Abstract
Citrobacter rodentium causes epithelial hyperplasia and colitis and is used as a model for enteropathogenic and enterohemorrhagic Escherichia coli infections. Little or no mortality develops in most inbred strains of mice, but C3H and FVB/N mice exhibit fatal outcomes of infection. Here we test the hypothesis that decreased intestinal transport activity during C. rodentium infection results in fatality in C3H/HeOu and FVB/N mice. Susceptible strains were compared to resistant C57BL/6 mice and to inbred strains SWR and SJL of Swiss origin, which have not been previously characterized for outcomes of C. rodentium infection. Mortality in susceptible strains C3H/HeOu and FVB/N was associated with significant fluid loss in feces, a remarkable downregulation of Slc26a3 and carbonic anhydrase IV (CAIV) message and protein expression, retention of chloride in stool, and hypochloremia, suggesting defects in intestinal chloride absorption. SWR, SJL, and C57BL/6 mice were resistant and survived the infection. Fluid therapy fully prevented mortality in C3H/HeOu and FVB/N mice without affecting clinical disease. Common pathogenic mechanisms, such as decreased levels of expression of Slc26a3 and CAIV, affect intestinal ion transport in C. rodentium-infected FVB and C3H mice, resulting in profound electrolyte loss, dehydration, and mortality. Intestinal chloride absorption pathways are likely a potential target for the treatment of infectious diarrhea.
Acute infectious diarrhea is an important cause of morbidity and mortality, affecting mainly children in developing countries (29). Diarrhea may result in dehydration, the development of irreversible multiple-organ failure, and death from hypovolemic shock (22, 33). With the exception of intestinal pathogens that possess enterotoxins, the mechanisms responsible for intestinal dysfunction during infectious diarrhea remain incompletely understood. Mice are generally considered poor models for studying watery diarrhea caused by pathogenic agents that infect people. Thus, diarrheagenic Escherichia coli, Vibrio cholerae, Campylobacter jejuni, Shigella spp., and Salmonella spp., as well as rotavirus, either fail to efficiently infect mice or do not cause appreciable diarrhea except in infant mice or older mice that have been manipulated (germfree, antibiotic pretreated, iron loaded, or given gastric acid-reducing agents) to increase susceptibility (1, 5, 16, 26, 27, 36, 39, 43, 45, 47, 56). Thus, elucidating the pathogenesis of acute diarrheal illness is hampered by the lack of a suitable small-animal model.
Citrobacter rodentium is a naturally occurring murine pathogen used to model human infections with enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli. C. rodentium infects the large intestine and causes attaching and effacing lesions, colonic hyperplasia, and variable degrees of colitis in most inbred (referred to hereafter as wild-type) mouse strains (reviewed in references 30 and 37). Host strain background is an important factor in determining the outcome of infection. For years, C3H mice were the only wild-type strain known to consistently develop fatal C. rodentium infection (7, 52). The mechanism of susceptibility is not well understood. Several substrains of C3H mice are known to differ in their responses to endotoxin due to genetic differences in Toll-like receptor 4 (TLR4). Mortality in C. rodentium-infected C3H substrains occurs regardless of TLR4 status and has been attributed to systemic disseminated infection (7, 52). We recently demonstrated that infected adult FVB mice also develop fatal disease, making it the second wild-type strain with marked susceptibility to C. rodentium (10). However, in our studies, the high mortality rate for infected FVB mice was associated with a substantial downregulation of the expression of apical ion transporters and serum ion abnormalities and not with increased bacterial translocation or inflammation. This led us to propose that the susceptibility of FVB mice to C. rodentium infection is caused by severe dehydration and hypovolemia-induced organ failure rather than by bacteremia or colitis (9, 10).
The top gene targets affected by C. rodentium in FVB mice in our previous study (9) were solute carrier family 26 member 3 (Slc26a3), also known as downregulated in adenoma (Dra), and carbonic anhydrase (CA) IV (CAIV). Both Dra and CAIV are crucial for the normal homeostasis of chloride ions by maintaining the intestinal exchange of chloride and bicarbonate (15, 28). Thus, a mutation in DRA results in recessively inherited congenital chloride diarrhea (CLD), which is characterized by a loss of chloride ions in stools and life-threatening diarrhea due to a defect in Cl−/HCO3− exchange (23). Patients with CLD develop hypochloremia and hyponatremia, followed by hypokalemia and metabolic alkalosis (24). Lifelong therapy restores biochemical abnormalities and allows normal growth and development but does not affect the diarrhea itself. Studies of chloride absorption in CLD patients and their healthy siblings indicate that colonic chloride uptake is dependent on the luminal concentration of bicarbonate (24). The pool of bicarbonate required for ion-exchange activity is maintained by CAs. These enzymes are abundant and active in the colon, found in both the apical membrane (i.e., CAIV) and the cytoplasm (i.e., CAI and CAII) of enterocytes, and catalyze the reversible hydration/dehydration of CO2 and water, thereby supplying protons and bicarbonate for apical membrane ion exchangers (12). The administration of CA inhibitors results in a significant reduction of colonic chloride absorption (12, 48), confirming the functional relationship of CAs and DRA in intestinal ion homeostasis.
The goal of this study was to test the hypothesis that susceptibility to C. rodentium-induced mortality in C3H mice is similar to the mechanism in FVB mice, namely, decreased levels of expression of Dra, CAIV, and other genes involved in intestinal ion transport. The C3H/HeOuJ substrain (TLR4 sufficient and endotoxin sensitive [hereafter called C3H]) rather than C3H/HeJ (TLR4 deficient and endotoxin resistant) was chosen to avoid a known defect in innate immunity. Resistant C57BL/6 mice, which are well characterized regarding outcomes of C. rodentium infection, and Swiss-derived strains SWR and SJL, which have not been previously characterized for outcomes of C. rodentium infection, were also included in the study. Both C3H and FVB mice had decreased levels of expression of Dra and CAIV, decreased levels of colonic uptake of chloride, and fatal diarrhea. Fluid therapy intervention protected susceptible strains of mice from fatal C. rodentium infection and confirmed the role of Dra and CAIV in protection against dehydration and hypovolemia in fatal infectious diarrhea.
MATERIALS AND METHODS
Bacteria and media.
Lennox L broth (LB) and LB agar (Difco Laboratories, Detroit, MI) were used for the routine cultivation of bacteria. MacConkey lactose agar (Difco) supplemented with 40 μg/ml kanamycin was used for measuring bacterial loads in feces and tissues. Kanamycin-resistant C. rodentium strain DBS120 (pCRP1::Tn5) (10) was used for infections.
Mice.
