Abstract
Salmonella enterica, a gram-negative pathogen, causes a spectrum of human infections including enterocolitis and typhoid fever. We previously showed that Salmonella flagellin played a role in suppressing intestinal mucosal inflammation in a murine model of acute enterocolitis. In this study, we examined the role of flagellin in the typhoid-like systemic murine Salmonella infection by measuring bacterial proliferation, inflammation, leukocyte recruitment, and cellular apoptosis in Peyer's patches (PPs), mesenteric lymph node (MLN), and spleen. We found that relative to an isogenic wild-type (WT) strain, aflagellate Salmonella exhibited increased proliferation at 4 days postinfection in PPs and MLN but not spleen. The aflagellate mutant also elicited increased local and systemic secretion of inflammatory cytokines such as interleukin-1β, gamma interferon, and tumor necrosis factor alpha and enhanced surface expression of ICAM-1 on macrophages and dendritic cells (DCs). Furthermore, the recruitment of macrophages and DCs in PPs and MLN, but not spleen, was enhanced upon infection with aflagellate Salmonella. The relative differences between WT and aflagellate Salmonella were highly attenuated in Toll-like receptor 5-deficient (TLR5−/−) mice, indicating involvement of TLR5-dependent signaling. Interestingly, infection with the aflagellate mutant also resulted in decreased levels of T-cell apoptosis in PPs relative to infection with WT Salmonella. We postulate that the initial lack of detection of the aflagellate mutant in the mucosa permits increased proliferation within the host and enhances inflammatory signaling in nonepithelial cell types, which subsequently promotes leukocyte recruitment. In contrast, lack of difference in any disease parameter measured in the spleen likely reflects that Salmonella expression of flagellin is downregulated in this organ. Thus, the characteristic inflammatory pathology of Salmonella infection occurs only in PPs and to a lesser extent in MLN during the initial phases of infection and these early responses are dependent on TLR5.
Salmonella enterica is a food- and waterborne gram-negative pathogen that causes a range of infectious diseases in a variety of hosts. In humans, infection with Salmonella enterica serovar Typhi typically causes severe systemic illness while infection with serovar Typhimurium is commonly associated with self-limiting gastroenteritis. S. enterica serovar Typhimurium infections are a common cause of food poisoning in industrialized countries. While the infection is usually confined to the intestinal tract, it can sometimes result in severe complications and is a particular threat to immunocompromised persons. In contrast, systemic infections by S. enterica serovar Typhi, often referred to as “typhoid fever,” commonly cause severe, sometimes lethal, illness, making this bacterium a major menace in the developing world. Importantly, in contrast to Salmonella-induced gastroenteritis, systemic infections with S. enterica serovar Typhi are not typically associated with significant intestinal injury during early stages of disease.
The mechanisms of Salmonella infection in mice have been extensively studied. The most efficient route of systemic infection appears to be that, following ingestion and gastric passage, Salmonella reaches the small intestine lumen, where it crosses the epithelium overlaying mucosal lymphoid aggregates, or Peyer's patches (PPs). The epithelium overlaying PPs—the follicle-associated epithelium—contains specialized enterocytes, referred to as “microfold” cells (or M cells), that are adapted for uptake of particulate antigens and are exploited by Salmonella to breach the epithelial barrier (31). PPs are distributed all along the small intestine, predominantly in the distal ileum. Histologically, they are composed of a subepithelial dome region containing dendritic cells (DCs) and discrete B-cell-rich germinal centers with a surrounding T-cell region. Salmonella initially enters the PPs and replicates intracellularly within DCs. This provokes inflammatory processes resulting in recruitment and accumulation of monocytes/macrophages, neutrophils, and additional DCs (22, 31). Neutrophils and monocytes predominately accumulate in the subepithelial dome, whereas monocytes alone are found in the T-cell area (25). DCs present in the subepithelial dome region migrate to the T-cell region following initial invasion (31). Free bacteria or infected DCs then egress via the lymphatics to reach the mesenteric lymph node (MLN) and ultimately to systemic reticuloendothelial tissues of the spleen and liver.
As with most pathogens, Salmonella possesses a range of virulence factors that are necessary to invade and survive in the hostile environment encountered within the host. One of these virulence factors is the flagella responsible for the bacterial motility and chemotaxis, which allows the organism to move in response to environmental signals (23). The long helical filament of flagella is mainly composed of monomers of two distinct but related bacterial flagellin proteins, FliC and FljB (18). It is now well known that flagellin monomers are recognized by the innate immune system via extracellular Toll-like receptor 5 (TLR5), a member of the TLR family (21, 37). TLR5 is expressed on the basolateral surface of intestinal epithelial cell lines and human colon (8, 24, 37) and on CD11c+ lamina propria DCs (33). TLR5 is able to sense flagellin from a variety of flagellated pathogens, including enteropathogenic Escherichia coli and Salmonella. Additionally, flagellin released or shed from intracellular bacteria can be recognized by the cytoplasmic pattern recognition receptors IPAF (IL-1β-converting enzyme protease activating factor) and/or NAIP5 (neuronal apoptosis inhibitor protein 5), expressed inside antigen-presenting cells such as macrophages (7, 13, 17, 20, 29). Flagellin has been shown to potently induce inflammation via the NF-κB and mitogen-activated protein kinase pathways and stimulate the production of cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-8 (IL-8), IL-1β, and IL-18 (13, 26, 41), and thus represents a major proinflammatory determinant of Salmonella. We have shown that in in vitro epithelial systems, aflagellate Salmonella (FliC− FljB−) was virtually devoid of the potent proinflammatory signaling mediated by isogenic wild-type (WT) Salmonella (41). Additionally, we have demonstrated in an acute model of murine enterocolitis that aflagellate Salmonella, while showing suppressed inflammation and epithelial apoptosis at very early stages (6 to 24 h post oral infection), stimulated markedly increased mucosal inflammation and apoptosis at 48 h, suggesting a time-dependent attenuating effect of flagellin on both cellular inflammation and apoptosis (39). Interestingly flagellin is rather involved in the early innate response in the bovine ileal loop model (40). In contrast to studies of Salmonella enterocolitis in which many bacteria directly invade throughout the gut epithelium, the role of flagellin in systemic infection, in which infection occurs via the PPs, is not known.
