Abstract
The underlying mechanisms of the epidemiological association between influenza virus infections and Neisseria meningitidis invasive infections are not fully understood. Here we report that adhesion of N. meningitidis to human Hec-1-B epithelial cells is enhanced by influenza A virus (IAV) infection. A potential role of the viral neuraminidase (NA) in facilitating meningococcal adhesion to influenza virus-infected epithelial cells was examined. Expression of a recombinant IAV NA in Hec-1-B human epithelial cells increased the adhesion of strains of N. meningitidis belonging to the sialic acid-containing capsular serogroups B, C, and W135 but not to the mannosamine phosphate-containing capsular serogroup A. Adhesion enhancement was not observed with an inactive NA mutant or in the presence of an NA inhibitor (zanamivir). Furthermore, purified IAV NA was shown to cleave sialic acid-containing capsular polysaccharides of N. meningitidis. On the whole, our findings suggest that a direct interaction between the NA of IAV and the capsule of N. meningitidis enhances bacterial adhesion to cultured epithelial cells, most likely through cleavage of capsular sialic acid-containing polysaccharides. A better understanding of the association between IAV and invasive meningococcal infections should help to set up improved control strategies against these seasonal dual viral-bacterial infections.
Neisseria meningitidis is commonly found in the human naso-oropharynx, among other commensal bacterial species. Asymptomatic carriers represent about 10% of the population (41). In Europe and North America, cases of invasive infection leading to meningococcal disease (MD), mainly septicemia and meningitis, occur sporadically. The annual incidence of MD varies between 0.3 and 4.35 per 100,000 inhabitants (36). Both bacterial virulence factors (32) and host susceptibility factors (34, 38) contribute to the development of invasive infections, but the exact mechanisms involved remain largely unknown. The serogroups of N. meningitidis, which are defined by the nature of the capsular polysaccharide, are distributed differently among carried and disease-associated isolates (42). Serogroups B and C (whose capsules are composed of polymers of sialic acids) and serogroups Y and W135 (whose capsules are composed of repeated units of sialic acid with d-glucose and d-galactose, respectively [4]) are prominent in MD in Europe and North America. Serogroup A, whose capsule is composed of α1,6-linked N-acetylmannosamine-1-phosphate (18), is most frequently involved in MD epidemics in Africa.
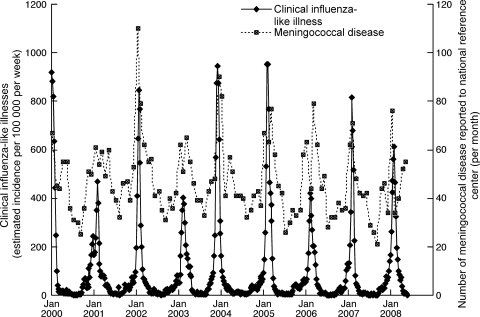
A number of clinical observations of MD occurring in patients with influenza have been reported (6, 13, 43). Epidemiological studies clearly showed a spatiotemporal association between influenza and N. meningitidis invasive infections (2, 14, 25). Recent data from the French National Reference Center for Meningococci and from the Sentinelles Network confirmed the overlap between the winter peaks of incidence of bacteriologically confirmed MD cases and influenza-like illnesses during the period from 2000 to 2008 (Fig. 1), in agreement with data published previously (14).
FIG. 1.
Invasive meningococcal infections and influenza-like illnesses recorded in France by the Reference Center for Meningococci and the Sentinelles Network from January 2000 to May 2008. Reporting of invasive meningococcal infections is mandatory. All invasive meningococcal isolates in France are sent to the National Reference Center for Meningococci for full characterization and typing. The general practitioners of the Sentinelles Network report on influenza-like illnesses on a weekly basis by sending patient deidentified data via the Internet to a GIS database (11). The monthly incidence of MD (right axis) and the weekly incidence of influenza-like illnesses (left axis) during the period of January 2000 to May 2008 are represented on the same graph.
The mechanisms by which influenza virus infection may favor bacterial superinfection with N. meningitidis, as well as with staphylococci, pneumococci, streptococci, and Haemophilus influenzae, have been investigated using cellular and animal models (15, 19, 22, 33). Virus-induced immune dysregulation, such as impairment of phagocytic functions or alterations in the production of cytokines, can be involved (12). In mice convalescing from influenza A virus (IAV) infection, the production of interleukin-10 in the lungs enhanced susceptibility to meningococcal (3) and to pneumococcal (37) superinfection. Influenza virus infection can also enhance bacterial adhesion by disrupting the respiratory epithelium or by increasing the accessibility or expression of membrane receptors (26). Conflicting results about the effects of influenza virus infection on meningococcal adherence to epithelial cells have been reported. One study reported that meningococci bind IAV-infected epithelial cells more efficiently than uninfected cells (10), whereas another concluded that coinfection with influenza B virus does not affect the association of meningococci with cultured human nasopharyngeal mucosa (29). Adhesion to epithelial cells in the nasopharynx is the early step enabling N. meningitidis to colonize the upper respiratory tract before it possibly goes through the epithelial barrier into the blood to induce bacteremia and reaches the meningeal spaces to induce meningitis. The adhesion process is associated with a downregulation of the capsule (9). We hypothesized that sialic acid-containing capsules could be a substrate for the neuraminidase (NA) of IAV and that this direct virus-bacterium interaction could play a major role in enhancing meningococcal adhesion to the respiratory epithelium. This hypothesis was tested on isolates belonging to various serogroups of N. meningitidis, using an in vitro model of adhesion to epithelial cells transiently expressing IAV-derived recombinant NAs.
MATERIALS AND METHODS
Cells, viruses, and infection protocol.