Eleven-week-old female FVB/N, SWR, SJL, C3H/HeOu, and C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME) or Taconic Laboratories (Germantown, NY). Mice were housed in polycarbonate microisolator cages within a barrier facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International and were viral antibody free for 11 murine viruses and negative for enteric Helicobacter spp., Salmonella spp., and C. rodentium as well as endoparasites and ectoparasites. Mice were maintained on pelleted rodent diet (LabDiet; Purina Mills, Inc., Richmond, IN) and water ad libitum. All experiments were approved by the MIT Committee on Animal Care. The data represent combined results from five independent experiments.
Infection.
Mice were inoculated by oral gavage with 100 μl of a bacterial culture grown overnight and concentrated 1:10 in 3% sodium bicarbonate or with 100 μl of sterile vehicle. Body weight loss and fecal shedding were determined as previously reported (10). For fluid therapy, infected mice received wet food from the day of inoculation and subcutaneous injections two to three times a day with 1 ml of lactated Ringer's solution (USP; Abbott Laboratories, Abbott Park, IL) from 3 days postinoculation (dpi) (C3H mice) or 6 dpi (FVB mice). Animals were euthanized with CO2 at predetermined times or when they lost ≥20% of body weight and/or exhibited severe clinical signs such as sunken eyes, hunched posture, reluctance to move, or recumbency. At necropsy, spleen, liver, colon, and cecum were collected, weighed, and cultured or subjected to histopathological analysis. Colon weight was expressed as a percentage of body weight. The most distal 5 mm of the colon was snap-frozen for subsequent RNA isolation and quantitative reverse transcription-PCR analysis. Group sizes were 5 to 10 mice for infected groups and 3 to 6 mice for uninoculated control groups. Results of five independent experiments are presented.
Measurement of serum endotoxin levels.
Serum endotoxin levels were measured by the quantitative Kinetic-QCL chromogenic Limulus amebocyte lysate assay according to the manufacturer's instructions (Cambrex Bio Science Walkersville, Inc., Walkersville, MD).
Histopathology.
Tissues were fixed in 10% formalin, paraffin embedded, processed routinely, sectioned at 5 μm, and stained with hematoxylin and eosin. Slides were scored for pathological lesions by a veterinary pathologist (B.H.R.) blinded to experimental groups. Inflammation, hyperplasia, dysplasia, edema, and epithelial defects within intestinal tissue sections were graded on a scale of 0 to 4 as described previously (10). The histologic colitis index was calculated as the sum of all scores, with 20 as a maximal possible index. Images were obtained using a Zeiss AxioScop 2 microscope equipped with an AxioCam HRc camera. Crypt length was measured by morphometry using quantitative computer-assisted image analysis (AxioVision software, version 4.6).
Real-time quantitative reverse transcription-PCR.
Quantitative gene expression analysis was performed as described previously (10). Briefly, RNA was isolated from colon tissue using Trizol reagent (Invitrogen, Carlsbad, CA). Five micrograms of RNA was used to generate cDNA, and levels of transcript were quantified with predesigned primers and probes (TaqMan gene expression assays) (Table 1) using an ABI Prism 7700 sequence detection system (Applied Biosystems, Branchburg, NJ). Transcript levels were normalized to the endogenous control glyceraldehyde-3-phosphate dehydrogenase and expressed as the change in expression levels compared with averaged expression in uninoculated FVB mice using the comparative threshold cycle method.
TABLE 1.
ABI predesigned gene expression assays (TaqMan)
| Gene | Designation(s) | TaqMan assay ID |
|---|---|---|
| Aquaporin 8 | Aqp8 | Mm00431846_m1 |
| ATPase, Na+/K+-transporting, beta 2 polypeptide | Atp1b2 | Mm00442612_m1 |
| CAI | CAI | Mm00486717_m1 |
| CAIV | CAIV | Mm00483021_m1 |
| Cystic fibrosis transmembrane conductance regulator homolog | Cftr, Abcc7 | Mm00445197_m1 |
| FBJ osteosarcoma oncogene B | FosB | Mm00500401_m1 |
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | Mm99999915_g1 |
| Solute carrier family 9, member 2 | Slc9a2, NHE2 | Mm01237137_m1 |
| Solute carrier family 26, member 3 | Slc26a3, Dra | Mm01291071_m1 |
Detection of Dra and CAIV expression by immunohistochemistry.
In situ expression of Dra and CAIV was performed as previously described (9). Control staining with normal rabbit or goat immunoglobulin G was routinely performed and was consistently negative. All of the immunohistochemistry analysis was performed on slides that were batched together to avoid the bias of variation in staining between the experiments. Colons were divided into four sections, with region “a” representing the most distal part of the colon, region “b” corresponding to the distal colon to mid-colon, region “c” corresponding to the mid-colon to proximal colon, and region “d” corresponding to the most proximal colon. The loss of protein expression was analyzed using a semiquantitative system. Grade “0” corresponded to normal staining, which is the apical expression of Dra in regions “a” through “c,” and predominantly apical, but also some cytosolic, expression of the surface colonic epithelium of CAIV throughout the entire large intestine (regions “a” through “d”). A partial loss of protein expression, visualized as a patchy signal as described previously (9), was graded as “1.” The general lack of expression with only occasional spots of positive staining was graded as “2,” whereas the complete absence of protein expression was graded as “3.” The cumulative lack of an expression index was calculated by adding the grades of expression from all regions of the colon.
Detection of water content in stool as a marker of diarrhea.
Feces were collected every 3 days and immediately weighed to define “wet weight.” Following incubation at 37°C for 72 h, feces were weighed to define “dry weight.” The water content in stool was expressed as a percentage of wet weight minus dry weight/wet weight and normalized to water content in uninfected mice of the corresponding strains. Dried samples were stored frozen until they were analyzed for chloride content.
Measurement of serum and fecal chloride levels.
Chloride levels were detected using a QuantiChrom chloride assay kit (BioAssay Systems, Hayward, CA). Serum samples were analyzed as recommended by the manufacturer. Fecal samples were processed as described previously (44). Briefly, dried fecal pellets were resuspended in water at 5 to 100 mg/ml, heated to 65°C for 30 min, and centrifuged at full speed for 10 min, and supernatant was used for chloride analysis.
Measurement of mucosal permeability by FITC-dextran.
Intestinal permeability was assayed at 9 dpi in C3H mice by the administration of 6 mg/10 g body weight of fluorescein isothiocyanate (FITC)-dextran (4 kDa; Sigma-Aldrich) by intragastric gavage, and serum was collected 4 h later. FITC-dextran concentrations were determined against a standard curve using a fluorescence plate reader (SpectraMax M2e; Molecular Devices Corporation, Sunnyvale, CA) with an excitation wavelength of 490 nm and an emission wavelength of 530 nm.
Statistical analysis.
