Abstract
Streptococcus agalactiae is a major pathogen in humans and animals. Virulence factors are often associated with mobile genetic elements, and their expression can be modulated by host factors. S. agalactiae harbors the genes for C5a peptidase (scpB) and Lmb on a composite transposon structure which is absent in many bovine isolates. To investigate whether these genes participate in the adaptation to human hosts, we determined the influence of human and bovine serum on the promoter activity of scpB and lmb by using fluorescence-activated cell sorter analysis. Culture in the presence of 1 to 50% human serum resulted in a dose-dependent induction of reporter gene activity for scpB but not lmb. Reporter gene activity was, however, unchanged following growth in fetal calf serum. Interestingly, a bovine strain did not display any induction of scpB by either bovine or human serum. Reverse transcription-PCR analysis was used to confirm differential induction of scpB in S. agalactiae and showed a similar induction of the Streptococcus pyogenes C5a peptidase gene scpA by human but not bovine serum. The specific induction of the streptococcal C5a peptidase by human serum corresponds to the absence of scpB in many bovine S. agalactiae isolates and underlines the importance of this virulence factor for human infections.
Streptococcus agalactiae (group B streptococci [GBS]) was initially identified as the causative agent of bovine mastitis. It later emerged as the major pathogen of bacterial sepsis and meningitis in neonates and has recently been recognized as an increasingly common cause of invasive disease in immunocompromised patients (16). Like other bacterial pathogens, e.g., Streptococcus pneumoniae (27), Enterococcus faecalis (33), and Streptococcus mutans (38), it has the ability to survive and multiply in various anatomical niches and body fluids of the host, including serum, which requires the expression and coordinate regulation of multiple pathogenicity factors.
Genes encoding virulence factors are often associated with mobile genetic elements and controlled by complex regulatory networks (13). The C5a peptidase of S. agalactiae is a surface-associated serine protease that plays an important role in virulence of S. agalactiae (6, 11, 12). The structure of this protein has recently been solved (9). It cleaves the chemotactic complement component C5a (4, 20, 39), binds to fibronectin, and contributes to cellular invasion (1, 11). Following the identification of the gene in Streptococcus pyogenes (44, 45), a nearly identical gene was found in S. agalactiae (12). Together with the detection of flanking mobile genetic elements, the finding suggests horizontal gene transfer of the C5a peptidase gene among pyogenic streptococci (8, 17). Interestingly, all human but only some bovine S. agalactiae isolates possess the scpB gene (14, 17). Genetic polymorphisms alter the functional activity of C5a peptidase (5) but do not affect its ability to bind fibronectin (40). Overall, its conserved nucleotide sequence in several β-hemolytic streptococcal species, its ubiquitous expression, and its surface localization make it a good vaccine candidate for S. agalactiae, as well as S. pyogenes, infections (10, 21, 29, 32).
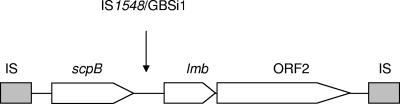
Immediately downstream of scpB lies lmb, the gene encoding a surface-associated lipoprotein of S. agalactiae that mediates binding to human laminin (37) and is involved in the invasion of brain endothelial cells (41). Similar to the findings for scpB, the nucleotide sequences of lmb of S. pyogenes and S. agalactiae are >98% identical (15, 37, 42). Both genes are part of a composite transposon structure which is flanked by insertion sequence (IS) elements (Fig. 1) and appears to build a functional unit that is lost or acquired en bloc (8, 17).
FIG. 1.
Genetic structure of the chromosomal region encoding the surface proteins ScpB and Lmb in S. agalactiae strain O90R (17). The three genes are located within a putative composite transposon flanked by IS elements. The IS element IS1548 or the intron GBSi1 is inserted between scpB and lmb in other S. agalactiae lineages.
Based on the observation that the entire transposon structure is found in the vast majority of human S. agalactiae strains but in only some bovine isolates, we were interested in the question of whether scpB and lmb specifically contribute to human pathogenicity. For this purpose, we determined if the genes are part of the bacterial system of adaptation to various host compartments by measuring transcription activity in the presence of human or bovine serum.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains and plasmids used in this study are listed in Table 1. The S. agalactiae strain O90R (ATCC 12386) was used for amplifying the promoter sequences of scpB and lmb, for constructing the mutant strains O90RscpBProm and O90RlmbProm, for preparation of RNA for LightCycler experiments, and together with strain O90RΔscpBΔlmb, for growth monitoring. The S. agalactiae strains O90RscpBProm and O90RlmbProm were used for fluorescence-activated cell sorter (FACS) analysis. In addition to the human wild-type strain O90R, the bovine S. agalactiae strain BSU77, the rgfC mutant strain O90RΔrgfC, and the previously described S. agalactiae clinical isolates AC475 (35), BSU147, and BSU191 (7) served as hosts for the scpB reporter gene plasmid. The Streptococcus pyogenes strain BSU316 is a clinical isolate that was used to determine mRNA levels of scpA by reverse transcription-PCR (RT-PCR) analysis. Escherichia coli strain DH5α served as a host for recombinant pBSU409 plasmids. Streptococcal strains were cultured in Todd-Hewitt broth (THB) (Oxoid) supplemented with 0.5% yeast extract (THY) or on THY agar at 37°C. Mutant strains harboring pBSU409 plasmids were cultured in medium containing spectinomycin (120 μg/ml) at a temperature of 37°C. E. coli strain DH5α was cultured in LB (Luria-Bertani) medium or on LB agar at 37°C. Mutant E. coli strains harboring pBSU409 plasmids were cultured in medium containing spectinomycin (100 μg/ml) at a temperature of 37°C.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| E. coli DH5α | endA1 hsdR17 supE44 ΔlacU169 (φ80lacZΔM15) recA1 gyrA96 thi-1 relA1 | Boehringer Ingelheim |
| S. agalactiae strains | ||
| O90R (ATCC 12386) | R. Lancefield grouping strain, human isolate | American Type Culture Collection |
| AC475 | Clinical isolate | 35 |
| BSU147 | Clinical isolate | 7 |
| BSU191 | Clinical isolate | 7 |
| BSU77 | Bovine isolate (Gi11d) | Gießen collection (C. Lämmler) |
| O90RΔscpBΔlmb | O90R derivative, scpB-lmb deletion mutant | 17 |
| O90RΔrgfC | O90R derivative, rgfC deletion mutant | This study |
| O90RpBSU409 | O90R derivative harboring pBSU409 without insert | This study |
| O90RscpBProm | O90R derivative harboring pBSU409scpBProm | This study |
| O90RlmbProm | O90R derivative harboring pBSU409lmbProm | This study |
| S. pyogenes BSU316 | Clinical isolate | Ulm collection |
| Plasmids | ||
| pAT28 | Sprori pUC ori pAmβ1 | 43 |
| pEGFP | Amr, source of egfp | Clontech Laboratories |
| pBSU409 | pAT28 derivative, novel MCS, carrying a promotorless egfp gene | This study |
| pBSU409scpBProm | pBSU409 derivative carrying the promoter region of scpB | This study |
| pBSU409lmbProm | pBSU409 derivative carrying the promoter region of lmb | This study |
Sp, spectinomycin; Am, ampicillin.