Hec-1-B cells (a human endometrial carcinoma epithelial cell line) were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin. For N. meningitidis adhesion assays, Hec-1-B cells were plated in the absence of antibiotics. Influenza virus A/WSN/33 (H1N1) was kindly provided by P. Palese (Mount Sinai Hospital, New York, NY). Influenza viruses A/Paris/2165/2000 (H1N1) and A/Paris/908/97 (H3N2) were isolated by the National Influenza Centre (France) and amplified by three serial passages on MDCK cells. Confluent monolayers of Hec-1-B cells in 12-well plates were incubated with 400 μl of viral suspension for 1 h at 35°C with gentle shaking. One milliliter of RPMI 1640 medium supplemented with 2% FCS was added. Cells were further incubated at 35°C for 5 h and then assayed for NA expression, NA activity, and N. meningitidis adhesion.
Plasmids and transfection protocol.
The sequences encoding the NAs of the A/Paris/650/2004 (H1N1) and A/Paris/908/97 (H3N2) viruses were amplified from the viral RNAs by reverse transcription-PCR and cloned into the pCI expression vector (Promega) as described earlier (28). The A/Paris/650/2004 isolate is closely related to the A/Paris/2165/2000 isolate (>99% protein homology for NA). Two independent plasmidic clones were selected for each of the corresponding pCI-N1-650 and pCI-N2-908 constructs. The Y406F mutation was introduced into the pCI-N1-650 plasmid by site-directed mutagenesis, using a QuikChange site-directed mutagenesis kit (Stratagene). All constructs were verified by sequencing using a BigDye Terminator sequencing kit and an automated sequencer (Perkin Elmer). Subconfluent Hec-1-B cells in 12-well plates were transfected with 2 μg of the pCI-NA expression vectors, using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's recommendations. At 24 h posttransfection, cells were assayed for NA expression, NA activity, and N. meningitidis adhesion.
Assays for NA expression and activity.
The presence of the viral NA at the surfaces of infected or transfected Hec-1-B cells was detected by an indirect immunofluorescence assay, using rabbit polyclonal antibodies against the A/New Caledonia/20/99 (H1N1) or A/Wisconsin/67/2005 (H3N2) virus and a FACSCalibur fluorocytometer (Becton Dickinson), as described previously (28).
A fluorimetric enzymatic assay was used to measure the sialidase activity in suspensions of Hec-1-B cells transiently expressing NA, as described previously (28). Briefly, the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA; Sigma) was added, at final concentrations ranging from 5 to 100 μM, to cells resuspended in MES buffer (33 mM morpholineethanesulfonic acid, pH 6.5, 120 mM NaCl, 4 mM CaCl2) in black 96-well plates at 37°C, and fluorescence was monitored every 45 s (excitation wavelength, 330 nm; and emission wavelength, 450 nm). The kinetic parameters Vmax and Km were calculated by fitting the data to the appropriate Michaelis-Menten equations, using the Levenberg-Marquardt algorithm as provided in the commercially available KaleidaGraph software package (Synergy Software).
N. meningitidis strains and adhesion assay.
N. meningitidis strains LNP20790, LNP20342, LNP8013 clone 12, and LNP19995 are clinical isolates of serogroups A, B, C, and W135, respectively, that were received at the National Reference Center for Meningococci. Strain SFCT7 is a capsule-deficient mutant of LNP8013 clone 12 obtained by insertional inactivation of the ctrA gene (44). Meningococci were grown on GCB medium (Difco) containing Kellogg supplements (17).
In order to infect monolayers of Hec-1-B cells previously infected with an IAV strain or transfected with a pCI-NA plasmid, N. meningitidis inocula were adjusted to a concentration of 5 × 106 CFU/ml in RPMI 1640 medium supplemented with 10% FCS and added to the cells at a multiplicity of infection (MOI) of 10 CFU per cell. After 4 h of incubation at 37°C, the supernatants were collected. Five successive washes of the cells were performed, and the washes were pooled together with the corresponding supernatant for bacterial counts. Hec-1-B cells and cell-associated bacteria were lifted off the plates and resuspended in 0.5 ml of RPMI 1640 medium supplemented with 10% FCS. The numbers of bacteria in the supernatant and in the cell-associated fraction were determined using a Petroff-Hausser counting chamber. Bacterial viability was controlled by plating bacteria on GCB medium. No significant proportion of dead bacteria was observed in the adherent fraction collected after a 4-hour incubation with NA-expressing cells (data not shown).
The level of adhesion was defined as the ratio of the number of cell-associated bacteria to the number of bacteria in the supernatant. When indicated, zanamivir (Glaxo Smith Kline) was added to the bacterial suspension at a final concentration of 0.5 μM upon infection. Bacterial quantification was also achieved by measuring the number of meningococcal genomes by real-time PCR, using a set of previously described primers (35) and LightCycler technology (Roche) according to the manufacturer's recommendations. For each meningococcal serogroup, a standard curve was obtained using 10-fold serial dilutions, from 109 to 103 bacteria/ml, of the appropriate meningococcal suspension.
Meningococcal adhesion was also assessed by an indirect immunofluorescence assay. Cells were fixed with phosphate-buffered saline-4% paraformaldehyde for 20 min and then incubated with a mixture of a rabbit polyclonal antibody against the A/New Caledonia/20/99 (H1N1) influenza virus and an anti-PorA mouse monoclonal antibody directed against the major outer membrane porin of N. meningitidis (NIBSC, United Kingdom), diluted 1/300 and 1/200, respectively. After subsequent incubation with a mixture of a fluorescein-coupled anti-mouse immunoglobulin G (IgG) (diluted 1/200; Pharmingen) and an Alexa Fluor 555-coupled anti-rabbit IgG (diluted 1/200; Molecular Probes) secondary antibody, the samples were analyzed under a fluorescence microscope (Leica).
Exposure of N. meningitidis to purified IAV NA.
