internal deletion of 5 amino acids (aa) in AB7, has a known function. The remainder consist of hypothetical proteins including a putative export protein (CAB502, with a 49-aa truncation in AB7) and a conserved membrane protein (CAB852 fused with the hypothetical protein CAB853 in AB7). Orthologs of the CAB852/CAB853 fusion have been previously described within the rrn-nqrF intergenic segment of C. abortus (15).
Analysis of the NTG-induced SNPs in the mutants.
Neither deletions nor insertions were identified in either 1B or 1H compared to AB7, consistent with previous observations of nucleotide conversions predominantly occurring as the result of NTG mutagenesis (16). Most (73%) of the SNPs were GC-to-AT conversions, confirming the known mutagenic bias of NTG. A total of 22 randomly distributed mutant-specific SNPs were identified (Fig. 1; Table 1). All but two SNPs were found in both mutants, suggesting that 1B, possessing the additional two unique SNPs, is derived from 1H. Ten of these mutations, including those unique to 1B, induced synonymous coding changes, while three were located in intergenic regions potentially affecting promoters of CAB186, CAB645, and CAB646. The remaining nine nonsynonymous mutations (Table 2) have the potential to affect the function of the encoded protein(s) and as such may contribute to strain attenuation individually or cumulatively.
TABLE 1.
Mutations unique to 1H and 1B
| Genomic positiona | SNPb | 1H | 1B | PCR verifiedc | Mutation classd | Original amino acid | Mutant amino acid | CDSe | Upstream/downstream locusf | Upstream/downstream distanceg |
|---|---|---|---|---|---|---|---|---|---|---|
| 110655 | C to A | + | + | Syn | R | R | CAB096 | NAh | NA | |
| 147575 | C to T | + | + | Syn | I | I | CAB139 | NA | NA | |
| 164301 | C to T | + | + | Yes | Non | E | K | CAB153 | NA | NA |
| 189518 | G to A | + | + | Yes | Non | V | I | CAB175 | NA | NA |
| 205074 | C to T | + | + | Intg | NA | NA | NA | CAB185/CAB186 | 160/38 | |
| 241667 | G to A | + | + | Yes | Non | D | N | CAB220 | NA | NA |
| 247417 | G to A | + | + | Syn | P | P | CAB227 | NA | NA | |
| 312724 | C to A | + | + | Non | *i | L | CAB273 | NA | NA | |
| 323028 | G to A | − | + | Yes | Syn | L | L | CAB281 | NA | NA |
| 328034 | C to T | + | + | Yes | Non | W | * | CAB283 | NA | NA |
| 335181 | T to C | + | + | Intg | NA | NA | NA | CAB287/CAB288 | 21/102 | |
| 358346 | C to T | + | + | Yes | Non | E | K | CAB308 | NA | NA |
| 429384 | T to C | + | + | Syn | V | V | CAB373 | NA | NA | |
| 453290 | A to C | + | + | Syn | L | L | CAB394 | NA | NA | |
| 542479 | A to G | − | + | Yes | Syn | L | L | CAB469 | NA | NA |
| 716144 | C to T | + | + | Yes | Non | E | K | CAB622 | NA | NA |
| 731338 | C to T | + | + | Yes | Non | G | D | CAB636 | NA | NA |
| 744408 | C to T | + | + | Intg | NA | NA | NA | CAB645/CAB646 | 146/147 | |
| 754284 | G to A | + | + | Yes | Non | P | S | CAB648 | NA | NA |
| 891683 | A to T | + | + | Syn | S | S | CAB772 | NA | NA | |
| 974264 | G to A | + | + | Syn | L | L | CAB842 | NA | NA | |
| 1036695 | A to C | + | + | Syn | A | A | CAB887 | NA | NA |
Position of an SNP, the preceding nucleotide of an insertion, or the first nucleotide of a deletion on the S26/3 genome.
Observed nucleotide conversion.
Result for verification of SNPs by PCR sequencing.
Effect of mutation on protein sequence or location outside a CDS: nonsynonymous (Non) or synonymous (Syn) codon change or intergenic location (Intg).
CDS designation.
Genes upstream and downstream of an intergenic mutation.