General DNA techniques.
Standard recombinant DNA techniques were employed for nucleic acid preparation and analysis. Genomic streptococcal DNA was isolated as described previously (30). Plasmid DNA was isolated and purified by using a QIAprep spin miniprep kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. PCR was carried out with Taq polymerase according to the manufacturer's protocol (Roche Diagnostics, Mannheim, Germany) with 35 cycles of amplification steps of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C. PCR products were sequenced on an ABI Prism 310 genetic analyzer using an ABI Prism BigDye Terminator cycle sequencing kit, version 1.1 (Applied Biosystems, Weiterstadt, Germany). S. agalactiae strain O90R was transformed according to an established protocol (31).
Construction of pBSU409.
To investigate the promoter activity of scpB and lmb, a novel enhanced green fluorescent protein (EGFP) reporter plasmid for streptococci was constructed. Vector pAT28 (43) and cloning vector pEGFP (Clontech Laboratories, Mountain View, CA) were used for the construction of pBSU409. Both plasmids were digested with the restriction enzymes BamHI and XbaI. The DNA fragment carrying the egfp sequence was isolated from DNA agarose gel by using a QIAquick gel extraction kit (Qiagen, Hilden, Germany). After insertion of egfp into pAT28, the multiple cloning site (MCS) was modified by using a DNA fragment consisting of the oligonucleotides pAT28-EGFP5 and pAT28-EGFP6 (Table 2). Annealing of both oligonucleotides generates an artificial DNA fragment with ends compatible with the restriction enzymes BamHI and SacI. Following the digestion of the first pAT28-EGFP construct with the two enzymes, the novel artificial MCS fragment was inserted into the vector. Correct construction of the novel MCS site was determined by PCR using primers flanking the MCS site (pAT28-3 and pAT28-EGFP4) (Table 2) and sequencing of the resulting PCR product.
TABLE 2.
Oligonucleotide primers and LightCycler hybridization probes
| Primer | Descriptiona | Sequenceb |
|---|---|---|
| pAT28-3 | Control of recombination | 5′ GTTGTGTGGAATTGTGAGCGG 3′ |
| pAT28-EGFP4 | Control of recombination | 5′ CCTTGAAGAAGATGGTGCGC 3′ |
| pAT28-EGFP5 | Construction of the novel MCS | 5′ CAGGCCTACTAGTCGTACG 3′ |
| pAT28-EGFP6 | Construction of the novel MCS | 5′ GATCCGTACGACTAGTAGGCCTGAGCT 3′ |
| scpBProm1 | Construction of O90RscpBProm | 5′ GCGCCGGAATTCGGAGGTCACAAAATAAGCAACTC 3′ |
| scpBProm2 | Construction of O90RscpBProm | 5′ GCGCGGATCCGTGTCGTCCTTTCATGATG 3′ |
| lmbProm1 | Construction of O90RlmbProm | 5′ GCGCCGGAATTCGTATTACTTAAACTGATAAGTAGC 3′ |
| lmbProm2 | Construction of O90RlmbProm | 5′ GCGCGGATCCGTACCTCCTCAATTATAATTTAACC 3′ |
| rgfCdel1 | Construction of rgfC deletion | 5′ TGGCACAAGCTTGAAGCATTTAGCAGAAGG 3′ |
| rgfCdel2 | Construction of rgfC deletion | 5′ CCCATCCACTAAACTTAAACAAAGCTATCTCTAAGATGG 3′ |
| rgfCdel3 | Construction of rgfC deletion | 5′ TGTTTAAGTTTAGTGGATGGGCTATTGCAACAAGTAGCC3′ |
| rgfCdel4 | Construction of rgfC deletion | 5′ CCGCGGATCCTAGAAACCATTGGACGCG 3′ |
| scpBLC1for | RT-PCR experiments, scpB | 5′ ACCAGCCATATCTTCAACAACA 3′ |
| scpBLC1rev | RT-PCR experiments, scpB | 5′ AACATCGGCAACATTCTCAAC 3′ |
| lmbLC1for | RT-PCR experiments, lmb | 5′ TTCGCAATAGCATGAGCAA 3′ |
| lmbLC1rev | RT-PCR experiments, lmb | 5′ AGAAGCAGAGCAACTAACTGAAGA 3′ |
| gyrA-F | LightCycler primer, reference gene, gyrA in GBS | 5′ CCTGGTCCAGATTTTCCTAC 3′ |
| gyrA-R | LightCycler primer, reference gene, gyrA in GBS | 5′ TGCGCTTTTCTTGTGCTA 3′ |
| gyrA-FL | LightCycler hybridization probe, reference gene, gyrA in GBS | CCAGTTTCATAGGCACGGTGAA-FL |
| gyrA-LC | LightCycler hybridization probe, reference gene, gyrA in GBS | R640-ACCTGAACGTCCCATCACCAAG-PH |
| gyrA-R2 | LightCycler primer, reference gene, gyrA in GAS | 5′ CACTCCTTCACGGCTAGATT 3′ |
| gyrA S | LightCycler primer, reference gene, gyrA in GAS | 5′ CTGGACCTGACTTTCCG 3′ |
| gyrA-FL2 | LightCycler hybridization probe, reference gene, gyrA in GAS | ATTGACGCCATAGGGAAATTCAGTAACC-FL |
| gyrA-LC2 | LightCycler hybridization probe, reference gene, gyrA in GAS | R640-CAATACGTTCCCGACCTGTTTGAGTTG-PH |
| scpB-se | LightCycler primer, scpB | 5′ CAGCTGAGGTCACAATGCTAAC 3′ |
| scpB-as | LightCycler primer, scpB | 5′ GCTCAAGCTATCAGAGATGCTGTC 3′ |
| scpB-FL | LightCycler hybridization probe, scpB | GGTTGGCGTAAGCTAGTGCAGCA-FL |
| scpB-LC | LightCycler hybridization probe, scpB | R640-TACCAAAGCTCATATTAATCACCTTAGCTCCC-PH |
| lmb-fw | LightCycler primer, lmb | 5′ GCCTTGTGTGACTTCCATATCTT 3′ |
| lmb-as | LightCycler primer, lmb | 5′ TGATGTGAGGATGATCCAATCAG 3′ |
| lmb-FL | LightCycler hybridization probe, lmb | CGCGTCATAAATAGCTGCCACATCATTTACA-FL |
| lmb-LC | LightCycler hybridization probe, lmb | R640-ACGGTTCAAAGGAATGAATGCCTGCA-PH |
GAS, group A streptococci.