The NA purified from the X31 reassortant virus A/Aichi/2/68 (H3 and N2 segments) × A/PR/8/34 (remaining segments) (30) was kindly provided by J. Skehel (MRC, National Institute for Medical Research, Mill Hill, United Kingdom). The sialidase activity of the purified NA preparation was determined using the fluorigenic substrate MUNANA as described above. One unit of purified NA is defined as the amount of enzyme liberating 1 μmol of methyl-umbelliferone per min at pH 6.5 and at 37°C.
Capsule hydrolysis by the NA was tested by enzyme-linked immunosorbent assay (ELISA). Four meningococcal strains, LNP20790, LNP20342, LNP8013 clone 12, and LNP19995 (serogroups A, B, C, and W135, respectively), were suspended in MES buffer, pH 6.5 (33 mM MES, 120 mM NaCl, and 4 mM CaCl2), at a density of 108 CFU/ml in the absence or presence of various concentrations of NA (0.125 to 0.0315 U/ml). After 4 h of incubation at 37°C, bacteria were centrifuged, and 100 μl of each supernatant was used to coat a 96-well ELISA plate, which was dried and washed before incubation with serogroup-specific antibodies as previously described (1).
RESULTS
Increased adhesion of N. meningitidis to IAV-infected epithelial cells.
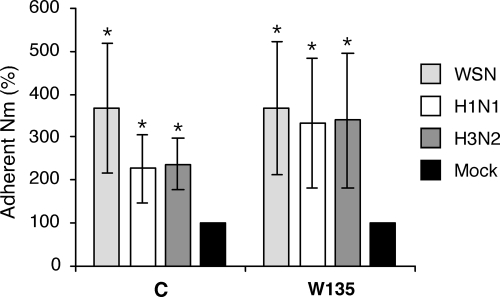
We first tested whether the level of adhesion of N. meningitidis to Hec-1-B epithelial cells was altered if the cells were previously infected by IAV. Subconfluent monolayers of Hec-1-B cells were either mock infected or infected at an MOI of 5 to 10 PFU/cell with the IAV strain A/WSN/33 (H1N1) or with the recent human isolates A/Paris/2165/2000 (H1N1) and A/Paris/908/97 (H3N2). At 5 h postinfection, cells were washed and superinfected at an MOI of 10 CFU/cell with N. meningitidis serogroup C strain LNP8013 clone 12 or the serogroup W135 strain LNP19995. After 4 h of incubation at 37°C, the levels of adhesion of both strains of N. meningitidis to Hec-1-B cells significantly increased on IAV-infected cells compared to those on mock-infected cells (P < 0.05) (Fig. 2). For a given strain of N. meningitidis, the levels of adhesion on Hec-1-B cells infected with each of the three IAV strains mentioned above were not significantly different.
FIG. 2.
Levels of adhesion of N. meningitidis (Nm) on IAV-infected epithelial cells. Hec-1-B cells were infected at an MOI of 5 to 10 PFU/cell with the A/WSN/33 (WSN), A/Paris/650/2004 (H1N1), or A/Paris/908/97 (H3N2) IAV or were mock infected. At 5 h postinfection, wild-type N. meningitidis strains of serogroup C and W135 were added to the cells at an MOI of 10 CFU/cell. After 4 h of incubation at 37°C, the levels of adhesion of N. meningitidis to Hec-1-B cells were determined as described in Materials and Methods. The levels of adhesion measured on cells infected with WSN (light gray bars), H1N1 (white bars), and H3N2 (dark gray bars) viruses are expressed as percentages of the level measured on mock-infected cells (black bars). The results are expressed as means ± standard deviations for three (two of which were performed in duplicate) and four (three of which were performed in duplicate) independent experiments for the serogroup C and W135 strains, respectively. Asterisks indicate P values of <0.05 (Student's t test).
Increased adhesion of N. meningitidis to epithelial cells transiently expressing recombinant IAV NA.
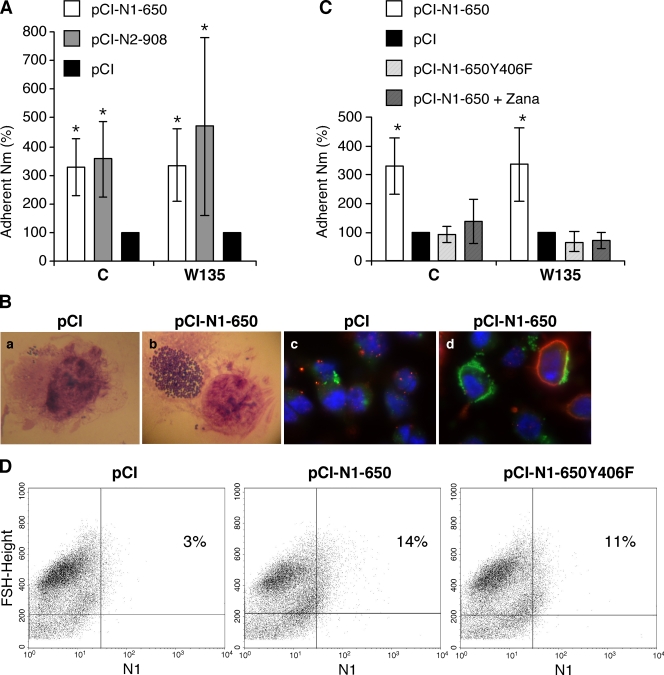
The contribution of viral NA to the enhancement of N. meningitidis adhesion to IAV-infected cells was examined. Hec-1-B cells were transfected with plasmids allowing the expression of the NAs derived from the A/Paris/650/2004 (H1N1) and A/Paris/908/97 (H3N2) viruses (plasmids pCI-N1-650 and pCI-N2-P908, respectively) or mock transfected with the pCI plasmid. At 48 h posttransfection, a fraction of the cells was used to control the efficiency of transfection. Expression of the recombinant NAs was detected at the cell surface by an indirect immunofluorescence assay and fluorescence-activated cell sorter (FACS) analysis (Fig. 3D) or by measuring the sialidase activity of the NA as described in Materials and Methods (data not shown). Adhesion levels of both the LNP8013 clone 12 and LNP19995 strains of N. meningitidis appeared significantly increased on pCI-NA-transfected cells compared to mock-transfected cells (P < 0.05) (Fig. 3A). As observed under the microscope, clusters of bacteria associated with pCI-NA-transfected cells were larger than those associated with mock-transfected cells (Fig. 3B, panels a and b) and were more frequent (data not shown). Notably, an immunofluorescence assay using antibodies directed against influenza virus NA and against the porin of N. meningitidis showed that large clusters of bacteria were associated with NA-expressing transfected cells but also with the surfaces of neighboring cells with undetectable expression of the NA (Fig. 3B, panel d). Such cell surface association of N. meningitidis was not observed on the mock-transfected cells (Fig. 3B, panel c). Cell-associated bacteria were mainly extracellular, as indicated by the very low percentage of viable bacteria (<0.1%) evaluated after incubation with gentamicin (100 mg/liter) for 1 h (data not shown).