Nucleotide distance of the SNP from the upstream and downstream genes.
NA, not applicable.
*, stop codon.
TABLE 2.
Mutated proteins in 1B and 1H
| CDS designation | Common namea | Genomic positionb | Original amino acidc | Mutant amino acidd |
|---|---|---|---|---|
| CAB153 | Putative sigma-54-dependent response regulator | 164301 | E | K |
| CAB175 | Putative export protein | 189518 | V | I |
| CAB220 | Putative serine hydroxymethyltransferase | 241667 | D | N |
| CAB273 | Polymorphic outer membrane protein (pseudogene) | 312724 | *e | L |
| CAB283 | Polymorphic outer membrane protein | 328034 | W | * |
| CAB308 | Putative lipoprotein | 358346 | E | K |
| CAB622 | Methionyl-tRNA synthetase | 716144 | E | K |
| CAB636 | Putative phosphate starvation-inducible protein | 731338 | G | D |
| CAB648 | Valyl-tRNA synthetase | 754284 | P | S |
Common/annotated denomination.
SNP position on the reference genome.
Original amino acid residue resulting from the SNP.
Mutant amino acid residue resulting from the SNP.
*, stop codon.
(i) PmpG mutations.
Mutations were identified in two different pmpG alleles, CAB283 and CAB273. The replacement of a single stop codon with a leucine residue in CAB273 is likely not significant, as CAB273 is a pseudogene with numerous other truncating stop codons. A mutation in the complete, uninterrupted CAB283 pmpG allele, however, introduces a stop at W866X, resulting in the loss of 512 aa, or 37% of the predicted PmpG protein. The truncated segment encompasses most of the predicted autotransporter barrel domain, which would prevent proper insertion of the protein in the outer membrane.
(ii) CAB153/putative sigma-54-dependent response regulator.
The E4K substitution found in CAB153, encoding a putative sigma-54-dependent response regulator, is unlikely to cause any significant functional alteration, as the same substitution is observed in Chlamydia pneumoniae (10, 20, 27), suggesting that this region of the protein is unessential for function or that the protein's activity can tolerate the mutation.
(iii) CAB175/putative export protein.
The V722I substitution in CAB175 is similarly unlikely to alter function, as the same substitution is found in orthologs of C. pneumoniae, Chlamydia muridarum, and C. felis (not shown). Additionally, alignment with the sequence of the SecD domain in which the mutation occurs reveals similar sequence variation at the same position.
(iv) CAB308/putative lipoprotein.
The E350K substitution in CAB308 does not reside within a characterized domain of the predicted lipoprotein. BLAST analysis shows that all Chlamydiaceae orthologs maintain the glutamate residue and that flanking sequences are highly conserved, suggesting the possibility that the resulting +2 charge modification of the mutant protein may affect an uncharacterized domain that is important for function.
(v) CAB220/putative serine hydroxymethyltransferase.
The D248N mutation in CAB220 occurs within the serine hydroxymethyltransferase domain (PFAM00464) of the predicted gene product. Comparative analysis of 250 orthologs by BLAST-P reveals a single ortholog (43% identity) in Anaeromyxobacter dehalogenans containing an asparagine at that site. Only 50 orthologs had a residue other than glutamate or aspartate, and of these, 42 were lysine, 3 were arginine, and 2 were histidine residues (data not shown), suggesting that selective pressure exists to maintain a charged residue at this position.
(vi) CAB622/methionyl-tRNA synthetase.
BLAST-P analysis indicates that the E70K mutation in CAB622 occurs within a conserved domain of the predicted protein. However, while nearly 75% of the orthologs maintained either a glutamate or an aspartate at that position, two were found with a lysine residue at the same site as the mutated residue in CAB622 (not shown).
(vii) CAB648/valyl-tRNA synthetase.
The nonconservative P444S substitution in CAB648 encoding a predicted valyl-tRNA synthetase does not occur within either of the two motifs associated with ATP binding by the enzyme. Alignment with the consensus sequence of the ValRS catalytic core domain (cd00817) places a serine residue at the position corresponding to the mutant serine residue. Furthermore, an alignment of known valyl-tRNA synthetases shows relatively reduced sequence conservation in this segment compared to that in other segments of the domain.