Data are presented as mean values ± standard errors of the means (SEM) except where indicated. Statistical analyses were performed with GraphPad PRISM, version 4.0 (GraphPad Software, Inc., San Diego, CA). Survival Kaplan-Meier curves were analyzed by log-rank test and χ2 analysis to determine median survival times. Statistical differences were determined by using a nonparametric Kruskal-Wallis test followed by a Mann-Whitney U test, one-way analysis of variance followed by a Student's t test or Tukey's multiple-comparison test, or a two-way analysis of variance test followed by the Bonferroni posttest whenever appropriate. Analysis of gene expression was performed on transformed data. A P value of less than 0.05 was regarded as being statistically significant.
RESULTS
C3H and FVB mice, but not SWR, SJL, or C57BL/6 mice, develop fatal disease in response to C. rodentium infection.
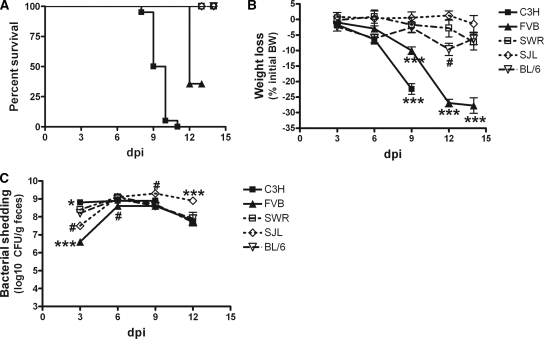
As expected, C3H mice infected with C. rodentium developed overt disease leading to 100% mortality at 8 to 11 dpi (Fig. 1A). FVB mice were also susceptible and demonstrated 64.3% mortality. The median survival times were 9.5 and 12 days for C3H and FVB mice, respectively (P < 0.0001). SWR, SJL, and C57BL/6 mice were resistant to infection and survived throughout the 2-week period postinoculation.
FIG. 1.
Experimental inoculation of animals with C. rodentium causes severe disease in C3H and FVB mice but not in SWR, SJL, or C57BL/6 mice. (A) Mortality of infected C3H mice and FVB mice, but not infected SWR, SJL, and C57BL/6 mice, was observed after 7 dpi (P < 0.0001). (B) Significant body weight (BW) loss was observed for infected C3H mice and FVB mice by 9 dpi (P < 0.005). Data were normalized to body weights of uninfected mice of the corresponding strains and are presented as mean differences ± SEM. (C) Fecal bacterial counts were highest in C3H mice and lowest in FVB mice at 3 dpi. Levels of fecal bacterial shedding were comparable between the strains on days 9 and 12, except for SJL mice, which had a delayed clearance of infection (P < 0.0001). Data were log transformed and are presented as mean differences ± SEM. *, P < 0.05; ***, P < 0.001 (compared with three other inbred strains). #, P < 0.05 compared with one or two other inbred strains.
Clinical disease in C3H mice was associated with a decrease in body weight reaching a 22.4% ± 1.7% loss by 9 dpi (P < 0.001) (Fig. 1B). Body weight loss in FVB mice was somewhat less severe but still greater than that in resistant strains, reaching 10.0% ± 1.2% by 9 dpi (P < 0.01). By 12 to 14 dpi, body weight loss in FVB mice was more profound, reaching more than a 27% loss of initial body weight (P < 0.001 compared with SWR, SJL, and C57BL/6 mice). The changes in body weight in SJL, SWR, or C57BL/6 mice fluctuated within a range of 5 to 10% of the initial body weight and were not significantly different between these strains.
Strain-dependent differences in fecal bacterial shedding were observed early during infection. At 3 dpi, the greatest number of bacteria was isolated from the feces of C3H mice, and the least bacteria were isolated from FVB mice (P < 0.0001) (Fig. 1C). Levels of C. rodentium in SJL mice at 3 dpi were less than those in C3H mice but greater than those in FVB mice (P < 0.01). As infection progressed, fecal shedding became comparable between the strains, except for delayed clearance in SJL mice.
Systemic bacterial translocation.
Because the susceptibility of C3H mice to C. rodentium infection was previously linked to a high bacterial burden in the colon and increased translocation of bacteria compared to other inbred strains (52), we cultured C. rodentium bacteria from colon and extraintestinal tissues. Colonic bacterial counts increased more slowly in FVB mice, consistent with the delayed increase in fecal bacterial load (P < 0.005) (Fig. 2A). By 9 dpi, colonic counts were lower in C57BL/6 mice than in C3H and SWR but not FVB mice (P < 0.05). Although the number of bacteria recovered was low, C3H mice had significantly more bacterial translocation to the liver at 4 and 9 dpi and to the spleen at 4 dpi, whereas C57BL/6 mice had the lowest spleen counts at 9 dpi (P < 0.0005) (Fig. 2B and C). Bacterial translocation was comparable between FVB, SJL, SWR, and C57BL/6 mice at 14 dpi (data not shown). Except in the case of moribund C3H and FVB mice, serum endotoxin levels were low and not significantly different between all groups of mice (P > 0.05) (Fig. 2D).
FIG. 2.
Colonic loads of C. rodentium and extraintestinal dissemination of infection. (A to C) Bacterial counts in colon increased more slowly in FVB mice, consistent with a delayed increase in fecal bacterial shedding. By 9 dpi, colonic counts were lower for C57BL/6 mice than for C3H and SWR but not FVB mice. C3H mice demonstrated significantly higher levels of bacterial translocation to the liver at 4 and 9 dpi and to spleen at 4 dpi, whereas C57BL/6 mice had the lowest spleen counts at 9 dpi. Data were log transformed and are presented as mean differences ± SEM. The dashed line indicates the limit of detection. *, P < 0.05; **, P < 0.01 (compared with three other inbred strains). #, P < 0.05 compared with one or two other inbred strains. (D) Levels of serum endotoxin were low and not different between groups (P > 0.05). Data are presented as mean differences ± SEM. EU, endotoxin units.
Intestinal lesions in inbred mice infected with C. rodentium.
Grossly, the distal half of the colon was rigid and thickened, and there was a prominent cecal patch in most of the strains. FVB mice, on the other hand, developed thickening throughout the entire colon but did not have a prominent cecal patch.