FIG. 3.
Levels of adhesion of N. meningitidis (Nm) on epithelial cells transiently expressing a recombinant IAV NA. Hec-1-B cells were transfected with the pCI-N1-650 and pCI-N2-908 plasmids, allowing the expression of the NAs derived from the A/Paris/650/2004 and A/Paris/908/97 IAVs, respectively (A and B), with the pCI-N1-650 plasmid in the absence or presence of 0.5 μM of the NA inhibitor zanamivir (pCI-N1-650 + Zana) (C), or with plasmid pCI-N1-650-Y406F, allowing the expression of an inactive mutant NA (C and D), or they were mock transfected with the pCI plasmid (A to D). (A to C) At 48 h posttransfection, wild-type N. meningitidis strains of serogroups C and W135 were added to the cells at an MOI of 10 CFU/cell. (A and C) After 4 h of incubation at 37°C, the levels of adhesion of N. meningitidis to Hec-1-B cells were determined as described in Materials and Methods. The levels of adhesion are expressed as percentages (100% represents the levels measured on mock-infected cells [black bars]). The results are expressed as means ± standard deviations for four (pCI-N1-650 and pCI) or two (pCI-N2-908, pCI-N1-650-Y406F, and pCI-N1-650 + Zana) independent experiments performed in duplicate. Asterisks indicate P values of <0.05 (Student's t test). (B) Hec-1-B cells transfected with the indicated plasmids and cell-associated bacteria were observed under a microscope before the bacteria were lifted off the plates for quantification of the adhesion level (a and b). Alternatively, cells were fixed and incubated with a mixture of a rabbit polyclonal antibody against the A/New Caledonia/20/99 (H1N1) influenza virus and an anti-PorA mouse monoclonal antibody for the detection of the viral NA (red staining) and the meningococci (green staining) by an indirect immunofluorescence assay (c and d). (D) A fraction of Hec-1-B cells transfected with the indicated plasmids was analyzed by flow cytometry for surface expression of the recombinant NA, using a rabbit polyclonal antibody directed against the A/New Caledonia/20/99 (H1N1) influenza virus. The percentages of NA-positive stained cells are indicated.
We then tested whether the increase in meningococcal adhesion was related to the enzymatic activity of the viral NA. Hec-1-B cells were transfected with the pCI-N1-650 plasmid and incubated in the presence or absence of 0.5 μM of zanamivir, a competitive inhibitor of influenza virus NAs. In parallel, Hec-1-B cells were transfected with the pCI-N1-650Y406F plasmid, encoding a mutant NA with no detectable sialidase activity. Again, the adhesion levels of the LNP8013 clone 12 and LNP19995 strains of N. meningitidis were significantly increased on pCI-N1-650- compared to pCI-transfected cells incubated in the absence of zanamivir (Fig. 3C). However, in the presence of zanamivir or on cells expressing the inactive Y406F mutant NA, the levels of adhesion were in the same range as those measured on pCI-transfected control cells (Fig. 3C). The Y406F mutant NA was found by FACS analysis to be expressed at the cell surface at similar levels to those of the wild-type NA (Fig. 3D). These findings were confirmed when a quantitative PCR assay specific for the crgA meningococcal gene (35) was used instead of a Petroff-Hausser counting chamber to determine the numbers of LNP19995 bacteria present in the supernatant and in the cell-associated fraction (Table 1).
TABLE 1.
Real-time PCR-based determination of levels of adhesion of N. meningitidis strain LNP19995 (serogroup W135) to epithelial cells transiently expressing a recombinant IAV NA
| Exptl group | Adhesion level (%)d | P valuee |
|---|---|---|
| pCI-transfected cells | 10.3 ± 3.7 | |
| pCI-N1-650-transfected cellsa | 28.3 ± 10.9 | <0.02 |
| pCI-N1-650-Y406F-transfected cellsb | 10.5 ± 2.4 | NS |
| pCI-N1-650-transfected cells treated with zanamivirc | 11.0 ± 1.4 | NS |
pCI-N1-650-transfected cells transiently expressed the NA derived from the A/Paris/650/2004 virus.
pCI-N1-650-Y406F-transfected cells transiently expressed an A/Paris/650/2004-derived mutant NA with no detectable sialidase activity.
Cells transiently expressing the NA derived from the A/Paris/650/2004 virus were incubated in the presence of 0.5 μM zanamivir.
The level of adhesion was defined as the ratio of the number of cell-associated bacterial genomes to the number of bacterial genomes in the supernatant, as determined using real-time PCR. Results are given as means ± standard deviations for four independent determinations.
Adhesion levels on pCI- and pCI-N1-650- or pCI-N1-650-Y406F-transfected cells were compared using paired Student's t test. NS, nonsignificant.