(viii) CAB636/PhoH-like protein.
The nonconservative G410D substitution in CAB636 may be significant, as all of the closest orthologs have a glycine, serine, alanine, or valine residue at the same position (data not shown). CAB636 encodes a putative phosphate starvation-inducible protein, based on its sequence similarity to Escherichia coli PhoH, which has been shown to be induced under phosphate-limiting conditions and to bind ATP (11). Members of the Pho regulon, including PhoH, are upregulated under phosphate-limiting conditions by PhoB. BLAST analysis of C. abortus identified two E. coli PhoB orthologs, CAB153 (30% identity), the putative sigma-54-dependent response regulator which is also altered in the mutants (see above), and CAB977 (31% identity).
DISCUSSION
Comparison of wild-type strains AB7 and S26/3 allowed investigation of C. abortus genomic variation. With the exception of CAB789, encoding a 30S ribosomal protein, no gene with a known function displays an SNP affecting more than one amino acid change between the two wild-type strains. Additionally, all pseudogenes are conserved in the two isolates. However, the number of mutations in proteins with unknown function is significant and could lead to important functional differences between these strains. Overall, alterations identified in AB7 relative to S26/3 are consistent with general intraspecies variation.
Comparative analysis of the parent and mutant strains provided genome-level verification of the NTG-induced GC-to-AT conversion bias and of NTG causing SNPs rather than deletions and/or insertions. Although the number of mutations induced by NTG mutagenesis was low (22 SNPs), there was no apparent bias in the numbers of synonymous SNPs, intergenic SNPs, and nonsynonymous SNPs (10, 3, and 9, respectively), which suggests that the small C. abortus genome tolerates mutations.
While the CGR method used here does identify most SNPs (>95%) as well as insertions and deletions, some mutations may be missed. Hence, we cannot rule out the possibility of a missed SNP in 1H leading to the observed instability of this attenuated mutant. However, it is less likely that the mutation(s) responsible for the virulence-attenuated phenotype of both mutants could have been missed, since such mutations would have to have been missed simultaneously in 1H and 1B. Another possible reason for the observed differential properties of the two mutants (21, 24) is that the two “silent” mutations identified as unique to 1B affect a regulatory sequence or alter protein folding. Kimchi-Sarfaty et al. have recently reported that alteration of a codon to a rare codon can delay translation, leading to alterations in protein folding (12). Analysis of C. abortus codon usage via the Codon Usage Database (http://www.kazusa.or.jp/codon/) shows that the conversion from CTT (21.54/1,000) to the less common CTC codon (12.07/1,000) in CAB469 of 1B could represent such a case. Notwithstanding this possibility, the mutations identified in CDSs and annotated genes offer some intriguing insights into the possible basis or bases for the attenuated virulence phenotype and conversely into C. abortus pathogenesis. Moreover, genomic sequence information specific to 1B will provide powerful diagnostic tools for identifying wild-type C. abortus-infected animals in C. abortus 1B-vaccinated flocks. Because of the uncertainty surrounding genotypic and phenotypic features of 1H, the following discussion is principally focused on the 1B mutant, i.e., the virulence-attenuated strain currently used as a live vaccine.
NTG-induced virulence-attenuating mutations of 1B do not cause loss of a central function, as the mutant replicates normally in McCoy cells, producing plaques of the same size and morphology as those of AB7 plaques when grown at 35 to 38°C (21). It is only when strains are grown at 39.5°C that any growth difference is observed, suggesting that a genotypic alteration(s) of the mutant becomes critical only at the higher temperature of the ovine host. Three protein mutations appear most likely to lead to the observed phenotype: the W-to-X translational stop mutation of CAB283, producing a truncated, secretion-incompetent PmpG protein; the E-to-K mutation in CAB308, potentially encoding an altered membrane-destabilizing lipoprotein; and the combined E-to-K and G-to-D mutations, potentially altering the functions of CAB153 and CAB636, two key proteins of the Pho regulon in other bacteria.
