Abstract
The Duffy binding-like (DBL) domains are common adhesion modules present in Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) variants, which are responsible for immune evasion and cytoadherence. Knowledge about how immune responses are acquired against polymorphic DBL domains of PfEMP1 can aid in the development of vaccines for malaria. A recombinant DBLα domain, encoded by R29 var1, which binds complement receptor 1 to mediate rosetting by the P. falciparum laboratory strain R29, was expressed in Escherichia coli, renatured by oxidative refolding to its native form, and purified to homogeneity. Antibody levels in 704 plasmas obtained from residents of areas of different levels of malaria endemicity in Orissa (India) and Manhiça (Mozambique) were assessed by enzyme-linked immunosorbent assay. The refolded DBLα domain was pure, homogeneous, and functional in that it bound human erythrocytes with specificity and was capable of inhibiting rosetting. The proportion of individuals who had measurable anti-DBLα immunoglobulin G responses was low in areas of low malaria endemicity in Orissa (6.7%) but high in areas of high endemicity in Orissa (87.5%) and Manhiça (74.5%). Seroprevalence and antibody levels against the recombinant protein increased with the age of inhabitants from areas with high transmission rates (P < 0.001). Half of the children in these areas had seroconverted by the age of 5 years. These findings suggest that in spite of the extreme polymorphism of PfEMP1 DBLα domains, the acquisition of specific antibodies is rapid and age related and reflects the reduced risk of malaria in areas with high transmission rates. Further studies are required to elucidate the role of these antibodies in protection from malaria.
Individuals residing in areas where Plasmodium falciparum malaria is endemic gradually develop clinical immunity with age following repeated infections (17). Passive transfer experiments have shown that immunoglobulin G (IgG) antibodies play a major role in the mechanisms of protection against malaria (9, 10). Naturally acquired IgGs with specificity for variant surface antigens (VSA) expressed on the surfaces of P. falciparum-infected erythrocytes (IE) are believed to contribute to the regulation of parasite densities in a manner that decreases the incidence of clinical disease (4, 13, 15, 19).
The most studied VSA, P. falciparum erythrocyte membrane protein 1 (PfEMP1), is a family of large (∼250 to 350 kDa) (2), polymorphic proteins that are encoded in each parasite genome by ∼60 different var genes (12). Switches in var gene expression allow parasites to evade host immunity (18). PfEMP1 mediates the binding of IE to host endothelial cell receptors, to uninfected erythrocytes to mediate rosetting, and to platelets to form clumps of IE enabling sequestration of the parasite at different sites in the host (21). Sequestration in some internal organs has been implicated in progression to severe disease manifestations, such as cerebral and placental malaria (23).
PfEMP1 proteins are composed of a variable number of adhesive domains of two types, namely, Duffy binding-like (DBL) domains and cysteine-rich interdomain regions (34). DBL sequences are extremely polymorphic, probably reflecting the intensity of immune pressure on PfEMP1 proteins at the IE surface. Although these domains average <50% amino acid identity (11), they can still be classified into six different types (α, β, γ, δ, ɛ, and X) based on the presence of conserved sequence motifs, including disulfide-linked cysteines (34). Certain DBLα domains harbor adhesive functions associated with virulent phenotypes. It has been shown that the DBLα domain is involved in the formation of rosettes (7, 31), a cytoadhesion phenotype that is associated with cerebral malaria (5, 23, 30, 36). The DBLα domain encoded by R29 var1, the var gene expressed by the rosetting parasite R29, binds complement receptor 1 (CR1) on erythrocytes to mediate the formation of rosettes (31). The CR1 binding residues map to the 233-amino-acid central stretch of the DBLα domain (20).
Current research efforts seek to determine whether specific PfEMP1 variants containing related adhesive domains with conserved structures are associated with severe disease. Such conserved adhesion-related protein structures could then be targeted therapeutically or prophylactically across parasite isolates to protect against severe malaria. The association between naturally acquired antibodies against VSA (4, 13, 19), which are dominantly represented by PfEMP1 molecules, and protection against clinical malaria in regions of endemicity argues for the inclusion of PfEMP1 domains in the development of malaria vaccines. Despite this apparent role in the development of antimalarial immunity, the use of PfEMP1 in vaccine development is hampered by the extensive polymorphism in the var gene family. Nevertheless, evidence supporting the utilization of the DBLα domain as a vaccine candidate is accumulating (8, 22). DBLα is an attractive candidate because it is one of the most conserved domains of PfEMP1 (11). Understanding naturally acquired immune responses to DBLα can aid in the development of malaria vaccines based on this domain.
Here we describe methods to produce the central, functional region of the R29 var1-encoded DBLα domain in its correctly folded conformation. The recombinant DBLα (rDBLα) domain was used to assess the presence of naturally acquired anti-DBLα antibodies in plasma samples collected from individuals residing in areas of different levels of endemicity in India and Mozambique. We show that individuals from areas with high rates of malaria transmission rapidly develop antibodies against DBLα as a function of age.
MATERIALS AND METHODS
Expression of rDBLα.