Overall, our results suggest that the sialidase activity of the NA expressed at the cell surface plays a major role in promoting the adhesion of N. meningitidis strains of serogroups C and W135 to pCI-NA-transfected cells.
Evidence for direct action of IAV NA on polysialic acid-containing capsules of N. meningitidis.
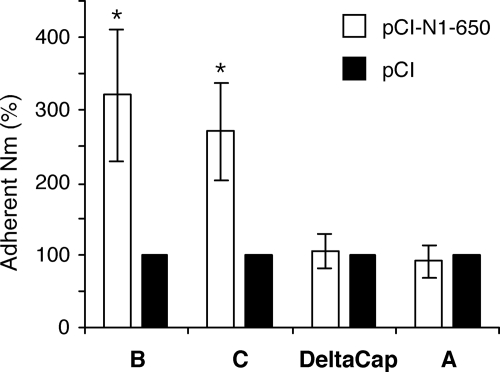
The N. meningitidis strains of serogroups C and W135 used in our initial set of experiments are characterized by a capsule which is composed of polysialic acids. The same type of experiment was performed in the presence of a strain of serogroup B (LNP20342) whose capsule is also made of polysialic acids and a strain of serogroup A (LNP20790) whose capsule is made of (α1,6)-linked N-acetylmannosamine 1 phosphate. In addition, a capsule-deficient mutant of LNP8013 clone 12 (DeltaCap) (44) was tested. Increased levels of adhesion were measured for the serogroup B strain as well as for the serogroup C strain on pCI-N1-650-transfected Hec-1-B cells compared to mock-transfected cells (Fig. 4). In contrast, the levels of adhesion measured for the serogroup A and DeltaCap strains were not increased on cells transfected with the NA-encoding plasmid pCI-N1-650 (Fig. 4).
FIG. 4.
Levels of adhesion of different serogroups of N. meningitidis (Nm) on epithelial cells transiently expressing a recombinant IAV NA. Hec-1-B cells were transfected with the pCI-N1-650 plasmid or mock transfected with the pCI plasmid. At 48 h posttransfection, N. meningitidis strains of serogroups A, B, and C, including a capsule-deficient mutant of the serogroup C strain (DeltaCap), were added to the cells at an MOI of 10 CFU/cell. After 4 h of incubation at 37°C, the levels of adhesion of N. meningitidis to Hec-1-B cells were determined as described in Materials and Methods. For a given strain of N. meningitidis, the level of adhesion measured on cells transfected with the pCI-N1-650 plasmid (white bars) is expressed as the percentage of the level measured on mock-transfected cells (black bars). The results are expressed as means ± standard deviations for three (serogroup B) or four (serogroups A and C and strain DeltaCap) independent experiments performed in duplicate. Asterisks indicate P values of <0.05 (Student's t test).
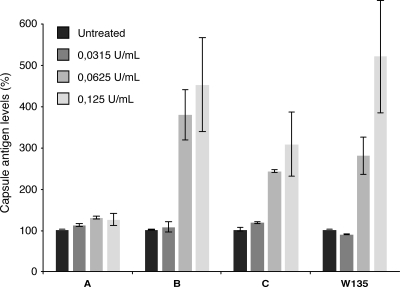
These observations prompted us to test whether the viral NA could exert a direct action on N. meningitidis capsules consisting of polysialic acids. The LNP20790, LNP20342, LNP8013 clone 12, and LNP19995 strains (108 bacteria per assay) were incubated for 4 h at 37°C with 0.125, 0.0625, or 0.0315 U/ml of NA purified from influenza virus strain X31 (kindly provided by J. Skehel). The solubilized capsule antigens were detected by an ELISA using serogroup-specific monoclonal antibodies, as described in Materials and Methods. Incubation with purified NA resulted in a dose-dependent increase in the amount of free capsular antigens released from strains of serogroups B and C but not from the serogroup A strain (Fig. 5). The release of capsular antigens was dependent on NA sialidase activity, as it was not observed in the presence of 1 mM zanamivir (data not shown).
FIG. 5.
Activity of purified IAV NA on N. meningitidis capsules. A total of 108 N. meningitidis bacteria of serogroup A, B, C, or W135 were incubated for 4 h at 37°C with various concentrations of NA purified from the IAV strain X31, as indicated. The solubilized capsule antigens were detected by an ELISA using serogroup-specific antibodies, as described in Materials and Methods. For a given strain of N. meningitidis, the levels of capsular antigens measured in NA-treated samples (gray bars) are expressed as percentages of the level measured in mock-treated samples (black bars). The results are expressed as means and standard deviations for duplicates and are representative of one of two (serogroup A), two (serogroup B), six (serogroup C), and three (serogroup W135) independent experiments.
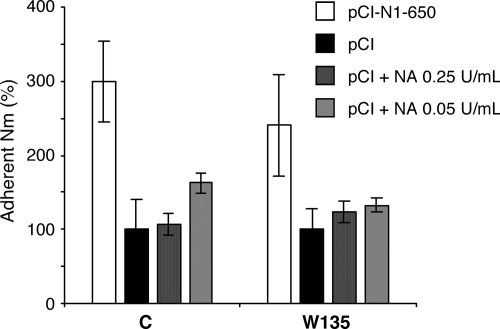
Finally, we checked whether the increased adhesion of N. meningitidis to epithelial cells transiently expressing the viral NA could be mediated by an effect of the enzyme on the cell surface, as previously reported for the pneumococci in a mouse model (21). To this end, Hec-1-B cells mock transfected with the pCI plasmid were incubated with 0.05 or 0.25 U/ml of purified NA for 1.5 h at 37°C. As monitored by flow cytometry analysis using the specific Maackia amurensis and Sambucus nigra lectins (23), the NA-treated cells expressed lower levels of α2,3- and α2,6-linked sialic acids, respectively, than the control cells (data not shown). After extensive washing of the cells, the adhesion of N. meningitidis strains LNP8013 clone 12 and LNP19995 was assayed as described above. The levels of adhesion of both strains on NA-treated cells were not increased compared to those on untreated cells, whereas they were increased on the control cells transfected with the pCI-N1-650 plasmid (Fig. 6).