Members of the pmp family encode a group of predicted autotransported proteins that may be important in pathogenesis, including the ability to evade host immune responses by antigenic variation (5, 7, 18, 29, 31). The observation that 1B grows normally in vitro suggests that the loss of PmpG does not affect invasion, replication, or lysis of the host cell. Indeed, the mutant phenotype supports a role for PmpG specific to survival at elevated temperatures or when the bacterium is subjected to immune pressure during infection of live animals. Although the possibility exists for other pmpG genes to complement the CAB283 mutation, our results support the idea that the absence of the CAB283 PmpG protein at the surface of C. abortus contributes to the observed virulence attenuation.
The CAB308-encoded putative lipoprotein is found in all members of the Chlamydiaceae and displays less than 30% identity to CDSs in other bacteria, suggesting that this protein is Chlamydia specific and as such may be critical for chlamydial biology. Sequence alignment of the putative lipoprotein within the Chlamydiaceae reveals that the region encompassing the mutation is well conserved, suggesting that it may be critical for function. It is therefore possible that the E-to-K charge shift in CAB308 could hinder the ability of this protein to interact with a putative ligand and that the effects of such a mutation would be more pronounced at higher temperatures.
The final protein mutations of interest encompass two potentially related CDSs, CAB636 and CAB153, respectively encoding orthologs of PhoH and PhoB of E. coli. The activator PhoB protein upregulates genes of the Pho regulon during phosphate starvation. Although the similarity of CAB153 and PhoB is limited, it is tempting to speculate that the observed E-to-K mutation in CAB153 could affect regulation of chlamydial pho promoters, including that of the phoH ortholog, CAB636. Functional disruption owing to the G-to-D mutation in mutant CAB636 could be further exacerbated by disrupted transcription mediated by the PhoB-like CAB153. However, as no function has been identified for PhoH in E. coli, the impact of the Pho protein mutations on chlamydial biology remains entirely speculative.
An intriguing possible explanation for the virulence attenuation observed in 1B results from an SNP in the intergenic space predicted to include the promoter of CAB645 encoding the predicted single-copy chlamydial pyruvate kinase. In preliminary experiments, CAB645 transcript was detected at both 37°C and 39.5°C in the parent AB7 strain but only at 37°C in the 1B mutant. Although transcript amounts were small in these experiments (data not shown), this result, if confirmed, would suggest that expression of this essential central metabolism gene is downregulated in the mutant at the animal's body temperature of 39.5°C, with downstream negative consequences for replication and transcriptional activity during infection. It is also an intriguing possibility that the same intergenic SNP could affect transcription of the divergently transcribed CAB646 at 39.5°C. Reduced expression of the putative SOS response nuclease encoded by CAB646 could lead to reduced survival during stressed conditions, such as elevated temperatures.
This study has yielded some intriguing insights into chlamydial pathogenesis and revealed that the attenuation observed in the virulence-attenuated C. abortus mutant is not the result of a single critical mutation but rather the cumulative and complex effect of several. The proposed reduced transcription of the predicted pyruvate kinase gene and the implied impact on central metabolism support a role for this mutation as a direct cause of virulence attenuation. Mutations affecting surface proteins may then contribute to the virulence-attenuated phenotype by simultaneously diminishing the pathogenic potential and enhancing the immunogenic properties of live vaccine C. abortus.
Supplementary Material
Acknowledgments
This work was supported by NIH grant RO1 AI51472 to G. S. A. Myers and P. M. Bavoil. Laurel Burall was supported during some of this study by a postdoctoral fellowship from the Training Program in Oral and Craniofacial Biology, NIH/NIDCR T32 DE07309-08.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 13 July 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aitken, I. D., I. E. Anderson, and G. W. Robinson. 1986. Ovine chlamydial abortion: limitation of inactivated vaccine, p. 55-65. In I. D. Aitken (ed.), Chlamydial disease of ruminants. Commission of the European Communities, Luxembourg.