FL, fluorescein; R640, LightCycler Red640 N-succinimide ester; PH, 3′ phosphate.
Generation of scpB and lmb reporter gene constructs.
The promoter regions of scpB and lmb were amplified with the primers scpBProm1 and scpBProm2 (Table 2) and lmbProm1 and lmbProm2 (Table 2), respectively. The resulting PCR products and the vector pBSU409 were digested with the enzymes BamHI and EcoRI, ligated, and transformed into E. coli cells. Correct construction of the plasmids was controlled through PCR with primers flanking the insertion site (pAT28-3 and pAT28-EGFP4) (Table 2) and sequencing of the PCR products. Recombinant plasmids harboring the scpB or lmb promoter sequences upstream of the egfp gene were transformed into the S. agalactiae wild-type strain O90R.
Construction of the rgfC deletion mutant.
For construction of the rgfC deletion mutant, the plasmid pGhost5 (2) was used. DNA regions upstream and downstream of the intended deletion site were amplified by PCR with the primer combination rgfCdel1 and rgfCdel2 and the primer combination rgfCdel3 and rgfCdel4 (Table 2). The resulting PCR products were cloned into pGhost5, transferred into the chromosome of strain O90R, and subsequently mobilized as described in reference 18. The resulting mutant strain, O90RΔrgfC, harbors a deletion of nucleotides 32 to 1276 of the 1,335 nucleotides of rgfC. Correct deletion of the targeted rgfC region was confirmed by DNA sequencing.
FACS analysis.
The recombinant S. agalactiae strains carrying the scpB reporter gene construct were incubated in 5 ml of THY medium containing spectinomycin (120 μg/ml) and in 5 ml of spectinomycin-containing THY medium supplemented with increasing amounts (1 to 50%) of serum (human serum, heat-inactivated human serum, fetal calf serum, and heat-inactivated fetal calf serum). Human serum was obtained from PAA Laboratories GmbH in Pasching, Austria, and fetal calf serum from Biochrom, AG, in Berlin, Germany. Blood was subjected to a natural clotting process and centrifuged to remove cells and fibrin. According to company information, serum was collected from healthy donors and represents pools from more than 50 individuals with endotoxin levels of ≤20 endotoxin units/ml. In addition, two different pools of human serum collected and pooled at our institute from 10 donors for each pool were used to study the induction of scpB transcription. In these pools, blood was subjected to a natural clotting process and centrifuged to remove cells and fibrin. The cultures were grown to stationary phase overnight, pelleted, washed, and resuspended in phosphate-buffered saline (PBS). Aliquots were analyzed by FACS (FACSCalibur; Becton Dickinson Immunocytometry Systems, San Jose, CA) until 10,000 fluorescent events were counted. Side and forward scatter data were collected using linear amplifiers (forward scatter, EOO, and side scatter, 400), and fluorescence data were collected using logarithmic amplifiers (fluorescence channel 1, 700). For assays testing the influence of fibronectin and C5a, the S. agalactiae strain O90RscpBProm was incubated in spectinomycin containing THY medium supplemented with 140 μg/ml of fibronectin (Sigma-Aldrich GmbH, Steinheim, Germany) or 10 to 200 ng/ml of C5a (Sigma-Aldrich). The cultures were grown to stationary phase overnight, pelleted, washed, and resuspended in PBS. Aliquots were analyzed by FACS as described above.
RNA preparation and analysis.
Total RNA was prepared from S. agalactiae strain O90R and S. pyogenes strain BSU316 grown to an optical density at 600 nm (OD600) of 1.0 in 100 ml of THY medium and in 100 ml of THY medium supplemented with 10% serum (human serum, heat-inactivated human serum, fetal calf serum, or heat-inactivated fetal calf serum). Cells were lysed mechanically with glass beads in a cell disrupter (Bio 101, Vista, CA). Purification of RNA was achieved with an RNeasy mini kit (Qiagen, Hilden, Germany) as instructed by the manufacturer. For RT-PCR experiments, 4 ng of DNase-treated RNA was used as a template (OneStep RT-PCR kit; Qiagen, Hilden, Germany). The RT reaction was carried out at 55°C for 30 min. After PCR activation at 95°C for 15 min, PCR was carried out with 28 cycles of amplification steps of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C. The RT-PCR kit was used according to the manufacturer's instructions; RT-PCR was performed with 30 pmol of each primer. To control for DNA contamination of RNA samples, 4 ng of DNase-treated RNA was used as a template in a PCR without the RT step under the above-described conditions. PCRs were carried out with the same primers used for the LightCycler experiments (Table 2). RNase-free water served as a negative and DNA as a positive control for the RT-PCR.
LightCycler experiments.