FIG. 6.
Levels of adhesion of N. meningitidis (Nm) on epithelial cells pretreated with purified IAV NA. Hec-1-B cells were transfected with the pCI-N1-650 or pCI plasmid. The pCI-transfected cells were incubated with 0.05 or 0.25 U/ml of purified NA for 1 h 30 min at 37°C (pCI + NA, as indicated) or were mock treated (pCI). After four washes, wild-type N. meningitidis strains of serogroups C and W135 were added to the cells at an MOI of 10 bacteria per cell. After 4 h of incubation at 37°C, the levels of adhesion of N. meningitidis to Hec-1-B cells were determined as described in Materials and Methods. The levels of adhesion measured on pCI-N1-650-transfected cells (white bars) and on NA-treated cells (gray bars) are expressed as percentages of the levels measured on mock-treated cells (black bars). The results are expressed as the means of duplicates in a single experiment.
DISCUSSION
Adhesion of meningococci to the upper respiratory tract epithelium is the first step of the pathophysiological process which leads to asymptomatic carriage, and possibly to further invasion and dissemination via blood (38). Meningococcal adhesion to target epithelial cells is a highly regulated mechanism. Initial contact between bacteria and the epithelial cell is followed by downregulation of expression of type 4 pili and of the capsule to allow so-called “intimate adhesion” between bacteria and target cells (9).
Here we show that a direct interaction between the NAs of IAVs and the capsule of N. meningitidis can enhance bacterial adhesion to cultured epithelial cells. Influenza virus NA was previously shown by McCullers and colleagues to increase the sensitivity to pneumococcal infection (20, 21, 27). In this case, the NA seemed to exert its effect on the target cells, possibly by stripping sialic acids and thus unmasking receptors at the cell surface (21). We found no evidence for any effect of cell surface pretreatment with purified NA on meningococcal adhesion. This could result from insufficient digestion of sialic acids at the cell surface. In our experiments, the recombinant viral NA was expressed at the surfaces of transfected epithelial cells, as indicated by FACS analysis and immunofluorescence assays. Meningococci were associated not only with the surfaces of NA-expressing cells but also with some neighboring cells with no detectable NA at the cell surface. This observation suggested that the overall increased adhesion levels of meningococci on cells transfected with the NA expression vector were unlikely to be due to an NA-mediated bridging between the cell surface and the sialic acid-containing meningococci. On the other hand, no increase in adhesion was observed on cells transiently expressing an inactive NA mutant or in the presence of an NA inhibitor. Taken together, these data suggest that rather than acting as a cellular receptor or mediating attachment to other cell surface ligands, the viral NA enhances N. meningitidis adhesion by another mechanism. The proadhesion effect of influenza virus NA was dependent on its enzymatic activity and on the nature of the capsular polysaccharide of N. meningitidis. Altogether, our observations strongly suggest a direct enzymatic action of the NA on the sialic acid capsules of serogroups B, C, and W135, which consist of α2,8-, α2,9-, and α2,6-linked sialic acids, respectively. The most common substrates of IAV NA are α2,6 (as in the serogroup W135 capsule)- and α2,3-linked sialic acids (24), although cleavage of α2,8-linked sialic acids (as in the serogroup B capsule) has also been reported (7). To our knowledge, there are no available data on NA recognition of α2,9-linked sialic acids, which are typical of serogroup C capsules. However, molecular modeling studies showed that the active site of influenza virus N9 NA has enough space to accommodate substrates bearing the four types of glycosidic linkages (39). Whatever the serogroup, the exosialidase activity of the viral NA is unlikely to lead to a complete disruption of the capsule. More probably, enhanced adherence of meningococci to epithelial cells as a result of the hydrolysis of sialic acid-containing capsules by the viral NA could correspond to the unmasking of subcapsular bacterial factors which interact with cell membrane receptors (2). Viral NA expressed at the peak of infection and/or residual NA present at the respiratory epithelium surface after the peak of infection could be involved.
Our findings provide for the first time a mechanistic clue to the ancient observation of an association between influenza virus infection and invasive meningococcal infections. They do not exclude the possibility that additional molecular mechanisms, such as influenza virus-mediated upregulation of a cellular receptor for N. meningitidis, hemagglutinin-mediated binding of bacterial sialic acids, or NA-mediated stripping of cellular sialic acids, also contribute to increased bacterial adhesion. Cellular receptors reported for meningococcal proteins include CD46, a human transmembrane glycoprotein involved in complement regulation (16), and the CD66 molecule, which could mediate interactions between Opa-expressing bacteria and human cells (40). The impact of influenza virus infection on the expression and accessibility of these receptors remains to be addressed. Less specific mechanisms could also contribute to enhanced adhesion of N. meningitidis. Increased concentrations of fibrinogen have been shown to increase the adherence of Staphylococcus aureus (8) and group A streptococcus (31) to IAV-infected cell cultures by docking the bacterial capsule to the cell surface. Hyperemia and edema occurring at the site of influenza virus infections could lead to locally increased fibrinogen concentrations in vivo. Immunological factors are also likely to contribute to the association between IAV and N. meningitidis infections, as indicated by experiments performed with an animal model of sequential IAV and N. meningitidis infections (3). They could represent the underlying mechanism for the observed lag of about 7 days between the peaks of IAV and N. meningitidis infections (14).