The binding site of the R29 var1-encoded DBLα domain has been mapped to the central region including amino acids 133 to 366 (Cys residues 5 to 12) (20). A DNA fragment (nucleotides 399 to 1098) encoding R29 DBLα (20, 31) fused to a hexahistidine tag at the C-terminal end was amplified by a PCR using primers 5′-GCA TGC CAT GGA TAG AAA TTT AGA ATA TTT GAT C-3′ and 5′-CGA GTG TCG ACT CAG TGA TGG TGA TGG TGA TGA CGT GGA CAA TTT AAA TCT ATA AAG-3′, with a plasmid containing a DNA fragment encoding DBLα of P. falciparum R29 as the template. The PCR product was cloned into Escherichia coli expression vector pET28a(+) (Novagen). The insert as well as junctions between the vector and insert was sequenced using an ABI 310 automated DNA sequencer (Applied Biosystems). E. coli BL21(DE3) cells (Novagen) were transformed with the construct and used for expression of rDBLα by induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After 4 h of growth at 37°C, E. coli cells were harvested and lysed by sonication, and inclusion bodies were collected by centrifugation and solubilized in 10 mM Tris, pH 8.0, containing 6 M guanidine-hydrochloride. rDBLα was purified from solubilized inclusion bodies under denaturing conditions by metal-affinity chromatography using a nickel-nitrilotriacetic acid column as described by the manufacturer (Qiagen). The column was washed with equilibration buffer at pH 6.3, and bound protein was eluted with elution buffer at pH 4.3.
Refolding of rDBLα.
Purified, denatured rDBLα was refolded by 100-fold dilution in a buffer containing 50 mM phosphate buffer, pH 6.5, 2 mM cysteine, 0.67 mM cystamine dihydrochloride, 16 mM β-cyclodextrin, 0.4 mM Triton X-100, 1 M urea, and 0.5 M arginine at a final concentration of recombinant protein of 60 mg/ml. Refolding was allowed to proceed for 36 h at 10°C. The refolding solution was dialyzed for 48 h against dialysis buffer (50 mM phosphate buffer, pH 6, 1 M urea) to remove arginine. Refolded rDBLα was loaded on an SP-Sepharose column (Pharmacia) equilibrated with 50 mM phosphate buffer, pH 6, and the bound protein was eluted with a linear gradient of NaCl (100 mM NaCl to 1 M NaCl). Fractions containing refolded rDBLα were pooled, and rDBLα was further purified by gel filtration chromatography using a preparative-grade Superdex 75 column (Pharmacia) with 50 mM phosphate buffer, pH 6.0, containing 150 mM NaCl as the running buffer. Refolded rDBLα was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before and after reduction with β-mercaptoethanol and was detected by Coomassie blue and silver staining.
Analysis of refolded rDBLα by reverse-phase chromatography.
Refolded rDBLα was loaded on a reverse-phase C8 column. The gradient used for elution was developed using buffer A (0.05% trifluoroacetic acid in water) and buffer B (0.05% trifluoroacetic acid in 90% acetonitrile and 10% water). The column was initially equilibrated with 95% buffer A and 5% buffer B and reached a composition of 90% buffer A and 10% buffer B in 10 min and a final composition of 5% buffer A and 95% buffer B in another 30 min.
Functional characterization of refolded rDBLα.
Blood collected in 10% citrate phosphate dextrose was washed three times in RPMI 1640. Ten micrograms of rDBLα was incubated for 1 h at room temperature with 2 × 108 normal erythrocytes or human erythrocytes expressing low levels of CR1 (kindly provided by Karina Yazdanbaksh, New York Blood Centre, New York, NY) in a final volume of 600 μl of RPMI 1640 containing 1% fetal calf serum. The reaction mixture was layered over dibutylphthalate (Sigma) and centrifuged to collect erythrocytes. Bound protein was eluted from the erythrocytes with 300 mM NaCl, separated by SDS-PAGE, and detected by Western blotting using a mouse monoclonal antibody against pentahistidine (Qiagen). Plasmodium vivax region II (PvRII) of the Duffy binding protein, which was previously shown to bind Duffy antigen receptor for chemokines on erythrocytes by this method (33), was used as a positive control. The intensities of the bands were quantified by densitometry scanning.
Rosette reversal by rDBLα.
A P. falciparum R29 culture at 5% parasitemia and with a rosetting frequency of 60 to 70% was stained with 20 μg/ml ethidium bromide for 5 min at 37°C, washed twice with incomplete RPMI 1640, and resuspended at 4% hematocrit in complete RPMI with 10% heat-inactivated human serum. Twenty-five microliters of parasite suspension was incubated at 37°C for 30 min with doubling concentrations of rDBLα (0.4 μg/ml to 200 μg/ml) in a final volume of 50 μl. The frequency of rosette formation was assessed by fluorescence microscopy in triplicate experiments. Two hundred infected erythrocytes were counted in each experiment, with the binding of two or more uninfected erythrocytes constituting a rosette. Rosetting at each rDBLα concentration was expressed as a percentage of the rosetting frequency in control R29 cultures with no added protein. The same amounts of PvRII were used as a negative control.
Study areas and subjects.