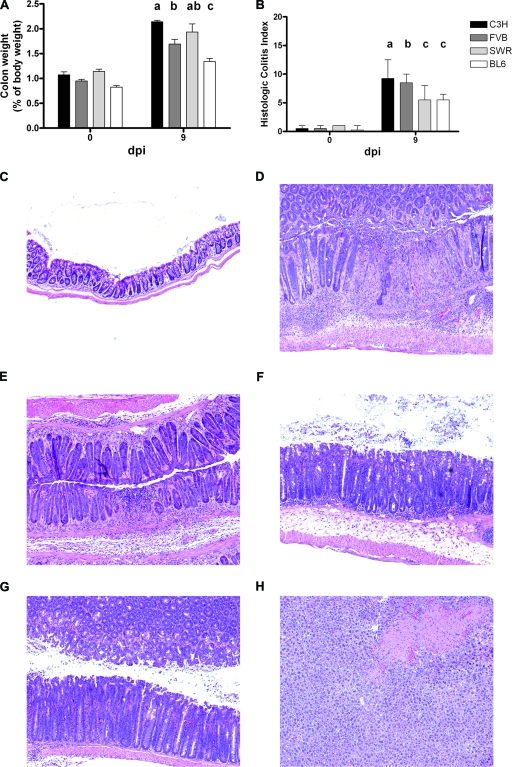
Colonic weight was increased in all infected mice (Fig. 3A) and was greatest in C3H mice and least in C57BL/6 mice at 9 dpi (P < 0.05). Increased colonic weight was initially less pronounced in FVB mice, corresponding to the delay in colonization, but exceeded that in other strains by 14 dpi (P < 0.0001) (data not shown).
FIG. 3.
Colonic lesions in C. rodentium-infected mice. (A) Increased colon weight in C. rodentium-infected mice. Data were normalized to body weight and are presented as mean differences ± SEM. Groups indicated by different letters (a, b, and c) are significantly different (P < 0.05). (B) Cumulative disease indices of colonic lesions. Median lines and ranges are presented. Groups indicated by different letters (a, b, and c) are significantly different (P < 0.0001). (C to F) Microscopic lesions in C. rodentium-infected mice. (C) Normal colon from an uninoculated C3H mouse. (D and E) Transmural colitis, ulcer, and blood congestion in C3H (D) and FVB (E) mice at 9 dpi. (F and G) Hyperplasia with mild to moderate colitis in SWR (F) and C57BL/6 (G) mice at 9 dpi. (H) Although SJL mice had colonic lesions comparable to those of SWR and C57BL/6 mice (data not shown), they also developed mild to moderate liver lesions characterized by focal to multifocal hepatocellular coagulative necrosis at 14 dpi. Shown is hematoxylin and eosin staining. Original magnifications are ×100.
Histologically, infected mice of all strains developed variable degrees of hyperplasia and inflammation, minimal dysplasia, mild edema, and a wide range of epithelial defects in the distal colon. By 9 dpi, crypt length had increased from 152.0 ± 9.5 μm to 306.5 ± 7.7 μm in C3H mice and from 153.3 ± 5.8 μm to 301.5 ± 9.7 μm in FVB mice (P > 0.05 between strains). Hyperplasia in these susceptible strains was greater than that in the resistant SWR and C57BL/6 mice (from 167.4.0 ± 7.0 μm to 275.4 ± 7.4 μm and from 152.8 ± 3.5 μm to 264.8 ± 9.1 μm, respectively) (P < 0.05). The histologic colitis index (Fig. 3B) was highest for C3H mice, intermediate for FVB mice, and lowest for SWR and C57BL/6 mice at 9 dpi (P < 0.05). Differences in disease index were attributable mainly to more severe inflammation, edema, and epithelial defects in C3H and FVB mice (P < 0.0001) (data not shown), which often included frank ulcers and erosions (Fig. 3C to G). Most of the FVB and C3H mice, and a few of the SWR mice, had gland herniation into gut-associated lymphoid tissue. Colonic lesions in SJL mice were comparable to those in SWR and C57BL/6 mice at 14 dpi (data not shown). However, by 14 dpi, the majority of SJL mice (80%) developed mild to moderate multifocal hepatic lesions, including coagulative necrosis (Fig. 3H). In addition, SJL mice demonstrated profound splenomegaly in response to infection (P < 0.001) (data not shown), whereas mild, if any, increases in spleen weight were observed for other strains (P > 0.05) (data not shown). Because of significant extraintestinal changes (hepatic lesions and splenomegaly), SJL mice were not included in subsequent analyses.
Susceptible mice have a profound downregulation of the Dra and CA genes, involved in apical chloride uptake and exchange with bicarbonate.
As expected, infection caused decreased levels of expression of downregulated in adenoma (Dra [Slc26a3]), the aquaporin gene Aqp8, sodium-proton exchanger (NHE2 [slc9a2]), CAI, and CAIV in all inbred strains (P < 0.01) (Fig. 4). Expression levels of the sodium/potassium-transporting ATPase Atp1b2 and the Fos-related transcriptional factor Fosb increased in infected inbred mice compared with those of uninoculated controls (P < 0.01), except that Fosb was not significantly upregulated in C57BL/6 mice. Decreased levels of expression of the cystic fibrosis transmembrane conductance regulator Cftr were marginal in infected animals and not significant in FVB mice (P > 0.05 compared with uninfected controls). Of the genes examined, only Dra, CAI, and CAIV were differentially expressed between susceptible and resistant strains of mice. Notably, the expression of Dra was negatively correlated with colonic crypt height (r = −0.9; P < 0.0001), suggesting that Dra could play a role in intestinal epithelial hyperproliferation.
FIG. 4.
Effects of C. rodentium infection on expression of genes involved in colonic ion and water transport. The expression of genes was normalized to that for uninoculated FVB mice. Data are presented as median (center line), upper and lower quartile (box), and range. Groups indicated by different letters (a, b, and c) are significantly different (P < 0.05 after log transformation).
To further validate these observations, we analyzed the expression of DRA and CAIV in colonic tissues of uninfected mice and mice at 9 dpi by immunohistochemistry (Fig. 5). Consistent with our previously reported results (9), uninoculated mice of all strains had positive staining for both proteins mainly at the upper crypt and surface epithelium of the colon. C. rodentium-infected FVB and C3H mice demonstrated a partial to complete lack of CAIV expression in the distal colon and a patchy signal characterized by the lack of staining adjacent to areas with normal protein expression levels in middle segments of the colon. CAIV expression levels remained normal in the proximal colon of infected C3H and FVB mice. Infected SWR mice showed normal CAIV expression levels throughout the large intestine, with the exception of the patchy phenotype at the very distal end. Infected C57BL/6 mice showed some lack of CAIV expression in the distal colon and normal expression levels in the rest of the areas (P < 0.0001). Compared with CAIV epithelial staining, the loss of DRA expression was more pronounced in all strains infected with C. rodentium, although there were still differences between susceptible and resistant strains of mice (P < 0.0001). Infected C3H and FVB mice completely lost DRA expression throughout the large intestine, while resistant strains had residual expression in the distal colon and essentially normal expression levels in the mid- to proximal colon (Fig. 5).
FIG. 5.