- 2.Aitken, I. D., M. J. Clarkson, and K. Linklater. 1990. Enzootic abortion of ewes. Vet. Rec. 126136-138. [DOI] [PubMed] [Google Scholar]
- 3.Boumedine, K. S., and A. Rodolakis. 1998. AFLP allows the identification of genomic markers of ruminant Chlamydia psittaci strains useful for typing and epidemiological studies. Res. Microbiol. 149735-744. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 311161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson, J. H., S. F. Porcella, G. McClarty, and H. D. Caldwell. 2005. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect. Immun. 736407-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalmers, W. S., J. Simpson, S. J. Lee, and W. Baxendale. 1997. Use of a live chlamydial vaccine to prevent ovine enzootic abortion. Vet. Rec. 14163-67. [DOI] [PubMed] [Google Scholar]
- 7.Crane, D. D., J. H. Carlson, E. R. Fischer, P. Bavoil, R. C. Hsia, C. Tan, C. C. Kuo, and H. D. Caldwell. 2006. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc. Natl. Acad. Sci. USA 1031894-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faye, P., A. Charton, C. Mage, C. Bernard, and C. Le Layec. 1972. Proprietés hémagglutinantes du virus de l'avortement enzootique des petits ruminants (souches de Rakeia d'origine ovine et caprine). Bull. Acad. Vet. Fr. 45169-173. [Google Scholar]
- 9.Grimwood, J., and R. S. Stephens. 1999. Computational analysis of the polymorphic membrane protein superfamily of Chlamydia trachomatis and Chlamydia pneumoniae. Microb. Comp. Genomics 4187-201. [DOI] [PubMed] [Google Scholar]
- 10.Kalman, S., W. Mitchell, R. Marathe, C. Lammel, J. Fan, R. W. Hyman, L. Olinger, J. Grimwood, R. W. Davis, and R. S. Stephens. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 21385-389. [DOI] [PubMed] [Google Scholar]
- 11.Kim, S. K., K. Makino, M. Amemura, H. Shinagawa, and A. Nakata. 1993. Molecular analysis of the phoH gene, belonging to the phosphate regulon in Escherichia coli. J. Bacteriol. 1751316-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimchi-Sarfaty, C., J. M. Oh, I. W. Kim, Z. E. Sauna, A. M. Calcagno, S. V. Ambudkar, and M. M. Gottesman. 2007. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315525-528. [DOI] [PubMed] [Google Scholar]
- 13.Li, D., A. Vaglenov, T. Kim, C. Wang, D. Gao, and B. Kaltenboeck. 2005. High-yield culture and purification of Chlamydiaceae bacteria. J. Microbiol. Methods 6117-24. [DOI] [PubMed] [Google Scholar]
- 14.Linklater, K. A., and D. A. Dyson. 1979. Field studies on enzootic abortion of ewes in south east Scotland. Vet. Rec. 105387-389. [DOI] [PubMed] [Google Scholar]
- 15.Liu, Z., R. Rank, B. Kaltenboeck, S. Magnino, D. Dean, L. Burall, R. D. Plaut, T. D. Read, G. Myers, and P. M. Bavoil. 2007. Genomic plasticity of the rrn-nqrF intergenic segment in the Chlamydiaceae. J. Bacteriol. 1892128-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucchesi, P., M. Carraway, and M. G. Marinus. 1986. Analysis of forward mutations induced by N-methyl-N′-nitro-N-nitrosoguanidine in the bacteriophage P22 mnt repressor gene. J. Bacteriol. 16634-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers, G. S., D. Parker, K. Al-Hasani, R. M. Kennan, T. Seemann, Q. Ren, J. H. Badger, J. D. Selengut, R. T. Deboy, H. Tettelin, J. D. Boyce, V. P. McCarl, X. Han, W. C. Nelson, R. Madupu, Y. Mohamoud, T. Holley, N. Fedorova, H. Khouri, S. P. Bottomley, R. J. Whittington, B. Adler, J. G. Songer, J. I. Rood, and I. T. Paulsen. 2007. Genome sequence and identification of candidate vaccine antigens from the animal pathogen Dichelobacter nodosus. Nat. Biotechnol. 25569-575. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen, A. S., G. Christiansen, and S. Birkelund. 2001. Differential expression of Pmp10 in cell culture infected with Chlamydia pneumoniae CWL029. FEMS Microbiol. Lett. 203153-159. [DOI] [PubMed] [Google Scholar]