Salmonella has been shown to induce several types of programmed cell death (6, 11), a fundamental mechanism necessary to eliminate infected, damaged, or superfluous cells. Apoptotic programmed cell death is characterized by the condensation of the nucleus and the cytoplasm and the fragmentation of the cell, leading to the formation of apoptotic bodies eliminated by macrophages, thus preventing release of noxious cellular contents (6). However, any type of cell death can also become deleterious and cause pathological tissue damage when it is prolonged or amplified, as often occurs during infection with a pathogen such as Shigella (42). Salmonella is able to induce apoptosis in several cell types, including both epithelial cells and leukocytes (16, 22, 30).
In this study, we examine the role of Salmonella flagellin in a typhoid fever model in C57BL/6 mice. We report that a mutant lacking flagellin is able to replicate more efficiently in PPs and to a lesser extent in the MLN, leading to an increase in inflammatory mediator expression and leukocyte recruitment in these tissues. We also observed that this effect does not occur in the spleen. Furthermore, the aflagellate mutant decreases T-cell apoptosis at day 4 postinfection via TLR5-independent mechanisms.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are the Salmonella enterica serovar Typhimurium WT strain SL3201, which is mouse virulent and resistant to nalidixic acid (14, 27), and the aflagellate fliC fljB (fliC::Tn10 fljB5001::MudJ) mutant, which is resistant to kanamycin (9, 27). For infection studies, bacteria were grown for 12 h at 37°C under microaerophilic conditions in Luria-Bertani (LB) broth. Bacteria were washed once in phosphate-buffered saline (PBS) and resuspended in PBS to yield ∼3 × 109 CFU/ml.
Mouse typhoid fever model.
TLR5−/− mice backcrossed with C57BL/6 mice (38) as well as the WT littermates were used between 8 and 10 weeks of age. Mice were infected as previously described (25) with the following modifications. Food and water were removed 4 h before the infection. One hundred microliters of 5% NaHCO3 was inoculated by oral gavage to neutralize gastric acidity, and 10 min after this treatment, 3 × 108 CFU in 100 μl was inoculated by oral gavage. In all experiments, the number of inoculated bacteria was determined by plating on LB agar. For the coinfection experiment, equal numbers (1.5 × 108 CFU) of both strains were inoculated, and mice were sacrificed 4 days postinfection. Blood was collected, and PPs, MLN, and spleen were removed. One-third of PPs, MLNs, and spleens were either fixed in 10% buffered neutral formalin and embedded in paraffin or embedded in OCT (optimal cutting temperature) medium and frozen at −80°C. Two-thirds of these organs were kept to be further analyzed by flow cytometry.
Flow cytometry analysis.
PPs, MLNs, and spleens were treated as previously described (25) for fluorescence-activated cell sorter (FACS) analysis with the following modifications. Cells of these organs were chopped and then treated with 1.7 mg/ml collagenase IV (Worthington) in 500 μl Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 1% Na pyruvate, and 1% glutamine for 30 min at 37°C. Cellular dissociation was achieved by thorough pipetting. Ten microliters of this suspension was lysed with 1% Triton X-100, and various dilutions were plated on LB agar containing either 30 μg/ml nalidixic acid for the WT strain or 45 μg/ml kanamycin for the aflagellate mutant. After a first centrifugation, the remaining supernatants were recovered and frozen for subsequent determination of cytokines. Cells were then washed three times in FACS buffer (1× PBS, 2% fetal bovine serum, 0.1% sodium azide) and filtered. Spleen was additionally treated 5 min on ice by 0.14 M NH4Cl to lyse red blood cells. Fc receptors were blocked for 10 min by incubation with 200 μg/ml mouse immunoglobulin G (Sigma) and stained with different antibodies for 20 min: CD86 (clone GL1; BD Pharmingen), CD11c (clone HL3; BD Pharmingen), ICAM-1 (clone 3E2; BD Pharmingen), F4/80 (clone BM8; Caltag), or CD4 (clone RM4-5; Caltag). If necessary, streptavidin-conjugated secondary antibody conjugated with either phycoerythrin (PE) or allophycocyanin (APC) (Caltag) was used to detect biotinylated ICAM-1 and F4/80. Staining with annexin V-fluorescein-5-isothiocyanate (FITC) and propidium iodide (PI) for apoptosis was performed using the apoptosis detection kit (Molecular probes) according to the manufacturer's instructions. Cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems). Data were analyzed with FlowJo software.
Cytokine measurement.
The level of cytokines was measured by enzyme-linked immunosorbent assay using either Quantikine immunoassay kit (gamma interferon [IFN-γ], IL-1β, and IL-12p70) or the Duo Set enzyme-linked immunosorbent assay development kit (TNF-α) (R&D Systems) according to the manufacturer's instructions.
Histological analyses.
Paraffin-embedded sections were stained for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) by the in situ cell death detection kit (Roche Diagnostics Corp.) according to the manufacturer's instructions. Briefly, after a treatment for 30 min at 60°C, sections were rehydrated by being dipped for 3 min twice in xylenes, once in 100% ethanol, once in 95% ethanol, once in 70% ethanol, and once in water. A permeabilization step of 10 min in 0.1% Triton X-100-0.1% Na citrate was performed, and the sections were stained with the TUNEL reaction mixture for 1 h at 37°C.