Our observations suggest a direct interaction between the viral NA and the bacterial capsule. Such a direct interaction between IAV and bacteria has not been described previously and may contribute to the association between IAV infection and meningococcal disease. IAVs represent a major global threat. Not only can IAVs be highly pathogenic by themselves, but they also can induce bacterial, cellular, and immune alterations which favor a range of invasive infections by otherwise commensal bacteria of the respiratory tract, including meningococci. Recent records on fatality rates during the influenza pandemics, particularly the 1918 pandemic, underscore the large impact of bacterial superinfections (5). Reducing the burden of influenza by vaccination and by the use of NA inhibitors could certainly help to prevent a significant proportion of these dual viral-bacterial syndromes.
Acknowledgments
We are grateful to J. Skehel (MRC, National Institute for Medical Research, Mill Hill, United Kingdom) for providing purified NA and for useful comments on the manuscript. We thank N. Escriou for providing polyclonal antibodies specific for the A/Wisconsin/67/2005 virus.
M.A.R.W. was supported by a fellowship from the Institut Pasteur.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 15 June 2009.
REFERENCES
- 1.Abdillahi, H., and J. T. Poolman. 1988. Typing of group-B Neisseria meningitidis with monoclonal antibodies in the whole-cell ELISA. J. Med. Microbiol. 26177-180. [PubMed] [Google Scholar]
- 2.Abramson, J. S. 1988. The pathogenesis of bacterial infections in infants and children: the role of viruses. Perspect. Biol. Med. 3263-72. [DOI] [PubMed] [Google Scholar]
- 3.Alonso, J. M., A. Guiyoule, M. L. Zarantonelli, F. Ramisse, R. Pires, A. Antignac, A. E. Deghmane, M. Huerre, S. van der Werf, and M. K. Taha. 2003. A model of meningococcal bacteremia after respiratory superinfection in influenza A virus-infected mice. FEMS Microbiol. Lett. 22299-106. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee, A. K., H. J. Jennings, C. P. Kenny, A. Martin, and I. C. Smith. 1976. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can. J. Biochem. 541-8. [DOI] [PubMed] [Google Scholar]
- 5.Brundage, J. F. 2006. Cases and deaths during influenza pandemics in the United States. Am. J. Prev. Med. 31252-256. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright, K. A., D. M. Jones, A. J. Smith, J. M. Stuart, E. B. Kaczmarski, and S. R. Palmer. 1991. Influenza A and meningococcal disease. Lancet 338554-557. [DOI] [PubMed] [Google Scholar]
- 7.Corfield, A. P., M. Wember, R. Schauer, and R. Rott. 1982. The specificity of viral sialidases. The use of oligosaccharide substrates to probe enzymic characteristics and strain-specific differences. Eur. J. Biochem. 124521-525. [PubMed] [Google Scholar]
- 8.Davison, V. E., and B. A. Sanford. 1982. Factors influencing adherence of Staphylococcus aureus to influenza A virus-infected cell cultures. Infect. Immun. 37946-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deghmane, A. E., D. Giorgini, M. Larribe, J. M. Alonso, and M. K. Taha. 2002. Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol. Microbiol. 431555-1564. [DOI] [PubMed] [Google Scholar]
- 10.El Ahmer, O. R., M. W. Raza, M. M. Ogilvie, D. M. Weir, and C. C. Blackwell. 1999. Binding of bacteria to HEp-2 cells infected with influenza A virus. FEMS Immunol. Med. Microbiol. 23331-341. [DOI] [PubMed] [Google Scholar]
- 11.Flahault, A., T. Blanchon, Y. Dorleans, L. Toubiana, J. F. Vibert, and A. J. Valleron. 2006. Virtual surveillance of communicable diseases: a 20-year experience in France. Stat. Methods Med. Res. 15413-421. [DOI] [PubMed] [Google Scholar]
- 12.Hament, J. M., J. L. Kimpen, A. Fleer, and T. F. Wolfs. 1999. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol. Med. Microbiol. 26189-195. [DOI] [PubMed] [Google Scholar]
- 13.Harrison, L. H., C. W. Armstrong, S. R. Jenkins, M. W. Harmon, G. W. Ajello, G. B. Miller, Jr., and C. V. Broome. 1991. A cluster of meningococcal disease on a school bus following epidemic influenza. Arch. Intern. Med. 1511005-1009. [PubMed] [Google Scholar]
- 14.Hubert, B., L. Watier, P. Garnerin, and S. Richardson. 1992. Meningococcal disease and influenza-like syndrome: a new approach to an old question. J. Infect. Dis. 166542-545. [DOI] [PubMed] [Google Scholar]
- 15.Jakab, G. J., G. A. Warr, and M. E. Knight. 1979. Pulmonary and systemic defenses against challenge with Staphylococcus aureus in mice with pneumonia due to influenza A virus. J. Infect. Dis. 140105-108. [DOI] [PubMed] [Google Scholar]
- 16.Kallstrom, H., M. K. Liszewski, J. P. Atkinson, and A. B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25639-647. [DOI] [PubMed] [Google Scholar]
- 17.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and D. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 851274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, T. Y., E. C. Gotschlich, F. T. Dunne, and E. K. Jonssen. 1971. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J. Biol. Chem. 2464703-4712. [PubMed] [Google Scholar]
- 19.Mahmud, M. I., R. Jennings, and C. W. Potter. 1979. The infant rat as a model for assessment of the attenuation of human influenza viruses. J. Med. Microbiol. 1243-54. [DOI] [PubMed] [Google Scholar]
- 20.McCullers, J. A. 2006. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullers, J. A., and K. C. Bartmess. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 1871000-1009. [DOI] [PubMed] [Google Scholar]