- 19.Plommet, M., N. Bosseray, F. Lantier, F. Bernard, P. Pardon, and A. Rodolakis. 1987. Simultaneous vaccination by three living attenuated strains of Brucella, Salmonella and Chlamydia. Vaccine 527-32. [DOI] [PubMed] [Google Scholar]
- 20.Read, T. D., R. C. Brunham, C. Shen, S. R. Gill, J. F. Heidelberg, O. White, E. K. Hickey, J. Peterson, T. Utterback, K. Berry, S. Bass, K. Linher, J. Weidman, H. Khouri, B. Craven, C. Bowman, R. Dodson, M. Gwinn, W. Nelson, R. DeBoy, J. Kolonay, G. McClarty, S. L. Salzberg, J. Eisen, and C. M. Fraser. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 281397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodolakis, A. 1983. In vitro and in vivo properties of chemically induced temperature-sensitive mutants of Chlamydia psittaci var. ovis: screening in a murine model. Infect. Immun. 42525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodolakis, A., and F. Bernard. 1984. Vaccination with temperature sensitive mutant of Chlamydia psittaci against enzootic abortion of ewes. Vet. Rec. 114193-194. [DOI] [PubMed] [Google Scholar]
- 23.Rodolakis, A., F. Bernard, and A. Souriau. 1989. Stability and safety of a chlamydial live vaccine against enzootic abortion of ewes, p. 92-97. In A. Meheus and R. E. Spier (ed.), Vaccines for sexually transmitted diseases. Butterworths, London, United Kingdom.
- 24.Rodolakis, A., and A. Souriau. 1983. Response of ewes to temperature sensitive mutants of Chlamydia psittaci (var. ovis) obtained by NTG mutagenesis. Ann. Rech. Vet. 14155-161. [PubMed] [Google Scholar]
- 25.Rodolakis, A., and A. Souriau. 1986. Response of goats to vaccination with temperature-sensitive mutants of Chlamydia psittaci obtained by nitrosoguanidine mutagenesis. Am. J. Vet. Res. 472627-2631. [PubMed] [Google Scholar]
- 26.Rodolakis, A., and A. Souriau. 1989. Variations in the virulence of strains of Chlamydia psittaci for pregnant ewes. Vet. Rec. 12587-90. [DOI] [PubMed] [Google Scholar]
- 27.Shirai, M., H. Hirakawa, M. Kimoto, M. Tabuchi, F. Kishi, K. Ouchi, T. Shiba, K. Ishii, M. Hattori, S. Kuhara, and T. Nakazawa. 2000. Comparison of whole genome sequences of Chlamydia pneumoniae J138 from Japan and CWL029 from USA. Nucleic Acids Res. 282311-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souriau, A., N. Bosseray, A. Rodolakis, F. Lantier, and M. Plommet. 1988. Anti-chlamydial immunity in ewes conferred by vaccination with a combination of three live Chlamydia, Brucella and Salmonella vaccines. Vet. Rec. 12312-32. [DOI] [PubMed] [Google Scholar]
- 29.Tan, C., J. K. Spitznagel, H.-Z. Shou, R. Hsia, and P. M. Bavoil. 2006. The polymorphic membrane protein gene family of the Chlamydiaceae, p. 195-218. In P. M. Bavoil and P. B. Wyrick (ed.), Chlamydia, genomics and pathogenesis. Horizon Bioscience, Norwich, Norfolk, United Kingdom.
- 30.Thomson, N. R., C. Yeats, K. Bell, M. T. Holden, S. D. Bentley, M. Livingstone, A. M. Cerdeno-Tarraga, B. Harris, J. Doggett, D. Ormond, K. Mungall, K. Clarke, T. Feltwell, Z. Hance, M. Sanders, M. A. Quail, C. Price, B. G. Barrell, J. Parkhill, and D. Longbottom. 2005. The Chlamydophila abortus genome sequence reveals an array of variable proteins that contribute to interspecies variation. Genome Res. 15629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viratyosin, W., L. A. Campbell, C. C. Kuo, and D. D. Rockey. 2002. Intrastrain and interstrain genetic variation within a paralogous gene family in Chlamydia pneumoniae. BMC Microbiol. 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wills, J. M., T. J. Gruffydd-Jones, S. J. Richmond, R. M. Gaskell, and F. J. Bourne. 1987. Effect of vaccination on feline Chlamydia psittaci infection. Infect. Immun. 552653-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.