Frozen sections (6 μm) were air-dried for 30 min before being fixed with acetone (10 min at −20°C). For TUNEL staining, frozen sections were immediately fixed with 4% paraformaldehyde (20 min at room temperature). Sections were blocked for 1 h in 3% bovine serum albumin prior to being stained by monoclonal antibodies (MAbs). The following MAbs were used: CD11b (clone M1/70; BD Pharmingen), F4/80 (clone BM8; Caltag), Gr1 (clone 1A8; BD Pharmingen), B220 (clone RA3-6B2; BD Pharmingen), CD3a (clone145-2C11; BD Pharmingen), CD8a (clone 53-6.7; BD Pharmingen), CD11c (clone HL3; BD Pharmingen), CD4 (clone RM4-5; Caltag), and anti-Salmonella CSA-1 (KPL). TUNEL staining of frozen sections was performed according to the protocol developed by Vijay-Kumar et al. (39).
Paraffin-embedded sections were also stained for poly(ADP-ribose) polymerase (PARP) using a MAb against cleaved PARP (Asp214) (Cell Signaling) according to the manufacturer's instructions. Sections were rehydrated as for TUNEL staining and placed in antigen retrieval buffer (10 mM Na citrate, pH 6) in a decloaking chamber for 10 min. Sections were then blocked for 1 h in a mixture of 1× Tris-buffered saline, 3% bovine serum albumin, and 0.1% Triton X-100 before being probed with anti-PARP overnight at 4°C. After three washes in 1× Tris-buffered saline-0.1% Triton X100, sections were treated for 1 h at room temperature with horseradish peroxidase (Amersham)-conjugated anti-rabbit immunoglobulin G and revealed with fast diaminobenzidine (Sigma) for 20 min.
Statistical analysis.
The statistical method used to analyze results was Student's t test with two-tailed distribution with GraphPad Prism.
RESULTS
Deletion of the Salmonella flagellin eventuates in an increased bacterial load in the PPs and MLN but not spleen.
Oral S. enterica serovar Typhimurium infection induces a systemic disease in mice reminiscent of human typhoid fever (10, 32). To begin characterizing the role of flagellin in this systemic model of Salmonella infection, we enumerated bacteria in PPs, MLN, and spleen. C57BL/6 mice from our animal facility (littermates of TLR5−/− mice) were inoculated by gavage and sacrificed 4 days after the challenge, and PPs, MLN, and spleen were recovered. Viable bacteria in the PPs, MLN, and spleen were quantified. We found that the number of salmonellae in PP and MLN infected by the aflagellate mutant (FliC− FljB−) was significantly higher than those of mice treated with the WT strain (three- and fivefold, respectively) (Fig. 1A). In contrast, no significant difference in the bacterial load (less than twofold) was observed between spleens of mice treated with the WT strain and those of mice treated with the aflagellate mutant, suggesting that the mechanisms that allow aflagellate bacteria to proliferate more extensively were no longer acting in the spleen.
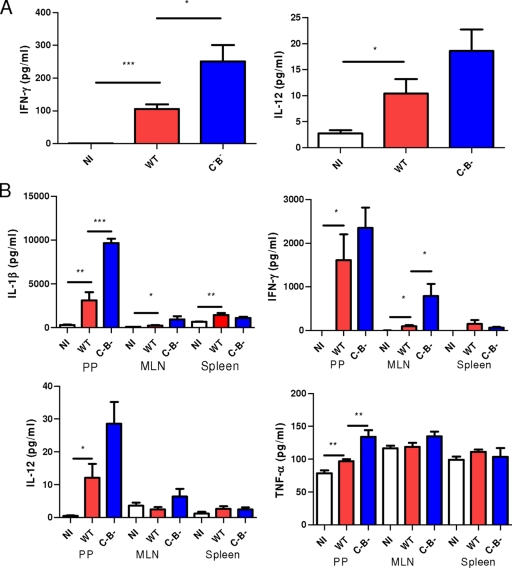
FIG. 1.
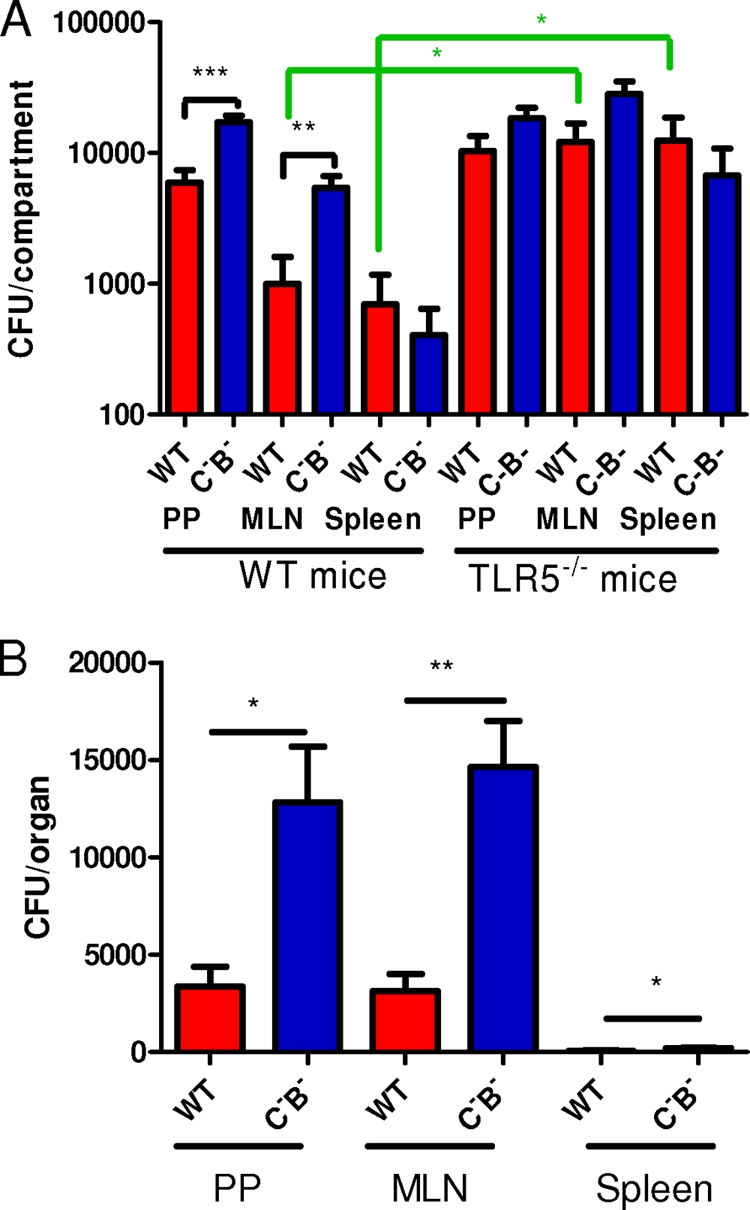
The bacterial load in PPs and MLN increases in the absence of flagellin. (A) In a systemic model at day 4 postinfection, PP, MLN, and spleen cells were isolated from WT and TLR5−/− mice infected with WT S. enterica serovar Typhimurium or an aflagellate mutant (C−B−) and plated on petri dishes, and the bacterial load was determined. Data are representative of at least five individually analyzed mice. (B) A similar experiment in which WT mice were infected with equal numbers of bacteria of both strains was performed. PP, MLN, and spleen cells recovered from the animals were plated on petri dishes containing 30 μg/ml nalidixic acid for the WT strain and 45 μg/ml kanamycin for the aflagellate mutant, and the bacterial load was determined. Data are representative of at least four individually analyzed mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further study the role of flagellin in the proliferation of the aflagellate mutant, we performed a coinfection experiment in which WT mice were infected with an equal number of bacteria of both the WT strain and the flagellate mutant in a systemic model. As shown