To quantify RT-PCR results, LightCycler experiments were performed. Real-time RT-PCRs were carried out with RNA as a template and hybridization probes for detection. The LightCycler RNA amplification kit hybridization probes (Roche Diagnostics, Mannheim, Germany) were used as instructed by the manufacturer. Primers and hybridization probes (Table 2) were present in a final concentration of 0.5 and 0.2 μM, respectively. Magnesium chloride was added to a final concentration of 7 mM. Amplification was carried out using a LightCycler program with an elongation time of 10 s and an annealing temperature of 61°C for scpB and 59°C for lmb. Standard curves were generated by using 40, 8, 4, 2, and 1 ng of RNA as the template in the RT-PCR. Two samples (4 ng) were prepared, and the mean of both values was used in subsequent calculations. The efficiency (E) of the reaction was determined with the equation E = 10−1/slope. Subsequently, the mRNA ratio was calculated using the following formula: E(target)Δcp(target)/E(ref)Δcp(ref). E(target) represents the efficiency of the gene of interest (scpB or lmb), whereas E(ref) represents the efficiency of the reference gene. Δcp(target) refers to the difference between two measurements of the crossing points of the gene of interest, whereas Δcp(ref) stands for the difference between the crossing points of the reference gene for two RNA samples. The gyrase A gene gyrA was chosen as the reference gene, due to its stable expression under different environmental conditions and growth phases. The quantification results are expressed as the number of mRNA copies of the gene of interest per mRNA copy of gyrA.
Growth curves.
To determine if the C5a peptidase gene is required for survival of S. agalactiae in human serum, S. agalactiae strain O90R and strain O90RΔscpBΔlmb, in which the entire composite transposon structure is deleted, were used. Both strains were grown at 37°C in THY or THY medium supplemented with 10% and 50% of human serum and 10% and 50% of fetal calf serum. Cultures were monitored for 8 h by measuring the OD600 every hour.
RESULTS AND DISCUSSION
Construction of pBSU409.
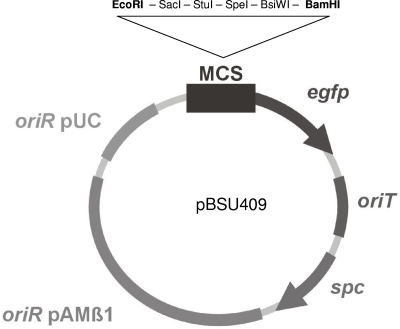
A novel EGFP reporter gene construct for use in streptococci was constructed to test the influence of serum on scpB and lmb. The promotorless egfp gene was cloned downstream of the MCS of pAT28 (43). To eliminate complementary sequences within the vector that caused difficulties in initial cloning experiments, the MCS of the construct was modified. The MCS of pAT28 (EcoRI, SacI, KpnI, SmaI, and BamHI) was replaced by a novel MCS site consisting of the following restriction enzyme sites: EcoRI, SacI, StuI, SpeI, BsiWI, and BamHI (Fig. 2). The novel vector pBSU409 was used as a reporter plasmid for subsequent FACS analysis.
FIG. 2.
Structure of the novel EGFP reporter plasmid pBSU409. Arrows indicate the direction of transcription. oriR, origin of replication; oriT, origin of transfer; spc, spectinomycin resistance gene.
Promoter activity of scpB and lmb in the presence of serum.
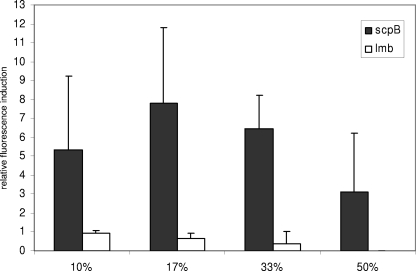
Experiments to study promoter activity of scpB and lmb in the presence of serum were carried out with the reporter plasmid pBSU409. The promoter sequences of scpB and lmb were inserted upstream of the promotorless copy of the egfp gene (Fig. 2). Recombinant plasmids were transformed into the S. agalactiae wild-type strain O90R, resulting in the two strains O90RscpBProm and O90RlmbProm. To determine the influence of human serum on the expression of scpB and lmb, strains O90RscpBProm and O90RlmbProm were cultured in THY medium supplemented with increasing amounts of human serum. Cultures were grown to stationary phase overnight and analyzed by FACS. A clear induction by human serum could be observed for scpB (Fig. 3), whereas no influence on the expression of lmb was detected (Fig. 3).
FIG. 3.
Induction of scpB and lmb reporter gene activity in S. agalactiae strains O90RscpBProm and O90RlmbProm following culture in THY medium supplemented with various concentrations of pooled human serum. Shown are mean values above baseline (controls were set to 1) and standard deviations from three independent experiments. The amount of serum supplementation is indicated. EGFP fluorescence was measured by FACS analysis.
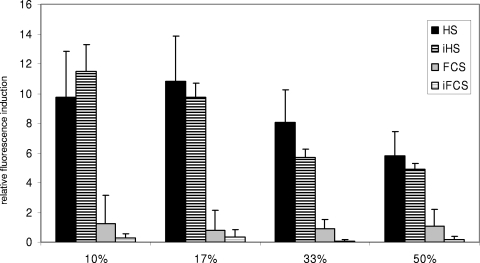
To investigate if the results were specific for human serum, the experiments were repeated using fetal calf serum as a stimulus. Compared with the reporter gene activity observed following the exposure to human serum, fetal calf serum did not show any influence on the expression of scpB or lmb. The values were indistinguishable from those for controls. Experiments using heat-inactivated human serum were performed with the scpB reporter construct in strain O90R and the rgfC deletion mutant, to further analyze the inducing factor in human serum. Heat inactivation of the serum samples did not destroy its ability to induce the expression of scpB (Fig. 4 and 5). However, in some preparations using the wild-type strain O90R, a reduced activity was noted (Fig. 5).
FIG. 4.
Induction of scpB reporter gene activity in strain O90RΔrgfC, which harbors a deletion of the rgfC histidine kinase, following culture in THY medium supplemented with different serum stimuli. The amount of serum supplementation is indicated. Shown are mean values above baseline (controls were set to 1) and standard deviations from three independent experiments. HS, pooled human serum; iHS, heat-inactivated pooled human serum; FCS, fetal calf serum; iFCS, heat-inactivated fetal calf serum.
FIG. 5.
