Abstract
Loa loa is a filarial nematode that infects humans. The adults live in subcutaneous tissues and produce microfilariae that live for several weeks in the blood circulation in order to be transmitted to another person via blood meals of a dipterian vector. As microfilariae live in continuous contact with plasma, it is obvious that they evade the complement system. We studied markers of complement activation and signs of complement regulation on Loa loa microfilariae in vivo. The microfilariae were isolated from anticoagulated blood samples of a Loa loa-infected Caucasian patient. C1q and some mannose-binding lectin but only a limited amount of C3b or C4b fragments and practically no C5 or C5b-9 were present on the microfilariae. The covalently microfilaria-bound C3 and C4 depositions were mainly inactive iC3b, C3c, and iC4b fragments indicating that microfilariae had regulated complement activation in vivo. Also, in vitro deposition of C3b onto the microfilariae upon serum exposure was limited. The patient-isolated microfilariae were found to carry the host complement regulators factor H and C4b-binding protein on the outermost layer, so called sheath. The microfilaria-bound factor H was functionally active. Binding of the complement regulators to the microfilariae was confirmed in vitro using 125I-labeled factor H and C4b-binding protein. In conclusion, our study shows that Loa loa microfilariae block complement activation and acquire the host complement regulators factor H and C4b-binding protein in blood circulation. This is the first time that binding of complement regulators onto nonviral pathogens has been demonstrated to occur in humans in vivo.
Loa loa is a filarial parasite and the causative agent of human loiasis. This nematode has adapted well to its human host, as the adults can migrate in subcutaneous tissues for at least 15 years (15). During its whole adult life this helminth can produce microfilariae (MF) in peripheral blood, and these can circulate in blood for several weeks before transmission to the vector. Loiasis is transmitted by a dipteran vector (Chrysops spp.). The infective larvae can enter human subcutaneous tissues through a bite wound when the vector feeds again after the first blood meal. The larvae develop into adults and mate in subcutaneous tissues, resulting in production of sheathed MF after a minimum of 5 months. The MF are found mainly in peripheral blood (34).
The prevalence of loiasis is high in the regions of endemicity in western and central Africa, where 20 to 40% of the population is microfilaremic (10). The majority of the infected individuals are asymptomatic, but a significant proportion of patients have symptoms such as calabar swellings, pruritis, secondary dermal lesions, and a subconjunctival eye passage of the adult worm (33, 37). In addition, serious sequelae such as endomyocardial fibrosis, renal complications, and encephalitis have also been reported (1).
The main functions of the complement system are to eliminate foreign organisms that come in contact with plasma or other body fluids, either by direct effects or by enhancement of the acquired humoral immune response. Depending on the activator, complement can be activated through three pathways: the classical pathway (CP), the alternative pathway (AP), and the lectin pathway (LP). Upon activation, these pathways lead to the terminal pathway and formation of membrane attack complexes on the target cell (31).
Complement activation is regulated by a variety of complement regulatory proteins. Most of the regulators are membrane bound, while two major regulators, complement factor H (CFH) and C4b-binding protein (C4BP), are plasma proteins found in high concentrations (12, 17, 47). All the three pathways lead to activation of C3, the central molecule of the complement cascade (8). Activation of C3 to C3b results in release of an anaphylatoxin, C3a (20, 21), while the C3b fragment can attach covalently to the target surface to start the AP amplification or to promote activation of the CP or LP (32). Inactivation of C3b to inactive C3b (iC3b) is carried out by serine protease factor I (FI), which needs a cofactor such as CFH (6, 8). In addition to the cofactor activity, CFH can also downregulate generation of C3b by two other means (11, 46).
In the CP and LP, activation of the component C4 is essential. Upon activation, C4b is attached covalently to the target surface (27, 45) and can form an active C3-convertase, C4b2a, which is essential for propagation of the CP and LP (5). This step is regulated in plasma by complement regulatory protein C4BP, which acts as a cofactor for FI in degradation of C4b to inactive C4b (iC4b) or as an accelerator of the decay of C4b2a (23, 40, 44).
Since MF of Loa loa are able to live and migrate in blood for weeks, they are obviously able to resist elimination by complement, but the mechanisms are largely unknown. One immune evasion mechanism of Loa loa is known to be induction of T-cell anergy (25), but nothing is known about evasion of innate or humoral immunity. A few complement resistance mechanisms have been reported for other helminths. Most of these are associated with physical barriers of macroscopic worms, but some helminths are known to have specific molecules mediating complement evasion or ligands that acquire host complement regulators on their surfaces (22). So far, two human helminth parasite structures have been shown to acquire host CFH on their surfaces, the echinococcal cyst wall and MF of a filarial nematode, Onchocerca volvulus (7, 30). So far there are no reports of acquisition of C4BP onto any pathogenic helminth. Several pathogenic bacteria and yeasts and a few viruses are known to utilize acquisition of host CFH or C4BP to evade complement attack (49). Four of these microbes, Streptococcus pyogenes, Borrelia burgdorferi, relapsing fever Borrelia, and Candida albicans, can acquire both CFH and C4BP on their surface (3, 28, 29). So far, all the reports where CFH or C4BP acquisition on microbes has been reported have been based on in vitro experiments only.
The aim of our study was to analyze whether patient-derived Loa loa MF carry any markers of complement attack or signs of cessation of the complement cascade. The MF showed C1q deposits, as was expected since the patient had antifilarial antibodies. Despite that, however, only limited amounts of C3 or C4 fragments and practically no C5 or C5b-9 could be detected on MF. The covalently bound C3 or C4 fragments were mainly iC3b, C3c, or iC4b. Most importantly we show that MF had acquired CFH and C4BP on their surfaces in vivo. In conclusion, for the first time we show acquisition of soluble complement regulators on pathogenic microbes in the human body. Our results suggest that acquisition of complement regulators CFH and C4BP from human plasma on MF could at least partially explain the prolonged survival of MF in circulation.