Individuals were enrolled for the study at Sundargarh District in Orissa (Eastern India) in March 2004 and at Manhiça District (Southern Mozambique) in 2007. The characteristics of the areas have been described in detail elsewhere (16, 32). Briefly, Sundargarh District is characterized by variations in transmission patterns over short geographic distances. The annual incidence of malaria cases per 1,000 persons is higher in villages located in forested areas (284.1) than in villages located in deforested plain areas (31.2) (32). The estimated entomological inoculation rate (EIR) in 2001 to 2003 was 113.5 infective bites per person per year in forest areas and 5.1 infective bites per person per year in plain areas (32). Manhiça District is characterized by perennial malaria transmission, mostly attributable to P. falciparum, with some seasonality. The estimated EIR in 2002 was 38 infective bites per person per year (1). In Sundargarh District, 539 volunteers (434 people from three forest villages [San Dulakudar, Rangamati, and Benuam] and 105 people from two plain villages [Chikatmati and Bhalupatra]) aged 1 to 84 years were enrolled in the survey. In Manhiça District, 60 children (aged 1 to 5 years) and 105 adults (aged 15 to 73 years) attending the Manhiça District Hospital with signs and symptoms of malaria were enrolled. Plasmas were isolated from heparin-anticoagulated blood samples. Parasitemic persons were treated following national guidelines at the time of the study. All study participants or their parents or guardians gave informed consent. The study protocol was approved by the ethical committee of the National Institute of Malaria Research, Indian Council of Medical Research, New Delhi, India, the National Mozambican Ethics Review Committee, and the Hospital Clinic of Barcelona Ethics Review Committee.
Measurement of antibodies against rDBLα by ELISA.
IgG levels in the collected plasmas were determined by an enzyme-linked immunosorbent assay (ELISA). MaxiSorp immunoplates (Nunc) were coated with 200 ng per well of rDBLα in 0.05 M carbonate-bicarbonate buffer by overnight incubation at 4°C. Plates were washed with 0.05% Tween 20 in phosphate-buffered saline (PBS-Tween), blocked with 2% bovine serum albumin in PBS-Tween for 8 h at 4°C, and washed with PBS-Tween, and plasma samples (1:200) were added in duplicate, along with a positive control plasma (a pool of plasmas from eight adults with lifelong exposure to malaria) and 10 negative control plasmas from nonexposed European adults. Plates were incubated overnight at 4°C, washed, and incubated for 1 h with peroxidase-conjugated goat anti-human IgG secondary antibodies (Sigma, St. Louis, MO) at a dilution of 1:30,000. After washing of the plates three times with PBS-Tween, 100 μl of a phosphate solution with 0.012% H2O2 substrate and o-phenylenediamine was added to each well. The colorimetric reaction was stopped with 25 μl of 3 M H2SO4 after 5 min, and the optical density (OD) was measured at 492 nm. Plasmas from Manhiça were also tested at a 1:200 dilution for IgG recognition of merozoite surface protein 1 (MSP-119; 19-kDa fragment, from strain 3D7), erythrocyte-binding antigen 175 (EBA-175; region F2, from strain CAMP), and apical membrane antigen 1 (AMA-1; full ectodomain, from strain 3D7) produced in E. coli following similar procedures to those for rDBLα. Antibody responses were expressed in arbitrary units (AU) to account for day-to-day variation and were calculated as follows: (ODsample/ODpositive control) × 100. The cutoff for positivity was defined as the AU value 3 standard deviations above the arithmetic mean for the negative control plasmas (33.0 for DBLα, 53.3 for MSP-1, 31.1 for EBA-175, and 20.3 for AMA-1).
Statistical analysis.
Mean rosetting frequencies at different rDBLα concentrations were compared to the rosetting frequency in control R29 cultures with no added protein by using Student's t test. Fisher's test was used to compare proportions of antibody responders in different age groups (1 to 2.5, 2.5 to 5, 5 to 7.5, 7.5 to 15, 15 to 25, 25 to 35, and >35 years), whereas the differences in the immunoglobulin levels (AU) were compared using the Kruskal-Wallis test. P values of <0.05 were considered to indicate statistical significance.
RESULTS
Biochemical, biophysical, and functional characterization of refolded and purified rDBLα.