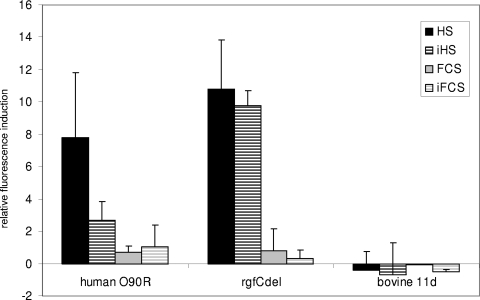
Induction of scpB reporter gene activity in the human S. agalactiae strains O90R and O90RΔrgfC (rgfCdel) and the bovine strain Gi11d following culture in THY medium supplemented with different serum stimuli at a concentration of 17%. Shown are mean values above baseline (controls were set to 1) and standard deviations from three independent experiments. HS, pooled human serum; iHS, heat-inactivated pooled human serum; FCS, fetal calf serum; iFCS, heat-inactivated fetal calf serum.
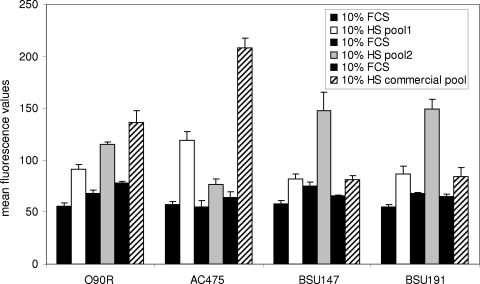
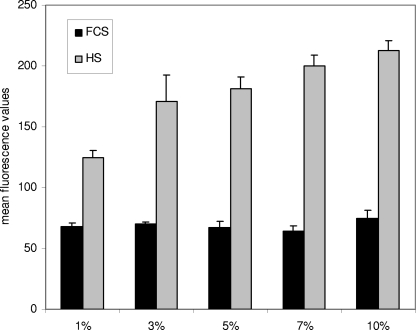
To see if the results obtained for strain O90R using a commercial preparation of human serum could also be observed in clinical strains of S. agalactiae with different serum preparations, we transformed the scpB reporter gene construct into the clinical strains AC475, BSU147, and BSU191. All of these strains were then grown in the presence of 10% of the commercial serum preparation and two independent pools of human serum collected at our institute. Growth in the presence of 10% fetal calf serum served as a control. Induction of scpB transcription was present in all of these strains (Fig. 6); while some variation in the amount of induction could be observed, depending on the strain and the source of serum used, it was clearly shown that induction is present in different human S. agalactiae strains stimulated with different sources of human serum. Whether the observed induction of scpB is dose dependent was investigated by exposing strain AC475 harboring the scpB reporter gene construct to human serum from our commercial supplier in concentrations ranging from 1 to 10%. Stimulation with fetal calf serum served as a control in this case. In contrast to the results with serum concentrations above 10%, a clear dose-dependent induction could be observed (Fig. 7), starting with concentrations of serum supplementation as low as 1%.
FIG. 6.
Induction of C5a peptidase transcription in different S. agalactiae strains using various pools and sources of human serum. All strains shown were transformed by plasmid pBSU409 harboring the scpB reporter gene construct (pBSU409scpBProm). Strains were grown at 37°C overnight in THY broth supplemented with 10% human serum as indicated or fetal calf serum as a control. Mean fluorescence values were measured by FACS analysis. Shown are means and standard deviations from five independent experiments. HS, human serum; FCS, fetal calf serum.
FIG. 7.
Dose-dependent induction of C5a peptidase transcription in S. agalactiae strain AC475 harboring plasmid pBSU409scpBProm. Bacteria were cultivated overnight at 37°C in THY broth supplemented with human serum in concentrations as indicated. Fetal calf serum served as a control. Shown are mean fluorescence values and standard deviations from five independent experiments as measured by FACS analysis. HS, human serum; FCS, fetal calf serum.
The gene scpB is generally present in human strains but often absent in bovine strains. To determine whether induction of scpB by human serum can be seen in bovine strains carrying the gene scpB, the scpB promoter construct was transferred into the bovine S. agalactiae strain 11d. Measurement of EGFP fluorescence after exposure of this construct to human serum, inactivated human serum, fetal calf serum, and inactivated fetal calf serum in various concentrations did not reveal any induction of the gene in the bovine background (Fig. 5).
Our studies were carried out to further investigate the role of the composite transposon structure encoding lmb and the C5a peptidase gene in S. agalactiae in human infections. S. agalactiae strains lacking the composite transposon structure are almost exclusively of bovine origin (14, 17), indicating a specific importance of the genes carried on this element for human infections. Lmb is a surface lipoprotein mediating attachment to immobilized human laminin (37), while the C5a peptidase of S. agalactiae is anchored to the cell surface via the LPXTG motif (25). The C5a peptidase is a well-characterized virulence factor of pyogenic streptococci, with crucial roles in the inhibition of host defenses, the binding of fibronectin, and the invasion of streptococci into eukaryotic cells (4, 5, 6, 11, 12, 20, 39, 40; for a comprehensive review of the topic, see reference 26). Its role as a virulence factor in S. pyogenes has been examined by showing that scpA-negative mutants are cleared more rapidly from infected tissue and from the nasopharynx in S. pyogenes mouse models (21, 23, 22). But its role as a virulence factor has been challenged recently (19), which may be connected to an ongoing controversy regarding the species specificity of C5a peptidase cleavage. For the C5a peptidase of S. agalactiae, it has been shown that it cleaves human C5a but has no activity on the C5a of other species, including mice (3), while a broader range of activity on the C5a of different species has been demonstrated for the S. pyogenes C5a peptidase (22). Our finding of a species-specific induction of the scpB gene by human but not bovine serum seems to support the results of studies showing a very restricted species-specific activity of the C5a peptidase, but we looked at induction of the gene and not the enzymatic activity of the C5a peptidase. Species-specific induction of scpB does not, however, extend to the lmb gene present on the transposon structure. The expression of lmb was not affected by exposure to sera from different hosts, indicating that scpB and lmb are regulated by different mechanisms, a finding that is consistent with the presence of a putative promoter region directly upstream of lmb (37).
Induction of C5a peptidase expression in an S. agalactiae rgfC knockout mutant.