MATERIALS AND METHODS
Source of MF.
The MF were obtained from a 43-year-old generally healthy Caucasian male who was originally admitted to a hospital due to African spotted fever. Loiasis was diagnosed from blood smears drawn to exclude malaria infection. The patient most probably had acquired loiasis while collecting insects as a tourist in Cameroon 2 years earlier (H. Siikamäki et al., unpublished data). The blood samples for MF isolation from this patient were drawn after the symptoms of spotted fever had subsided and before the antihelminth treatment was started. In the samples used, the MF concentration was 500 to 1,000/ml. Presence of antifilarial antibodies in the patient serum was tested using Acanthocheilonema viteae as an antigen (performed at the Swiss Tropical Institute, Basel, Switzerland, by Hanspeter Marti et al.).
Isolation of MF.
EDTA- or heparin-anticoagulated venous blood samples were collected at the Helsinki University Central Hospital. EDTA was used in the sample collection to exclude any ex vivo activation of complement on the MF. The samples were subjected to purification and concentration using a modified Percoll gradient method (1.130 g/ml; Fluka BioChemica, Sweden) as previously described (4). Briefly, an iso-osmolar Percoll solution was prepared by mixing nine parts of Percoll and one part of 2.5 M sucrose. Iso-osmolar Percoll was diluted with 0.25 M sucrose to obtain 25, 30, 35, 40, and 45% solutions (densities of 1.055, 1.062, 1.068, 1.074, and 1.080 g/ml, respectively). To obtain a gradient, 2 ml of each solution was layered in a 15-ml plastic tube starting with the 45% solution. On top of the gradient, 2 ml of phosphate-buffered saline (PBS)-diluted blood was added at a 1:2 ratio. The tubes were centrifuged at 400 × g for 35 min at 22°C, and the MF-containing layer on top of the 45% layer was collected and washed three times with 1 ml of PBS. In our study nearly all MF restored in EDTA- or heparin-anticoagulated blood on ice maintained their vitality, i.e., capacity for motility upon warming, for 2 weeks, and some did so for even 50 days, while larvae kept in RPMI-1 medium maintained their motility for no more than 2 weeks. In the C3b deposition and binding assays in the study, the MF were freshly isolated and kept on ice until used within 48 h. For Western blotting and cofactor assays, the MF were stored at −130°C and thawed immediately prior to the experiments.
Complement proteins.
CFH was from Calbiochem, and C4BP, purified from plasma in complex with protein S, was obtained as a kind gift from A. Blom, Lund, Sweden. C3b was prepared from plasma-purified C3 using trypsin as described previously (24, 41). C4b and iC3b were purchased from Calbiochem. The iC4b-C4c mixture used was prepared from C4b (100 μg/ml) by incubation with 2 μg/ml of FI (Calbiochem), 1 μg/ml of C4BP, and 5 μg/ml of soluble complement receptor 1 (T Cell Sciences Inc., Needham, MA) in PBS for 2 h at 37°C.
Western blotting.
EDTA-blood-derived MF were purified with Percoll gradients as described above. The purified MF were subjected to sequential elutions using low-pH buffer (0.1 M glycine-HCl, pH 2.3) and Laemmli sample buffer under reduced conditions. Finally, we made a homogenate of the resulting MF in the presence of 1% detergent-Triton X-100 and Complete protease inhibitor cocktail (Roche, Germany). As a control we used zymosan A yeast particles (Sigma) that were incubated in fresh human serum for 60 min at 37°C and washed prior to elutions and homogenization. Homogenization of both MF and zymosan was performed mechanically on ice using a UniForm homogenizer (Jencons, United Kingdom).
The MF homogenate; the zymosan homogenate; normal human serum (NHS); and complement proteins C1q, mannose-binding lectin (MBL), C3, C3b, iC3b, C4b, iC4b, C5, CFH, and C4BP were run in 5 to 15% gradient (C4 fragments and C5), 5% (MBL), or 10% (all the other proteins) sodium dodecyl sulfate (SDS)-polyacrylamide gels under nonreducing (MBL, CFH, C4BP, C4, and C5) or reducing (all the other proteins) conditions, followed by electrotransfer onto nitrocellulose membranes. The nitrocellulose membranes were blocked with 3% skim milk in PBS (1 h at 22°C). After being washed three times, the membranes were incubated with primary antibodies for 24 h at 4°C. The primary antibodies used were rabbit anti-C1q (Dako), mouse anti-MBL 131-1 (Statens Serum Institute, Copenhagen, Denmark), rabbit anti-C3c (Dako), sheep anti-C3 (ICN Biochemicals), goat anti-C4 (Cappel, Germany), goat anti-C5 (Quidel), goat anti-CFH (Calbiochem), rabbit anti-C4BP (a kind gift from A. Blom, Lund, Sweden), or mouse anti-C5b-9 antibody directed against a neoepitope formed by poly(C9) in the terminal complement complex (Quidel). After washings, the bound antibodies were incubated with peroxidase-conjugated donkey anti-goat, rabbit anti-mouse, donkey anti-sheep, or goat anti-rabbit immunoglobulin G (IgG) (Jackson Immunoresearch) and detected using the enhanced chemiluminescence method.
Direct protein-binding assay.
Binding of CFH and C4BP to MF was analyzed with MF collected from both EDTA (40 MF/assay)- and heparin (100 MF/assay)-anticoagulated blood. The washed MF were diluted in 50 μl of PBS and incubated for 30 min at 37°C with 125I-labeled CFH, C4BP, or bovine serum albumin (BSA) with continuous shaking (350 rpm). The specific activities of the radiolabeled proteins were 88, 2.3, and 0.4 × 106 cpm/μg, respectively. The incubated samples were centrifuged at 10,000 × g for 3 min through 250 μl of 20% sucrose and frozen for 30 min. Pellets and supernatants were cut into separate tubes, and radioactivity was measured with a gamma counter. The ratios of radioactivity in pellet versus in pellet and supernatant were calculated after subtracting the background radioactivity obtained with samples where no MF were used. The experiment was performed twice in duplicate, and the difference between the mean values was analyzed using Student's t test to obtain the P values.