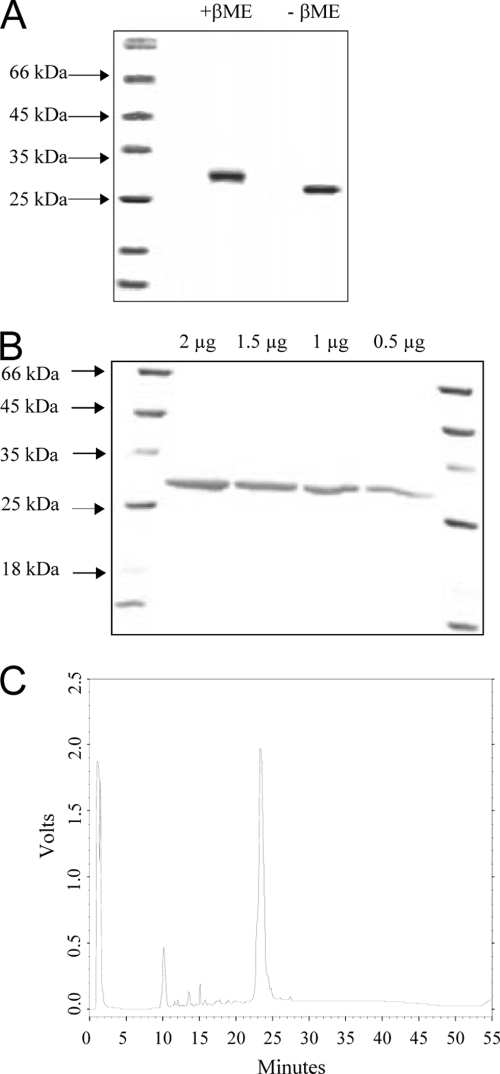
rDBLα expressed in E. coli accumulates in inclusion bodies as insoluble aggregates. Misfolded rDBLα was solubilized in 6 M guanidine-HCl, purified under denaturing conditions by metal-affinity chromatography, and refolded by the method of rapid dilution. Cysteine and cystamine were used to allow disulfide bond shuffling during refolding. Conditions such as the concentration of rDBLα and pH were optimized to maximize yields of refolded rDBLα. Ion-exchange chromatography using an SP-Sepharose column and gel filtration chromatography using a Superdex 75 column were used to purify rDBLα monomers to homogeneity after they refolded. rDBLα was separated by SDS-PAGE and detected by Coomassie blue (Fig. 1A) and silver (Fig. 1B) staining. rDBLα migrated with an apparent molecular mass of ≈27 kDa in denaturing SDS-PAGE gels, as predicted by the calculated molecular mass of DBLα. Densitometry scanning of silver-stained SDS-PAGE gels indicated that the purity of refolded rDBLα was >95%. Refolded rDBLα migrated slower in SDS-PAGE gels after reduction with β-mercaptoethanol, indicating the presence of disulfide bonds (Fig. 1A). The mobility of purified rDBLα in a Superdex 75 column was consistent with an apparent molecular mass of ≈27 kDa (data not shown), indicating that purified rDBLα does not contain aggregates or multimers. Refolded rDBLα eluted in a single, symmetric peak by reverse-phase chromatography on a C8 column, suggesting that purified rDBLα is conformationally homogeneous (Fig. 1C).
FIG. 1.
Refolded and purified rDBLα minimal domain. (A) Mobility of refolded and purified DBLα by SDS-PAGE before and after reduction with β-mercaptoethanol (β-ME). (B) Silver-stained SDS-PAGE gel of refolded and purified rDBLα. Different quantities (0.5, 1.0, 1.5, and 2.0 μg) of refolded and purified rDBLα were reduced, denatured, separated by SDS-PAGE, and detected by silver staining. (C) Reverse-phase chromatography profile of refolded and purified rDBLα.
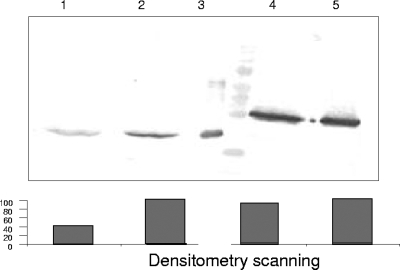
Erythrocyte binding assays were used to test the functional activity of refolded rDBLα. After incubation of refolded rDBLα with normal or CR1-deficient human erythrocytes to allow binding, bound protein was eluted, separated by SDS-PAGE, and detected by Western blotting using a monoclonal antibody against pentahistidine (Fig. 2). Refolded rDBLα bound to normal human erythrocytes. Densitometry scanning showed that binding of rDBLα to CR1-deficient erythrocytes was reduced 60% compared to binding to normal erythrocytes, whereas binding of PvRII to normal and CR1-deficient erythrocytes was similar (Fig. 2).
FIG. 2.
Erythrocyte binding assay of refolded and purified rDBLα with CR1-deficient human erythrocytes (lane 1) and normal human erythrocytes (lane 2). Lanes 4 and 5 show the erythrocyte binding assay of PvRII with CR1-deficient and normal erythrocytes, respectively. Lane 3 shows rDBLα (1 μg).
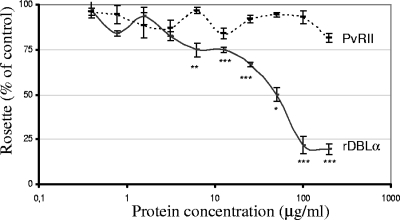
The ability of rDBLα to reverse rosetting of P. falciparum R29 was also tested. rDBLα reversed rosetting of P. falciparum R29 in a dose-dependent manner (Fig. 3). The rosetting frequency in the absence of rDBLα was 58% (standard deviation, 3%), whereas the rosetting frequency in the presence of 200 μg/ml of rDBLα was 11.3% (standard deviation, 3.1%). Differences in rosetting frequency were statistically significant between control parasites with no added protein and parasites incubated with a minimum of 6.25 μg/ml rDBLα (P < 0.01). No statistically significant reversal of rosetting was observed with similar amounts of PvRII (Fig. 3).
FIG. 3.
Dose-dependent inhibition of R29 rosetting by rDBLα. Rosetting at each DBLα concentration (continuous line) is expressed as the percentage of rosette frequency compared to that for control strain R29 with no added protein. PvRII (discontinuous line) was used as a negative control. Data shown are means and standard deviations for triplicate experiments with each concentration. Values that are statistically different from the control value by Student's t test are asterisked (*, P < 0.001; **, P < 0.01; ***, P < 0.05).