Expression of CAIV and DRA in situ. (A) Expression of the CAIV and DRA proteins in colonic segments was detected using semiquantitative analysis as described in Materials and Methods. Uninoculated mice of all strains had normal levels of expression and were excluded from the analysis. CAIV was normally expressed throughout the whole large intestine (regions “a” through “d”), whereas DRA was usually not expressed in the most proximal colon (region “d”). Susceptible C3H and FVB mice were characterized by a more significant loss of CAIV and DRA expression throughout the intestine than resistant SWR and C57BL/6 mice during C. rodentium infection (P < 0.0001; n = 3 mice per group). Medians and ranges are shown. *, P < 0.05; ***, P < 0.001; ns, not significant. (B) Representative images for grading of CAIV (a through d) and DRA (e through h) protein expression. Grades “0” (a and e), “1” (b and f), “2” (c and g), and “3” (d and h) are presented. Arrows indicate apical DRA expression. Original magnifications are ×200.
Susceptible inbred mice develop chloride diarrhea following C. rodentium infection.
Because both Dra and CAIV contribute to chloride absorption, we predicted that a profound downregulation of these genes would cause alterations in chloride homeostasis. Indeed, upon infection with C. rodentium, susceptible C3H and FVB mice showed a retention of chloride in feces (Fig. 6A), accompanied by severe hypochloremia (Fig. 6B), whereas resistant SWR and C57BL/6 mice maintained normal fecal and serum chloride levels (P < 0.0001). Delayed kinetics of chloride abnormalities in FVB mice were consistent with the kinetics of bacterial infection and disease progression in this strain. The expression of Dra was negatively correlated with fecal chloride levels and positively correlated with serum chloride levels (r = −0.75 and r = 0.65, respectively; P < 0.0002). Our previously reported results showed that serum bicarbonate is not affected by the infection (9); hence, bicarbonate levels were not measured in this study.
FIG. 6.
C. rodentium infection induces chloride diarrhea in susceptible mice. (A and B) Infection resulted in chloride retention in feces (A) and hypochloremia (B) in C3H and FVB mice at 9 and 12 dpi, respectively (P < 0.001). Resistant SWR and C57BL/6 mice did not demonstrate abnormal fecal or serum chloride concentrations. Data are presented as mean differences ± SEM. (C) C3H and FVB mice demonstrated significant water loss in stool, reaching more than a 20% loss of initial fecal water content as disease progressed. The diarrheal response of resistant strains was comparable and less dramatic (P < 0.005). Data were normalized to water content in uninfected mice of the corresponding strains and are presented as mean differences ± SEM. The dashed line indicates the 20% water loss threshold. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with three other inbred strains). #, P < 0.05 compared with one or two other inbred strains.
Abnormalities in intestinal ion transport following C. rodentium infection were accompanied by the development of diarrhea in all animals, although the most marked responses were observed in C3H and FVB mice (P < 0.0001) (Fig. 6C). The fecal water content increased from 56.9% ± 0.6% in uninfected mice to 82.6% ± 0.9% in C3H mice at 9 dpi, indicating a rapid development of severe diarrhea (>25% loss of water in stool). Similarly, the fecal water content increased from 58.7% ± 0.6% in uninfected mice to 81.3% ± 1.5% in FVB mice at 12 dpi (>20% loss of water in stool). This is a conservative estimate, because infected C3H and FVB mice with severe diarrhea do not pass well-formed feces, resulting in an underestimation of fecal water content for these mice. Water contents for the other strains were comparable (from 58 to 62% for uninfected SWR, SJL, and C57BL/6 mice to 72 to 78% at 12 dpi) and did not exceed 20% fecal water loss. The water content in feces was highly correlated with the expression of Dra (r = −0.9; P < 0.0001). Although we did not perform such an analysis for CAIV expression, similar correlations between CAIV mRNA levels and chloride content in feces and serum, as well as fecal water loss, are likely and warrant further investigation.
Fluid therapy protects susceptible strains of mice from fatal infection.
The administration of fluid therapy fully prevented mortality in both susceptible strains without affecting body weight or fecal water loss, bacterial shedding, colon weight, or the histologic colitis index (Fig. 7 and data not shown). By preventing mortality, we were able to monitor bacterial shedding in C3H mice until eradication of infection. This occurred earlier than in FVB mice (P < 0.01) but comparably to other inbred strains (data not shown), supporting our hypothesis that susceptibility to fatal C. rodentium infection in C3H mice is not due to a defect in bacterial clearance. Although recoveries of C. rodentium from colon, spleen, and liver were comparable between infected mice regardless of fluid administration (P > 0.05), moribund animals that required euthanasia did have slightly more extraintestinal translocation (Fig. 7E). These mice were also the only animals with elevated serum levels of FITC-dextran; otherwise, barrier functions were comparable among all groups of control, infected, and fluid-treated mice (P > 0.05) (Fig. 7F).
FIG. 7.
Effects of fluid therapy on disease outcome for susceptible mice. (A) Mortality of C. rodentium-infected C3H and FVB mice was fully prevented by fluid administration (P < 0.0001). (B) Body weight loss in infected C3H and FVB mice was not affected by fluid administration (P > 0.05), although it was delayed in infected FVB mice (P < 0.05 at 5 to 10 dpi). Data are presented as mean differences ± SEM. (C) Levels of fecal bacterial shedding were comparable between fluid-treated and untreated groups for both strains (P > 0.05). C. rodentium counts were lower in FVB mice at 3 dpi and higher at 18 dpi than in C3H mice (*, P < 0.05). Data were log transformed and are presented as mean differences ± SEM. The dashed line indicates the limit of detection. (D) No differences in stool water losses were observed between infected fluid-treated and untreated animals (P > 0.05), although the diarrheal response was more rapid in C3H mice than in FVB mice (***, P < 0.001). In addition, uninfected C3H mice had lower stool water contents in the beginning of the experiment than did uninoculated FVB mice (*, P < 0.05). Data are presented as mean differences ± SEM. (E) Fluid therapy did not affect bacterial recovery from tissues of C3H mice (P > 0.05). Data were log transformed, and each dot corresponds to an individual animal. Lines indicate mean values. (F) Mucosal permeability was measured by the detection of FITC-dextran in serum after administration by intragastric intubation. No differences between groups of C3H mice were found (P > 0.05). Each dot corresponds to an individual animal, and lines indicate mean values. C, uninoculated controls; I, infected untreated mice; IF, infected mice treated with fluid therapy intervention.