Immunofluorescence microscopy.
Deposition of complement regulators CFH and C4BP on patient-derived MF was analyzed by immunofluorescence microscopy. The Percoll sucrose gradient-isolated MF (approximately 50/assay) were washed five times with PBS, followed by 60 min of incubation at 37°C with gentle shaking with primary goat anti-CFH (Calbiochem) or rabbit anti-C4BP antibodies at a concentration of 20 μg/ml in a 50-μl sample volume. After three washes with 0.5% BSA in PBS, the MF were diluted in 100 μl of PBS, and Alexa-conjugated goat anti-rabbit, donkey anti-goat, or rabbit anti-mouse IgG (Molecular Probes, The Netherlands) were added at a 1:100 dilution. After incubation for 30 min at 37°C (with shaking at 350 rpm) the MF were washed three times with 0.5% BSA in PBS and analyzed using an Olympus BX50 fluorescence microscope.
Quantitation of iC3b/C3b deposition.
An immunofluorescence-based method was used to quantify C3b depositions on MF as previously described (16). Briefly, approximately 150 MF in 50 μl of PBS were incubated with 50 μl of various dilutions of NHS (0, 7, 14, or 20%) in PBS for 1 h at 37°C with continuous shaking (350 rpm). Extensively washed zymosan A particles (Sigma-Aldrich) (100 μg/ml) were used as a positive control and human red blood cells (0.01%) as a negative control. The controls (50 μl) were incubated with 50 μl of various concentrations of NHS (0, 1, 2, 3, 4, 5, or 20%). Zymosan A was also incubated with 50 μl of 7 and 14% NHS with or without heat inactivation at 56°C for 30 min. All the samples were washed twice with 1 ml of PBS containing 0.025% Tween 20 prior to incubation with a 1:50 dilution of rabbit anti-C3c (Dako A/S, Denmark) for 1 h at 22°C in 50 μl (350 rpm). After being washed twice with PBS containing 0.025% Tween 20, the samples were incubated with a 1:100 dilution of Alexa 488-conjugated goat anti-rabbit IgG (Molecular Probes) for 30 min at 22°C in 50 μl (350 rpm). After the last washings, each sample was resuspended in 200 μl of 25% glycerol in PBS and transferred to 96-well plates. The fluorescence intensity in each well was measured using a 1423 Victor-2 multilabel counter (Wallac, Finland) with a 485-nm/535-nm filter. The experiment was performed twice in duplicate, and the difference between the mean values was analyzed using Student's t test to obtain the P values. The correlation coefficient (r2) was calculated after linear regression analysis.
Cofactor assay.
The functionality of the surface-bound CFH on MF was analyzed by a cofactor assay using MF isolated from EDTA-anticoagulated blood. Approximately 75 MF were incubated for 1 h at 37°C with 100,000 cpm 125I-labeled C3b and 15 μg/ml FI in veronal-buffered saline, pH 7.4. The specific activity of the radiolabeled C3b was 5.6 × 106 cpm/μg. To control the assay, 125I-labeled C3b was incubated without MF but with 15 μg/ml FI in the presence (positive control) or absence (negative control) of 10 μg/ml CFH. All the samples were run on a 10% SDS-polyacrylamide gel under reducing conditions, fixed, dried, and detected using autoradiography.
RESULTS
Loa loa MF show only limited complement activation in vivo.
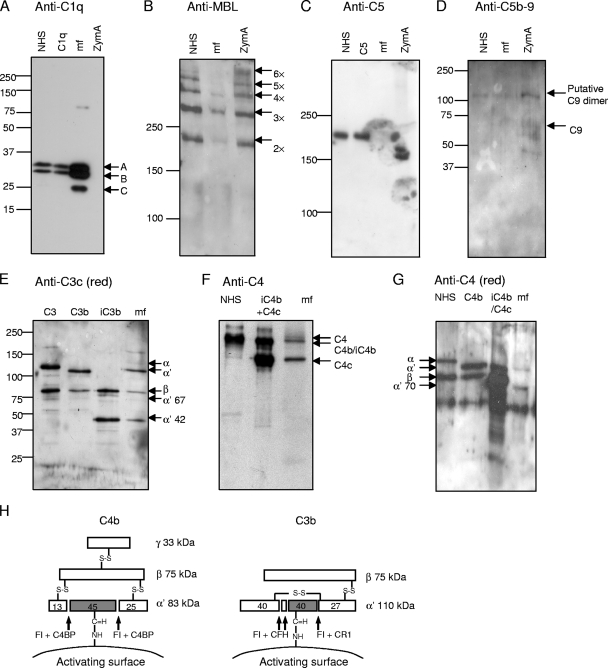
To study complement activation on Loa loa MF, we analyzed whether MF taken from patient blood carry signs of initiation or propagation of the three complement pathways. First, the presence of C1q, MBL, C3, C4, C5, and C9 neoepitopes (an indicator of C5b-9 complexes) in MF homogenates or acidic MF elutes was analyzed. C1q and some MBL but no C5 or C9-neoepitope were detected (Fig. 1A to D). Fragments of the α′ chain of C3 indicated that some C3b but mainly iC3b existed in MF homogenate (Fig. 1E). Also, mainly C4c and C4b/iC4b could be detected (Fig. 1F). The presence of C4b was excluded by analyzing fragments of the C4 α′ chain in an elution under reducing conditions (Fig. 1G). Since only small amount of intact α′ chain of C3b or C4b were found, it is evident that only small amount of active C3b or C4b could be found on the MF surface.
FIG. 1.