- 22.McCullers, J. A., and J. E. Rehg. 2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186341-350. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros, R., N. Escriou, N. Naffakh, J. C. Manuguerra, and S. van der Werf. 2001. Hemagglutinin residues of recent human A(H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes. Virology 28974-85. [DOI] [PubMed] [Google Scholar]
- 24.Mochalova, L., V. Kurova, Y. Shtyrya, E. Korchagina, A. Gambaryan, I. Belyanchikov, and N. Bovin. 2007. Oligosaccharide specificity of influenza H1N1 virus neuraminidases. Arch. Virol. 1522047-2057. [DOI] [PubMed] [Google Scholar]
- 25.Moore, P. S., J. Hierholzer, W. DeWitt, K. Gouan, D. Djore, T. Lippeveld, B. Plikaytis, and C. V. Broome. 1990. Respiratory viruses and mycoplasma as cofactors for epidemic group A meningococcal meningitis. JAMA 2641271-1275. [PubMed] [Google Scholar]
- 26.Peltola, V. T., and J. A. McCullers. 2004. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr. Infect. Dis. J. 23S87-S97. [DOI] [PubMed] [Google Scholar]
- 27.Peltola, V. T., K. G. Murti, and J. A. McCullers. 2005. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 192249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rameix-Welti, M. A., F. Agou, P. Buchy, S. Mardy, J. T. Aubin, M. Veron, S. van der Werf, and N. Naffakh. 2006. Natural variation can significantly alter the sensitivity of influenza A (H5N1) viruses to oseltamivir. Antimicrob. Agents Chemother. 503809-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read, R. C., L. Goodwin, M. A. Parsons, P. Silcocks, E. B. Kaczmarski, A. Parker, and T. J. Baldwin. 1999. Coinfection with influenza B virus does not affect association of Neisseria meningitidis with human nasopharyngeal mucosa in organ culture. Infect. Immun. 673082-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell, R. J., L. F. Haire, D. J. Stevens, P. J. Collins, Y. P. Lin, G. M. Blackburn, A. J. Hay, S. J. Gamblin, and J. J. Skehel. 2006. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 44345-49. [DOI] [PubMed] [Google Scholar]
- 31.Sanford, B. A., V. E. Davison, and M. A. Ramsay. 1982. Fibrinogen-mediated adherence of group A streptococcus to influenza A virus-infected cell cultures. Infect. Immun. 38513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoen, C., J. Blom, H. Claus, A. Schramm-Gluck, P. Brandt, T. Muller, A. Goesmann, B. Joseph, S. Konietzny, O. Kurzai, C. Schmitt, T. Friedrich, B. Linke, U. Vogel, and M. Frosch. 2008. Whole-genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 1053473-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki, M., Y. Higashiyama, K. Tomono, K. Yanagihara, H. Ohno, Y. Kaneko, K. Izumikawa, Y. Miyazaki, Y. Hirakata, Y. Mizuta, T. Tashiro, and S. Kohno. 2004. Acute infection with influenza virus enhances susceptibility to fatal pneumonia following Streptococcus pneumoniae infection in mice with chronic pulmonary colonization with Pseudomonas aeruginosa. Clin. Exp. Immunol. 13735-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparling, P. F. 2002. A plethora of host factors that determine the outcome of meningococcal infection. Am. J. Med. 11272-74. [DOI] [PubMed] [Google Scholar]
- 35.Taha, M. K., J. M. Alonso, M. Cafferkey, D. A. Caugant, S. C. Clarke, M. A. Diggle, A. Fox, M. Frosch, S. J. Gray, M. Guiver, S. Heuberger, J. Kalmusova, K. Kesanopoulos, A. M. Klem, P. Kriz, J. Marsh, P. Molling, K. Murphy, P. Olcen, O. Sanou, G. Tzanakaki, and U. Vogel. 2005. Interlaboratory comparison of PCR-based identification and genogrouping of Neisseria meningitidis. J. Clin. Microbiol. 43144-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trotter, C. L., M. Chandra, R. Cano, A. Larrauri, M. E. Ramsay, C. Brehony, K. A. Jolley, M. C. Maiden, S. Heuberger, and M. Frosch. 2007. A surveillance network for meningococcal disease in Europe. FEMS Microbiol. Rev. 3127-36. [DOI] [PubMed] [Google Scholar]
- 37.van der Sluijs, K. F., L. J. van Elden, M. Nijhuis, R. Schuurman, J. M. Pater, S. Florquin, M. Goldman, H. M. Jansen, R. Lutter, and T. van der Poll. 2004. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J. Immunol. 1727603-7609. [DOI] [PubMed] [Google Scholar]
- 38.van Deuren, M., P. Brandtzaeg, and J. W. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veluraja, K., M. X. Suresh, T. H. Christlet, and Z. A. Rafi. 2001. Molecular modeling of sialyloligosaccharide fragments into the active site of influenza virus N9 neuraminidase. J. Biomol. Struct. Dyn. 1933-45. [DOI] [PubMed] [Google Scholar]
- 40.Virji, M., K. Makepeace, D. J. Ferguson, and S. M. Watt. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol. Microbiol. 22941-950. [DOI] [PubMed] [Google Scholar]
- 41.Yazdankhah, S. P., and D. A. Caugant. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53821-832. [DOI] [PubMed] [Google Scholar]
- 42.Yazdankhah, S. P., P. Kriz, G. Tzanakaki, J. Kremastinou, J. Kalmusova, M. Musilek, T. Alvestad, K. A. Jolley, D. J. Wilson, N. D. McCarthy, D. A. Caugant, and M. C. Maiden. 2004. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J. Clin. Microbiol. 425146-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young, L. S., F. M. LaForce, J. J. Head, J. C. Feeley, and J. V. Bennett. 1972. A simultaneous outbreak of meningococcal and influenza infections. N. Engl. J. Med. 2875-9. [DOI] [PubMed] [Google Scholar]
- 44.Zarantonelli, M. L., M. Szatanik, D. Giorgini, E. Hong, M. Huerre, F. Guillou, J. M. Alonso, and M. K. Taha. 2007. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect. Immun. 755609-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]