The two-component regulator system rgf has been shown to inhibit transcription of C5a peptidase in S. agalactiae (36). To investigate whether the induction of the gene by serum is regulated by this system, we transferred the plasmid construct into the rgfC mutant strain O90RΔrgfC and tested the resulting strain for scpB promoter activity in the presence of serum. The histidine kinase of the rgf regulator system is encoded by rgfC. As shown by the results in Fig. 4 and 5, the strain displayed an induction of scpB in FACS analysis following exposure to human serum that was greater than the induction observed in the wild-type strain. In accordance with the results for the wild-type strain, the induction of scpB by human serum was still present after heat inactivation of the serum sample and could be demonstrated for a wide range of different serum concentrations (Fig. 4). These results are consistent with the previously described increased transcription of scpB in rgf mutants (36), but since the inducing effect of human serum is clearly present in the regulator mutant, rgf is most likely not responsible for the regulation of increased scpB transcription following exposure to serum.
Promoter activity of scpB in the presence of fibronectin and C5a.
The C5a peptidase encoded by scpB specifically cleaves the human complement component C5a and mediates binding to fibronectin. To test if one or both of these molecules represent the inducing factor in human serum, FACS analysis with the EGFP reporter gene construct was carried out. Strain O90RscpBProm was incubated overnight in regular growth medium (THY) supplemented with 140 μg/ml fibronectin. The amount of fibronectin added is consistent with the physiological concentration of fibronectin in human serum (28). The reporter gene activity was compared to the activity of the construct after growth in medium without fibronectin supplementation. Strain O90RscpBProm cultured in THY medium supplemented with 33% human serum served as a positive control. The reporter gene activities of the strain grown with or without fibronectin supplementation were, however, indistinguishable (data not shown). In similar experiments, we tested whether C5a stimulates scpB. FACS analysis was carried out following supplementation of the culture medium with 10 to 200 ng of C5a per ml. As a positive control, O90RscpBProm was incubated in THY medium supplemented with 33% human serum. Based on the physiological concentrations of C5a in serum (34), this medium contains approximately 4 ng of C5a per ml. Cultures were grown to stationary phase overnight, and the whole experiment was done in triplicate. No induction of scpB by C5a supplementation was noted (data not shown), indicating that the scpB-inducing factor in human serum is neither fibronectin nor C5a. Together with the experiments using heat-inactivated serum, these experiments were performed to characterize the unknown factor in human serum that is responsible for the induction of scpB transcription. C5a and fibronectin both represent interaction partners of the C5a peptidase (1, 36, 20, 39). In particular, the results for C5a are consistent with the fact that the inducing factor is not destroyed by heat. The elucidation of the structure of C5a peptidase led to the hypothesis that a eukaryotic molecule binds to the RGD sites present in C5a as a conformational trigger that is required for efficient binding of S. agalactiae to eukaryotic cells (9). It is possible that the putative conformational trigger is present in human serum and represents the same molecule that induces the transcription of the C5a peptidase. In any case, the data from our experiments and the data from the C5a peptidase structure are consistent with the hypothesis that the C5a peptidase is a key molecule in the complex interactions of streptococci with their host. Further biochemical characterization of the signal that is recognized by S. agalactiae will be carried out in a separate investigation.
Determination of mRNA levels of scpB and lmb by real-time RT-PCR.
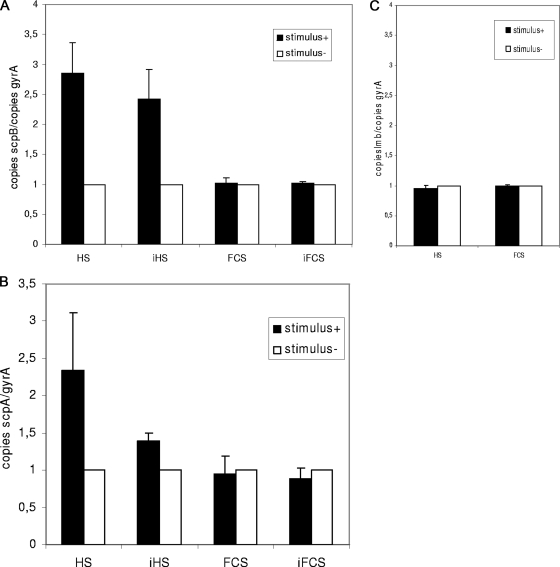
Quantitative real-time RT-PCR experiments were carried out in order to confirm the results of FACS analysis. Whereas FACS analysis was based on the expression of the reporter gene egfp in the recombinant S. agalactiae strains O90RscpBProm and O90RlmbProm, the LightCycler experiments were based on transcript levels to quantify the mRNA of streptococcal wild-type strain O90R. We investigated the influence of human serum, heat-inactivated human serum, fetal calf serum, and heat-inactivated fetal calf serum on the expression of scpB (Fig. 8A) and the influence of human serum and fetal calf serum on the expression of lmb (Fig. 8C). Streptococcal wild-type strain O90R was incubated in THY medium and in THY medium supplemented with 10% serum. The cultures were grown to an OD600 of 1.0. Total RNA was prepared, and RT-PCRs were carried out as detailed in Materials and Methods. Using the LightCycler, we quantified the amounts of scpB and lmb mRNA in samples without stimulus and samples that were grown in the presence of different serum stimuli. Each experiment was done in triplicate. Quantitative real-time RT-PCRs revealed an approximately threefold-greater amount of scpB mRNA in the presence of human serum and an approximately two- to threefold-greater induction of scpB following exposure to heat-inactivated human serum. With fetal calf serum and heat-inactivated fetal calf serum, no differences in the amount of scpB mRNA could be detected. Consistent with results from the FACS analysis, no influence of human serum and fetal calf serum on the amount of lmb mRNA was found. Taken together, we were able to verify the results of FACS analysis for scpB and lmb with quantitative real-time RT-PCRs.
FIG. 8.
Quantitative transcript analysis of the S. agalactiae virulence genes scpB (A) and lmb (C) and the S. pyogenes gene scpA (B) by LightCycler RT-PCR. ScpB, lmb, and scpA transcripts were quantified in relation to the number of gyrA transcripts in each sample. Transcription of scpB, lmb, and scpA was determined in samples without stimulus (−) and in samples that were grown in the presence of different serum stimuli (+). Sera were used as indicated. Values from experiments without stimulus were set to 1. Measurements from three separate RNA preparations were used to calculate the means ± standard deviations. HS, human serum; iHS, heat-inactivated human serum; FCS, fetal calf serum; iFCS, heat-inactivated fetal calf serum.