Deposition of complement components C1q, MBL, and C5; C5b-9 complexes; and fragments of C3 and C4 on the surface of Loa loa MF. The presence of those molecules on MF was analyzed after subjecting purified patient blood-derived MF to either acidic elution (A, B, and C) or elution with reducing buffer (G) or after homogenizing MF (D, E, and F). The samples obtained were used in SDS-polyacrylamide gel electrophoresis under either reducing (A, D, E, and G) or nonreducing (B, C, and F) conditions and subsequent Western blotting with antibodies against the molecules as indicated above each panel (the anti-C5b-9 panel was blotted with anti-C9 neoepitope antibody). As positive controls, complement components C1q (0.3 μg/ml); C5 (1 μg/ml); C3, C3b, and iC3b (0.02 μg each); C4b and iC4b (0.04 μg each); and NHS were used (diluted 1:500 for panel A, 1:100 for panel B, 1:200 for panel C, and 1:1,000 for panels F and G). NHS was used as a negative control for C5b-9 deposition (D). NHS-exposed zymosan A (ZymA) was used as a positive control in the deposition of the complement components MBL (B) and C5b-9 complex (D) and as a negative control in the deposition of C1q (A). The mobility of molecular size markers is indicated on the left sides. Mobilities of the C1q subunits A, B, and C (A); the oligomers of MBL (B); the activated C5b-9 associated C9 complexes (D); the fragments of C3 (E); and the fragments of C4 (F and G) are indicated. A schematic presentation of C4b and C3b fragments and the cleavage sites of FI is shown in panel H.
Complement activation is restricted on Loa loa MF in vitro.
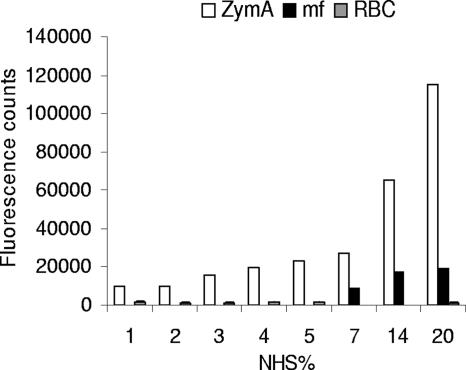
Since some, although a small amount of, intact α′ chain of C3b could be detected on MF (Fig. 1E), we next wanted to determine whether MF act as AP activators or nonactivators. In the assay, either MF, a known AP activator (zymosan A [35, 36]), or a known AP nonactivator (human erythrocytes) was incubated in nonimmune serum. Binding of C3b/iC3b was detected using anti-C3c antibody in a fluorometric assay. Loa loa MF showed restricted C3b/iC3b depositions in 7 to 20% NHS compared to zymosan A, which showed over fivefold more deposition (P < 0.05) (Fig. 2). No C3b/iC3b depositions were formed on human erythrocytes under the same conditions compared to deposition on zymosan A in 3 to 20% NHS (P < 0.05). The C3b deposition on MF and zymosan surfaces correlated with the concentration of NHS (r2 = 0.956).
FIG. 2.
Activation of the AP of complement on Loa loa MF in vitro. Patient blood-isolated Loa loa MF, complement-activating zymosan A yeast particles (ZymA), and complement-nonactivating human red blood cells (RBC) were exposed to increasing concentrations of NHS. The amount of surface-bound C3b and iC3b molecules was measured by staining the cells or MF with rabbit anti-C3c antibody and Alexa 488-conjugated secondary antibody followed by detection of the bound fluorescence using a fluorometer. The fluorescence intensity obtained without any NHS incubation was subtracted as background. The results are shown as means for parallel samples assayed in duplicate.
Loa loa MF acquire plasma complement regulators in vivo.
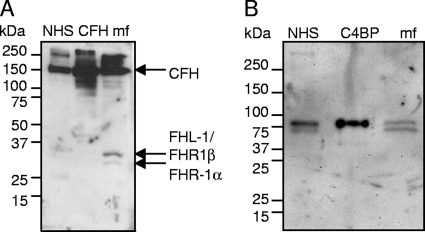
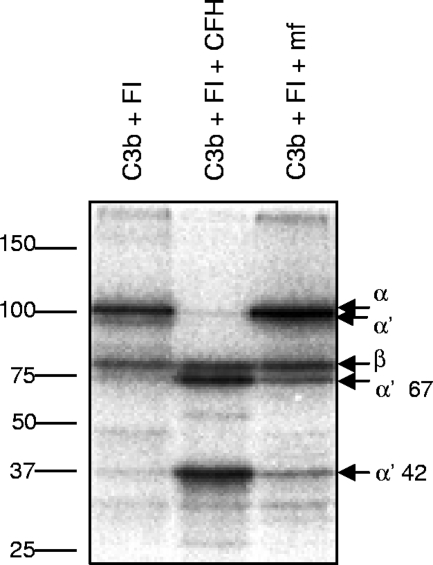
Since C1q and MBL were found to bind to MF, while only small amount of C3b or C4b could be detected, we next analyzed whether MF were able to acquire plasma complement regulators CFH and C4BP on their surface. Freshly isolated and thoroughly washed MF were homogenized, and samples were used in Western blotting analysis with anti-CFH and anti-C4BP antibodies. Both CFH and C4BP were detected in the samples (Fig. 3), indicating that these plasma proteins were attached to the MF surface while these organisms were living in human plasma in vivo. In order to detect whether the bound CFH was functionally active as a cofactor for FI, we performed a cofactor assay using radiolabeled C3b. The cleavage of the α′ chain to α′ 67- and α′ 42-kDa fragments indicated that CFH on the surface of the MF is functionally active (Fig. 4).
FIG. 3.