Recognition of rDBLα by plasmas from individuals residing in areas where P. falciparum is endemic.
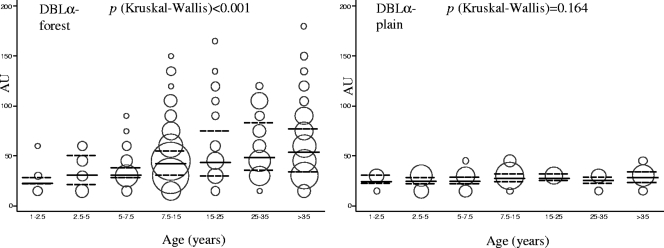
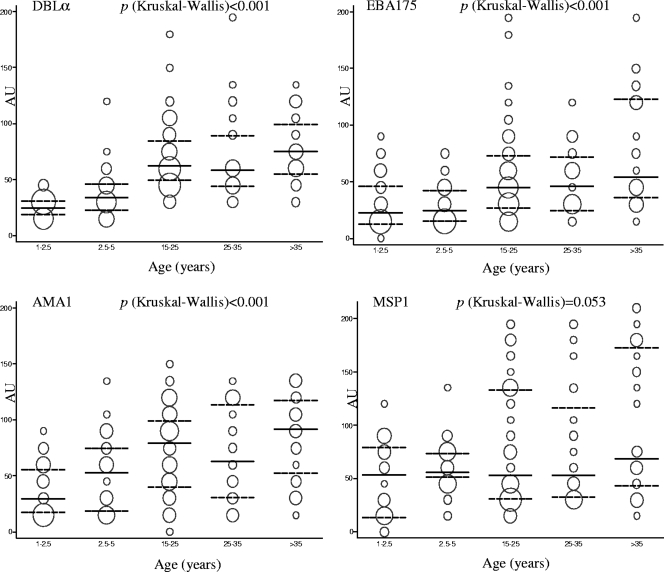
There were marked differences among the three study areas with respect to the proportion of individuals who had measurable anti-rDBLα IgG responses (Table 1). Seropositivity for rDBLα was significantly higher for residents in the forest villages of the Sundargarh District in Orissa (380/434 samples; 87.5%) than for residents in deforested plain areas of the Sundargarh District (7/105 samples; 6.7%) with lower malaria transmission rates (P < 0.001). Similarly, the level of anti-rDBLα IgGs was higher for the population from forest areas in Orissa than for those in plain villages (Fig. 4) (P < 0.001). In plain villages, no statistically significant difference between age groups was observed in recognition of rDBLα (Table 1) or in the level of IgGs (Fig. 4). However, in the population from forest villages, rDBLα IgG seroprevalence (Table 1) and levels of antibodies (Fig. 4) increased with age. Compared to the youngest children (1 to 2.5 years), the prevalence of rDBLα IgG was similar for 2.5- to 5-year-old children (P = 0.371) and increased significantly in 5- to 7.5-year-old children (P = 0.018). In Manhiça (Mozambique), average recognition was 74.5% (123/165 samples) for rDBLα, 53.9% (89/165 samples) for MSP-1, 59.3% (98/165 samples) for EBA-175, and 84.8% (140/165 samples) for AMA-1 (Table 1). The prevalence of seropositive individuals (Table 1) and the level of antibodies (Fig. 5) were found to increase with age for rDBLα, EBA-175, and AMA-1 but not for MSP-1 (Table 1; Fig. 5). Compared to the youngest children (1 to 2.5 years), the first significant increase in antibody levels was found at 2.5 to 5 years for rDBLα (P = 0.004) and at 15 to 25 years for AMA-1 and EBA-175 (P < 0.001) (Fig. 5). Similarly, seroprevalences for rDBLα were significantly higher in 2.5- to 5-year-old Mozambican children (53.3%) than in the youngest children (16.7%; P = 0.006), whereas seroprevalences were found to increase later in life (15 to 25 years) for AMA-1 (P = 0.001) and EBA-175 (P = 0.003).
TABLE 1.
Proportions of IgG responders against rDBLα, MSP-1, EBA-175, and AMA-1 for forest and plain villages in Orissa (India) and Manhiça (Mozambique)a
| Age group (yr) | Orissa plain
|
Orissa forest
|
Manhiça
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | No. (%) of responders to rDBLα | n | No (%) of responders to rDBLα | n | No. (%) of responders
|
||||
| rDBLα | MSP-1 | EBA-175 | AMA-1 | ||||||
| 1-2.5 | 7 | 0 (0) | 6 | 2 (33.3) | 30 | 5 (16.7) | 15 (50.0) | 10 (33.3) | 20 (66.7) |
| 2.5-5 | 20 | 0 (0) | 17 | 10 (58.8) | 30 | 16 (53.3) | 17 (56.7) | 14 (46.7) | 22 (73.3) |
| 5-7.5 | 15 | 1 (6.7) | 33 | 28 (84.8) | |||||
| 7.5-15 | 28 | 4 (14.3) | 170 | 150 (88.2) | |||||
| 15-25 | 9 | 0 (0) | 36 | 33 (91.2) | 54 | 53 (98.1) | 27 (50.0) | 37 (68.5) | 51 (94.4) |
| 25-35 | 8 | 0 (0) | 62 | 57 (91.9) | 24 | 23 (95.8) | 11 (45.8) | 15 (62.5) | 21 (87.5) |
| >35 | 18 | 2 (11.1) | 110 | 100 (90.9) | 27 | 26 (96.3) | 19 (70.4) | 22 (81.5) | 26 (96.3) |
P values for differences in seroprevalence between age groups (Fisher's test) were 0.388, <0.001, <0.001, 0.382, 0.001, and 0.001 for the Orissa plain and forest groups and the Manhiça rDBLα, MSP-1, EBA-175, and AMA-1 groups, respectively.