DISCUSSION
C. rodentium infection of mice has been used to study epithelial hyperplasia, tumor promotion, mucosal inflammation, and innate and adaptive immunity. However, there is growing interest in using this model to study diarrheal disease. Alterations in tight-junction integrity with increased intestinal mucosal permeability (19, 21, 31), mislocalization of aquaporin water channels (20), and interactions of Na+/H+ exchanger regulatory factor 1 with the C. rodentium Map protein (46) in infected mice have been documented. Here we confirm and extend data from our previously reported studies (9) of the role of Dra and CAIV in protection from fatal diarrheal disease. Two genetically unrelated susceptible strains developed body weight loss and mortality that were associated with a >20% loss of fecal water but not with differences in the numbers of C. rodentium bacteria shed in feces or in the colon compared to resistant strains of mice. Fluid therapy was fully protective for both susceptible strains without affecting body weight loss, fecal shedding of bacteria, or the number of C. rodentium cells in the colon. Although some bacterial translocation was observed for mice at the peak of infection, no differences in serum endotoxin or FITC-dextran levels were found between infected and uninoculated mice regardless of strain background. A subset of the infected mice that had to be euthanized due to severe morbidity tended to have elevated serum endotoxin levels, although they were still much lower than levels detected for sepsis (25). Similar findings were observed for serum FITC-dextran levels, suggesting that the loss of barrier function may be a consequence of hypovolemia and irreversible organ failure in this model, consistent with previous findings that hemorrhagic shock leads to increased gut permeability (42, 54), although changes in barrier function earlier in the course of infection, highlighted by differences in FITC translocation, cannot be excluded.
Of eight genes which we previously implicated in the pathogenesis of fatal diarrhea in susceptible inbred FVB mice (9), only Dra, CAIV, and CAI were differentially expressed between C. rodentium-susceptible and -resistant strains. All three genes play an important role in chloride and bicarbonate exchange and fluid homeostasis (15, 28), and patients with autosomal recessive DRA mutations fail to resorb chloride in the ileum and colon and suffer from life-threatening diarrheal disease even though they absorb fluid normally in the jejunum (24). EPEC was recently shown to inhibit chloride absorption by reducing the surface expression of DRA in intestinal epithelial cells (18), and here we demonstrate that infected mice had markedly reduced levels of colonic surface epithelial expression of Dra. The absence of Dra expression was associated with significant increases in fecal water and chloride levels, as well as hypochloremia, consistent with electrolyte abnormalities found in CLD patients (24). The loss of Dra expression is apparently sufficient to account for the retention of chloride and water, leading to diarrhea in C. rodentium-infected mice, since it was also observed for Dra-deficient mice (44). However, a role for CAs, enzymes that reversibly catalyze the conversion of carbon dioxide and water to protons and bicarbonate (12), in diarrhea cannot be excluded. CAs, including CAIV, also may affect the colonic absorption of sodium and chloride independently of their catalytic activity either by changing vesicular trafficking of anion exchangers (11, 13) or as a result of direct interactions with ion cotransporters (4, 48). While such an interaction with Dra has not been observed (4, 48), our results show a concordance in Dra and CAIV mRNA levels and Dra and CAIV protein expression in infected C3H and FVB mice. This finding suggests that DRA and CAs may be transcriptionally coregulated and indicates that the role of CAs in chloride uptake and fatal infectious diarrhea warrants further investigation. CAIV may have activities other than providing bicarbonate for apical anion exchange mediated by DRA. A recent report implicates CAIV in apical buffering rather than in bicarbonate fluxes, at least in corneal endothelium (49). In addition to chloride/bicarbonate antiport, DRA has been shown to exchange chloride and hydroxide ions (35), providing yet another possible link for DRA and CAIV in infectious fatal diarrhea.
It is not clear how Dra and CA expression levels are regulated during infection with attaching and effacing pathogens. Although it was implicated in our previous study (9), levels of Fosb expression were comparable between infected C3H, FVB, and SWR mice, although they were lower in C57BL/6 mice. It seems that other transcription factors regulate the expression of intestinal transporters. Hepatic nuclear factor 4, Yin Yang (YY1), and GATA zinc finger proteins were recently identified as being the main transcription factors regulating the intestine-specific expression of Dra (3). Colonic CAI is regulated by the homeodomain transcription factor Cdx2 (14), but the factors controlling the expression of CAIV are not yet known. Likewise, the role of inflammation in intestinal ion homeostasis remains unclear. Proinflammatory signals have been implicated in the suppression of colonic ion transporters, including DRA and CAs (6, 34, 41, 53), but this has not been consistently observed in every study (3). In susceptible and resistant mice, differences in levels of expression of Dra and CAIV were found to be independent of inflammatory status and did not correlate with the expression of gamma interferon and other proinflammatory cytokines (9, 10; this report). Interestingly, little or no decrease in CAIV or DRA expression was observed for mice with acute or chronic dextran sulfate sodium-induced colitis by immunohistochemistry except in areas with severe dysplasia or inflammation, where it was slightly reduced, corresponding to grades of 0.5 and 1, respectively (data not shown). Additional studies will be needed to determine if different forms of colitis have distinct effects on Dra and CA expression.
In addition to susceptible mouse C3H and FVB strains, we characterized C. rodentium infection of two inbred strains of Swiss mice that have not been previously studied. Infected SWR and SJL mice developed little morbidity and survived throughout the 14 days of the study. Resistance to fatal disease was comparable to what was previously reported for C57BL/6 mice (7, 52). SJL mice, although resistant to fatal disease, had delayed clearance of infection and profound splenomegaly and liver lesions despite having comparable intestinal lesions. Although SJL mice are considered to be immunocompetent, they have high circulating levels of T cells, defective T-cell receptor-induced interleukin-4 and immunoglobulin E production, elevated levels of interleukin-12p40 expression by antigen-presenting cells, and a high incidence of spontaneous B-cell-type lymphomas (2, 8, 51, 55). In light of these abnormalities and the high degree of relatedness between SWR and FVB/N mice (50), SWR may serve as a better resistant Swiss strain for direct comparisons with fatal infectious diarrhea in FVB mice.
In conclusion, our data indicate that susceptible C3H and FVB mice die from severe dehydration due to C. rodentium-induced diarrhea. Fatal diarrhea in these animals is most likely caused by impaired chloride absorption as a result of a marked downregulation of the anion exchanger Dra and the CAs CAIV and CAI. Mortality can be prevented by fluid therapy, is independent of a systemic manifestation of increased intestinal permeability, and is not associated with bacterial counts or disseminated infection. Although oral rehydration therapy is an effective means to prevent mortality from diarrheal disease (40), compliance may be an issue due to continued diarrhea in children undergoing treatment (17), and severe cases of EPEC infection do not respond to oral fluid resuscitation (38). Therefore, novel targets for the treatment of infectious diarrhea are needed. C. rodentium infection of FVB and C3H mice provides an excellent model for investigating the pathogenesis of fatal infectious diarrhea, particularly with regard to intestinal ion transport, chloride bicarbonate exchange, and decreased levels of expression of DRA and CAIV.
Acknowledgments
We thank Lisiane B. Meira and Leona D. Samson for colonic tissues from mice with dextran sulfate sodium-induced colitis (32), Kathy Cormier for technical help with immunohistochemistry, and the MIT Division of Comparative Medicine for help with mouse husbandry.