Analysis of human complement regulators CFH (A) and C4BP (B) bound on the patient EDTA blood-derived Loa loa MF. The MF homogenate and the positive controls NHS (diluted 1:1,000), 1.5 μg of CFH (A), and 0.02 μg of C4BP (B) were subjected to SDS-polyacrylamide gel electrophoresis using 5 to 15% gradient gels under reducing conditions and used for Western blotting with anti-CFH (A) or anti-C4BP (B) antibodies. The presence of 155-kDa CFH (A) and the 70-kDa α-chain of C4BP (B) can be detected in the MF preparations. The mobility of the molecular size marker is indicated on the left.
FIG. 4.
Analysis of cofactor activity of Loa loa MF-bound CFH. The MF were isolated from EDTA-anticoagulated blood by the Percoll gradient method and washed. The cofactor activity of the MF-bound CFH was analyzed by incubating the MF with 125I-labeled C3b and FI (C3b+FI+MF). The positive control sample contained 125I-labeled C3b, FI, and CFH and the negative control only 125I-labeled C3b and FI. The mobility of the molecular weight markers is shown on the left, and the identities of the fragments of C3b are indicated on the right.
Factor H and C4BP are bound to the sheath of Loa loa MF.
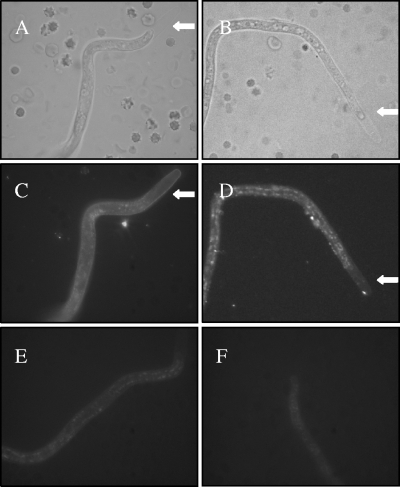
Next we wanted to localize CFH and C4BP on the Loa loa MF surface. For this purpose, we exposed freshly isolated MF to immunofluorescence microscopy using antibodies specific for CFH and C4BP. Staining with anti-CFH antibody resulted in homogeneous staining of the entire length of the sheath of the organism (Fig. 5C). With anti-C4BP antibody a dual staining pattern was observed: a homogeneous staining of the sheath and a patchy pattern covering the entire MF with only minimal amount of patches at both ends (Fig. 5D).
FIG. 5.
Localization of CFH and C4BP bound on Loa loa MF. The MF were enriched from EDTA-blood by the Percoll gradient method, washed, and subjected to immunofluorescence staining using polyclonal goat anti-CFH (C) or rabbit anti-C4BP (D) antibody and Alexa 488-conjugated secondary antibodies. The photographs were taken from representative MF using bright-field (A and B) or fluorescence (C and D) microscopy. Control photographs with only the secondary antibodies are shown in panels E and F. The typical extended sheath in the anterior end of Loa loa MF is indicated with a arrows in panels A, B, C, and D.
Purified complement regulators CFH and C4BP bind to Loa loa MF in vitro.
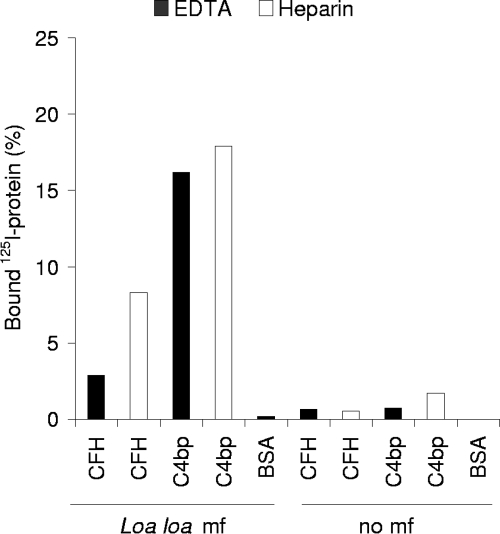
In order to verify that Loa loa MF can bind human CFH and C4BP, we studied binding of 125I-labeled regulatory proteins to MF in vitro. For this assay we isolated and intensively washed MF from both EDTA- and heparin-anticoagulated blood samples, followed by incubation with 125I-labeled CFH or C4BP. Binding of both CFH and C4BP on MF compared to the negative control samples was detected (P < 0.05) (Fig. 6). Clearly, more CFH (P < 0.05) and slightly more C4BP (P > 0.05) bound to the heparin-anticoagulated sample. However, the quantity of MF in the heparin-anticoagulated blood sample was approximately twice the quantity in the EDTA-anticoagulated blood sample. Several microbes use heparin-binding sites on CFH to bind to it, but the result that exposure of MF to heparin does not inhibit the interaction between MF and CFH indicates that heparin-binding sites and MF-binding sites on CFH are probably distinct. Addition of various concentrations of heparin to the reaction mixture was not, however, done in this study. Compared to the samples where radiolabeled FH or C4BP was used, no binding was detected when radiolabeled BSA was used (P < 0.05), indicating that MF do not blindly bind any protein under the conditions used.
FIG. 6.
Loa loa MF bind purified CFH and C4BP. The MF were isolated from EDTA-anticoagulated blood (40 MF/tube) or heparin-anticoagulated blood (100 MF/tube) and incubated with 125I-labeled CFH, C4BP, or BSA for 30 min. The unbound proteins were separated from the bound ones by centrifugation through 20% sucrose. Radioactivity in the pellets and supernatants was measured using a gamma counter. The experiment was performed twice in duplicate, and the mean values are indicated.
DISCUSSION
The filarial parasite Loa loa has an exceptionally long life span in human tissues, as it can live up to 15 years or more without leading to an effective immunological defense by the host (9, 15). Loa loa MF also live for weeks in the blood of the patients (2). It has previously been proposed that Loa loa MF are able to activate the AP of complement, but the activation has no effect on the larval viability (48). In this work we showed that complement is activated on MF in vivo but that the activation is efficiently restricted by inactivation of the key complement component C3b. Furthermore, not only do we show that MF bind complement regulators in vitro but, most importantly, for the first time it is also shown that acquisition of the plasma regulators on an invasive microbe occurs in patient blood in vivo.