in Fig. 1B, the number of aflagellate bacteria recovered from the PPs, MLN, and spleen was significantly higher than the number of WT bacteria. To verify that this difference is not due to a defect of growth of the WT strain, we performed a similar experiment in vitro. LB medium was inoculated with equal numbers of bacteria of both the wild-type strain and the aflagellate mutant. No difference of growth was observed (data not shown), suggesting that both strains grow similarly. Taken together, these results suggest that the aflagellate mutant may have a greater proliferation due to an absence of recognition by the innate immune system.
Aflagellate Salmonella enhances the release of inflammatory mediators.
We next determined whether mice infected with the aflagellate mutant showed a modified production of inflammatory cytokines as a response to the increased bacterial load. We measured TNF-α, IL-1β, IL-12p70, and IFN-γ in the serum of noninfected and infected mice at day 4 postinfection. The aflagellate Salmonella increased significantly the level of IFN-γ in the serum (Fig. 2A). We also determined the level of these different cytokines in PPs, MLN, and spleen at day 4 postinfection (see Materials and Methods). The level of TNF-α, IL-1β, and IL-12p70 was significantly increased in PPs infected with the aflagellate mutant compared to those treated with the WT strain (Fig. 2B). Only IFN-γ was highly increased (8.5-fold) in MLN of mice infected with the aflagellate mutant, whereas no difference was observed in the spleen when mice were infected with the aflagellate mutant (Fig. 2B). Thus, in accordance with its ability to achieve greater levels of colonization 4 days postinfection, aflagellate Salmonella elicits greater cytokine production at this time point.
FIG. 2.
The level of cytokines in serum and organs at day 4 postinfection increases in the typhoid fever model when mice are treated with the aflagellate mutant. (A) Level of IFN-γ and IL-12 in the serum of noninfected (NI) mice or mice infected with WT S. enterica serovar Typhimurium or an aflagellate mutant (C−B−). Data are representative of at least six individually analyzed mice. (B) PP, MLN, and spleen cells were dissociated with collagenase, and the level of cytokines was determined in the cell supernatant (see Materials and Methods). Data are representative of at least seven individually analyzed mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Aflagellate Salmonella increases the emigration of leukocytes into PPs and MLN.
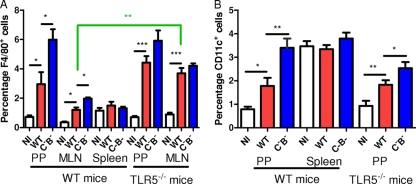
Since we found that the inflammatory cytokine level was increased by infection with the aflagellate mutant, we next examined whether the recruitment of leukocytes was also modified during this systemic infection model. To assess leukocyte infiltration to intestinal lymphoid tissue, we measured via flow cytometry the percentages of cells in the PPs, MLN, and spleen that were macrophages and DCs. As shown in Fig. 3, the percentage of macrophages and DCs of mice administered WT Salmonella was higher than that of noninfected mice (two- to fourfold). Furthermore, a significantly greater increase in levels of macrophages and DCs was observed when mice were infected with the aflagellate mutant (Fig. 3B). In contrast, no significant difference in the percentages of macrophages and DCs was observed in the spleen between mice treated with the WT strain and those treated with the aflagellate mutant (<1.5-fold) as well as between noninfected and WT-treated mice (Fig. 3).
FIG. 3.
The absence of flagellin increases the percentage of macrophages and DCs. PP, MLN, and spleen cells of WT and TLR5−/− mice noninfected (NI) or infected with WT S. enterica serovar Typhimurium or an aflagellate mutant (C−B−) were isolated at day 4 postinfection and stained for F4/80 (A; macrophages) and CD11c (B; DCs) for flow cytometric analysis. Data are representative of at least four individually analyzed mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Aflagellate Salmonella increases ICAM-1 expression.