FIG. 4.
ELISA reactivity (AU) against rDBLα by age group in forest and plain villages of Orissa (India). Continuous lines represent the medians, and discontinuous lines represent the 25th and 75th percentiles. The areas of the circles are proportional to the numbers of observations.
FIG. 5.
ELISA reactivity (AU) against rDBLα, EBA-175, AMA-1, and MSP-1 by age group in Manhiça (Mozambique). Continuous lines represent the medians, and discontinuous lines represent the 25th and 75th percentiles. The areas of the circles are proportional to the numbers of observations.
DISCUSSION
Here we report methods to produce the minimal functional domain of DBLα encoded by R29 var1 in its correctly folded conformation. Our results confirm that the region of DBLα required by P. falciparum R29 to mediate rosetting maps to the central 233-amino-acid region of DBLα encoded by R29 var1, which includes cysteines 5 to 12 (20). Refolded E. coli DBLα is pure, homogeneous, and functional. First, the purity of DBLα was >95%, as determined by densitometry scanning of silver-stained SDS-PAGE gels. Second, refolded E. coli DBLα eluted in a single peak during reverse-phase chromatography on a C8 column, confirming that it was composed of a homogeneous population of conformers. Third, the migration profile of refolded DBLα by gel filtration chromatography indicates that it is monomeric. Fourth, DBLα exhibited a reduction-sensitive shift in mobility by SDS-PAGE, indicating that intramolecular disulfide bonds had formed. Fifth, rDBLα bound specifically to CR1, as CR1-deficient erythrocytes bound the recombinant protein less efficiently than normal erythrocytes did, indicating that rDBLα was folded in its functional conformation. Binding to CR1-deficient erythrocytes was not null, as the copy number of CR1 on CR1-deficient erythrocytes is ∼10 to 20% that on normal erythrocytes (K. Yazdanbaksh, personal communication). Finally, we have shown that rDBLα is capable of reversing rosette formation in a dose-dependent manner. This is in contrast to a previous study (25) showing that bacterially produced DBLα domains were unable to disrupt rosettes. This discrepancy suggests that recombinant proteins produced through denaturation of inclusion bodies may not reflect the correct conformation and that methods to refold the recombinant protein are needed to obtain functional DBL domains (3, 33).
Recombinant refolded DBLα was recognized by plasmas from individuals residing in regions of India and Mozambique with high rates of malaria transmission, indicating that DBLα contains B-cell epitopes that are naturally exposed to the host immune system. In the Manhiça population, IgGs against MSP-1 were found to be the least seroprevalent (53.9%), followed by IgGs against EBA-175 (59.3%), DBLα (74.5%), and AMA-1 (84.8%). This is in the range of previously reported seroprevalences of IgGs against recombinant merozoite antigens (26-29) and DBLα domains (24) in areas of malaria endemicity. Major differences in the acquisition of DBLα-specific antibodies were observed between different malaria transmission settings. A significant proportion (75 to 87%) of individuals living in areas with high rates of malaria transmission in India (EIR, 110 infective bites per person per year) and Mozambique (EIR, 38 infective bites per person per year) had developed antibodies directed against DBLα. However, residents of an area in India with a low rate of malaria transmission (EIR, 5 infective bites per person per year) showed poor recognition of rDBLα (seroprevalence of 6.7%). This is in accordance with a previous report (24) showing a strong correlation between the immune status of plasma donors and reactivity with rDBLα domains. In the areas with high rates of transmission, the proportion of rDBLα IgG-seropositive results increased significantly with age, similar to what was observed in Manhiça and other African settings for AMA-1 (35) and EBA-175 (27) but not for MSP-1 (26). However, increases in IgGs during the first 5 years of life were more accentuated for DBLα than for AMA-1 and EBA-175, as only rDBLα IgG levels and prevalences were found to increase significantly during early childhood. Seroconversion against DBLα was found to occur later in the population from forest areas of Orissa (5 to 7.5 years) than in inhabitants from Manhiça (2.5 to 5 years), but this may have been due to the small number of young Indian children included in the study (n = 23), which might have reduced the power of the comparisons. The age-related buildup of antibodies to DBLα in areas with high rates of transmission is presumably due to repeated exposure to infection by P. falciparum. It is interesting that the age at which children acquire antibodies against DBLα coincides with the age at which clinical immunity would be predicted to occur in Orissa forest areas (32) and Manhiça (14). This suggests that antibodies to DBLα represent a marker of age-acquired immunity against P. falciparum.