In this study it was found that Loa loa MF activate complement to some extent. It is known that MF activate one of the three C activation pathways, the AP (48), and our results using Western blotting of blood-isolated MF (Fig. 1E) and in vitro incubation of MF in serum (Fig. 2) are concordant with this. On the basis of our results it seems that MF also activate the CP, since a relatively large amount C1q could be detected on the patient isolated-MF and some C4b fragments could also be detected. Activation of the CP on MF is logical, since the patient, similar to Loa loa patients in general, had antifilarial antibodies in his serum. The role of the exposure of MF to the antibody-initiated CP for survival of MF in different Loa loa-infected patients requires further studies, however. It also remains possible that the LP is activated on MF, but it is likely that the LP is not of major importance, since an MBL-binding yeast preparation bound much more MBL than MF did (Fig. 1A and B).
The presence of inactivated fragments showed that there was evidently downregulation of both C4b and C3b molecules on these organisms. C4b and especially C3b are central in the complement cascade and therefore probably the most efficient targets for controlling the cascade. This is well exemplified by the fact that most human complement regulators work on this level: CFH, CFH-like protein-1, CD35, CD46, CD55, and CRIg act on C3b, and C4BP, CD35, CD46, and CD55 act on C4b. Thus, it is not surprising that pathogenic organisms such as Loa loa MF also control complement activation at that level. We found out that MF acquire host CFH and C4BP on their surface in vivo (Fig. 3 and 5). To exclude to possible role of nonspecific binding of antibodies in the antisera used, the immunofluorescence results were confirmed using an assay where radiolabeled proteins were used in vitro instead of detecting the bound regulators with antibodies (Fig. 6). We also found that CFH is able to maintain its cofactor activity when bound on the MF surface (Fig. 4). Both CFH and C4BP act as complement regulators in plasma in several ways. Both act as a cofactor for FI in cleavage of either C3b or C4b and in accelerating dissociation of one of the C3 convertases (C3bBb or C4b2a). In addition, CFH can compete with factor B in binding to C3b. Since CFH and C4BP act not only by leading to proteolysis of C3b and C4b, some uncleaved C3b and C4b can be tolerated on CFH/C4BP-binding surfaces without propagation of the cascade. This explains why we were unable to detect MF-bound C5 or C5b-9 (Fig. 1C and D) despite the presence of some, although a small amount of, noninactivated C3b and C4b on MF (Fig. 1E to G).
Our observations from immunofluorescence studies showed that CFH and C4BP bind to the sheath layer of Loa loa MF. It is logical that the regulators bind to the sheath, the outermost structure of MF that is in continuous contact with plasma. It is also known that the sheath is involved in downregulation of inflammation caused by MF in general (18). Both CFH and C4BP are known to bind to certain proteins and polyanionic carbohydrates such as heparin. It remains open whether the structure(s) binding CFH and C4BP on Loa loa MF are proteins or carbohydrates of the sheath, for example, a sulfated proteoglycan (42). Since heparin does not seem to inhibit the interactions (Fig. 6), it is less likely that a similar polyanionic carbohydrate is responsible for acquisition of the complement regulators onto MF. One of the four previously described pathogens that bind both CFH and C4BP, S. pyogenes uses a single surface protein for the interactions (3, 19). CFH and C4BP share common structural features, since both consist of similar domains (so-called short consensus repeat or complement control protein domains) and have binding sites for the same molecules such as heparin, C3b, streptococcal M protein, and C-reactive protein (38, 43). Therefore it is possible that they share the same binding molecule on Loa loa MF, but this remains to be determined.
We found that Loa loa MF utilize host CFH and C4BP for protection against complement attack. We have previously been involved in showing that another filarial parasite, Onchocerca volvulus, is able to acquire host CFH in vitro (30). It is possible and even probable, however, that MF use some other complement evasion mechanisms as well. It is possible that those mechanisms act on the same level of the cascade, but it might also be that the other mechanisms act on different steps. One other parasitic worm, Schistosoma mansoni, expresses a serine protease that cleaves C3, C3b, and C9 and in addition a protein called schistosoma complement inhibitor protein that prevents C5b-9 formation (14, 26). Since practically no C5 or C5b-9 was detected on Loa loa MF, a regulator of the terminal pathway (from the C5 level on) might also be involved. Binding of the late components is, however, likely to be unimportant with elimination of MF in any case, as we explain below, and therefore we did not analyze the terminal pathway in detail.
The main defense functions of complement are opsonization, direct lysis of the target by C5b-9n complexes, and release of anaphylatoxic and chemotactic peptides. In the case of Loa loa MF, however, it is very unlikely that the formation of the C5b-9n complexes could damage the surface of MF due to the thick and firm layers of chitin, proteins, and carbohydrates in the sheath that surround the outermost lipid bilayer of MF (39). Therefore, the most effective defense against MF must be inhibition of opsonization and chemotaxis and subsequent attack by eosinophils. In our patient, similar to Loa loa cases in general, practically no attached leukocytes were seen on MF in blood smears (data not shown). This fits well with inefficiency of complement attack against MF as the obvious prerequisite for MF survival in blood. Further questions are whether MF in general can be attacked by complement or whether opsonization with complement components can lead to any damage to the surface structures. Since some AP activation occurs on MF as shown in previous studies (48) and this report (Fig. 1), it seems clear that the MF surface could be opsonized from a biochemical point of view. The generated C3b could thereby clearly enhance attachment of eosinophils (and neutrophils) to MF, followed by indirect damage to MF if C3b was not downregulated. We suggest that the presence of CFH and C4BP on the MF surface leads to enhanced inactivation of C3b and C4b. This in turn can result in lower deposition of complement opsonins and cell-mediated damage to MF. Simultaneously, regulation of these molecules results in the delayed release of the two potent anaphylatoxins, C3a and C5a, involved in enhancing vascular permeability, vasoconstriction, chemotaxis, and activation of inflammatory cells, thereby contributing to a decreased inflammatory response (13, 20). On the basis of these mechanisms, the overall effect of complement evasion is likely to prolong survival of Loa loa MF in human blood and thereby to increase the probability of MF obtaining access to the vector.