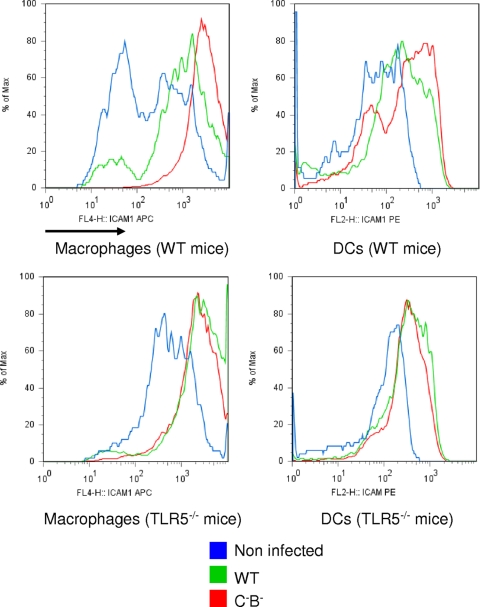
As we found that leukocyte recruitment was increased in PPs and MLN, we examined whether markers involved in leukocyte migration were enhanced in the typhoid model. ICAM-1 expression is controlled by inflammation and is involved in leukocyte recruitment and retention. We observed that, relative to mice infected with WT Salmonella, macrophages recovered from PPs of mice infected with the aflagellate mutant showed enhanced ICAM-1 expression on their surface (Fig. 4). Similarly, DCs from mice infected with aflagellate Salmonella also exhibited elevated ICAM-1 expression (Fig. 4). Interestingly, ICAM-1 expression on macrophages or DCs was not modified in MLN and spleen when mice were infected with the WT strain or the aflagellate mutant (data not shown). Collectively, these data indicate that the aflagellate Salmonella proliferated to a greater degree and were more efficient in provoking local and systemic inflammation, and therefore more virulent in extramucosal tissues. This is analogous to our previous finding with increased parameters of cellular injury in the mucosa during short-term enterocolitis with the same aflagellate mutant (39).
FIG. 4.
The absence of flagellin increases ICAM-1 expression on macrophages and DCs in a systemic model. PP cells of wild-type and TLR5−/− mice noninfected (NI) or infected with WT S. enterica serovar Typhimurium or an aflagellate mutant (C−B−) were isolated at day 4 postinfection, gated on F4/80 or CD11c, and stained for ICAM-1 for flow cytometric analysis. Data are representative of at least six individually analyzed mice.
TLR5 is involved in the modulation of Salmonella dissemination and inflammatory activation by flagellin.
To investigate whether the increased bacterial loads observed in the PPs and MLN resulted from the aflagellate bacterium's evasion of TLR5 signaling, we measured the relative abilities of WT- and aflagellate Salmonella to colonize these sites in mice lacking TLR5. We observed that, relative to WT littermates, TLR5−/− mice exhibited modestly higher levels of WT Salmonella in the PPs (about 2-fold) and markedly higher levels in the MLN (about 10-fold). Moreover, the relative difference between WT and aflagellate bacteria was strikingly diminished in TLR5−/− mice (3-fold to 1.8-fold in the PPs and 5.5-fold to 2-fold in the MLN) (Fig. 1). This is in contrast to infection in the WT mouse background, where the aflagellate mutant markedly overproliferated compared to the WT Salmonella, indicating that this growth-enhancing effect of the aflagellate condition in a normal mouse was recapitulated by flagellate Salmonella during infection in a mouse with the inability to perceive extracellular flagellin (Fig. 1). Thus, a substantial portion, but likely not all, of the increased colonization attained by aflagellate Salmonella likely reflects the lack of activation of TLR5 by the aflagellate strain. Additionally, we also observed an increased percentage of macrophages in PPs and MLN of TLR5−/− mice compared to those of WT mice (Fig. 3). Similarly, the difference in ICAM-1 expression observed between the WT strain and the aflagellate mutant on WT macrophages and DCs was not observed in TLR5−/− mice (Fig. 4), and the difference in the percentage of macrophages and DCs in PPs and MLN was also strongly attenuated in TLR5−/− mice treated with the aflagellate mutant (Fig. 3). Taken together, these results suggest that TLR5 is involved in the ability to sense invasive WT Salmonella, control bacterial numbers, and, as a consequence, mount an inflammatory response.
Aflagellate Salmonella decreases T-cell apoptosis in PPs in the systemic model.
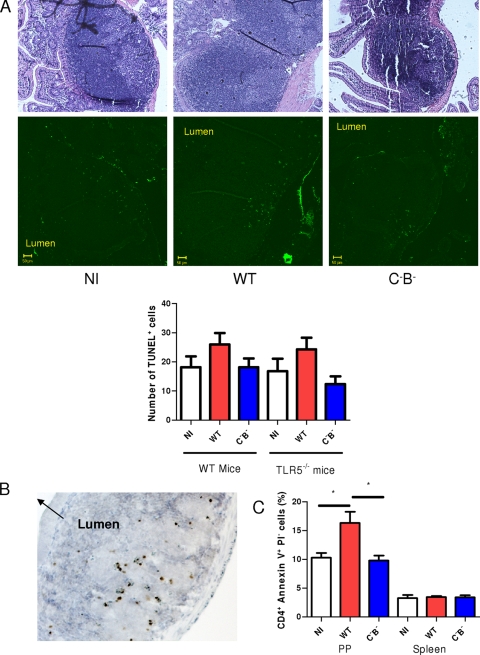
Programmed cell death is another characteristic cellular response to Salmonella infection. We had previously observed increased epithelial apoptosis in a short-term enterocolitis model (39) and sought to determine if similar events were occurring in systemic infection. We thus investigated whether PP cells were undergoing apoptosis during typhoid-like infection. PPs and spleen were recovered 4 days postinfection, paraffin embedded, and stained with TUNEL, which detects DNA strand breaks occurring during apoptosis or pyroptosis. As shown in Fig. 5A, the number of cells undergoing cell death was slightly lower in the PPs of mice infected with the aflagellate mutant than in those treated with the WT Salmonella. In contrast, no difference in the number of apoptotic cells was observed in spleen (data not shown). To confirm that cells stained by TUNEL were undergoing apoptosis, PPs were stained for the detection of cleaved PARP, a polymerase inactivated by caspase during apoptosis, and cells expressing an inactivated PARP were indeed present in the germinal center of PPs (Fig. 5B).
FIG. 5.