Transcription analysis of scpA in Streptococcus pyogenes.
The C5a peptidase gene is highly conserved among β-hemolytic streptococci from the pyogenic group, and the respective genes of S. agalactiae and the strictly human pathogen S. pyogenes are virtually identical (12). To investigate whether similar induction phenomena following exposure to serum could be observed in other streptococci, scpA transcription was measured in S. pyogenes by real time RT-PCR analysis. Similar to the results for S. agalactiae, human but not bovine serum caused an increase in transcription of the gene. Consistent with the results obtained for S. agalactiae, this activation was still present to some extent after heat inactivation of the pooled human sera that we tested in our experiments (Fig. 8B). In a previous study, proteome analysis of S. pyogenes following exposure to human plasma (24) revealed an increase in the expression of C5a peptidase, which is consistent with our data and the hypothesis that the induction of C5a peptidase expression by human serum represents a host-specific adaptation mechanism of human pyogenic streptococcal strains.
Growth of the wild-type strain and scpB deletion mutant in human serum.
Based on our results showing altered scpB expression in the presence of human serum, we were interested in seeing whether the growth of an scpB deletion mutant is impaired in THY medium containing human serum. Streptococcal wild-type strain O90R and strain O90RΔscpBΔlmb, with a deletion of the entire composite transposon structure (17), were used in this experiment. Both strains were cultured in THY medium supplemented with 10% and 50% human serum or fetal calf serum, and growth was monitored over a period of 8 h. No differences between growth kinetics of the two strains could be observed (data not shown). These data suggest that C5a peptidase activity is not necessary for the growth and survival of S. agalactiae in the presence of human serum.
In summary, we found a dose-dependent, host-specific induction of the C5a peptidase gene by human but not bovine serum in S. agalactiae and S. pyogenes bacteria. These results correspond to the absence of scpB in many bovine S. agalactiae isolates and underline the importance of the gene for human infections.
Acknowledgments
The work of U.G.-T. was supported by a grant from the IZKF-Ulm (IZKF-H1) and by a grant from the state of Baden-Württemberg, and the work of B.S. was supported in part by a grant from the DFG (Sp 511/5-1).
We thank Melanie Kuhn for expert technical assistance.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 8 June 2009.
REFERENCES
- 1.Beckmann, C., J. D. Waggoner, T. O. Harris, G. S. Tamura, and C. E. Rubens. 2002. Identification of novel adhesins from group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect. Immun. 702869-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas, I., A. Gruss, D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 1753628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohnsack, J. F., J. K. Chang, and H. R. Hill. 1993. Restricted ability of group B streptococcal C5a-ase to inactivate C5a prepared from different animal species. Infect. Immun. 611421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnsack, J. F., K. W. Mollison, A. M. Buko, J. C. Ashworth, and H. R. Hill. 1991. Group B streptococci inactivate complement component C5a by enzymic cleavage at the C-terminus. Biochem. J. 273635-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnsack, J. F., S. Takahashi, L. Hammitt, D. V. Miller, A. A. Aly, and E. E. Adderson. 2000. Genetic polymorphisms of group B streptococcus scpB alter functional activity of a cell-associated peptidase that inactivates C5a. Infect. Immun. 685018-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohnsack, J. F., K. Widjaja, S. Ghazizadeh, C. E. Rubens, D. R. Hillyard, C. J. Parker, K. H. Albertine, and H. R. Hill. 1997. A role for C5 and C5a-ase in the acute neutrophil response to group B streptococcal infections. J. Infect. Dis. 175847-855. [DOI] [PubMed] [Google Scholar]
- 7.Brimil, N., E. Barthell, U. Heindrichs, M. Kuhn, R. Lutticken, and B. Spellerberg. 2006. Epidemiology of Streptococcus agalactiae colonization in Germany. Int. J. Med. Microbiol. 29639-44. [DOI] [PubMed] [Google Scholar]
- 8.Broker, G., and B. Spellerberg. 2004. Surface proteins of Streptococcus agalactiae and horizontal gene transfer. Int. J. Med. Microbiol. 294169-175. [DOI] [PubMed] [Google Scholar]
- 9.Brown, C. K., Z. Y. Gu, Y. V. Matsuka, S. S. Purushothaman, L. A. Winter, P. P. Cleary, S. B. Olmsted, D. H. Ohlendorf, and C. A. Earhart. 2005. Structure of the streptococcal cell wall C5a peptidase. Proc. Natl. Acad. Sci. USA 10218391-18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, Q., S. Debol, H. Lam, R. Eby, L. Edwards, Y. Matsuka, S. B. Olmsted, and P. P. Cleary. 2002. Immunization with C5a peptidase or peptidase-type III polysaccharide conjugate vaccines enhances clearance of group B streptococci from lungs of infected mice. Infect. Immun. 706409-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, Q., D. Stafslien, S. S. Purushothaman, and P. Cleary. 2002. The group B streptococcal C5a peptidase is both a specific protease and an invasin. Infect. Immun. 702408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmouryguina, I., A. Suvorov, P. Ferrieri, and P. Cleary. 1996. Conservation of the C5a peptidase genes in group A and B streptococci. Infect. Immun. 642387-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter, P. A., and V. J. DiRita. 2000. Bacterial virulence gene regulation: an evolutionary perspective. Annu. Rev. Microbiol. 54519-565. [DOI] [PubMed] [Google Scholar]
- 14.Dmitriev, A., L. Tkacikova, A. Suvorov, M. Kantikova, I. Mikula, and A. Totolyan. 1999. Comparative genetic study of group B streptococcal strains of human and bovine origin. Folia Microbiol. (Praha) 44449-453. [DOI] [PubMed] [Google Scholar]
- 15.Elsner, A., B. Kreikemeyer, A. Braun-Kiewnick, B. Spellerberg, B. A. Buttaro, and A. Podbielski. 2002. Involvement of Lsp, a member of the LraI-lipoprotein family in Streptococcus pyogenes, in eukaryotic cell adhesion and internalization. Infect. Immun. 704859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farley, M. M. 2001. Group B streptococcal disease in nonpregnant adults. Clin. Infect. Dis. 33556-561. [DOI] [PubMed] [Google Scholar]