In conclusion, our study shows that Loa loa MF acquire host complement regulators CFH and C4BP in vivo and restrict complement activation in an efficient way. This is the first report showing that acquisition of host C regulators occurs on a nonviral microbe during the infection. In addition this report adds to the knowledge concerning immune evasion of sheathed MF in general.
Acknowledgments
We thank Marjatta Ahonen, Kirsti Widing, and the medical laboratory technologists of the Parasitological Unit of HUSLAB (especially E. Tyyni) for excellent technical assistance. We thank Anna Blom for providing us with C4BP and anti-C4BP and Heli Siikamäki (Helsinki University Central Hospital, Finland) for patient contacts.
This work was financially supported by grants from the Academy of Finland (projects 201506, 202529, and 216251), The Helsinki University Central Hospital Funds, The Sigrid Jusélius Foundation, and The Finnish Cultural Foundation.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 15 June 2009.
REFERENCES
- 1.Ajuh, P. M., J. P. Akue, P. Boutin, S. Everaere, and T. G. Egwang. 1995. Loa loa: structural diversity of a 15-kDa repetitive antigen. Exp. Parasitol. 81145-153. [DOI] [PubMed] [Google Scholar]
- 2.Bain, O., and S. Babayan. 2003. Behaviour of filariae: morphological and anatomical signatures of their life style within the arthropod and vertebrate hosts. Filaria J. 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berggård, K., E. Johnsson, E Morfeldt, J. Persson, M. Stålhammar-Carlemalm, and G. Lindahl. 2001. Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Mol. Microbiol. 42539-551. [DOI] [PubMed] [Google Scholar]
- 4.Chandrashekar, R., U. R. Rao, G. R. Rajasekariah, and D. Subrahmanyam. 1984. Separation of viable microfilariae free of blood cells on Percoll gradients. J. Helminthol. 5869-70. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, N. R. 1975. Enzymatic activity of the second component of complement. Biochemistry 144245-4251. [DOI] [PubMed] [Google Scholar]
- 6.Davis, A. E., III, and R. A. Harrison. 1982. Structural characterization of factor I mediated cleavage of the third component of complement. Biochemistry 215745-5749. [DOI] [PubMed] [Google Scholar]
- 7.Diaz, A., A. Ferreira, and R. B. Sim. 1997. Complement evasion by Echinococcus granulosus: sequestration of host factor H in the hydatid cyst wall. J. Immunol. 1583779-3786. [PubMed] [Google Scholar]
- 8.Ekdahl, K. N., U. R. Nilsson, and B. Nilsson. 1990. Inhibition of factor I by diisopropylfluorophosphate. Evidence of conformational changes in factor I induced by C3b and additional studies on the specificity of factor I. J. Immunol. 1444269-4274. [PubMed] [Google Scholar]
- 9.Eveland, L. K., V. Yermakov, and M. Kenney. 1975. Loa loa infection without microfilaraemia. Trans. R. Soc. Trop. Med. Hyg. 69354-355. [DOI] [PubMed] [Google Scholar]
- 10.Fain, A. 1981. Epidemiology and pathology of loaiasis. Ann. Soc. Belg. Med. Trop. 61277-285. [PubMed] [Google Scholar]
- 11.Farries, T. C., T. Seya, R. A. Harrison, and J. P. Atkinson. 1990. Competition for binding sites on C3b by CR1, CR2, MCP, factor B and factor H. Complement Inflamm. 730-41. [DOI] [PubMed] [Google Scholar]
- 12.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 27250-53. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez, H. N., and T. E. Hugli. 1978. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J. Biol. Chem. 2536955. [PubMed] [Google Scholar]
- 14.Fishelson, Z. 1995. Novel mechanisms of immune evasion by Schistosoma mansoni. Mem. Inst. Oswaldo Cruz. 90289-292. [DOI] [PubMed] [Google Scholar]
- 15.Gems, D. 2000. Longevity and ageing in parasitic and free-living nematodes. Biogerontology 1289-307. [DOI] [PubMed] [Google Scholar]
- 16.Giacomin, P. R., H. Wang, D. L. Gordon, and L. A. Dent. 2004. Quantitation of complement and leukocyte binding to a parasitic helminth species. J. Immunol. Methods 289201-210. [DOI] [PubMed] [Google Scholar]
- 17.Gigli, I., T. Fujita, and V. Nussenzweig. 1979. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc. Natl. Acad. Sci. USA 766596-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammerberg, B., Y. Rikihisa, and M. W. King. 1984. Immunoglobulin interactions with surfaces of sheathed and unsheathed microfilariae. Parasite Immunol. 6421-434. [DOI] [PubMed] [Google Scholar]
- 19.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 851657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hugli, T. E., and H. J. Müller-Eberhard. 1978. Anaphylatoxins: C3a and C5a. Adv. Immunol. 261-53. [DOI] [PubMed] [Google Scholar]
- 21.Isenman, D. E. 1983. The role of the thioester bond in C3 and C4 in the determination of the conformational and functional states of the molecule. Ann. N. Y. Acad. Sci. 421277-290. [DOI] [PubMed] [Google Scholar]
- 22.Jokiranta, T. S., L. Jokipii, and S. Meri. 1995. Complement resistance of parasites. Scand. J. Immunol. 429-20. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita, T., M. E. Medof, K. Hong, and V. Nussenzweig. 1986. Membrane-bound C4b interacts endogenously with complement receptor CR1 of human red cells. J. Exp. Med. 1641377-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koistinen, V., S. Wessberg, and J. Leikola. 1989. Common binding region of complement factors B, H and CR1 on C3b revealed by monoclonal anti-C3d. Complement Inflamm. 6270-280. [DOI] [PubMed] [Google Scholar]