Our observations are in agreement with the concept of rapidly developing antibody responses against conserved epitopes in PfEMP1 DBLα domains in spite of their extreme polymorphism (24, 34). The finding that one-half of the 2.5- to 5-year-old children from Mozambique recognized rDBLα suggests that they were exposed frequently, and possibly early in life, to parasites expressing PfEMP1 variants with epitopes similar to those contained in the recombinant protein. This may suggest that the R29 var1-encoded DBLα minimal domain contains conserved epitopes which transcend sequence diversity (6, 22, 24). Whether antibodies targeting such epitopes of DBLα confer protection against P. falciparum malaria remains to be determined.
Acknowledgments
We thank the people of the Sundargarh District, Orissa (India), and of Manhiça (Mozambique) for participating in the study. We also thank the staffs of the National Institute of Malaria Research in Rourkela and the Manhiça District Hospital for their collaboration in the project. The work of the clinical officers, field supervisors, and data manager was critical for the successful completion of the study.
This study received financial support from the Department of Biotechnology, Government of India, and the Instituto de Salud Carlos III (PI060016). The Manhiça Health Research Center receives core support from the Spanish Agency for International Cooperation. C.E.C. was a recipient of a Wellcome Trust international senior research fellowship, and A.M. was supported by the Instituto de Salud Carlos III (CP-04/00220).
The funding sources did not have any involvement in study design, collection, analysis, and interpretation of data, in writing of the report, or in the decision to submit the paper for publication. The researchers were independent of the funders.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 22 June 2009.
REFERENCES
- 1.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 3641411-1420. [DOI] [PubMed] [Google Scholar]
- 2.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 8277-87. [DOI] [PubMed] [Google Scholar]
- 3.Bir, N., S. S. Yazdani, M. Avril, C. Layez, J. Gysin, and C. E. Chitnis. 2006. Immunogenicity of Duffy binding-like domains that bind chondroitin sulfate A and protection against pregnancy-associated malaria. Infect. Immun. 745955-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson, J., H. Helmby, A. V. Hill, D. Brewster, B. M. Greenwood, and M. Wahlgren. 1990. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 3361457-1460. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay, R., A. Sharma, V. K. Srivastava, S. S. Pati, S. K. Sharma, B. S. Das, and C. E. Chitnis. 2003. Plasmodium falciparum infection elicits both variant-specific and cross-reactive antibodies against variant surface antigens. Infect. Immun. 71597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Q., A. Barragan, V. Fernandez, A. Sundstrom, M. Schlichtherle, A. Sahlen, J. Carlson, S. Datta, and M. Wahlgren. 1998. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J. Exp. Med. 18715-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Q., F. Pettersson, A. M. Vogt, B. Schmidt, S. Ahuja, P. Liljestrom, and M. Wahlgren. 2004. Immunization with PfEMP1-DBL1alpha generates antibodies that disrupt rosettes and protect against the sequestration of Plasmodium falciparum-infected erythrocytes. Vaccine 222701-2712. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, S., G. I. McGregor, and S. Carrington. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192733-737. [DOI] [PubMed] [Google Scholar]
- 10.Druilhe, P., and J. L. Perignon. 1994. Mechanisms of defense against P. falciparum asexual blood stages in humans. Immunol. Lett. 41115-120. [DOI] [PubMed] [Google Scholar]
- 11.Flick, K., and Q. Chen. 2004. var genes, PfEMP1 and the human host. Mol. Biochem. Parasitol. 1343-9. [DOI] [PubMed] [Google Scholar]
- 12.Frank, M., and K. Deitsch. 2006. Activation, silencing and mutually exclusive expression within the var gene family of Plasmodium falciparum. Int. J. Parasitol. 36975-985. [DOI] [PubMed] [Google Scholar]
- 13.Giha, H. A., T. Staalsoe, D. Dodoo, C. Roper, G. M. Satti, D. E. Arnot, L. Hviid, and T. G. Theander. 2000. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol. Lett. 71117-126. [DOI] [PubMed] [Google Scholar]
- 14.Guinovart, C., Q. Bassat, B. Sigauque, P. Aide, J. Sacarlal, T. Nhampossa, A. Bardaji, A. Nhacolo, E. Macete, I. Mandomando, J. J. Aponte, C. Menendez, and P. L. Alonso. 2008. Malaria in rural Mozambique. I. Children attending the outpatient clinic. Malar. J. 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta, S., K. Trenholme, R. M. Anderson, and K. P. Day. 1994. Antigenic diversity and the transmission dynamics of Plasmodium falciparum. Science 263961-963. [DOI] [PubMed] [Google Scholar]
- 16.INDEPTH. 2002. Population and health in developing countries. International Development Research Centre, Ottawa, Canada.