- 17.Franken, C., G. Haase, C. Brandt, J. Weber-Heynemann, S. Martin, C. Lammler, A. Podbielski, R. Lutticken, and B. Spellerberg. 2001. Horizontal gene transfer and host specificity of beta-haemolytic streptococci: the role of a putative composite transposon containing scpB and lmb. Mol. Microbiol. 41925-935. [DOI] [PubMed] [Google Scholar]
- 18.Gottschalk, B., G. Broker, M. Kuhn, S. Aymanns, U. Gleich-Theurer, and B. Spellerberg. 2006. Transport of multidrug resistance substrates by the Streptococcus agalactiae hemolysin transporter. J. Bacteriol. 1885984-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidalgo-Grass, C., I. Mishalian, M. Dan-Goor, I. Belotserkovsky, Y. Eran, V. Nizet, A. Peled, and E. Hanski. 2006. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. EMBO J. 254628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, H. R., J. F. Bohnsack, and E. Z. Morris. 1988. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J. Immunol. 1413551-3556. [PubMed] [Google Scholar]
- 21.Ji, Y., B. Carlson, A. Kondagunta, and P. P. Cleary. 1997. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A streptococcus. Infect. Immun. 652080-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji, Y., L. McLandsborough, A. Kondagunta, and P. P. Cleary. 1996. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 64503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji, Y., N. Schnitzler, E. DeMaster, and P. Cleary. 1998. Impact of M49, Mrp, Enn, and C5a peptidase proteins on colonization of the mouse oral mucosa by Streptococcus pyogenes. Infect. Immun. 665399-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson, B. P., F. Levander, U. von Pawel-Rammingen, T. Berggard, L. Bjorck, and P. James. 2005. The protein expression of Streptococcus pyogenes is significantly influenced by human plasma. J. Proteome Res. 42302-2311. [DOI] [PubMed] [Google Scholar]
- 25.Lalioui, L., E. Pellegrini, S. Dramsi, M. Baptista, N. Bourgeois, F. Doucet-Populaire, C. Rusniok, M. Zouine, P. Glaser, F. Kunst, C. Poyart, and P. Trieu-Cuot. 2005. The SrtA sortase of Streptococcus agalactiae is required for cell wall anchoring of proteins containing the LPXTG motif, for adhesion to epithelial cells, and for colonization of the mouse intestine. Infect. Immun. 733342-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindahl, G., M. Stalhammar-Carlemalm, and T. Areschoug. 2005. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin. Microbiol. Rev. 18102-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marra, A., J. Asundi, M. Bartilson, S. Lawson, F. Fang, J. Christine, C. Wiesner, D. Brigham, W. P. Schneider, and A. E. Hromockyj. 2002. Differential fluorescence induction analysis of Streptococcus pneumoniae identifies genes involved in pathogenesis. Infect. Immun. 701422-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosher, D. F. 1984. Physiology of fibronectin. Annu. Rev. Med. 35561-575. [DOI] [PubMed] [Google Scholar]
- 29.Park, H.-S., and P. P. Cleary. 2005. Active and passive intranasal immunizations with streptococcal surface protein C5a peptidase prevent infection of murine nasal mucosa-associated lymphoid tissue, a functional homologue of human tonsils. Infect. Immun. 737878-7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pospiech, A., and B. Neumann. 1995. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 11217-218. [DOI] [PubMed] [Google Scholar]
- 31.Ricci, M. L., R. Manganelli, C. Berneri, G. Orefici, and G. Pozzi. 1994. Electrotransformation of Streptococcus agalactiae with plasmid DNA. FEMS Microbiol. Lett. 11947-52. [DOI] [PubMed] [Google Scholar]
- 32.Santillan, D. A., M. E. Andracki, and S. K. Hunter. 2008. Protective immunization in mice against group B streptococci using encapsulated C5a peptidase. Am. J. Obstet. Gynecol. 198114.e1-e6. [DOI] [PubMed] [Google Scholar]
- 33.Shepard, B. D., and M. S. Gilmore. 2002. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect. Immun. 704344-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soto, E., K. Richani, R. Romero, J. Espinoza, T. Chaiworapongsa, J. K. Nien, S. Edwin, Y. M. Kim, J. S. Hong, L. Goncalves, and M. Mazor. 2005. Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J. Matern. Fetal Neonatal Med. 17247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spellerberg, B., B. Pohl, G. Haase, S. Martin, J. Weber-Heynemann, and R. Lütticken. 1999. Identification of genetic determinants for the hemolytic activity of Streptococcus agalactiae by ISS1 transposition. J. Bacteriol. 1813212-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, and R. Lutticken. 2002. rgf encodes a novel two-component signal transduction system of Streptococcus agalactiae. Infect. Immun. 702434-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lütticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svensater, G., B. Sjogreen, and I. R. Hamilton. 2000. Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology 146107-117. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi, S., Y. Nagano, N. Nagano, O. Hayashi, F. Taguchi, and Y. Okuwaki. 1995. Role of C5a-ase in group B streptococcal resistance to opsonophagocytic killing. Infect. Immun. 634764-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamura, G. S., J. R. Hull, M. D. Oberg, and D. G. Castner. 2006. High-affinity interaction between fibronectin and the group B streptococcal C5a peptidase is unaffected by a naturally occurring four-amino-acid deletion that eliminates peptidase activity. Infect. Immun. 745739-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenenbaum, T., B. Spellerberg, R. Adam, M. Vogel, K. S. Kim, and H. Schroten. 2007. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 9714-720. [DOI] [PubMed] [Google Scholar]
- 42.Terao, Y., S. Kawabata, E. Kunitomo, I. Nakagawa, and S. Hamada. 2002. Novel laminin-binding protein of Streptococcus pyogenes, Lbp, is involved in adhesion to epithelial cells. Infect. Immun. 70993-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1990. A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and in Gram-positive bacteria. Nucleic Acids Res. 184296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wexler, D. E., D. E. Chenoweth, and P. P. Cleary. 1985. Mechanism of action of the group A streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 828144-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wexler, D. E., R. D. Nelson, and P. P. Cleary. 1983. Human neutrophil chemotactic response to group A streptococci: bacteria-mediated interference with complement-derived chemotactic factors. Infect. Immun. 39239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]