- 25.Leroy, E., S. Baize, G. Wahl, T. G. Egwang, and A. J. Georges. 1997. Experimental infection of a nonhuman primate with Loa loa induces transient strong immune activation followed by peripheral unresponsiveness of helper T cells. Infect. Immun. 651876-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marikovsky, M., M. Parizade, R. Arnon, and Z. Fishelson. 1990. Complement regulation on the surface of cultured schistosomula and adult worms of Schistosoma mansoni. Eur. J. Immunol. 20221-227. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto, M., K. Nagaki, H. Kitamura, S. Kuramitsu, S. Nagasawa, and T. Seya. 1989. Probing the C4-binding site on C1s with monoclonal antibodies. Evidence for a C4/C4b-binding site on the gamma-domain. J. Immunol. 1422743-2750. [PubMed] [Google Scholar]
- 28.Meri, T., S. J. Cutler, A. M. Blom, S. Meri, and T. S. Jokiranta. 2006. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect. Immun. 744157-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meri, T., A. M. Blom, A. Hartmann, D. Lenk, S. Meri, and P. F. Zipfel. 2004. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect. Immun. 726633-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meri, T., T. S. Jokiranta, J. Hellwage, A. Bialonski, P. F. Zipfel, and S. Meri. 2002. Onchocerca volvulus microfilariae avoid complement attack by direct binding of factor H. J. Infect. Dis. 1851786-1793. [DOI] [PubMed] [Google Scholar]
- 31.Müller-Eberhard, H. J. 1985. Transmembrane channel-formation by five complement proteins. Biochem. Soc. Symp. 50235-246. [PubMed] [Google Scholar]
- 32.Müller-Eberhard, H. J., and O. Gotze. 1972. C3 proactivator convertase and its mode of action. J. Exp. Med. 1351003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noireau, F., J. D. Apembet, A. Nzoulani, and B. Carme. 1990. Clinical manifestations of loiasis in an endemic area in the Congo. Trop. Med. Parasitol. 4137-39. [PubMed] [Google Scholar]
- 34.Padgett, J. J., and K. H. Jacobsen. 2008. Loiasis: African eye worm. Trans. R. Soc. Trop. Med. Hyg. 102983-989. [DOI] [PubMed] [Google Scholar]
- 35.Pillemer, L., L. Blum, I. H. Lepow, O. A. Ross, E. W. Todd, and A. C. Wardlaw. 1954. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science 120279-285. [DOI] [PubMed] [Google Scholar]
- 36.Pillemer, L., L. Blum, I. H. Lepow, L. Wurz, and E. W. Todd. 1956. The properdin system and immunity. III. The zymosan assay of properdin. J. Exp. Med. 1031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinder, M. 1988. Loa loa--a neglected filaria. Parasitol. Today 4279-284. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez de Cordoba, S., J. Esparza-Gordillo, E. Goicoechea de Jorge, M. Lopez-Trascasa, and P. Sanchez-Corral. 2004. The human complement factor H: functional roles, genetic variations and disease associations. Mol. Immunol. 41355-367. [DOI] [PubMed] [Google Scholar]
- 39.Selkirk, M. E., M. Yazdanbakhsh, D. Freedman, M. L. Blaxter, E. Cookson, R. E. Jenkins, and S. A. Williams. 1991. A proline-rich structural protein of the surface sheath of larval Brugia filarial nematode parasites. J. Biol. Chem. 26611002-11008. [PubMed] [Google Scholar]
- 40.Seya, T., J. R. Turner, and J. P. Atkinson. 1986. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J. Exp. Med. 163837-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sim, E., and R. B. Sim. 1981. Binding of fluid-phase complement components C3 and C3b to human lymphocytes. Biochem. J. 198509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson, M. G., and B. R. Laurence. 1972. Histochemical studies on microfilariae. Parasitology 6461-88. [DOI] [PubMed] [Google Scholar]
- 43.Sjöberg, A. P., L. A. Trouw, F. D. McGrath, C. E. Hack, and A. M. Blom. 2006. Regulation of complement activation by C-reactive protein: targeting of the inhibitory activity of C4b-binding protein. J. Immunol. 1767612-7620. [DOI] [PubMed] [Google Scholar]
- 44.Von Zabern, I., E. L. Bloom, V. Chu, and I. Gigli. 1982. The fourth component of human complement treated with amines or chaotropes or frozen-thawed (C4b-like C4): interaction with C4 binding protein and cleavage by C3b/C4b inactivator. J. Immunol. 1281433-1438. [PubMed] [Google Scholar]
- 45.Vorup-Jensen, T., J. C. Jensenius, and S. Thiel. 1998. MASP-2, the C3 convertase generating protease of the MBLectin complement activating pathway. Immunobiology 199348-357. [DOI] [PubMed] [Google Scholar]
- 46.Weiler, J. M., M. R. Daha, K. F. Austen, and D. T. Fearon. 1976. Control of the amplification convertase of complement by the plasma protein beta1H. Proc. Natl. Acad. Sci. USA 733268-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whaley, K., and S. Ruddy. 1976. Modulation of the alternative complement pathways by beta 1 H globulin. J. Exp. Med. 1441147-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yates, J. A., G. I. Higashi, A. Lowichik, T. C. Orihel, R. C. Lowrie, Jr., and M. L. Eberhard. 1985. Activation of the alternative complement pathway in normal human serum by Loa loa and Brugia malayi infective stage larvae. Acta Trop. 42157-163. [PubMed] [Google Scholar]
- 49.Zipfel, P. F., R. Würzner, and C. Skerka. 2007. Complement evasion of pathogens: common strategies are shared by diverse organisms. Mol. Immunol. 443850-3857. [DOI] [PubMed] [Google Scholar]