An aflagellate mutant of S. enterica serovar Typhimurium decreases apoptosis in PPs in WT mice in a systemic model. (A) PPs and spleen of mice noninfected (NI) or infected with WT S. enterica serovar Typhimurium or an aflagellate mutant (C−B−) retrieved at 4 days postinfection were stained with TUNEL or hematoxylin and eosin stain. Data are representative of at least seven individually analyzed mice. (B) PP of a mouse infected with the aflagellate Salmonella was stained for the detection of PARP. (C) At day 4 postinfection, cells were isolated from PPs of mice noninfected (NI) or infected with WT S. enterica serovar Typhimurium or an aflagellate mutant. They were gated for CD4 and stained with annexin V and PI for flow cytometric analysis. Data are representative of at least three individually analyzed mice. *, P < 0.05.
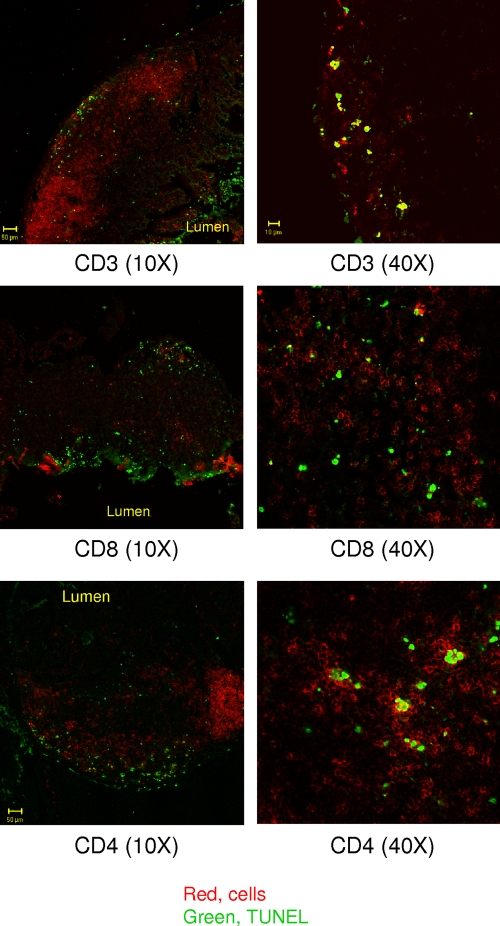
In order to determine the cell type that undergoes apoptosis, we stained PP frozen sections using both TUNEL and antibody markers for specific subsets of leukocytes. Our data showed that apoptotic cells in PPs were not macrophages (CD11b+, F4/80+), DCs (CD11c+), neutrophils (Gr1+), or B cells (B220+) (data not shown), but rather T cells (CD3+, CD4+, or CD8+) (Fig. 6). Interestingly, although some CD8+ cells were also apoptotic, they do not seem to be the main target of Salmonella (Fig. 6).
FIG. 6.
PP cells undergoing apoptosis are T cells. PPs from mice infected with an aflagellate Salmonella mutant were double stained with antibodies for a subset of cells (CD3, CD8, or CD4) and for TUNEL.
To further examine Salmonella-induced apoptosis of T cells, we performed an analysis by flow cytometry. PP cells gated on CD4 were stained for annexin V and PI. Annexins are able to bind to negatively charged phospholipids that are exposed when cells undergo apoptosis, while PI stains permeable dead cells. The number of apoptotic CD4+ cells (annexin V+ PI−) from PPs infected with the WT strain was significantly decreased compared to that from noninfected PPs, suggesting that Salmonella infection induces CD4+ cell apoptosis (Fig. 5C). PP cells of mice treated with a Salmonella lacking flagellin contained a significantly smaller number of CD4+ cells in early apoptosis than those infected by the WT strain (Fig. 5C). No difference was observed in the spleen. Taken together, these results thus suggest that flagellin makes a significant contribution to the apoptosis of germinal center T cells induced by WT Salmonella in a mouse model of typhoid.
Since the presence of flagellin on Salmonella modifies T-cell apoptosis, we decided to determine whether bacteria could act directly on T cells. The localization of bacteria in PPs was thus examined. Bacteria were observed at different places in the PPs and not only in germinal center regions where apoptosis occurs (data not shown), suggesting that their action may be indirect rather than direct. We also investigated whether TLR5 was involved in the T-cell apoptosis. We still observed a decreased number of TUNEL+ stained cells when TLR5−/− mice were infected with the aflagellate mutant (Fig. 5A), suggesting that TLR5 is not involved in this process.
DISCUSSION
In this report, we showed that aflagellate Salmonella generated an aggravated systemic disease, manifested by increased expression of inflammatory mediators, adhesion molecules such as ICAM-1, and consequent leukocyte recruitment. The number of bacteria recovered from PPs and MLN (but not spleen) in the systemic model was enhanced when mice were infected with the aflagellate mutant.
In a previously published model of Salmonella enterocolitis, we showed that aflagellate Salmonella exhibited a decreased inflammatory response in the first 6 to 12 h post-oral infection, presumably because of the lack of perception on the part of the epithelia. However, by 48 h, the aflagellate bacteria elicited markedly greater mucosal inflammation and epithelial apoptosis, secondary to increased bacterial load and secondary inflammatory events in phagocytes in the lamina propria (39). In the present work, we report similar events in lymphoid and reticuloendothelial tissues in a systemic model. We hypothesize that the increased bacterial load of the aflagellate bacteria, likely given a “head start” in proliferation due to initial lack of detection by TLR5 present in the epithelial layer, accounts for the difference in pathogenic effect in nonepithelial tissues, with the exception of the spleen.