- 17.Koch, R. 1900. Zweiter bericht über die thatigkeit der malaria expedition. Dtsch. Med. Wochenschr. 2688-90. [Google Scholar]
- 18.Kraemer, S. M., and J. D. Smith. 2006. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 9374-380. [DOI] [PubMed] [Google Scholar]
- 19.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231150-153. [DOI] [PubMed] [Google Scholar]
- 20.Mayor, A., N. Bir, R. Sawhney, S. Singh, P. Pattnaik, S. K. Singh, A. Sharma, and C. E. Chitnis. 2005. Receptor-binding residues lie in central regions of Duffy-binding-like domains involved in red cell invasion and cytoadherence by malaria parasites. Blood 1052557-2563. [DOI] [PubMed] [Google Scholar]
- 21.Miller, L. H., D. I. Baruch, K. Marsh, and O. K. Doumbo. 2002. The pathogenic basis of malaria. Nature 415673-679. [DOI] [PubMed] [Google Scholar]
- 22.Moll, K., F. Pettersson, A. M. Vogt, C. Jonsson, N. Rasti, S. Ahuja, M. Spangberg, O. Mercereau-Puijalon, D. E. Arnot, M. Wahlgren, and Q. Chen. 2007. Generation of cross-protective antibodies against Plasmodium falciparum sequestration by immunization with an erythrocyte membrane protein 1-duffy binding-like 1 alpha domain. Infect. Immun. 75211-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newbold, C., P. Warn, G. Black, A. Berendt, A. Craig, B. Snow, M. Msobo, N. Peshu, and K. Marsh. 1997. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 57389-398. [DOI] [PubMed] [Google Scholar]
- 24.Oguariri, R. M., S. Borrmann, M. Q. Klinkert, P. G. Kremsner, and J. F. Kun. 2001. High prevalence of human antibodies to recombinant Duffy binding-like alpha domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect. Immun. 697603-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oguariri, R. M., D. Mattei, C. Tena-Tomas, A. C. Uhlemann, P. G. Kremsner, and J. F. Kun. 2003. Recombinant Duffy binding-like-alpha domains of Plasmodium falciparum erythrocyte membrane protein 1 elicit antibodies in rats that recognise conserved epitopes. Parasitol. Res. 90467-472. [DOI] [PubMed] [Google Scholar]
- 26.Okech, B. A., P. H. Corran, J. Todd, A. Joynson-Hicks, C. Uthaipibull, T. G. Egwang, A. A. Holder, and E. M. Riley. 2004. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect. Immun. 721557-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okenu, D. M., E. M. Riley, Q. D. Bickle, P. U. Agomo, A. Barbosa, J. R. Daugherty, D. E. Lanar, and D. J. Conway. 2000. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect. Immun. 685559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polley, S. D., T. Mwangi, C. H. Kocken, A. W. Thomas, S. Dutta, D. E. Lanar, E. Remarque, A. Ross, T. N. Williams, G. Mwambingu, B. Lowe, D. J. Conway, and K. Marsh. 2004. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23718-728. [DOI] [PubMed] [Google Scholar]
- 29.Quelhas, D., L. Puyol, L. Quinto, E. Serra-Casas, T. Nhampossa, E. Macete, P. Aide, A. Mayor, I. Mandomando, S. Sanz, J. J. Aponte, V. S. Chauhan, C. E. Chitnis, P. L. Alonso, C. Menendez, and C. Dobano. 2008. Impact of intermittent preventive treatment with sulfadoxine-pyrimethamine on antibody responses to erythrocytic-stage Plasmodium falciparum antigens in infants in Mozambique. Clin. Vaccine Immunol. 151282-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe, A., J. Obeiro, C. I. Newbold, and K. Marsh. 1995. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect. Immun. 632323-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe, J. A., J. M. Moulds, C. I. Newbold, and L. H. Miller. 1997. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388292-295. [DOI] [PubMed] [Google Scholar]
- 32.Sharma, S. K., P. K. Tyagi, K. Padhan, A. K. Upadhyay, M. A. Haque, N. Nanda, H. Joshi, S. Biswas, T. Adak, B. S. Das, V. S. Chauhan, C. E. Chitnis, and S. K. Subbarao. 2006. Epidemiology of malaria transmission in forest and plain ecotype villages in Sundargarh District, Orissa, India. Trans. R. Soc. Trop. Med. Hyg. 100917-925. [DOI] [PubMed] [Google Scholar]
- 33.Singh, S., K. Pandey, R. Chattopadhayay, S. S. Yazdani, A. Lynn, A. Bharadwaj, A. Ranjan, and C. Chitnis. 2001. Biochemical, biophysical, and functional characterization of bacterially expressed and refolded receptor binding domain of Plasmodium vivax Duffy-binding protein. J. Biol. Chem. 27617111-17116. [DOI] [PubMed] [Google Scholar]
- 34.Smith, J. D., G. Subramanian, B. Gamain, D. I. Baruch, and L. H. Miller. 2000. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 110293-310. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, A. W., J. F. Trape, C. Rogier, A. Goncalves, V. E. Rosario, and D. L. Narum. 1994. High prevalence of natural antibodies against Plasmodium falciparum 83-kilodalton apical membrane antigen (PF83/AMA-1) as detected by capture-enzyme-linked immunosorbent assay using full-length baculovirus recombinant PF83/AMA-1. Am. J. Trop. Med. Hyg. 51730-740. [DOI] [PubMed] [Google Scholar]
- 36.Treutiger, C. J., I. Hedlund, H. Helmby, J. Carlson, A. Jepson, P. Twumasi, D. Kwiatkowski, B. M. Greenwood, and M. Wahlgren. 1992. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am. J. Trop. Med. Hyg. 46503-510. [DOI] [PubMed] [Google Scholar]