This work was supported by Public Health Service grants P01 CA26731, T32 ES07020, and P30 ES02109. D.B. was supported by a National Institutes of Health graduate research fellowship.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 22 June 2009.
We dedicate this article to the memory of our valued teacher, colleague, and friend, David B. Schauer, who passed away from cardiac arrest during revision of the manuscript, leaving us all with a deep sense of sadness, loss, gratitude, and love.
REFERENCES
- 1.Allen, K. P., M. M. Randolph, and J. M. Fleckenstein. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect. Immun. 74869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alleva, D. G., E. B. Johnson, J. Wilson, D. I. Beller, and P. J. Conlon. 2001. SJL and NOD macrophages are uniquely characterized by genetically programmed, elevated expression of the IL-12(p40) gene, suggesting a conserved pathway for the induction of organ-specific autoimmunity. J. Leukoc. Biol. 69440-448. [PubMed] [Google Scholar]
- 3.Alrefai, W. A., X. Wen, W. Jiang, J. P. Katz, K. A. Steinbrecher, M. B. Cohen, I. R. Williams, P. K. Dudeja, and G. D. Wu. 2007. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am. J. Physiol. Gastrointest. Liver Physiol. 293G923-G934. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez, B. V., F. B. Loiselle, C. T. Supuran, G. J. Schwartz, and J. R. Casey. 2003. Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry 4212321-12329. [DOI] [PubMed] [Google Scholar]
- 5.Baqar, S., E. Burg, and J. R. Murphy. 2000. Mouse models of Campylobacter jejuni infection, p. 223-240. In O. Zak and M. A. Sande (ed.), Handbook of animal models of infection. Experimental models in antimicrobial chemotherapy. Academic Press, San Diego, CA.
- 6.Barmeyer, C., M. Harren, H. Schmitz, U. Heinzel-Pleines, J. Mankertz, U. Seidler, I. Horak, B. Wiedenmann, M. Fromm, and J. D. Schulzke. 2004. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 286G244-G252. [DOI] [PubMed] [Google Scholar]
- 7.Barthold, S. W., G. W. Osbaldiston, and A. M. Jonas. 1977. Dietary, bacterial, and host genetic interactions in the pathogenesis of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 27938-945. [PubMed] [Google Scholar]
- 8.Beutner, U., P. Launois, T. Ohteki, J. A. Louis, and H. R. MacDonald. 1997. Natural killer-like T cells develop in SJL mice despite genetically distinct defects in NK1.1 expression and in inducible interleukin-4 production. Eur. J. Immunol. 27928-934. [DOI] [PubMed] [Google Scholar]
- 9.Borenshtein, D., R. C. Fry, E. B. Groff, P. R. Nambiar, V. J. Carey, J. G. Fox, and D. B. Schauer. 2008. Diarrhea as a cause of mortality in a mouse model of infectious colitis. Genome Biol. 9R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borenshtein, D., P. R. Nambiar, E. B. Groff, J. G. Fox, and D. B. Schauer. 2007. Development of fatal colitis in FVB mice infected with Citrobacter rodentium. Infect. Immun. 753271-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charney, A. N., J. Alexander-Chacko, R. Gummaconda, and R. W. Egnor. 2002. Non-catalytic role of carbonic anhydrase in rat intestinal absorption. Biochim. Biophys. Acta 1573141-148. [DOI] [PubMed] [Google Scholar]
- 12.Charney, A. N., and P. C. Dagher. 1996. Acid-base effects on colonic electrolyte transport revisited. Gastroenterology 1111358-1368. [DOI] [PubMed] [Google Scholar]
- 13.Charney, A. N., R. W. Egnor, D. Henner, H. Rashid, N. Cassai, and G. S. Sidhu. 2004. Acid-base effects on intestinal Cl− absorption and vesicular trafficking. Am. J. Physiol. Cell Physiol. 286C1062-C1070. [DOI] [PubMed] [Google Scholar]
- 14.Drummond, F. J., J. Sowden, K. Morrison, and Y. H. Edwards. 1998. Colon carbonic anhydrase 1: transactivation of gene expression by the homeodomain protein Cdx2. FEBS Lett. 423218-222. [DOI] [PubMed] [Google Scholar]
- 15.Field, M. 2003. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Investig. 111931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankel, G., A. D. Phillips, M. Novakova, H. Field, D. C. Candy, D. B. Schauer, G. Douce, and G. Dougan. 1996. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 645315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs, G. J. 2001. A better oral rehydration solution? An important step, but not a leap forward. BMJ 32359-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill, R. K., A. Borthakur, K. Hodges, J. R. Turner, D. R. Clayburgh, S. Saksena, A. Zaheer, K. Ramaswamy, G. Hecht, and P. K. Dudeja. 2007. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J. Clin. Investig. 117428-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guttman, J. A., Y. Li, M. E. Wickham, W. Deng, A. W. Vogl, and B. B. Finlay. 2006. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 8634-645. [DOI] [PubMed] [Google Scholar]
- 20.Guttman, J. A., F. N. Samji, Y. Li, W. Deng, A. Lin, and B. B. Finlay. 2007. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell. Microbiol. 9131-141. [DOI] [PubMed] [Google Scholar]
- 21.Guttman, J. A., F. N. Samji, Y. Li, A. W. Vogl, and B. B. Finlay. 2006. Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo. Infect. Immun. 746075-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyton, A. C. 1991. Textbook of medical physiology, 8th ed. W. B. Saunders Company, Philadelphia, PA.