The bacterial loads in the spleen were similar when mice were infected with the WT and the aflagellate mutant, possibly indicating that spleen at 4 days postinfection is not yet the main infectious site (25). Schmitt et al. found that at early stages of a systemic infection model (days 6 to 8), the aflagellate mutant was slightly less capable of reaching the spleen than the WT strain and a similar number of bacteria was recovered from the spleen whether the mice were infected with the WT strain or the aflagellate mutant (28). These results are consistent with ours. The aflagellate mutant does not exhibit a modified capacity to survive within macrophages in vitro (data not shown and see reference 20), ruling out increased intracellular survival. However, another explanation may be that Salmonella flagellin gene expression is modulated throughout the infectious process. Interestingly, flagellin is not expressed at the early stage when bacteria are within epithelial cells or macrophages. Although flagellin is not expressed when present in macrophages during all stages of infection, it seems to be expressed in epithelial cells at a later stage of infection, suggesting that flagellin expression is indeed regulated by the stage of infection but also by the environment (2, 5, 12). Furthermore, the flagellin gene is expressed only in PPs and its expression dramatically decreases when bacteria reach the spleen during systemic salmonellosis (3, 4, 15, 19), suggesting that by the time Salmonella invades this organ, all organisms are functionally aflagellate. Indeed, we did not find flagellin in the spleen by Western blot analysis (data not shown). Thus, the absence of flagellin expression in the spleen could also account for the unchanged bacterial load observed in the spleen of mice infected with the WT strain and the aflagellate mutant.
Our past work in the epithelia showed that aflagellate Salmonella induced markedly greater mucosal apoptosis, an effect related to the inability of epithelial cells to upregulate antiapoptotic proteins and protect themselves from proapoptotic stimuli. In contrast, in our present data with systemic infection, apoptosis was reduced in lymphoid tissues infected by aflagellate bacteria. In this context, by 4 days, an inflammatory milieu is well established by secondary inflammatory events likely mediated by TLR2 and -4. Inflammatory signaling is generally accepted to have antiapoptotic effects by the transcriptional upregulation of a battery of antiapoptotic genes such as those coding for inhibitor of apoptosis proteins, A20, BCL family members, etc. We hypothesize that the decreased apoptosis seen in PPs infected with aflagellate Salmonella is a consequence of the enhanced local inflammation (itself a consequence of increased bacterial proliferation), possibly providing a degree of protection for cells within the lymphoid tissues. The lack of effect in the TLR5−/− animals is consistent with the model that inflammatory signaling by other TLRs (e.g., TLR2 and -4) mediated the indirect protection. Additionally, leukocytes may be perceiving flagellin via the intracellular cytoplasmic Nod-like receptors IPAF and NAIP, which are well known to induce potent flagellin-dependent cell death in DCs and macrophages (1, 7, 20, 36).
We did not find bacteria present in PPs interacting with apoptotic cells, suggesting that apoptotic stimulation is not a direct result of bacterial invasion. A previous study showed that when Salmonella is in contact with T cells, it decreases TCR expression of both CD4+ and CD8+ cells but does not enhance T-cell apoptosis (34, 35). This is consistent with our results suggesting that bacteria may act indirectly through antigen-presenting cells or by the production of cytokines.
Taken together, our results show that the Salmonella mutant lacking flagellin in the systemic model is able to disseminate and/or replicate more easily than the WT and indicate the key role played by flagellin during initial infection (before it is downregulated). Other Salmonella pathogen-associated molecular pattern proteins such as lipopolysaccharide and peptidoglycan would be available to induce the production of inflammatory regulators (e.g., cytokines), which subsequently increases ICAM-1 expression and leukocyte migration. This suggests that flagellin may have a suppressive role in the dissemination and/or replication of Salmonella through its recognition by the innate immune system. As a molecule detected by the mucosal innate immune system, flagellin allows the host to perceive a local invasion. This early warning induces classical acute inflammation that controls (if not eliminates) the infection. A component of the inflammatory response is the upregulation of antiapoptotic proteins that provide some degree of protection to cells in the site of infection and inflammation. A parallel detection and response system in infected leukocytes uses the IPAF/NAIP system to elicit cell death in this cell type. A property of flagellin, unique among pathogen-associated molecular pattern proteins, is that it is a protein and thus under transcriptional control. Thus, the pathogen has the ability to downregulate this potent inducer of inflammatory/apoptotic signaling. This is especially apparent in the spleen, where flagellin is downregulated, and no different effects were noted in the inflammatory parameters of WT versus aflagellate Salmonella infection. Thus, in systemic infection, the significance of flagellin-elicited inflammation may be relevant only in the initial mucosal and mesenteric phases.
Acknowledgments
The work described here was funded by grant NIH R01DK071604 to A.S.N.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 13 July 2009.