- 23.Hoglund, P., S. Haila, J. Socha, L. Tomaszewski, U. Saarialho-Kere, M. L. Karjalainen-Lindsberg, K. Airola, C. Holmberg, A. de la Chapelle, and J. Kere. 1996. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat. Genet. 14316-319. [DOI] [PubMed] [Google Scholar]
- 24.Holmberg, C., J. Perheentupa, and K. Launiala. 1975. Colonic electrolyte transport in health and in congenital chloride diarrhea. J. Clin. Investig. 56302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurley, J. C. 1995. Endotoxemia: methods of detection and clinical correlates. Clin. Microbiol. Rev. 8268-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klapproth, J. M., M. Sasaki, M. Sherman, B. Babbin, M. S. Donnenberg, P. J. Fernandes, I. C. Scaletsky, D. Kalman, A. Nusrat, and I. R. Williams. 2005. Citrobacter rodentium lifA/efa1 is essential for colonic colonization and crypt cell hyperplasia in vivo. Infect. Immun. 731441-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kordasti, S., C. Istrate, M. Banasaz, M. Rottenberg, H. Sjovall, O. Lundgren, and L. Svensson. 2006. Rotavirus infection is not associated with small intestinal fluid secretion in the adult mouse. J. Virol. 8011355-11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunzelmann, K., and M. Mall. 2002. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol. Rev. 82245-289. [DOI] [PubMed] [Google Scholar]
- 29.Lopez, A. D., C. D. Mathers, M. Ezzati, D. T. Jamison, and C. J. Murray. 2006. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 3671747-1757. [DOI] [PubMed] [Google Scholar]
- 30.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3333-340. [DOI] [PubMed] [Google Scholar]
- 31.Ma, C., M. E. Wickham, J. A. Guttman, W. Deng, J. Walker, K. L. Madsen, K. Jacobson, W. A. Vogl, B. B. Finlay, and B. A. Vallance. 2006. Citrobacter rodentium infection causes both mitochondrial dysfunction and intestinal epithelial barrier disruption in vivo: role of mitochondrial associated protein (Map). Cell. Microbiol. 81669-1686. [DOI] [PubMed] [Google Scholar]
- 32.Meira, L. B., J. M. Bugni, S. L. Green, C. W. Lee, B. Pang, D. Borenshtein, B. H. Rickman, A. B. Rogers, C. A. Moroski-Erkul, J. L. McFaline, D. B. Schauer, P. C. Dedon, J. G. Fox, and L. D. Samson. 2008. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 1182516-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mello, P. M., V. K. Sharma, and R. P. Dellinger. 2004. Shock overview. Semin. Respir. Crit. Care Med. 25619-628. [DOI] [PubMed] [Google Scholar]
- 34.Mizoguchi, E., R. J. Xavier, H. C. Reinecker, H. Uchino, A. K. Bhan, D. K. Podolsky, and A. Mizoguchi. 2003. Colonic epithelial functional phenotype varies with type and phase of experimental colitis. Gastroenterology 125148-161. [DOI] [PubMed] [Google Scholar]
- 35.Moseley, R. H., P. Hoglund, G. D. Wu, D. G. Silberg, S. Haila, A. de la Chapelle, C. Holmberg, and J. Kere. 1999. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am. J. Physiol. 276G185-G192. [DOI] [PubMed] [Google Scholar]
- 36.Mundy, R., F. Girard, A. J. FitzGerald, and G. Frankel. 2006. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol. Lett. 265126-132. [DOI] [PubMed] [Google Scholar]
- 37.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell. Microbiol. 71697-1706. [DOI] [PubMed] [Google Scholar]
- 38.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okuda, J., M. Fukumoto, Y. Takeda, and M. Nishibuchi. 1997. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect. Immun. 65428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao, M. C. 2004. Oral rehydration therapy: new explanations for an old remedy. Annu. Rev. Physiol. 66385-417. [DOI] [PubMed] [Google Scholar]
- 41.Renes, I. B., M. Verburg, D. J. Van Nispen, J. A. Taminiau, H. A. Buller, J. Dekker, and A. W. Einerhand. 2002. Epithelial proliferation, cell death, and gene expression in experimental colitis: alterations in carbonic anhydrase I, mucin MUC2, and trefoil factor 3 expression. Int. J. Colorect. Dis. 17317-326. [DOI] [PubMed] [Google Scholar]
- 42.Russell, D. H., J. C. Barreto, K. Klemm, and T. A. Miller. 1995. Hemorrhagic shock increases gut macromolecular permeability in the rat. Shock 450-55. [DOI] [PubMed] [Google Scholar]
- 43.Santos, R. L., R. M. Tsolis, A. J. Baumler, and L. G. Adams. 2003. Pathogenesis of Salmonella-induced enteritis. Braz. J. Med. Biol. Res. 363-12. [DOI] [PubMed] [Google Scholar]
- 44.Schweinfest, C. W., D. D. Spyropoulos, K. W. Henderson, J. H. Kim, J. M. Chapman, S. Barone, R. T. Worrell, Z. Wang, and M. Soleimani. 2006. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 28137962-37971. [DOI] [PubMed] [Google Scholar]
- 45.Shimamura, T., S. Tazume, K. Hashimoto, and S. Sasaki. 1981. Experimental cholera in germfree suckling mice. Infect. Immun. 34296-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson, N., R. Shaw, V. F. Crepin, R. Mundy, A. J. FitzGerald, N. Cummings, A. Straatman-Iwanowska, I. Connerton, S. Knutton, and G. Frankel. 2006. The enteropathogenic Escherichia coli type III secretion system effector Map binds EBP50/NHERF1: implication for cell signalling and diarrhoea. Mol. Microbiol. 60349-363. [DOI] [PubMed] [Google Scholar]
- 47.Singer, M., and P. J. Sansonetti. 2004. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J. Immunol. 1734197-4206. [DOI] [PubMed] [Google Scholar]
- 48.Sterling, D., B. V. Alvarez, and J. R. Casey. 2002. The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl−/HCO3− exchanger binds carbonic anhydrase IV. J. Biol. Chem. 27725239-25246. [DOI] [PubMed] [Google Scholar]
- 49.Sun, X. C., J. Li, M. Cui, and J. A. Bonanno. 2008. Role of carbonic anhydrase IV in corneal endothelial HCO3− transport. Investig. Ophthalmol. Vis. Sci. 491048-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taketo, M., A. C. Schroeder, L. E. Mobraaten, K. B. Gunning, G. Hanten, R. R. Fox, T. H. Roderick, C. L. Stewart, F. Lilly, C. T. Hansen, and P. A. Overbeek. 1991. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc. Natl. Acad. Sci. USA 882065-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang, J. C., F. C. Ho, A. C. Chan, and G. Srivastava. 1998. Clonality of lymphomas at multiple sites in SJL mice. Lab. Investig. 78205-212. [PubMed] [Google Scholar]
- 52.Vallance, B. A., W. Deng, K. Jacobson, and B. B. Finlay. 2003. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect. Immun. 713443-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, H., W. Jiang, E. E. Furth, X. Wen, J. P. Katz, R. K. Sellon, D. G. Silberg, T. M. Antalis, C. W. Schweinfest, and G. D. Wu. 1998. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am. J. Physiol. 275G1445-G1453. [DOI] [PubMed] [Google Scholar]
- 54.Yang, R., D. J. Gallo, J. J. Baust, S. K. Watkins, R. L. Delude, and M. P. Fink. 2002. Effect of hemorrhagic shock on gut barrier function and expression of stress-related genes in normal and gnotobiotic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283R1263-R1274. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimoto, T., A. Bendelac, J. Hu-Li, and W. E. Paul. 1995. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc. Natl. Acad. Sci. USA 9211931-11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, S., R. A. Kingsley, R. L. Santos, H. Andrews-Polymenis, M. Raffatellu, J. Figueiredo, J. Nunes, R. M. Tsolis, L. G. Adams, and A. J. Baumler. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 711-12. [DOI] [PMC free article] [PubMed] [Google Scholar]