REFERENCES
- 1.Chen, L. M., K. Kaniga, and J. E. Galan. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 211101-1115. [DOI] [PubMed] [Google Scholar]
- 2.Cummings, L. A., S. L. Barrett, W. D. Wilkerson, I. Fellnerova, and B. T. Cookson. 2005. FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J. Immunol. 1747929-7938. [DOI] [PubMed] [Google Scholar]
- 3.Cummings, L. A., W. D. Wilkerson, T. Bergsbaken, and B. T. Cookson. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61795-809. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez-Bernal, G., M. G. Pucciarelli, F. Ramos-Morales, M. Garcia-Quintanilla, D. A. Cano, J. Casadesus, and F. Garcia-del Portillo. 2004. Repression of the RcsC-YojN-RcsB phosphorelay by the IgaA protein is a requisite for Salmonella virulence. Mol. Microbiol. 531437-1449. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47103-118. [DOI] [PubMed] [Google Scholar]
- 6.Fink, S. L., and B. T. Cookson. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 731907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchi, L., A. Amer, M. Body-Malapel, T. D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat. Immunol. 7576-582. [DOI] [PubMed] [Google Scholar]
- 8.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 1671882-1885. [DOI] [PubMed] [Google Scholar]
- 9.Gillen, K. L., and K. T. Hughes. 1991. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J. Bacteriol. 1732301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govoni, G., and P. Gros. 1998. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm. Res. 47277-284. [DOI] [PubMed] [Google Scholar]
- 11.Guiney, D. G. 2005. The role of host cell death in Salmonella infections. Curr. Top. Microbiol. Immunol. 289131-150. [DOI] [PubMed] [Google Scholar]
- 12.Hautefort, I., A. Thompson, S. Eriksson-Ygberg, M. L. Parker, S. Lucchini, V. Danino, R. J. Bongaerts, N. Ahmad, M. Rhen, and J. C. Hinton. 2008. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell. Microbiol. 10958-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 14.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291238-239. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, E. A., and J. E. Galan. 2002. Immune response to Salmonella: location, location, location? Immunity 16325-328. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. M., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthoft, and M. F. Kagnoff. 1998. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 1021815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lightfield, K. L., J. Persson, S. W. Brubaker, C. E. Witte, J. von Moltke, E. A. Dunipace, T. Henry, Y. H. Sun, D. Cado, W. F. Dietrich, D. M. Monack, R. M. Tsolis, and R. E. Vance. 2008. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 91171-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macnab, R. M. 1992. Genetics and biogenesis of bacterial flagella. Annu. Rev. Genet. 26131-158. [DOI] [PubMed] [Google Scholar]
- 19.McSorley, S. J., S. Asch, M. Costalonga, R. L. Reinhardt, and M. K. Jenkins. 2002. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity 16365-377. [DOI] [PubMed] [Google Scholar]
- 20.Miao, E. A., C. M. Alpuche-Aranda, M. Dors, A. E. Clark, M. W. Bader, S. I. Miller, and A. Aderem. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 7569-575. [DOI] [PubMed] [Google Scholar]
- 21.Miao, E. A., E. Andersen-Nissen, S. E. Warren, and A. Aderem. 2007. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin. Immunopathol. 29275-288. [DOI] [PubMed] [Google Scholar]
- 22.Monack, D. M., D. Hersh, N. Ghori, D. Bouley, A. Zychlinsky, and S. Falkow. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos, H. C., M. Rumbo, and J. C. Sirard. 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12509-517. [DOI] [PubMed] [Google Scholar]
- 24.Rhee, S. H., E. Im, and C. Pothoulakis. 2008. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology 135518-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rydstrom, A., and M. J. Wick. 2007. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J. Immunol. 1785789-5801. [DOI] [PubMed] [Google Scholar]
- 26.Salazar-Gonzalez, R. M., and S. J. McSorley. 2005. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol. Lett. 101117-122. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt, C. K., S. C. Darnell, and A. D. O'Brien. 1996. The attenuated phenotype of a Salmonella typhimurium flgM mutant is related to expression of FliC flagellin. J. Bacteriol. 1782911-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt, C. K., J. S. Ikeda, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, and A. D. O'Brien. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 695619-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun, Y. H., H. G. Rolan, and R. M. Tsolis. 2007. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J. Biol. Chem. 28233897-33901. [DOI] [PubMed] [Google Scholar]
- 30.Sundquist, M., and M. J. Wick. 2009. Salmonella induces death of CD8α+ dendritic cells but not CD11cintCD11b+ inflammatory cells in vivo via MyD88 and TNFR1. J. Leukoc. Biol. 85225-234. [DOI] [PubMed] [Google Scholar]
- 31.Tam, M. A., A. Rydstrom, M. Sundquist, and M. J. Wick. 2008. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol. Rev. 225140-162. [DOI] [PubMed] [Google Scholar]
- 32.Tsolis, R. M., R. A. Kingsley, S. M. Townsend, T. A. Ficht, L. G. Adams, and A. J. Baumler. 1999. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 473261-274. [PubMed] [Google Scholar]
- 33.Uematsu, S., M. H. Jang, N. Chevrier, Z. Guo, Y. Kumagai, M. Yamamoto, H. Kato, N. Sougawa, H. Matsui, H. Kuwata, H. Hemmi, C. Coban, T. Kawai, K. J. Ishii, O. Takeuchi, M. Miyasaka, K. Takeda, and S. Akira. 2006. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 7868-874. [DOI] [PubMed] [Google Scholar]
- 34.van der Velden, A. W., M. K. Copass, and M. N. Starnbach. 2005. Salmonella inhibit T cell proliferation by a direct, contact-dependent immunosuppressive effect. Proc. Natl. Acad. Sci. USA 10217769-17774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Velden, A. W., J. T. Dougherty, and M. N. Starnbach. 2008. Down-modulation of TCR expression by Salmonella enterica serovar Typhimurium. J. Immunol. 1805569-5574. [DOI] [PubMed] [Google Scholar]
- 36.van der Velden, A. W., M. Velasquez, and M. N. Starnbach. 2003. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J. Immunol. 1716742-6749. [DOI] [PubMed] [Google Scholar]
- 37.Vijay-Kumar, M., J. D. Aitken, and A. T. Gewirtz. 2008. Toll like receptor-5: protecting the gut from enteric microbes. Semin. Immunopathol. 3011-21. [DOI] [PubMed] [Google Scholar]
- 38.Vijay-Kumar, M., C. J. Sanders, R. T. Taylor, A. Kumar, J. D. Aitken, S. V. Sitaraman, A. S. Neish, S. Uematsu, S. Akira, I. R. Williams, and A. T. Gewirtz. 2007. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Investig. 1173909-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijay-Kumar, M., H. Wu, R. Jones, G. Grant, B. Babbin, T. P. King, D. Kelly, A. T. Gewirtz, and A. S. Neish. 2006. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am. J. Pathol. 1691686-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter, S. E., P. Thiennimitr, S. P. Nuccio, T. Haneda, M. G. Winter, R. P. Wilson, J. M. Russell, T. Henry, Q. T. Tran, S. D. Lawhon, G. Gomez, C. L. Bevins, H. Russmann, D. M. Monack, L. G. Adams, and A. J. Baumler. 2009. Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype Typhimurium infection. Infect. Immun. 771904-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng, H., H. Wu, V. Sloane, R. Jones, Y. Yu, P. Lin, A. T. Gewirtz, and A. S. Neish. 2006. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 290G96-G108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5201-204. [DOI] [PubMed] [Google Scholar]